Abstract
The purpose of this study was to examine and compare the melanoma targeting and imaging properties of 64Cu-NOTA-GGNle-CycMSHhex {64Cu-1,4,7-triazacyclononane-1,4,7-triacetic acid-Gly-Gly-Nle-c[Asp-His-DPhe-Arg-Trp-Lys]-CONH2} and 64Cu-DOTA-GGNle-CycMSHhex {64Cu-1,4,7,10-tetraazacyclononane-1,4,7,10-tetraacetic acid-GGNle-CycMSHhex}. Two lactam bridge-cyclized peptides, NOTA-GGNle-CycMSHhex and DOTA-GGNle-CycMSHhex, were synthesized using fluorenylmethyloxy carbonyl (Fmoc) chemistry. The melanocortin-1 (MC1) receptor binding affinity of NOTA-GGNle-CycMSHhex was determined in B16/F1 melanoma cells and compared with DOTA-GGNle-CycMSHhex. The melanoma targeting and imaging properties of 64Cu-NOTA-GGNle-CycMSHhex and 64Cu-DOTA-GGNle-CycMSHhex were determined in B16/F1 melanoma-bearing C57 mice. NOTA-GGNle-CycMSHhex and DOTA-GGNle-CycMSHhex displayed comparable MC1 receptor binding affinities (1.6 vs. 2.1 nM). The substitution of DOTA with NOTA dramatically increased the melanoma uptake and decreased the renal and liver uptake of 64Cu-NOTA-GGNle-CycMSHhex. The tumor uptake of 64Cu-NOTA-GGNle-CycMSHhex was between 12.39 ± 1.61 and 12.71 ± 2.68 % ID/g at 0.5, 2 and 4 h post-injection. The accumulation of 64Cu-NOTA-GGNle-CycMSHhex activity in normal organs was lower than 1.02 % ID/g except for the kidneys 2, 4 and 24 h post-injection. The tumor/liver uptake ratios of 64Cu-NOTA-GGNle-CycMSHhex were 17.96, 16.95 and 8.02, whereas the tumor/kidney uptake ratios of 64Cu-NOTA-GGNle-CycMSHhex were 2.52, 3.60 and 5.74 at 2, 4 and 24 h post-injection, respectively. Greater than 91% of the injected radioactivity cleared through the urinary system by 2 h post-injection. The substitution of DOTA with NOTA resulted in a dramatic increase in melanoma uptake and decrease in renal and liver uptake of 64Cu-NOTA-GGNle-CycMSHhex compared to 64Cu-DOTA-GGNle-CycMSHhex. High melanoma uptake coupled with low accumulation in non-target organs suggested 64Cu-NOTA-GGNle-CycMSHhex as a lead radiolabeled peptide for melanoma imaging and therapy.
Keywords: Alpha-melanocyte stimulating hormone, 64Cu-labeled peptide, lactam bridge-cyclized peptide, positron emission tomography, melanoma imaging
INTRODUCTION
Melanoma is the most deadly skin cancer with an increased incidence worldwide over the past decade. The cancer statistics from the American Cancer Society predicted that 68,130 new melanoma cases would be diagnosed and 8,700 deaths of melanoma would occur in 2010 in the United States.1 There is a great need for better diagnostic strategies and more effective treatments for melanoma since no curative treatment exists for patients with metastatic melanoma. At the present time, the utilization of melanocortin-1 (MC1) receptor-targeting radiolabeled alpha-melanocyte stimulating hormone (α-MSH) peptides represents a very promising strategy for melanoma detection and treatment. This strategy takes advantage of fast distribution via blood circulation, receptor-targeting cancer tissue localization and rapid whole-body clearance of the radiolabeled α-MSH peptides. Both linear and cyclized α-MSH peptide radiopharmaceuticals have been reported to target the MC1 receptors2–6 for melanoma imaging and therapy.7–23
Over the past several years, we have developed two generations of novel lactam bridge-cyclized 111In-labeled α-MSH peptides for melanoma imaging using single photon emission computed tomography (SPECT).18–23 The first-generation peptides built upon the backbone of CycMSH {c[Lys-Nle-Glu-His-DPhe-Arg-Trp-Gly-Arg-Pro-Val-Asp], 12 amino acids} peptide,18–21 whereas the second-generation peptides were based on the construct of CycMSHhex {c[Asp-His-DPhe-Arg-Trp-Lys]-CONH2, 6 amino acids} peptide. 22–23 The MC1 receptor binding motif (His-DPhe-Arg-Trp) was retained in the 12-amino acid CycMSH moiety cyclized by a Lys-Asp lactam bridge, or in the 6-amino acid CycMSHhex moiety cyclized via an Asp-Lys lactam bridge. The radiometal chelator DOTA (1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid) was conjugated to the N-terminus of the CycMSH or CycMSHhex moiety with or without an amino acid linker for radiolabeling. The second-generation 111In-labeled CycMSHhex peptides exhibited enhanced melanoma uptake and reduced renal uptake than the first-generation 111In-labeled CycMSH peptides.18–23 For instance, 111In-DOTA-Nle-CycMSHhex displayed higher melanoma uptake (19.39 ± 1.65 % ID/g at 2 h post-injection) and lower renal uptake (9.52 ± 0.44 % ID/g at 2 h post-injection) compared to 111In-DOTA-GlyGlu-CycMSH in B16/F1 melanoma-bearing C57 mice.18–22 Recently, we have found that the introduction of the -GlyGly- linker between the DOTA and Nle-CycMSHhex moiety maintained high melanoma uptake (19.05 ± 5.04 % ID/g at 2 h post-injection) while reducing the renal and liver uptake of 111In-DOTA-GGNle-CycMSHhex by 42 and 68% compared to 111In-DOTA-Nle-CycMSHhex.23 The melanoma lesions could be clearly visualized by SPECT/CT using 111In-DOTA-GGNle-CycMSHhex as an imaging probe.23
We have been interested in expanding our melanoma imaging modality from SPECT to positron emission tomography (PET) because PET has some distinct advantages over other functional imaging modalities in terms of sensitivity, resolution and quantification. The combination of the outstanding imaging properties of PET with specific receptor-targeting properties of peptide radiopharmaceuticals offers an exciting opportunity for sensitive tumor-specific imaging. Copper-64 (t1/2=12.7 h, 17.4% β+, 40% β−) is an attractive PET radionuclide coupled with therapeutic properties due to its positron- and electron-emissions.24–26 Despite the fact that DOTA can form stable complexes with a variety of diagnostic and therapeutic radionuclides, DOTA can only form a moderately stable complex with 64Cu due to the demetallation of 64Cu-DOTA moiety in vivo. The dissociation of 64Cu from DOTA chelator generally resulted in high accumulation in non-target tissues such as liver.13,15,27–30 Alternatively, 1,4,7-triazacyclononane-1,4,7-triacetic acid (NOTA) could form a more stable complex with 64Cu than DOTA in vivo. It was reported that the 64Cu-NOTA-8-Aoc-BBN(7-14)NH2 displayed higher resistance to transmetallation reactions in vivo than 64Cu-DOTA-8-Aoc-BBN(7-14)NH2.27 64Cu-NOTA-8-Aoc-BBN(7-14)NH2 displayed considerably lower liver uptake than 64Cu-DOTA-8-Aoc-BBN(7-14)NH227,28 in PC-3 tumor-bearing mice, underscoring the advantage of NOTA for 64Cu conjugation compared to DOTA.
111In-DOTA-GGNle-CycMSHhex exhibited more favorable pharmacokinetic properties (comparable high tumor uptake, less renal and liver uptake) than 111In-DOTA-Nle-CycMSHhex in our previous reports.22,23 Hence, we managed to develop PET imaging probes building upon the GGNle-CycMSHhex peptide construct. In this study, we hypothesized that 64Cu-NOTA-GGNle-CycMSHhex would display more favorable melanoma targeting and pharmacokinetic properties than 64Cu-DOTA-GGNle-CycMSHhex. To examine the hypothesis, we synthesized NOTA-GGNle-CycMSHhex and DOTA-GGNle-CycMSHhex peptides. The MC1 receptor binding affinity of NOTA-GGNle-CycMSHhex was determined in B16/F1 melanoma cells and compared with DOTA-GGNle-CycMSHhex. Thereafter, we examined the biodistribution properties of 64Cu-NOTA-GGNle-CycMSHhex and 64Cu-DOTA-GGNle-CycMSHhex in B16/F1 melanoma-bearing C57 mice. Furthermore, we determined the melanoma imaging properties of 64Cu-NOTA-GGNle-CycMSHhex and 64Cu-DOTA-GGNle-CycMSHhex in B16/F1 melanoma-bearing C57 mice using small animal PET/CT.
EXPERIMENTAL SECTION
Chemicals and Reagents
Amino acid and resin were purchased from Advanced ChemTech Inc. (Louisville, KY) and Novabiochem (San Diego, CA). NO2AtBu ester was purchased from CheMatech Inc. (Dijon, France) for peptide synthesis. 125I-Tyr2-[Nle4, D-Phe7]-α-MSH {125I-(Tyr2)-NDP-MSH} was obtained from PerkinElmer, Inc. (Waltham, MA) for receptor binding assay. 64CuCl2 was purchased from Trace Life Sciences, Inc. (Dallas, TX) for radiolabeling. All other chemicals used in this study were purchased from Thermo Fischer Scientific (Waltham, MA) and used without further purification. B16/F1 murine melanoma cells were obtained from American Type Culture Collection (Manassas, VA).
Peptide Synthesis and Receptor Binding Assay
New NOTA-GGNle-CycMSHhex was synthesized using fluorenylmethyloxy carbonyl (Fmoc) chemistry. Briefly, the intermediate scaffold of Fmoc-Asp(O-2-PhiPr)-His(Trt)-DPhe-Arg(Pbf)-Trp(Boc)-Lys(Mtt) was synthesized on H2N-Novagel resin by an Advanced ChemTech multiple-peptide synthesizer (Louisville, KY). Generally, 70 μmol of resin, 210 μmol of each Fmoc-protected amino acid and NO2AtBu were used for the synthesis. The protecting groups of Mtt and 2-phenylisopropyl were removed by 2.5% of trifluoroacetic acid (TFA) for peptide cyclization. The cyclization reaction was achieved on the resin by an overnight reaction in N,N-dimethylformamide (DMF) using benzotriazole-1-yl-oxy-tris-pyrrolidino-phosphonium-hexafluorophosphate (PyBOP) as a coupling agent in the presence of N,N-diisopropylethylamine (DIPEA). After the cyclization, the moiety of NO2AtBu-CH2-Gly-Gly-Nle was coupled to the cyclic intermediate scaffold to yield NO2AtBu-CH2-Gly-Gly-Nle-Cyclic[Asp-His(Trt)-DPhe-Arg(Pbf)-Trp(Boc)-Lys] on the resin. All protecting groups were totally removed and the peptide was cleaved from the resin by treating with a mixture of trifluoroacetic acid (TFA), thioanisole, phenol, water, ethanedithiol and triisopropylsilane (87.5:2.5:2.5:2.5:2.5:2.5) for 2 h at 25 °C. The peptide was precipitated and washed with ice-cold ether four times, purified by reverse phase-high performance liquid chromatography (RP-HPLC) and characterized by liquid chromatography-mass spectrometry (LC-MS). DOTA-GGNle-CycMSHhex was synthesized, purified by RP-HPLC and characterized by LC-MS according to our published procedure.23 The MC1 receptor binding affinity (IC50 value) of NOTA-GGNle-CycMSHhex was determined in B16/F1 melanoma cells by in vitro competitive receptor binding assay according to our published procedure.23 The IC50 value of DOTA-GGNle-CycMSHhex was cited from our previous report23 for direct comparison.
Peptide Radiolabeling with 64Cu
Both 64Cu-NOTA-GGNle-CycMSHhex and 64Cu-DOTA-GGNle-CycMSHhex were prepared in a 0.5 M NH4OAc-buffered solution at pH 5.4. Briefly, 10 μL of 64CuCl2 (37–74 MBq in 0.05 M HCl aqueous solution), 10 μL of 1 mg/mL NOTA-GGNle-CycMSHhex or DOTA-GGNle-CycMSHhex aqueous solution and 200 μL of 0.5 M NH4OAc (pH 5.4) were added into a reaction vial and incubated at 75 °C for 1 h. After the incubation, 10 μL of 0.5% EDTA aqueous solution was added into the reaction vial to scavenge potential unbound 64Cu2+ ions. The radiolabeled complexes were purified to single species by Waters RP-HPLC (Milford, MA) on a Grace Vydac C-18 reverse phase analytical column (Deerfield, IL) using a 20-minute gradient of 18–28% acetonitrile in 20 mM HCl aqueous solution with a flow rate of 1.0 mL/min. Each purified peptide sample was purged with N2 gas at 25 °C for 20 min to remove the acetonitrile. The pH of final solution was adjusted to 7.4 with 0.1 N NaOH and sterile normal saline for animal studies. In vitro serum stabilities of both 64Cu-NOTA-GGNle-CycMSHhex and 64Cu-DOTA-GGNle-CycMSHhex were determined by incubation in mouse serum at 37 °C for 2 h and monitored for degradation by RP-HPLC.
Biodistribution Studies
All the animal studies were conducted in compliance with Institutional Animal Care and Use Committee approval. The melanoma targeting and pharmacokinetic properties of 64Cu-NOTA-GGNle-CycMSHhex and 64Cu-DOTA-GGNle-CycMSHhex were determined in B16/F1 melanoma-bearing C57 female mice (Harlan, Indianapolis, IN). The C57 mice were subcutaneously inoculated with 1×106 B16/F1 cells on the right flank for each mouse to generate B16/F1 tumors. The weights of tumors reached approximately 0.2 g 10 days post cell inoculation. Each melanoma-bearing mouse was injected with 0.037 MBq of 64Cu-NOTA-GGNle-CycMSHhex or 64Cu-DOTA-GGNle-CycMSHhex via the tail vein. Groups of five mice were sacrificed at 0.5, 2, 4 and 24 h post-injection, and tumors and organs of interest were harvested, weighed and counted. Blood values were taken as 6.5% of the whole-body weight. The specificities of the tumor uptake of 64Cu-NOTA-GGNle-CycMSHhex and 64Cu-DOTA-GGNle-CycMSHhex were determined by co-injecting 10 μg (6.07 nmol) of unlabeled NDP-MSH peptide at 2 h post-injection.
Melanoma Imaging with 64Cu-NOTA-GGNle-CycMSHhex and 64Cu-DOTA-GGNle-CycMSHhex
The melanoma imaging properties of 64Cu-NOTA-GGNle-CycMSHhex or 64Cu-DOTA-GGNle-CycMSHhex were determined in B16/F1 melanoma-bearing C57 mice using small animal PET/CT. Approximately 37.0 MBq of 64Cu-NOTA-GGNle-CycMSHhex or 64Cu-DOTA-GGNle-CycMSHhex was injected into each mouse via the tail vein. The mice were sacrificed for small animal PET and CT imaging 2 and 4 h post-injection. The 9-min CT imaging performed by Nano-SPECT/CT (Bioscan, Washington DC) was immediately followed by the PET imaging conducted by LabPET (Gamma Media-Ideas, Quebec, Canada) using the same animal bed. The PET data were reconstructed by maximum-likelihood expectation maximization (MLEM) reconstruction algorithms. The CT data were reconstructed, visualized and fused with the PET data using InVivoScope (Bioscan, Washington DC).
Statistical Analysis
Statistical analysis was performed using the Student’s t-test for unpaired data. A 95% confidence level was chosen to determine the significance of the difference in tumor, kidney and liver uptake between 64Cu-NOTA-GGNle-CycMSHhex with/without NDP-MSH co-injection, and between 64Cu-DOTA-GGNle-CycMSHhex with/without NDP-MSH co-injection. The differences at the 95% confidence level (p<0.05) were considered significant.
RESULTS
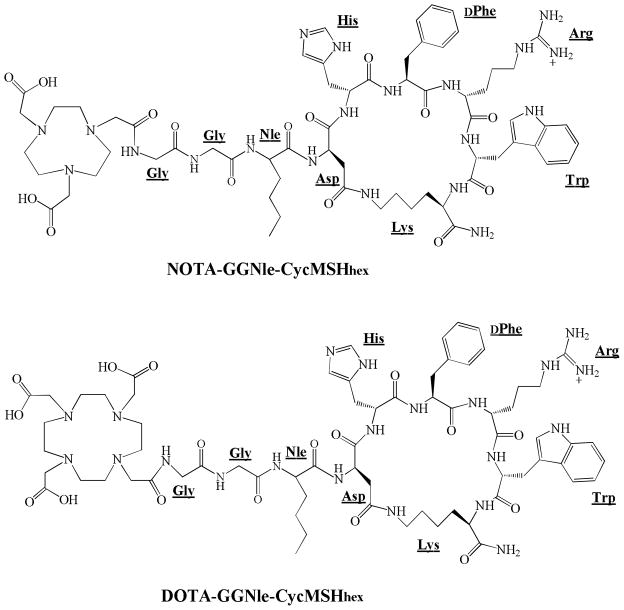
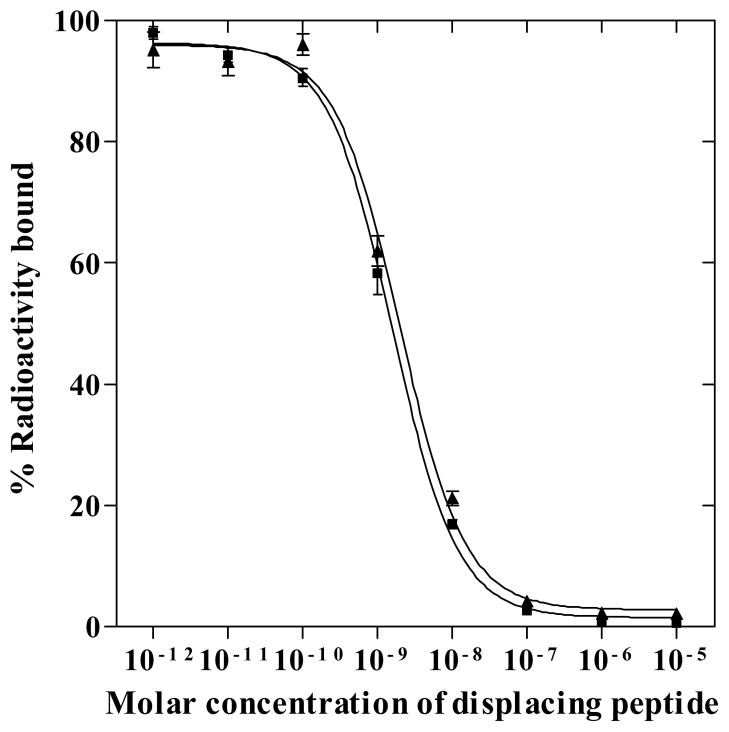
Novel NOTA-GGNle-CycMSHhex was synthesized and purified by RP-HPLC. NOTA-GGNle-CycMSHhex displayed greater than 90% chemical purity after HPLC purification. The identity of NOTA-GGNle-CycMSHhex was confirmed by electrospray ionization mass spectrometry. The calculated and found molecular weights of NOTA-GGNle-CycMSHhex were 1381 and 1381, respectively. Meanwhile, DOTA-GGNle-CycMSHhex was synthesized and characterized according to our published procedure23 for direct comparison. The schematic structures of NOTA-GGNle-CycMSHhex and DOTA-GGNle-CycMSHhex are shown in Figure 1. The competitive binding curve of NOTA-GGNle-CycMSHhex in B16/F1 cells is presented in Figure 2 and compared with DOTA-GGNle-CycMSHhex. The IC50 of NOTA-GGNle-CycMSHhex was 1.6 nM, which was comparable to that of DOTA-GGNle-CycMSHhex (2.1 nM).23
Figure 1.
Schematic structures of NOTA-GGNle-CycMSHhex and DOTA-GGNle-CycMSHhex.
Figure 2.
The in vitro competitive binding curves of NOTA-GGNle-CycMSHhex (■) and DOTA- GGNle-CycMSHhex (▲) in B16/F1 melanoma cells. The IC50 values of NOTA-GGNle- CycMSHhex and DOTA-GGNle-CycMSHhex were 1.6 and 2.1 nM, respectively. The data of DOTA-GGNle-CycMSHhex was cited from our previous report23 for comparison.
Both NOTA-GGNle-CycMSHhex and DOTA-GGNle-CycMSHhex were readily labeled with 64Cu in 0.5 M ammonium acetate at pH 5.4 with greater than 95% radiolabeling yields. 64Cu-NOTA-GGNle-CycMSHhex and 64Cu-DOTA-GGNle-CycMSHhex were completely separated from their excess non-labeled peptides by RP-HPLC. The specific activities of 64Cu-NOTA-GGNle-CycMSHhex and 64Cu-DOTA-GGNle-CycMSHhex were approximately 20,000 Ci/g and 20,000 Ci/g, respectively. The retention times of 64Cu-NOTA-GGNle-CycMSHhex and 64Cu-DOTA-GGNle-CycMSHhex were 18.5 and 18.8 min, respectively. Both 64Cu-NOTA-GGNle-CycMSHhex and 64Cu-DOTA-GGNle-CycMSHhex showed greater than 98% radiochemical purities after HPLC purification, and were stable in mouse serum at 37°C for 2 h. Only intact 64Cu-NOTA-GGNle-CycMSHhex and 64Cu-DOTA-GGNle-CycMSHhex were detected by RP-HPLC after 2 h of incubation in mouse serum.
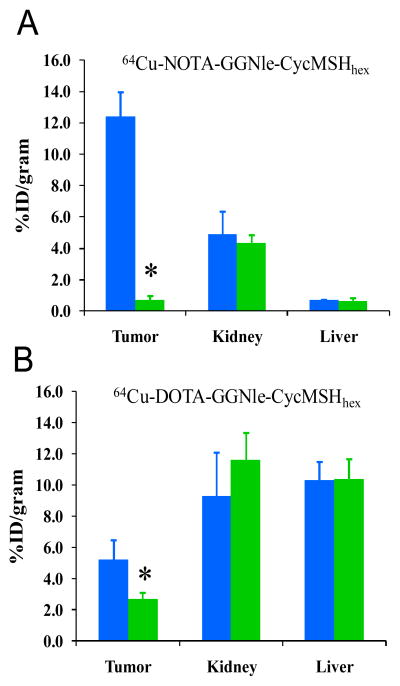
The melanoma targeting and pharmacokinetic properties of 64Cu-NOTA-GGNle-CycMSHhex and 64Cu-DOTA-GGNle-CycMSHhex were determined in B16/F1 melanoma-bearing C57 mice. The biodistribution results of 64Cu-NOTA-GGNle-CycMSHhex and 64Cu-DOTA-GGNle-CycMSHhex are presented in Tables 1 and 2. 64Cu-NOTA-GGNle-CycMSHhex exhibited rapid high melanoma uptake and prolonged tumor retention. The tumor uptake value of 64Cu-NOTA-GGNle-CycMSHhex was 12.51 ± 0.44 % ID/g at 0.5 h post-injection. 64Cu-NOTA-GGNle-CycMSHhex displayed similar high tumor uptake (12.39 ± 1.61 and 12.71 ± 2.68 % ID/g) at 2 and 4 h post-injection. Even at 24 h post-injection, there was 4.25 ± 0.32 % ID/g of 64Cu-NOTA-GGNle-CycMSHhex activity remained in the tumor. Approximately 94.2% of the tumor uptake of 64Cu-NOTA-GGNle-CycMSHhex was blocked by 10 μg (6.07 nmol) of non-radiolabeled NDP-MSH (p<0.05) (Figure 3), demonstrating that the tumor uptake was specific and MC1 receptor-mediated. Whole-body clearance of 64Cu-NOTA-GGNle-CycMSHhex was rapid, with 91.7% of the injected radioactivity cleared through the urinary system by 2 h post-injection. Normal organ uptake of 64Cu-NOTA-GGNle-CycMSHhex was lower than 1.02 % ID/g except for the kidneys at 2, 4 and 24 h post-injection. The liver uptake of 64Cu-NOTA-GGNle-CycMSHhex was only 0.69 ± 0.06 and 0.75 ± 0.17 % ID/g at 2 and 4 h post-injection. The kidney uptake was 9.28 ± 0.77 % ID/g at 0.5 h post-injection and decreased to 4.92 ± 1.43 % ID/g at 2 h post-injection. The renal uptake was only 0.74 ± 0.18 % ID/g at 24 h post-injection. Co-injection of NDP-MSH didn’t significantly reduce the renal uptake of 64Cu-NOTA-GGNle-CycMSHhex activity at 2 h post-injection, indicating that the renal uptake was not MC1 receptor-mediated. High tumor uptake and prolonged retention coupled with rapid whole-body clearance resulted in high tumor/blood and high tumor/normal organ uptake ratios achieved as early as 0.5 h post-injection. The tumor/liver uptake ratios of 64Cu-NOTA-GGNle-CycMSHhex were 11.37, 17.96, 16.95 and 8.02 at 0.5, 2, 4 and 24 h post-injection, whereas the tumor/kidney uptake ratios of 64Cu-NOTA-GGNle-CycMSHhex were 1.35, 2.52, 3.60 and 5.74 at 0.5, 2, 4 and 24 h post-injection.
Table 1.
Biodistribution of 64Cu-NOTA-GGNle-CycMSHhex in B16/F1 melanoma-bearing C57 mice. The data were presented as percent injected dose/gram or as percent injected dose (mean ± SD, n=5)
| Tissue | 0.5 h | 2 h | 4 h | 24 h |
|---|---|---|---|---|
| Percent injected dose/gram (%ID/g) | ||||
| Tumor | 12.51±0.44 | 12.39±1.61 | 12.71±2.68 | 4.25±0.32 |
| Brain | 0.10±0.03 | 0.04±0.02 | 0.07±0.04 | 0.04±0.03 |
| Blood | 1.59±0.19 | 0.33±0.10 | 0.30±0.11 | 0.10±0.04 |
| Heart | 0.89±0.29 | 0.24±0.13 | 0.41±0.24 | 0.13±0.09 |
| Lung | 2.05±0.26 | 0.56±0.09 | 0.71±0.17 | 0.37±0.11 |
| Liver | 1.10±0.18 | 0.69±0.06 | 0.75±0.17 | 0.53±0.07 |
| Spleen | 0.51±0.07 | 0.25±0.09 | 0.17±0.08 | 0.15±0.06 |
| Stomach | 0.65±0.15 | 0.76±0.27 | 1.02±0.17 | 0.22±0.04 |
| Kidneys | 9.28±0.77 | 4.92±1.43 | 3.53±0.57 | 0.74±0.18 |
| Muscle | 0.31±0.08 | 0.29±0.16 | 0.36±0.09 | 0.04±0.03 |
| Pancreas | 0.71±0.04 | 0.29±0.13 | 0.34±0.04 | 0.30±0.14 |
| Bone | 0.41±0.19 | 0.27±0.05 | 0.19±0.06 | 0.09±0.07 |
| Skin | 2.23±0.21 | 0.35±0.11 | 0.71±0.21 | 0.35±0.27 |
|
| ||||
| Percent injected dose (%ID) | ||||
| Intestines | 1.27±0.11 | 1.41±0.70 | 1.19±0.23 | 0.50±0.20 |
| Urine | 76.82±1.19 | 91.67±1.57 | 91.47±1.66 | 97.05±0.55 |
|
| ||||
| Tumor-to-normal-tissue uptake ratio | ||||
| Tumor/Blood | 7.87 | 37.55 | 42.37 | 42.50 |
| Tumor/Heart | 14.06 | 51.63 | 31.00 | 32.69 |
| Tumor/Kidneys | 1.35 | 2.52 | 3.60 | 5.74 |
| Tumor/Lung | 6.10 | 22.13 | 17.90 | 11.49 |
| Tumor/Liver | 11.37 | 17.96 | 16.95 | 8.02 |
| Tumor/Stomach | 19.25 | 16.30 | 12.46 | 19.32 |
| Tumor/Muscle | 40.35 | 42.72 | 35.31 | 106.25 |
Table 2.
Biodistribution of 64Cu-DOTA-GGNle-CycMSHhex in B16/F1 melanoma-bearing C57 mice. The data were presented as percent injected dose/gram or as percent injected dose (mean ± SD, n=5)
| Tissue | 0.5 h | 2 h | 4 h | 24 h |
|---|---|---|---|---|
| Percent injected dose/gram (%ID/g) | ||||
| Tumor | 5.61±0.91 | 5.20±1.28 | 5.25±1.22 | 5.96±0.36 |
| Brain | 0.27±0.03 | 0.39±0.22 | 0.17±0.06 | 0.29±0.06 |
| Blood | 2.33±0.54 | 1.03±0.19 | 0.81±0.33 | 1.07±0.17 |
| Heart | 2.22±0.25 | 1.75±0.13 | 1.45±0.13 | 2.23±0.10 |
| Lung | 3.76±0.54 | 3.93±0.64 | 4.39±0.36 | 4.45±0.51 |
| Liver | 11.59±1.77 | 10.33±1.18 | 9.61±1.34 | 7.23±0.58 |
| Spleen | 1.43±0.58 | 0.82±0.26 | 1.12±0.26 | 2.87±1.00 |
| Stomach | 2.83±0.84 | 4.78±0.96 | 5.09±0.69 | 1.21±0.14 |
| Kidneys | 14.44±1.85 | 9.30±2.81 | 7.45±0.96 | 5.39±0.42 |
| Muscle | 0.80±0.12 | 0.42±0.24 | 0.02±0.02 | 0.34±0.16 |
| Pancreas | 1.34±0.28 | 1.30±0.23 | 1.26±0.16 | 1.40±0.28 |
| Bone | 1.07±0.24 | 0.82±0.28 | 1.95±0.53 | 0.42±0.11 |
| Skin | 2.46±0.40 | 1.60±0.35 | 1.27±0.47 | 0.46±0.40 |
|
| ||||
| Percent injected dose (%ID) | ||||
| Intestines | 7.19±0.35 | 10.71±1.68 | 12.52±2.98 | 6.63±0.51 |
| Urine | 46.00±4.07 | 62.68±3.56 | 62.22±4.43 | 67.36±1.55 |
|
| ||||
| Tumor-to-normal-tissue uptake ratio | ||||
| Tumor/Blood | 2.41 | 5.05 | 6.48 | 5.57 |
| Tumor/Heart | 2.53 | 2.97 | 3.62 | 2.67 |
| Tumor/Kidneys | 0.39 | 0.56 | 0.70 | 1.11 |
| Tumor/Lung | 1.49 | 1.32 | 1.20 | 1.34 |
| Tumor/Liver | 0.48 | 0.50 | 0.55 | 0.82 |
| Tumor/Stomach | 1.98 | 1.09 | 1.03 | 4.93 |
| Tumor/Muscle | 7.01 | 12.38 | 262.50 | 17.53 |
Figure 3.
The tumor, kidney and liver uptake of 64Cu-NOTA-GGNle-CycMSHhex (A) and 64Cu-DOTA-GGNle-CycMSHhex (B) with (
 ) or without (
) or without (
 ) 10 μg of NDP-MSH blockade at 2 h post-injection. *p<0.05.
) 10 μg of NDP-MSH blockade at 2 h post-injection. *p<0.05.
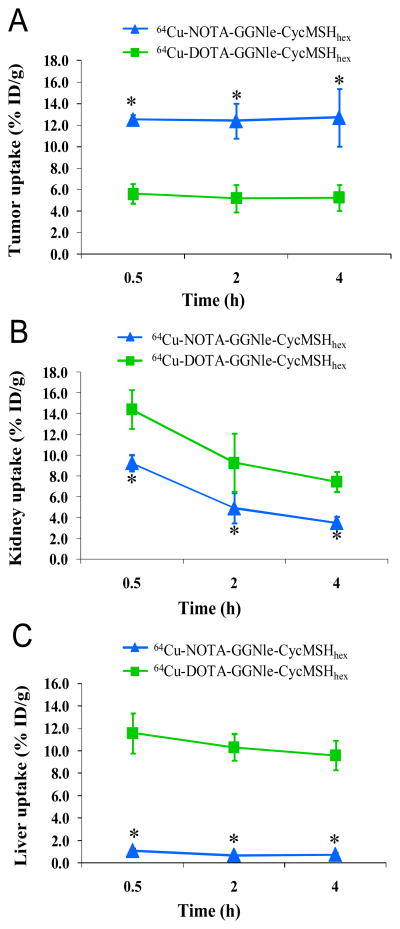
As we anticipated, 64Cu-DOTA-GGNle-CycMSHhex showed lower tumor uptake than 64Cu-NOTA-GGNle-CycMSHhex at 0.5, 2 and 4 h post-injection (Figure 4). The tumor uptake of 64Cu-DOTA-GGNle-CycMSHhex was 5.61 ± 0.91, 5.20 ± 1.28 and 5.25 ± 1.22 % ID/g at 0.5, 2 and 4 h post-injection, respectively. Co-injection of non-radioactive NDP-MSH blocked 47.9% of the tumor uptake at 2 h post-injection (p<0.05) (Figure 3), indicating that the partial tumor uptake of 64Cu-DOTA-GGNle-CycMSHhex was MC1 receptor-specific. Besides the lower tumor uptake, 64Cu-DOTA-GGNle-CycMSHhex displayed higher renal uptake than 64Cu-NOTA-GGNle-CycMSHhex at all time points investigated. The renal uptake of 64Cu-DOTA-GGNle-CycMSHhex was 14.44 ± 1.85, 9.30 ± 2.81, 7.45 ± 0.96 and 5.39 ± 0.42 % ID/g at 0.5, 2, 4 and 24 h post-injection, respectively. Co-injection of NDP-MSH didn’t significantly reduce the renal uptake of 64Cu-DOTA-GGNle-CycMSHhex activity at 2 h post-injection, indicating that the renal uptake was non-specific. Not surprisingly, 64Cu-DOTA-GGNle-CycMSHhex also displayed higher accumulation than 64Cu-NOTA-GGNle-CycMSHhex in normal organs such as liver, lung, heart and stomach at all the time points investigated. The liver uptake of 64Cu-DOTA-GGNle-CycMSHhex was 11.59 ± 1.77, 10.30 ± 1.18, 9.61 ± 1.34 and 7.23 ± 0.58 % ID/g at 0.5, 2, 4 and 24 h post-injection, respectively. High liver uptake associated with 64Cu-DOTA-GGNle-CycMSHhex activity was not receptor-mediated because co-injection of NDP-MSH didn’t significantly reduce the liver uptake of 64Cu-DOTA-GGNle-CycMSHhex at 2 h post-injection (Figure 3). In comparison with 64Cu-NOTA-GGNle-CycMSHhex, 64Cu-DOTA-GGNle-CycMSHhex exhibited dramatically lower tumor/kidney and tumor/liver uptake ratios (Figure 5) due to the decreased tumor uptake and increased renal and liver uptake. The tumor/kidney uptake ratios of 64Cu-DOTA-GGNle-CycMSHhex were 0.39, 0.56, 0.70 and 1.11 at 0.5, 2, 4 and 24 h post-injection, whereas the tumor/liver uptake ratios of 64Cu-DOTA-GGNle-CycMSHhex were 0.48, 0.50, 0.55 and 0.82 at 0.5, 2, 4 and 24 h post-injection.
Figure 4.
The comparison of tumor (A), kidney (B) and liver (C) uptake between 64Cu-NOTA-GGNle-CycMSHhex (
 ) and 64Cu-DOTA-GGNle-CycMSHhex (
) and 64Cu-DOTA-GGNle-CycMSHhex (
 ) at 0.5, 2 and 4 h post-injection. *p<0.05.
) at 0.5, 2 and 4 h post-injection. *p<0.05.
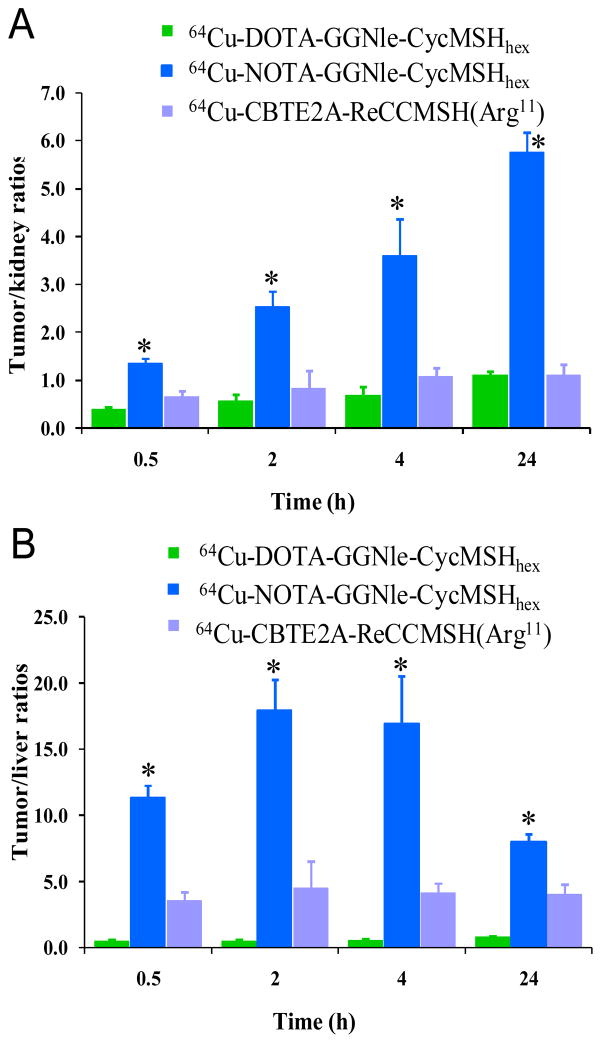
Figure 5.
The comparison of tumor/kidney (A) and tumor/liver (B) uptake ratios among 64Cu-DOTA- GGNle-CycMSHhex (
 ), 64Cu-NOTA-GGNle-CycMSHhex (
), 64Cu-NOTA-GGNle-CycMSHhex (
 ) and 64Cu-CBTE2A- ReCCMSH(Arg11) (
) and 64Cu-CBTE2A- ReCCMSH(Arg11) (
 ) at 0.5, 2, 4 and 24 h post-injection. *p<0.05, significance comparison between 64Cu-NOTA-GGNle-CycMSHhex and 64Cu-DOTA-GGNle-CycMSHhex, and between 64Cu-NOTA-GGNle-CycMSHhex and 64Cu-CBTE2A-ReCCMSH(Arg11). The tumor/kidney and tumor/liver uptake ratios of 64Cu-CBTE2A-ReCCMSH(Arg11) (
) at 0.5, 2, 4 and 24 h post-injection. *p<0.05, significance comparison between 64Cu-NOTA-GGNle-CycMSHhex and 64Cu-DOTA-GGNle-CycMSHhex, and between 64Cu-NOTA-GGNle-CycMSHhex and 64Cu-CBTE2A-ReCCMSH(Arg11). The tumor/kidney and tumor/liver uptake ratios of 64Cu-CBTE2A-ReCCMSH(Arg11) (
 ) were calculated based on the results published by Wei et al.14
) were calculated based on the results published by Wei et al.14
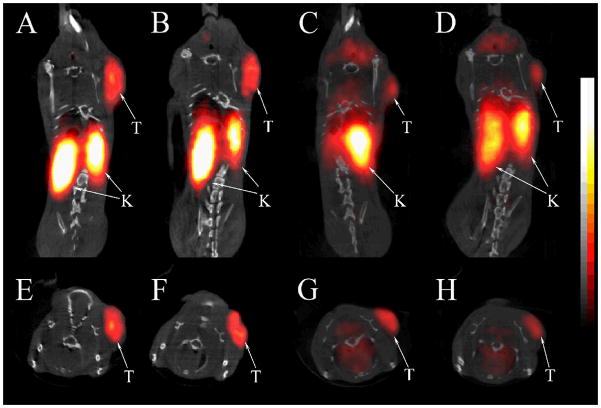
We further evaluated the melanoma imaging properties (melanoma visualization and imaging contrast) of 64Cu-NOTA-GGNle-CycMSHhex and 64Cu-DOTA-GGNle-CycMSHhex. The coronal and transversal PET/CT images are presented in Figure 6. The melanoma tumors were clearly visualized by PET/CT using either 64Cu-NOTA-GGNle-CycMSHhex or 64Cu-DOTA-GGNle-CycMSHhex as an imaging probe. However, 64Cu-NOTA-GGNle-CycMSHhex displayed higher tumor uptake and lower non-target organ accumulation compared to 64Cu-DOTA-GGNle-CycMSHhex. Both coronal and transversal images of 64Cu-NOTA-GGNle-CycMSHhex exhibited high tumor to normal organ uptake ratios except for the kidneys, which was consistent with the biodistribution results.
Figure 6.
Representative coronal (A, B, C, D) and transversal (E, F, G, H) PET/CT images of B16/F1 melanoma-bearing mice (12 days post cell inoculation) at 2 h (A, E, C, G) and 4 h (B, F, D, H) post-injection of 37.0 MBq of 64Cu-NOTA-GGNle-CycMSHhex (A, B, E, F) or 64Cu-DOTA- GGNle-CycMSHhex (C, D, G, H). The tumors (T) and kidneys (K) were highlighted with arrows on the images.
DISCUSSION
Both 64Cu-labeled DOTA-conjugated linear or rhenium-cyclized a-MSH peptides have been reported to target the MC1 receptors for successful melanoma imaging.13,15 However, the rhenium-cyclized 64Cu-DOTA-ReCCMSH(Arg11) {64Cu-DOTA-Re-[Cys3,4,10, D-Phe7, Arg11]a-MSH3–13}13 showed higher melanoma uptake than the linear 64Cu-DOTA-NAPamide {Ac-Nle-Asp-His-D-Phe-Arg-Trp-Gly-Lys(64Cu-DOTA)-NH2}15 at 2, 4 and 24 h post-injection. The melanoma uptake (8.80 ± 1.70 % ID/g) of 64Cu-DOTA-ReCCMSH(Arg11) was 1.9 times the melanoma uptake (4.63 ± 0.45 % ID/g) of 64Cu-DOTA-NAPamide at 2 h post-injection. Not surprisingly, both 64Cu-DOTA-ReCCMSH(Arg11) and 64Cu-DOTA-NAPamide displayed high liver uptake (7.3 vs. 11.0 % ID/g at 2 h post-injection) that could be attributed to the demetallation of 64Cu from the DOTA chelator in vivo. The in vivo stability of 64Cu-DOTA moiety was dramatically improved by using the CBTE2A {4,11-bis(carboxymethyl)-1,4,8,11-tetraazabicyclo[6.6.2]hexadecane} chelator. Compared to 64Cu-DOTA-ReCCMSH(Arg11), 64Cu-CBTE2A-ReCCMSH(Arg11) showed dramatically reduced liver uptake (1.57 ± 0.42 % ID/g) while maintained similar melanoma uptake (7.09 ± 3.20 % ID/g) at 2 h post-injection.14 Interestingly, the substitution of DOTA with NOTA considerably reduced the liver uptake of 64Cu-NOTA-8-Aoc-BBN(7-14)NH2 compared to 64Cu-DOTA-8-Aoc-BBN(7-14)NH2 in PC-3 tumor-bearing mice,27,28 suggesting that the NOTA was more suitable than DOTA for 64Cu conjugation. Therefore, based on the novel GGNle-CycMSHhex peptide construct we identified,23 we were interested in examining whether the replacement of DOTA with NOTA could enhance the melanoma uptake and reduce the liver uptake of 64Cu-NOTA-GGNle-CycMSHhex compared to 64Cu-DOTA-GGNle-CycMSHhex in this study.
In this study, we hypothesized that 64Cu-NOTA-GGNle-CycMSHhex would display more favorable melanoma targeting and pharmacokinetic properties than 64Cu-DOTA-GGNle-CycMSHhex. To examine the hypothesis, we evaluated NOTA-GGNle-CycMSHhex and DOTA-GGNle-CycMSHhex both in vitro and in vivo. The substitution of DOTA with NOTA slightly improved the MC1 receptor binding affinity of the peptide in B16/F1 melanoma cells (Figure 2). NOTA-GGNle-CycMSHhex exhibited 1.6 nM MC1 receptor binding affinity, whereas DOTA-GGNle-CycMSHhex showed 2.1 nM MC1 receptor binding affinity. Despite the slightly difference in receptor binding affinity between NOTA-GGNle-CycMSHhex and DOTA-GGNle-CycMSHhex, we observed dramatic differences in melanoma targeting and pharmacokinetic properties between their 64Cu-conjugates. 64Cu-NOTA-GGNle-CycMSHhex exhibited higher melanoma uptake than 64Cu-DOTA-GGNle-CycMSHhex in B16/F1 melanoma-bearing mice. The tumor uptake of 64Cu-NOTA-GGNle-CycMSHhex was 2.2, 2.4 and 2.4 times the tumor uptake of 64Cu-DOTA-GGNle-CycMSHhex at 0.5, 2 and 4 h post-injection, respectively (Tables 1 and 2, Figure 4). The lower melanoma uptake of 64Cu-DOTA-GGNle-CycMSHhex was likely due to the dissociation of 64Cu from the DOTA chelator in vivo. Interestingly, the co-injection of 10 μg of NDP-MSH blocked 94.2% of the tumor uptake of 64Cu-NOTA-GGNle-CycMSHhex, whereas the co-injection of 10 μg of NDP-MSH only blocked 47.9% of the tumor uptake of 64Cu-DOTA-GGNle-CycMSHhex (Figure 3). 64Cu-DOTA-GGNle-CycMSHhex showed 2.71 ± 0.42 % ID/g of non-specific melanoma uptake, which was similar to those of 64Cu-DOTA-ReCCMSH(Arg11) and 64Cu-DOTA-NAPamide.13,15
The substitution of DOTA with NOTA dramatically reduced the renal uptake of 64Cu-NOTA-GGNle-CycMSHhex compared to 64Cu-DOTA-GGNle-CycMSHhex (Figure 4). The renal uptake of 64Cu-NOTA-GGNle-CycMSHhex was only 64.3%, 52.9%, 47.3% and 13.7% of the renal uptake of 64Cu-DOTA-GGNle-CycMSHhex at 0.5, 2, 4 and 24 h post-injection, respectively. The increased tumor uptake and decreased renal uptake dramatically improved the tumor to kidney uptake ratios of 64Cu-NOTA-GGNle-CycMSHhex at all time points investigated in this study (Figure 5). The tumor to kidney uptake ratios of 64Cu-NOTA-GGNle-CycMSHhex were 3.5, 4.5, 5.1 and 5.2 times those of 64Cu-DOTA-GGNle-CycMSHhex at 0.5, 2, 4 and 24 h post-injection, respectively. Meanwhile, the substitution of DOTA with NOTA dramatically reduced the liver uptake by 14-fold to 0.69 ± 0.06 % ID/g at 2 h post-injection. The liver uptake of 64Cu-NOTA-GGNle-CycMSHhex was lower than 0.8 % ID/g after 2 h post-injection. The liver uptake of 64Cu-NOTA-GGNle-CycMSHhex was only 9.5%, 6.7%, 7.8% and 7.3% of the liver uptake of 64Cu-DOTA-GGNle-CycMSHhex at 0.5, 2, 4 and 24 h post-injection, respectively (Figure 4). Higher tumor uptake and lower liver uptake dramatically enhanced the tumor to liver uptake ratios of 64Cu-NOTA-GGNle-CycMSHhex at all time points investigated in this study (Figure 5). The tumor to liver uptake ratios of 64Cu-NOTA-GGNle-CycMSHhex were 23.7, 35.9, 30.8 and 9.8 times those of 64Cu-DOTA-GGNle-CycMSHhex at 0.5, 2, 4 and 24 h post-injection, respectively. Extremely low liver uptake of 64Cu-NOTA-GGNle-CycMSHhex indicated the in vivo stability of 64Cu-NOTA moiety in 64Cu-NOTA-GGNle-CycMSHhex peptide, which was consistent with the findings reported for 64Cu-NOTA-8-Aoc-BBN(7-14)NH227,28 and 64Cu-NOTA-STh peptides.29 As we anticipated, the improved in vivo stability of 64Cu-NOTA-GGNle-CycMSHhex also resulted in its low accumulation (<1.02 % ID/g) in non-target organs such as lung, stomach and heart after 2 h post-injection. Rapid whole-body clearance of 64Cu-NOTA-GGNle-CycMSHhex further confirmed its enhanced in vivo stability compared to 64Cu-DOTA-GGNle-CycMSHhex. Greater than 91% of 64Cu-NOTA-GGNle-CycMSHhex activity cleared out the body through urinary system, whereas only 63% of 64Cu-DOTA-GGNle-CycMSHhex activity excreted out the body via urinary system at 2 h post-injection.
At the present time, 64Cu-CBTE2A-ReCCMSH(Arg11) displayed the highest tumor/kidney ratio (1.11 at 24 h post-injection) and tumor/liver uptake ratio (4.52 at 2 h post-injection) among all reported 64Cu-labeled linear and cyclic a-MSH peptides.13–15 Remarkably, 64Cu-NOTA-GGNle-CycMSHhex displayed higher tumor uptake and lower renal and liver uptake than 64Cu-CBTE2A-ReCCMSH(Arg11) at all time points investigated in this study. The tumor uptake of 64Cu-NOTA-GGNle-CycMSHhex were 1.5, 1.7 and 1.7 times the tumor uptake of 64Cu-CBTE2A-ReCCMSH(Arg11) at 0.5, 2 and 4 h post-injection. The tumor/kidney uptake ratios of 64Cu-NOTA-GGNle-CycMSHhex were 2.0, 4.5, 3.4 and 5.2 times the tumor/kidney uptake ratios of 64Cu-CBTE2A-ReCCMSH(Arg11) at 0.5, 2, 4 and 24 h post-injection, whereas The tumor/liver uptake ratios of 64Cu-NOTA-GGNle-CycMSHhex were 3.1, 4.0, 4.1 and 2.0 times the tumor/liver uptake ratios of 64Cu-CBTE2A-ReCCMSH(Arg11) at 0.5, 2, 4 and 24 h post-injection (Figure 5). The increase in tumor uptake and decrease in renal and liver uptake of 64Cu-NOTA-GGNle-CycMSHhex were likely due to the structural difference between 64Cu-NOTA-GGNle-CycMSHhex and 64Cu-CBTE2A-ReCCMSH(Arg11). Despite the fact that the melanoma lesions could be visualized by PET/CT using either 64Cu-NOTA-GGNle-CycMSHhex or 64Cu-DOTA-GGNle-CycMSHhex as an imaging probe, 64Cu-NOTA-GGNle-CycMSHhex exhibited higher tumor imaging contrast than 64Cu-DOTA-GGNle-CycMSHhex. Higher melanoma uptake coupled with lower accumulation in normal organs suggested 64Cu-NOTA-GGNle-CycMSHhex as a lead radiolabeled peptide for future melanoma imaging and therapy. From the therapeutic perspective, the enhanced tumor/liver and tumor/kidney uptake ratios of 64Cu-NOTA-GGNle-CycMSHhex would potentially increase the absorbed dose to the tumor while keeping the liver and kidneys safe when treating the melanoma with 64Cu-NOTA-GGNle-CycMSHhex in future studies.
In conclusion, the melanoma targeting and imaging properties of 64Cu-NOTA-GGNle-CycMSHhex and 64Cu-DOTA-GGNle-CycMSHhex were determined in B16/F1 melanoma-bearing C57 mice in this study. The substitution of DOTA with NOTA dramatically increased the melanoma uptake and decreased the renal and liver uptake of 64Cu-NOTA-GGNle-CycMSHhex compared to 64Cu-DOTA-GGNle-CycMSHhex. High melanoma uptake coupled with low accumulation in non-target organs suggested 64Cu-NOTA-GGNle-CycMSHhex as a lead radiolabeled peptide for future melanoma imaging and therapy.
Acknowledgments
We thank Drs. Jianquan Yang, Fabio Gallazzi and Mr. Benjamin M. Gershman for their technical assistance. This work was supported in part by the NIH grant NM-INBRE P20RR016480/P20GM103451 and University of New Mexico HSC RAC Award. The image in this article was generated by the Keck-UNM Small Animal Imaging Resource established with funding from the W.M. Keck Foundation and the University of New Mexico Cancer Research and Treatment Center (NIH P30 CA118100).
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Siegrist W, Solca F, Stutz S, Giuffre L, Carrel S, Girard J, Eberle AN. Characterization of receptors for alpha-melanocyte-stimulating hormone on human melanoma cells. Cancer Res. 1989;49:6352–6358. [PubMed] [Google Scholar]
- 3.Tatro JB, Reichlin S. Specific receptors for alpha-melanocyte-stimulating hormone are widely distributed in tissues of rodents. Endocrinology. 1987;121:1900–1907. doi: 10.1210/endo-121-5-1900. [DOI] [PubMed] [Google Scholar]
- 4.Miao Y, Whitener D, Feng W, Owen NK, Chen J, Quinn TP. Evaluation of the human melanoma targeting properties of radiolabeled alpha-melanocyte stimulating hormone peptide analogues. Bioconjugate Chem. 2003;14:1177–1184. doi: 10.1021/bc034069i. [DOI] [PubMed] [Google Scholar]
- 5.Miao Y, Owen NK, Whitener D, Gallazzi F, Hoffman TJ, Quinn TP. In vivo evaluation of 188Re-labeled alpha-melanocyte stimulating hormone peptide analogs for melanoma therapy. Int J Cancer. 2002;101:480–487. doi: 10.1002/ijc.10640. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Cheng Z, Hoffman TJ, Jurisson SS, Quinn TP. Melanoma-targeting properties of 99mtechnetium-labeled cyclic alpha-melanocyte-stimulating hormone peptide analogues. Cancer Res. 2000;60:5649–5658. [PubMed] [Google Scholar]
- 7.Chen J, Cheng Z, Owen NK, Hoffman TJ, Miao Y, Jurisson SS, Quinn TP. Evaluation of an 111In-DOTA-rhenium cyclized α-MSH analog: a novel cyclic-peptide analog with improved tumor-targeting properties. J Nucl Med. 2001;42:1847–1855. [PubMed] [Google Scholar]
- 8.Froidevaux S, Calame-Christe M, Tanner H, Eberle AN. Melanoma targeting with DOTA-alpha-melanocyte-stimulating hormone analogs: structural parameters affecting tumor uptake and kidney uptake. J Nucl Med. 2005;46:887–895. [PubMed] [Google Scholar]
- 9.Froidevaux S, Calame-Christe M, Schuhmacher J, Tanner H, Saffrich R, Henze M, Eberle AN. A Gallium-labeled DOTA-α-melanocyte-stimulating hormone analog for PET imaging of melanoma metastases. J Nucl Med. 2004;45:116–123. [PubMed] [Google Scholar]
- 10.Froidevaux S, Calame-Christe M, Tanner H, Sumanovski L, Eberle AN. A novel DOTA-alpha-melanocyte-stimulating hormone analog for metastatic melanoma diagnosis. J Nucl Med. 2002;43:1699–1706. [PubMed] [Google Scholar]
- 11.Miao Y, Owen NK, Fisher DR, Hoffman TJ, Quinn TP. Therapeutic efficacy of a 188Re-labeled alpha-melanocyte-stimulating hormone peptide analog in murine and human melanoma-bearing mouse models. J Nucl Med. 2005;46:121–129. [PubMed] [Google Scholar]
- 12.Miao Y, Hylarides M, Fisher DR, Shelton T, Moore HA, Wester DW, Fritzberg AR, Winkelmann CT, Hoffman TJ, Quinn TP. Melanoma therapy via peptide-targeted alpha-radiation. Clin Cancer Res. 2005;11:5616–5621. doi: 10.1158/1078-0432.CCR-05-0619. [DOI] [PubMed] [Google Scholar]
- 13.McQuade P, Miao Y, Yoo J, Quinn TP, Welch MJ, Lewis JS. Imaging of melanoma using 64Cu- and 86Y-DOTA-ReCCMSH(Arg11), a cyclized peptide analogue of alpha-MSH. J Med Chem. 2005;48:2985–2992. doi: 10.1021/jm0490282. [DOI] [PubMed] [Google Scholar]
- 14.Wei L, Butcher C, Miao Y, Gallazzi F, Quinn TP, Welch MJ, Lewis JS. Synthesis and biologic evaluation of 64Cu-labeled rhenium-cyclized alpha-MSH peptide analog using a cross-bridged cyclam chelator. J Nucl Med. 2007;48:64–72. [PubMed] [Google Scholar]
- 15.Cheng Z, Xiong Z, Subbarayan M, Chen X, Gambhir SS. 64Cu-labeled alpha-melanocyte-stimulating hormone analog for MicroPET imaging of melanocortin 1 receptor expression. Bioconjugate Chem. 2007;18:765–772. doi: 10.1021/bc060306g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miao Y, Benwell K, Quinn TP. 99mTc- and 111In-labeled alpha-melanocyte-stimulating hormone peptides as imaging probes for primary and pulmonary metastatic melanoma detection. J Nucl Med. 2007;48:73–80. [PubMed] [Google Scholar]
- 17.Cheng Z, Chen J, Miao Y, Owen NK, Quinn TP, Jurisson SS. Modification of the structure of a metallopeptide: synthesis and biological evaluation of 111In-labeled DOTA-conjugated rhenium-cyclized alpha-MSH analogues. J Med Chem. 2002;45:3048–3056. doi: 10.1021/jm010408m. [DOI] [PubMed] [Google Scholar]
- 18.Miao Y, Gallazzi F, Guo H, Quinn TP. 111In-labeled lactam bridge-cyclized alpha-melanocyte stimulating hormone peptide analogues for melanoma imaging. Bioconjugate Chem. 2008;19:539–547. doi: 10.1021/bc700317w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo H, Shenoy N, Gershman BM, Yang J, Sklar LA, Miao Y. Metastatic melanoma imaging with an 111In-labeled lactam bridge-cyclized alpha-melanocyte-stimulating hormone peptide. Nucl Med Biol. 2009;36:267–276. doi: 10.1016/j.nucmedbio.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo H, Yang J, Gallazzi F, Prossnitz ER, Sklar LA, Miao Y. Effect of DOTA position on melanoma targeting and pharmacokinetic properties of 111In-labeled lactam bridge-cyclized a-melanocyte stimulating hormone peptide. Bioconjugate Chem. 2009;20:2162–2168. doi: 10.1021/bc9003475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo H, Yang J, Shenoy N, Miao Y. Gallium-67-labeled lactam bridge-cyclized alpha-melanocyte stimulating hormone peptide for primary and metastatic melanoma imaging. Bioconjugate Chem. 2009;20:2356–2363. doi: 10.1021/bc900428x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo H, Yang J, Gallazzi F, Miao Y. Reduction of the ring size of radiolabeled lactam bridge-cyclized alpha-MSH peptide resulting in enhanced melanoma uptake. J Nucl Med. 2010;51:418–426. doi: 10.2967/jnumed.109.071787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo H, Yang J, Gallazzi F, Miao Y. Effects of the amino acid linkers on melanoma-targeting and pharmacokinetic properties of Indium-111-labeled lactam bridge-cyclized a-MSH peptides. J Nucl Med. 2011;52:608–616. doi: 10.2967/jnumed.110.086009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis J, Laforest R, Buettner T, Song S, Fujibayashi Y, Connett J, Welch MJ. Copper-64-diacetyl-bis(N4-methylthiosemicarbazone): an agent for radiotherapy. Proc Natl Acad Sci USA. 2001;98:1206–1211. doi: 10.1073/pnas.98.3.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson CJ, Ferdani R. Copper-64 radiopharmaceuticals for PET imaging of cancer: advances in preclinical and clinical research. Cancer Biother Radiopharm. 2009;24:379–393. doi: 10.1089/cbr.2009.0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Z, Li ZB, Cao Q, Liu S, Wang F, Chen X. Small-animal PET of tumors with 64Cu-labeled RGD-bombesin heterodimer. J Nucl Med. 2009;50:1168–1177. doi: 10.2967/jnumed.108.061739. [DOI] [PubMed] [Google Scholar]
- 27.Prasanphanich AF, Nanda PK, Rold TL, Ma L, Lewis MR, Garrison JC, Hoffman TJ, Sieckman GL, Figueroa SD, Smith CJ. [64Cu-NOTA-8-Aoc- BBN(7-14)NH2] targeting vector for positron-emission tomography imaging of gastrin-releasing peptide receptor-expressing tissues. Proc Natl Acad Sci USA. 2007;104:12462–12467. doi: 10.1073/pnas.0705347104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffman TJ, Smith CJ. True radiotracers: Cu-64 targeting vectors based upon bombesin peptide. Nucl Med Biol. 2009;36:579–585. doi: 10.1016/j.nucmedbio.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 29.Liu D, Overbey D, Watkinson LD, Smith CJ, Figueroa SD, Hoffman TJ, Forte LR, Volkert WA, Giblin MF. Comparative evaluation of three 64Cu-Labeled E. coli heat-stable enterotoxin analogues for PET imaging of colorectal cancer. Bioconjugate Chem. 2010;21:1171–1176. doi: 10.1021/bc900513u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bass LA, Wang M, Welch MJ, Anderson CJ. In vivo transchelation of copper-64 from TETA-octreotide to superoxide dismutase in rat liver. Bioconjugate Chem. 2000;11:527–532. doi: 10.1021/bc990167l. [DOI] [PubMed] [Google Scholar]