Abstract
Objectives
To determine whether intensification with raltegravir improves endothelial function in antiretroviral-treated, HIV-infected individuals.
Design
Randomized, double-blinded, placebo-controlled study.
Methods
Fifty-six subjects with treatment-mediated viral suppression for at least one year were randomized to add raltegravir 400 mg twice daily or matching placebo for 24 weeks. The primary endpoint was the difference in rate of change in endothelial function (as assessed by flow-mediated vasodilation of the brachial artery [FMD]) from baseline to week 24 between the raltegravir and placebo groups. Linear mixed models were used to evaluate the association of treatment group with changes in FMD, immune activation, and measures of viral persistence.
Results
At baseline, the median CD4+ T cell count was 498 cells/mm3, nadir CD4+ T cell count was 191 cells/mm3, duration of HIV infection was 18 years, FMD was 3.3%, and hyperemic velocity (a marker of microvascular function) was 68.3 cm. There were no significant differences between treatment groups in rate of change in FMD (raltegravir group +0.032% per week, placebo group +0.023% per week; p=0.60). There were also no differences between treatment groups in rate of change in hyperemic velocity, immune activation, or viral persistence. In multivariable analysis, older age, longer duration of HIV infection, and current abacavir use were associated with lower FMD. Lower CD4+ T cell count and current abacavir use were associated with lower hyperemic velocity.
Conclusions
The addition of raltegravir to suppressive antiretroviral therapy did not have a significant impact on cardiovascular risk, as assessed by endothelial function (ClinicalTrials.gov NCT00843713).
Keywords: HIV, raltegravir intensification, endothelial function, flow-mediated vasodilation
INTRODUCTION
Highly active antiretroviral therapy (HAART) has been effective in decreasing morbidity and mortality associated with HIV infection 1. However, multiple studies have shown that HIV-infected patients remain at increased risk for cardiovascular events 2–7. Whether this increased risk is due to factors related to HIV disease (such as low level viral replication or persistent immune activation) or factors related to HAART remains to be determined.
Several raltegravir intensification studies have assessed whether low level viral replication persists in the setting of suppressive HAART 8–11. Although the studies differed in terms of patient population and outcome measures, they consistently found that intensification does not decrease plasma viremia as measured by ultrasensitive plasma HIV RNA assays. However, one study reported a significant decrease in viral replication as measured by an increase in 2-long terminal repeat (2-LTR) circles in peripheral blood mononuclear cells (PBMCs) 9, and another study showed a decrease in unspliced RNA in gut-associated lymphoid tissue (GALT) 8, suggesting that intensification may decrease viral replication if measured in cells and tissues.
To date, however, there have been no published studies examining whether treatment intensification affects end-organ disease, and more specifically whether it has the potential to decrease cardiovascular risk in treated patients. We therefore conducted a randomized, double-blinded, placebo-controlled study to assess whether raltegravir intensification in HAART-suppressed individuals decreases cardiovascular risk. Endothelial dysfunction, as measured by brachial flow-mediated vasodilation (FMD), has been shown to be independently predictive of both short- and long-term cardiovascular events 12,13. Hyperemic velocity, the stimulus for FMD, is a measure of microvascular function that has recently been shown to independently predict incident cardiovascular risk.14,15 Traditional cardiovascular risk factors are strongly related to hyperemic velocity, suggesting that the impaired FMD observed among these individuals is at least partly due to microvascular dysfunction.16 In the Framingham Study, systemic inflammation remained independently associated with reactive hyperemia after adjustment for traditional risk factors, in contrast to FMD.17 Among individuals with rheumatoid arthritis, macrovascular function (as measured by FMD) and microvascular function (as assessed by hyperemic velocity) were relatively independent of each other, suggesting there is differential regulation of endothelial function in these two vascular beds.18 Because HIV-infected individuals are in a chronic inflammatory state and since inflammation may impact macrovascular and microvascular function differentially, we also examined the effect of raltegravir intensification on hyperemic velocity as a secondary objective.
METHODS
Study participants
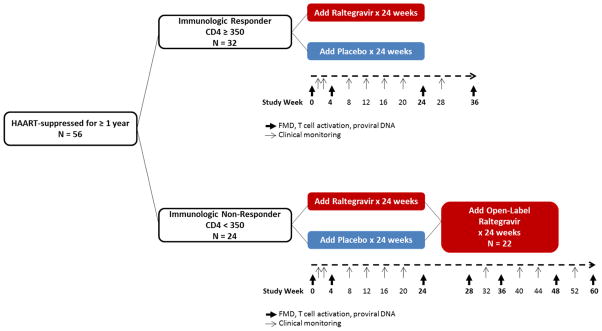
We performed a randomized, double-blinded, placebo-controlled study. Fifty-six HIV-infected, HAART- treated individuals with viral suppression for at least 1 year were randomized to add raltegravir 400 mg twice daily or matching placebo to their current suppressive HAART regimens for 24 weeks (Figure 1). Subjects were randomized in a 1:1 ratio using a computer-generated random allocation sequence; all authors were kept blinded to study group assignment until data collection and analyses were completed. Because we were interested in the impact of treatment intensification in individuals with low and high CD4+ T cell counts, we selected subjects based upon having either a CD4+ T cell count above or below 350 cells/mm3; this threshold has often been used by our group and others to define incomplete CD4+ T cell recovery 11,19,20. 32/56 subjects were “immunologic responders” (CD4+ ≥350 cells/mm3 and viral suppression for ≥1 year). The remaining subjects (24/56) were “immunologic non-responders” (CD4+ <350 cells/mm3 for ≥1 year despite viral suppression for ≥1 year) 11; 22/24 of the immunologic non-responders continued study participation in an optional extension study in which all 22 subjects (regardless of initial treatment assignment to raltegravir or placebo) received open-label raltegravir from weeks 24 to 48. Of these 22 subjects, 6 subjects chose to continue raltegravir indefinitely (through week 60).
FIGURE 1. Study schema.
56 HAART- suppressed subjects added raltegravir 400 mg twice daily or matching placebo to their current suppressive HAART regimens for 24 weeks. 32/56 subjects were “immunologic responders” (CD4+ ≥350 cells/mm3 and viral suppression for ≥1 year). The remaining subjects (24/56) were “immunologic non-responders” (CD4+ <350 cells/mm3 for ≥1 year despite viral suppression for ≥1 year) 11; 22/24 of the immunologic non-responders continued study participation in an extension study in which all 22 subjects received open-label raltegravir from weeks 24 to 48. Of these 22 subjects, 6 subjects chose to continue raltegravir indefinitely (through week 60).
HAART=highly active antiretroviral therapy. FMD=flow-mediated vasodilation.
All subjects provided written informed consent. This study was approved by the University of California San Francisco (UCSF) Committee on Human Research. Adherence to study drug was measured at every study visit by self-report and by pill-count. An independent Data Monitoring Committee comprised of three individuals from the scientific community met at 12, 24, 48, and 60 weeks after the enrollment of the first subject, and at 60 weeks after the enrollment of the last subject. No significant adverse events occurred during the study.
Endothelial function and hyperemic velocity
Flow-mediated vasodilation of the brachial artery and hyperemic velocity were performed at baseline and weeks 4, 24, and 36 (and at weeks 28, 48, and 60 for subjects who participated in the extension study). For FMD measurements, we used a 10MHz linear array probe in conjunction with the GE VividSeven Imaging System. To assess endothelium-dependent vasodilation, brachial artery diameter was measured under basal conditions and during reactive hyperemia following an ischemic stimulus. A blood pressure cuff was placed on the forearm and inflated to suprasystolic pressures for 5 minutes to induce forearm ischemia. The maximal increase in brachial artery diameter was assessed at 1 minute of reactive hyperemia. To assess endothelium-independent vasodilation, after 20 minutes of rest, brachial artery diameter was determined under basal conditions and following the administration of sublingual nitroglycerin (0.4 mg). Maximal dilation was assessed 3 minutes after the administration of sublingual nitroglycerin. Acquisition and analysis of the digitized images was performed using dedicated software (Information Integrity Inc.). Images were analyzed by a technician who was blinded to the subject’s HIV disease and treatment status. Hyperemic velocity was assessed as the peak velocity-time integral of the first complete velocity envelope obtained after cuff release; as this measurement reflects vasodilation of the microvasculature, higher levels represent improved vasodilation.
We previously performed repeated brachial artery reactivity studies on 25 HIV-infected subjects in order to define the test’s performance characteristics as performed at our center. Intra-observer reliability for measurement of brachial artery diameters was 0.972, which reflects an interclass correlation coefficient across all conditions of the study (i.e., baseline, reactive hyperemia, and both pre- and post-nitroglycerin administration).
T cell immunophenotyping
The percent of activated CD4+ and CD8+ T cells were measured in PBMCs at baseline and weeks 4 and 24 (and weeks 28 and 48 for subjects who participated in the extension study). PBMCs were isolated from whole blood, cryopreserved, and stored at the UCSF AIDS Specimen Bank. Markers of T cell activation (CD38 and HLA-DR) were measured using flow cytometry at the UCSF Core Immunology Laboratory, using previously described methods that have been optimized and validated for cryopreserved PBMCs 21. Briefly, cryopreserved PBMCs were rapidly thawed in warm media, counted on an Accuri C6 (BD Biosciences) with the Viacount assay (Millipore); average viability of thawed cells was 93% (range 61–98%; 80% of samples had a viability >90%). Cells were then washed, and stained with Aqua Amine Reactive Dye (AARD, Invitrogen) to discriminate dead cells and then stained with the following fluorescently-conjugated monoclonal antibodies: CD3-Pacific Blue, CD38-PE, HLA-DR-FITC (all BD Biosciences), CD4-PE Texas Red, and CD8-QDot 605 (Invitrogen). In each experiment a fluorescent-minus one control was included for CD38 and HLA-DR to determine the cut-off for positive staining. Stained cells were washed, fixed in 0.5% formaldehyde (Polyscience), and held at 4°C until analysis. Stained cells were run on a customized BD LSR II (BD Bioscience). 100,000 lymphocytes were collected for each sample. Data were compensated and analyzed using FlowJo (Tree Star) to determine the proportion of CD4+ and CD8+ T cells co-expressing CD38 and HLA-DR).
Virologic measurements
Ultrasensitive plasma HIV RNA was measured at baseline and week 12 (and weeks 24 and 36 for subjects who participated in the extension study) with an ultrasensitive assay with a lower limit of detection of <0.3 copies/mL 22.
Total proviral HIV DNA was measured from PBMCs at baseline and weeks 4 and 24 (and weeks 28 and 48 for subjects who participated in the extension study). Total proviral DNA was extracted from PBMCs using modifications of previously-described methods 23,24. This assay has an overall sensitivity of 1 copy/3 μg of input DNA, equivalent to approximately 450,000 PBMCs 25,26. All proviral DNA levels were normalized to per million CD4+ T cells (derived from the quantitation of human genomic DNA from a parallel real-time PCR amplification targeting a highly conserved region of the DQ-alpha locus, multiplied by the proportion of PBMCs that were CD4+ T cells at each timepoint).
Covariates
Candidate covariates of FMD and hyperemic velocity included demographics, comorbidities, cardiovascular risk factors, and HIV-specific risk factors. Comorbidities and cardiovascular risk factors included body mass index (BMI), hypertension, antihypertensive medication use, lipid lowering medication use, aspirin use, diabetes, smoking, high density lipoprotein cholesterol, low density lipoprotein cholesterol, total cholesterol, triglycerides, family history of cardiovascular disease, C-reactive protein, estimated glomerular filtration rate, testosterone use, and testosterone levels. HIV-related factors included duration of HIV infection, ultrasensitive plasma RNA, proviral DNA, current and nadir CD4+ T cell count, hepatitis C antibody status, history of opportunistic infection, lipodystrophy, and class of antiretroviral medications.
Statistical methods
We compared baseline demographic and clinical characteristics of subjects in the raltegravir and placebo groups using the Mann-Whitney U test for continuous variables and Fisher’s exact test for categorical variables. Linear mixed models with random intercepts and slopes were used to evaluate the association of treatment group with FMD and rates of change in FMD. Interaction terms between treatment group and time were used to determine whether the rate of change in FMD differed between raltegravir and placebo groups. As secondary analyses, linear mixed models were used to evaluate the association of treatment group with changes in immune activation, ultrasensitive plasma RNA, and proviral DNA.
Separate multivariable linear regression models were constructed for each outcome, adjusting for duration of raltegravir use as well as for demographics, comorbidities, cardiovascular risk factors, and HIV-specific risk factors. The relationship of time on study showed non-linear associations with some outcome measures; we therefore modeled time using linear splines, with potentially different slopes for weeks 0–28 and weeks 28–60. Factors forced in the full model included age, gender, and race. We used stepwise backward selection with a significance level of α=0.05 to remove candidate covariates that were not associated with the outcome. All statistical analyses were conducted with the SAS system, version 9.2 (SAS Institute, Inc.).
The primary endpoint was the difference in rate of change in FMD between the raltegravir and placebo groups at week 24. All 56 subjects contributed data to the primary FMD analyses; 6 additional subjects who did not have FMD performed contributed data to the secondary immunologic and virologic analyses. The sample size was determined based upon data from prior studies. In a randomized, controlled study of 60 HIV negative individuals with severe cardiovascular disease, subjects were treated with pravastatin vs. placebo for 6 weeks; the percent FMD was found to increase from 4.9±0.8% to 7.0±0.8% in the pravastatin group (p=0.02) 27. From a reproducibility study of FMD among healthy volunteers followed for 6 weeks, the mean standard deviation of the change in FMD was approximately 3% 28. Assuming the standard deviation in the change in FMD is as high as 3.5%, with 25 subjects in each arm, we would have 80% power (assuming a type I error of 5%) to detect a difference in FMD between groups of 2.8%. This effect size compares favorably to that observed in trials of 6 weeks of pravastatin in HIV-uninfected individuals (2.1%) 27.
RESULTS
Baseline characteristics
Fifty-six individuals (26 raltegravir, 30 placebo) were enrolled in the study. Baseline characteristics between raltegravir and placebo groups were similar (Table 1). The median age was 53 years and 95% were male. The median CD4+ T cell count was 498 cells/mm3. There was a trend towards the raltegravir group having a lower nadir CD4+ T cell count compared to the placebo group (135 vs. 270 cells/mm3, p=0.075).
TABLE 1.
Baseline Characteristics
| Raltegravir (N = 26) | Placebo (N = 30) | P-value | Overall (N = 56) | |
|---|---|---|---|---|
| Age (years) | 54 (47–59) | 53 (47–59) | 0.90 | 53 (47–59) |
| Female | 2 (8%) | 1 (3%) | 0.59 | 3 (5%) |
| Current CD4+ T cell count (cells/mm3) | 490 (242–719) | 498 (256–707) | 0.95 | 498 (246–713) |
| Nadir CD4 + T cell count (cells/mm3) | 135 (72–241) | 270 (95–389) | 0.075 | 191 (75–328) |
| Plasma HIV RNA < 75 copies/mL | 26 (100%) | 30 (100%) | N/A | 56 (100%) |
| FMD (%) | 2.9 (2.6–5.1) | 3.4 (2.4–5.1) | 0.68 | 3.3 (2.5–5.1) |
| Race | ||||
| Caucasian | 20 (77%) | 19 (63%) | 0.80 | 39 (70%) |
| African-American | 3 (12%) | 5 (17%) | 8 (14%) | |
| Latino | 1 (4%) | 2 (7%) | 3 (5%) | |
| Other | 2 (8%) | 4 (13%) | 6 (11%) | |
| Duration of HIV infection (years) | 19 (13–23) | 16 (13–20) | 0.21 | 18 (13–21) |
| HAART duration (years) | 7.4 (3.5–10.3) | 6.5 (2.3–11.4) | 0.57 | 6.5 (2.8–10.8) |
| NRTI use | 26 (100%) | 30 (100%) | N/A | 56 (100%) |
| NRTI duration (years) ** | 8.8 (5.6–11.9) | 6.2 (2.5–12.0) | 0.20 | 7.9 (3.4–12.0) |
| Abacavir use | 10 (38%) | 6 (20%) | 0.15 | 16 (29%) |
| NNRTI use | 14 (54%) | 21 (70%) | 0.27 | 35 (63%) |
| NNRTI duration (years) ** | 4.6 (3.1–9.9) | 3.8 (2.1–5.2) | 0.13 | 3.8 (2.2–5.9) |
| PI use | 20 (77%) | 22 (73%) | 0.99 | 42 (75%) |
| PI duration (years) ** | 7.8 (3.0–11.3) | 6.5 (2.5–10.6) | 0.56 | 6.5 (2.8–10.8) |
| History of CAD | 1 (4%) | 1 (3%) | 0.99 | 2 (4%) |
| Cigarette smoking | ||||
| Current | 4 (15%) | 9 (30%) | 0.28 | 13 (23%) |
| Past | 8 (31%) | 11 (37%) | 19 (34%) | |
| Never | 14 (54%) | 10 (33%) | 24 (43%) | |
| Diabetes mellitus | 0 | 1 (3%) | 0.99 | 1 (2%) |
| Hypertension | 11 (42%) | 8 (27%) | 0.27 | 19 (34%) |
| Statin use | 9 (35%) | 7 (23%) | 0.39% | 16 (29%) |
| Hyperlipidemia | 10 (38%) | 9 (30%) | 0.58 | 19 (34%) |
| LDL (mg/dL) | 95 (87–118) | 112 (90–131) | 0.15 | 104 (89–123) |
| HDL (mg/dL) | 50 (38–65) | 44 (38–55) | 0.43 | 46 (38–59) |
| Triglycerides (mg/dL) | 122 (90–161) | 132 (75–193) | 0.52 | 128 (80–169) |
| Total Cholesterol (mg/dL) | 180 (159–192) | 189 (162–214) | 0.27 | 181 (159–206) |
| hsCRP (mg/L) | 1.6 (0.6–3.2) | 1.9 (1.0–3.1) | 0.80 | 1.9 (0.6–3.1) |
| BMI (kg/m2) | 26 (23–29) | 26 (22–29) | 0.72 | 26 (23–29) |
| Hepatitis C Ab+ | 5 (19%) | 8 (27%) | 0.55 | 13 (23%) |
Data are presented as medians (interquartile range [IQR]) or numbers (percent).
Duration of ARV use among ever users.
FMD=flow-mediated vasodilation. HAART=highly active antiretroviral therapy. NRTI=nucleoside reverse transcriptase inhibitor. NNRTI=nonnucleoside reverse transcriptase inhibitor. PI=protease inhibitor. CAD=coronary artery disease. LDL=low density lipoprotein. HDL=high density lipoprotein. CRP=C-reactive protein. BMI=body mass index. N/A=not applicable.
Flow-mediated dilation and hyperemic velocity
At baseline, the median FMD was 3.3% (interquartile range [IQR] 2.5–5.1), the median endothelial independent vasodilation (nitroglycerin-mediated vasodilation) was 12.3% (IQR 9.4–15.8), and hyperemic velocity was 68.3 cm (IQR 48.7–100.1). The average rate of change in FMD over the first 24 weeks of the study was similar between the raltegravir (+0.032% per week, 95% confidence interval [CI]: 0.007, 0.058; p=0.014) and placebo (+0.023% per week, 95%CI: −0.001, 0.047; p=0.059) groups (p=0.60).
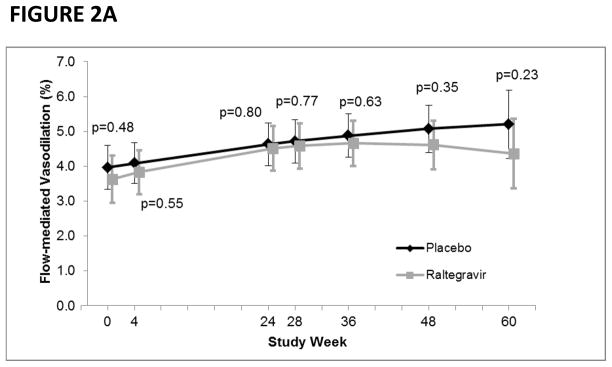
When including data from the extension study, the average rate of change in FMD across the entire 60-week study period was also similar between the raltegravir (+0.018% per week, 95%CI: 0.004, 0.032; p=0.011) and placebo (+0.023% per week, 95%CI: 0.009, 0.036; p=0.001) groups (p=0.66) (Figure 2A). With regards to endothelial independent vasodilation, the rate of change appeared to be greater in the raltegravir group (0.064% per week, 95%CI: 0.035, 0.093; p<0.0001) compared with the placebo group (0.033% per week, 95%CI: 0.0045, 0.061; p=0.023); however, it did not reach statistical significance (p=0.13). Similarly, the average rate of change in hyperemic velocity was similar between the raltegravir (+0.28 cm per week, 95%CI: 0.056, 0.50; p=0.014) and placebo (+0.47 cm per week, 95%CI: 0.26, 0.69; p<0.0001) groups (p=0.22) (Figure 2B). In an exploratory analysis, we examined whether raltegravir intensification had a unique effect on FMD in immunologic non-responders, but did not observe any differences in FMD between treatment groups at any visit.
FIGURE 2.
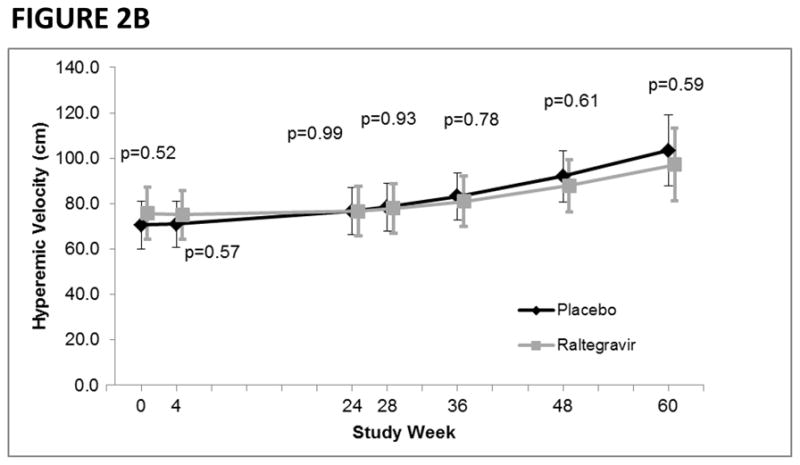
FIGURE 2A. Flow-mediated vasodilation (FMD) by study week and treatment group.
P-values denote differences in FMD between treatment groups.
FIGURE 2B. Hyperemic velocity by study week and treatment group.
P-values denote differences in hyperemic velocity between treatment groups.
In multivariable analysis, older age, longer duration of HIV infection, and current abacavir use were independently associated with lower FMD (Table 2A) while higher BMI was associated with higher FMD levels. In multivariable analysis, lower CD4+ T-cell count and current abacavir use were associated with lower hyperemic velocity (Table 2B). In an exploratory analysis, we examined whether raltegravir intensification had a unique effect on FMD in non-smokers and non-abacavir users; amongst 32 non-smokers and non-abacavir users, there were no differences in FMD between treatment groups at any visit.
TABLE 2A.
Factors Associated with flow-mediated vasodilation (FMD)
| Selected model: | Unadjusted* Estimate (95%CI), p-value |
Adjusted Estimate (95%CI), p-value |
|---|---|---|
| Time on study linear spline: | ||
| 0–28 weeks (effect per week) | 0.034 (0.018, 0.051), p<.0001 | 0.036 (0.018, 0.054), p<.0001 |
| 28–60 weeks (effect per week) | −0.0043 (−0.025, 0.017), p=0.69 | −0.007 (−0.030, 0.017), p=0.59 |
| Raltegravir duration (per week) | −0.0016 (−0.019, 0.016), p=0.86 | −0.001 (−0.015, 0.013), p=0.91 |
| Baseline age (per decade) | −0.83 (−1.30, −0.36), p=0.0005 | −0.75 (−1.18, −0.33), p=0.0005 |
| Female vs. Male | 0.29 (−1.46, 2.0), p=0.75 | −1.27 (−3.0, 0.46), p=0.15 |
| Duration of HIV infection (years) | −0.090 (−0.15, −0.030), p=0.0033 | −0.083 (−0.14, −0.024), p=0.0056 |
| Abacavir (current use) | −0.80 (−1.66, 0.056), p=0.067 | −0.99 (−1.73, −0.26), p=0.0078 |
| African-American vs. Caucasian | 0.087 (−1.05, 1.22), p=0.88 | −0.86 (−1.97, 0.25), p=0.13 |
| Other/Latino vs. Caucasian | −0.22 (−1.31, 0.86), p=0.69 | −0.26 (−1.15, 0.63), p=0.57 |
| BMI (kg/m2) | 0.090 (0.0046, 0.17), p=0.039 | 0.13 (0.039, 0.21), p=0.0043 |
Estimates from multivariable linear mixed models with random intercepts and slopes.
Unadjusted estimates control for time on study. In the unadjusted analysis, there was a trend toward FMD being associated with current CD4+ T cell count (0.41 per doubling of CD4+ T cell count, 95% CI [−0.055, 0.87], p=0.081). In the unadjusted analysis, there was no association between FMD and nadir CD4+ T cell count (0.097 per doubling of nadir CD4+ T cell count, 95% CI [−0.13, 0.32], p=0.40), FMD and current smoking status (−0.093, 95% CI [−1.04, 0.86], p=0.85), FMD and past smoking status (0.43, 95% CI [−0.39, 0.26], p=0.30), FMD and hypertension (−0.30, 95% CI [−1.14, 0.53], p=0.48), FMD and hypolipidemic use (0.087, 95% CI [−0.75, 0.92], p=0.84), or FMD and statin use (0.15, 95% CI [−0.73, 1.03], p=0.73).
BMI=body mass index.
TABLE 2B.
Factors Associated with Hyperemic Velocity
| Selected model: | Unadjusted* Estimate (95%CI), p-value |
Adjusted Estimate (95%CI), p-value |
|---|---|---|
| Time on study (per week) | 0.32 (0.13, 0.50), p=0.0007 | 0.30 (0.032, 0.57), p=0.028 |
| Raltegravir duration (per week) | 0.0045 (−0.26, 0.27), p=0.97 | 0.057 (−0.21, 0.32), p=0.67 |
| Baseline age (per decade) | −0.074 (−8.8, 8.6), p=0.99 | −3.0 (−10.7, 4.7), p=0.44 |
| Female vs. Male | 12.7 (−16.7, 42.2), p=0.40 | 2.6 (−24.6, 29.8), p=0.85 |
| Current CD4+ T cell count (cells/mm3) (per doubling) | 14.8 (8.1, 21.5), p<.0001 | 13.7 (6.9, 20.6), p<.0001 |
| Abacavir (current use) | −20.5 (−34.8, −6.3), p=0.0047 | −19.5 (−32.4, −6.7), p=0.0028 |
| African-American vs. Caucasian | 6.6 (−12.5, 25.8), p=0.50 | 2.2 (−15.8, 20.2), p=0.81 |
| Other/Latino vs. Caucasian | −9.8 (−28.0, 8.5), p=0.29 | −4.6 (−20.9, 11.6), p=0.58 |
Estimates from multivariable linear mixed models with random intercepts and slopes.
Unadjusted estimates control for time on study.
BMI=body mass index.
T cell activation and CD4+ T cell counts
At baseline, the median percent CD38+HLA-DR+ CD4+ T cells was 7.2%, and the median percent CD38+HLA-DR+ CD8+ T cells was 22.0%. The rate of change in immune activation was similar between the raltegravir and placebo groups. The average rate of change in percent CD38+HLA-DR+ CD4+ T cells over the first 24 weeks of the study was −0.041% per week (p=0.031) in the raltegravir group and −0.008% per week (p=0.64) in the placebo group (p=0.37). Between weeks 24 and 48, the rate of change in percent CD38+HLA-DR+ CD4+ T cells was +0.11% per week (p<0.0001) in the raltegravir group and +0.074% per week (p=0.0003) in the placebo group (p=0.63).
The proportion of CD8+ T cells co-expressing CD38 and HLA-DR has been shown to correlate with treatment-mediated immune recovery 29, mortality in the setting of treated HIV infection 30, and to differentiate different clinical phenotypes of HIV infection 31. The average rate of change in percent CD38+HLA-DR+ CD8+ T cells over the first 24 weeks of the study was +0.028% per week (p=0.57) in the raltegravir group and +0.023% per week (p=0.58) in the placebo group (p=0.72). Between weeks 24 and 48, the rate of change in percent CD38+HLA-DR+ CD8+ T cells was +0.087% per week (p=0.10) in the raltegravir group and +0.055% per week (p=0.29) in the placebo group (p=0.58).
The rate of change in peripheral CD4+ T cell count was similar between the raltegravir and placebo groups (p=0.67).
Ultrasensitive plasma RNA and proviral DNA
At baseline, 24/60 subjects had detectable plasma RNA levels using an ultrasensitive <0.3 copies/mL assay; the median baseline plasma RNA level was 0.2 (IQR 0.2, 0.4) copies/mL. There was no statistically significant difference in the proportion of subjects with undetectable plasma RNA at week 12 between the raltegravir and placebo groups (62% vs. 61%, respectively, p=0.99). Moreover, the average rate of change in ultrasensitive plasma RNA was similar between the raltegravir and placebo groups (p=0.40).
The median baseline proviral DNA level in PBMCs was 203 (IQR 21, 1188) copies/million CD4+ T cells. Moreover, the average rate of change in proviral DNA was similar between the raltegravir and placebo groups (p=0.51).
Finally, we found no statistically significant associations between FMD and percent CD38+HLA-DR+ CD4+ T cells, percent CD38+HLA-DR+ CD8+ T cells, proviral DNA, or ultrasensitive plasma RNA in unadjusted or fully adjusted analyses.
DISCUSSION
In this randomized, double-blinded, placebo-controlled study, we found that the addition of raltegravir to a suppressive antiretroviral regimen did not have a significant impact on cardiovascular risk, as assessed by endothelial function (FMD) or microvascular function (hyperemic velocity). In addition, in this expanded cohort which included immunologic responders we confirmed our earlier results that raltegravir intensification did not have a significant effect on immune activation, ultrasensitive plasma HIV RNA, or proviral HIV DNA 11.
While the precise mechanisms linking HIV disease and cardiovascular disease have not been clearly defined, several studies have suggested that viral replication and persistent immune activation may play a key role. HIV-infected individuals (even those receiving HAART) have dampened endothelial function as assessed by FMD compared to HIV uninfected individuals 32, and the initiation of HAART has been associated with a significant improvement in FMD 33. The importance of the relationship between plasma viremia and cardiovascular disease was further highlighted by the SMART study, in which continuous HAART was associated with reduced cardiovascular events as compared to intermittent or delayed HAART 34,35. Moreover, the link between CD4+ T cell count and cardiovascular events has been reported in two large cohorts 36,37. Finally, our own group has found a consistent relationship between nadir CD4+ T cell count and surrogate markers of cardiovascular risk, including carotid intima-media thickness 2, arterial stiffness 38, and endothelial function 39.
Initial treatment studies with raltegravir demonstrated significantly higher rates of decline in plasma HIV RNA compared to antiretroviral drugs in other drug classes 40, which led to widespread interest in the possibility of using raltegravir as an intensification agent. However, subsequent studies have for the most part shown no effect of raltegravir intensification on markers of viral replication or immune activation. For example, in the ACTG 5244 study, 12 weeks of raltegravir intensification did not reduce ultrasensitive plasma RNA levels 41. A prior analysis from the immunologic non-responder subset of our current study showed that in individuals with a CD4+ T cell count < 350 cells/mm3, raltegravir intensification did not result in a significant decrease in ultrasensitive plasma RNA, cell-associated RNA, proviral DNA, immune activation in PBMCs or GALT, or HIV-specific responses in PBMCs or GALT 11. Our current study adds to these findings by the inclusion of individuals with all CD4+ T cell counts and by also studying a subset of individuals for 48–60 weeks; however, intensification did not affect measures of viral persistence or immune activation.
Our baseline values for FMD and hyperemic velocity were much lower than values reported in the literature for HIV-uninfected men of a similar age (age 49 years, FMD 8.6%, hyperemia 121.6 cm) 14. Since treatment intensification in antiretroviral-treated individuals does not appear to provide much benefit in terms of vascular function, other novel adjunctive therapies will likely be needed. Of note, we observed that regardless of treatment group, individuals with a low baseline CD4+ T cell count (<350 cells/mm3) displayed a slow improvement in hyperemic velocity over time (data not shown). These data suggest that endothelial function may improve with longer term HAART, although it is unlikely that these measurements will ever improve to the level of the general population 2,4,5. In multivariable analysis, older age and longer duration of HIV infection were associated with more impaired FMD, and lower CD4+ T cell count was associated with more impaired hyperemic velocity. Collectively, our data add to the growing body of literature in support of earlier initiation of HAART 42–45. Finally, although the association of abacavir with cardiovascular disease remains an area of debate 46–49, we observed that current abacavir use was associated with lower FMD and hyperemic velocity, which is consistent with our previous report (involving a different cohort of subjects) on abacavir use being a risk factor for cardiovascular disease 46.
Limitations of this study include a modest sample size, limited number of female subjects, and multiplicity of analyses. However, this study expands upon prior studies of cardiovascular disease in treated HIV infection because it includes the assessment of endothelial function as well as microvascular function (hyperemic velocity); the latter has not been previously reported in the setting of HIV disease. Given that lower CD4+ T cell count was associated with lower hyperemic velocity but not FMD, and having a lower CD4+ T cell count on treatment has been associated with an increased risk of cardiovascular disease 39,42, one could speculate that the measurement of microvascular function is a more sensitive measure of future cardiovascular disease than FMD in the setting of treated HIV infection, or that it may identify a distinct type of vascular dysfunction that occurs in immunologic non-responders vs. responders; future studies will be needed to explore these hypotheses.
In this randomized, double-blinded, placebo-controlled study of 56 HAART-suppressed individuals, raltegravir intensification was not associated with any significant change in endothelial function, hyperemic velocity, immune activation, ultrasensitive plasma RNA, or proviral DNA. Older age, longer HIV duration, and current abacavir use were independently associated with lower FMD, while lower current CD4+ T cell count and current abacavir use were associated with lower microvascular function. Additional studies will be needed to identify mechanisms to decrease cardiovascular risk in the setting of treated HIV infection.
Acknowledgments
SOURCES OF FUNDING
This work was supported by grants from the NIH (K23AI075985, K24AI069994, AI052745, AI055273, RR 16482, R01 HL095130, R01 AI087145, R01 AI057020), American Foundation for AIDS Research (106710-40-RGRL, 107170-44-RGRL), the UCSF/Gladstone Institute of Virology & Immunology CFAR (P30 AI027763), the UCSF Clinical and Translational Research Institute Clinical Research Center (UL1 RR024131), the Center for AIDS Prevention Studies (P30 MH62246), the Center for HIV/AIDS Vaccine Immunology (U01 AI067854), and the CFAR Network of Integrated Systems (R24 AI067039).
HH and SGD have received research support from Merck, Inc. Study drug provided by Merck at no cost to this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
This study was presented at the 19th Conference on Retroviruses and Opportunistic Infections, March 2012, Seattle, Washington, USA (abstract #O-1002).
CONFLICTS OF INTEREST
RS, YW, KH, KM, RH, ES, SP, JNM, MPB, PYH: No conflicts.
References
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998 Mar 26;338(13):853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Hsue PY, Lo JC, Franklin A, et al. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation. 2004 Apr 6;109(13):1603–1608. doi: 10.1161/01.CIR.0000124480.32233.8A. [DOI] [PubMed] [Google Scholar]
- 3.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007 Jul;92(7):2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phillips AN, Neaton J, Lundgren JD. The role of HIV in serious diseases other than AIDS. Aids. 2008 Nov 30;22(18):2409–2418. doi: 10.1097/QAD.0b013e3283174636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsue PY, Hunt PW, Schnell A, et al. Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. Aids. 2009 Jun 1;23(9):1059–1067. doi: 10.1097/QAD.0b013e32832b514b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lo J, Abbara S, Shturman L, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. Aids. 2010 Jan 16;24(2):243–253. doi: 10.1097/QAD.0b013e328333ea9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seaberg EC, Benning L, Sharrett AR, et al. Association between human immunodeficiency virus infection and stiffness of the common carotid artery. Stroke. 2010 Oct;41(10):2163–2170. doi: 10.1161/STROKEAHA.110.583856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yukl SA, Shergill AK, McQuaid K, et al. Effect of raltegravir-containing intensification on HIV burden and T-cell activation in multiple gut sites of HIV-positive adults on suppressive antiretroviral therapy. Aids. 2010 Oct 23;24(16):2451–2460. doi: 10.1097/QAD.0b013e32833ef7bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buzon MJ, Massanella M, Llibre JM, et al. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat Med. 2010 Apr;16(4):460–465. doi: 10.1038/nm.2111. [DOI] [PubMed] [Google Scholar]
- 10.Gandhi RT, Zheng L, Bosch RJ, et al. The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: a randomized controlled trial. PLoS medicine. 2010;7(8) doi: 10.1371/journal.pmed.1000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hatano H, Hayes TL, Dahl V, et al. A randomized, controlled trial of raltegravir intensification in antiretroviral-treated, HIV-infected patients with a suboptimal CD4+ T cell response. J Infect Dis. 2011 Apr 1;203(7):960–968. doi: 10.1093/infdis/jiq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neunteufl T, Heher S, Katzenschlager R, et al. Late prognostic value of flow-mediated dilation in the brachial artery of patients with chest pain. The American journal of cardiology. 2000 Jul 15;86(2):207–210. doi: 10.1016/s0002-9149(00)00857-2. [DOI] [PubMed] [Google Scholar]
- 13.Gokce N, Keaney JF, Jr, Hunter LM, et al. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. Journal of the American College of Cardiology. 2003 May 21;41(10):1769–1775. doi: 10.1016/s0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- 14.Anderson TJ, Charbonneau F, Title LM, et al. Microvascular function predicts cardiovascular events in primary prevention: long-term results from the Firefighters and Their Endothelium (FATE) study. Circulation. 2011 Jan 18;123(2):163–169. doi: 10.1161/CIRCULATIONAHA.110.953653. [DOI] [PubMed] [Google Scholar]
- 15.Lind L, Berglund L, Larsson A, Sundstrom J. Endothelial function in resistance and conduit arteries and 5-year risk of cardiovascular disease. Circulation. 2011 Apr 12;123(14):1545–1551. doi: 10.1161/CIRCULATIONAHA.110.984047. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell GF, Parise H, Vita JA, et al. Local shear stress and brachial artery flow-mediated dilation: the Framingham Heart Study. Hypertension. 2004 Aug;44(2):134–139. doi: 10.1161/01.HYP.0000137305.77635.68. [DOI] [PubMed] [Google Scholar]
- 17.Vita JA, Keaney JF, Jr, Larson MG, et al. Brachial artery vasodilator function and systemic inflammation in the Framingham Offspring Study. Circulation. 2004 Dec 7;110(23):3604–3609. doi: 10.1161/01.CIR.0000148821.97162.5E. [DOI] [PubMed] [Google Scholar]
- 18.Sandoo A, Carroll D, Metsios GS, Kitas GD, Veldhuijzen van Zanten JJ. The association between microvascular and macrovascular endothelial function in patients with rheumatoid arthritis: a cross-sectional study. Arthritis Res Ther. 2011;13(3):R99. doi: 10.1186/ar3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunt PW, Martin JN, Sinclair E, et al. Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J Infect Dis. 2011 May 15;203(10):1474–1483. doi: 10.1093/infdis/jir060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lederman MM, Calabrese L, Funderburg NT, et al. Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J Infect Dis. 2011 Oct 15;204(8):1217–1226. doi: 10.1093/infdis/jir507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinclair E, Tan QX, Sharp M, et al. Protective immunity to cytomegalovirus (CMV) retinitis in AIDS is associated with CMV-specific T cells that express interferon- gamma and interleukin-2 and have a CD8+ cell early maturational phenotype. J Infect Dis. 2006 Dec 1;194(11):1537–1546. doi: 10.1086/508997. [DOI] [PubMed] [Google Scholar]
- 22.Palmer S, Wiegand AP, Maldarelli F, et al. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 2003 Oct;41(10):4531–4536. doi: 10.1128/JCM.41.10.4531-4536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee TH, el-Amad Z, Reis M, et al. Absence of HIV-1 DNA in high-risk seronegative individuals using high-input polymerase chain reaction. Aids. 1991 Oct;5(10):1201–1207. doi: 10.1097/00002030-199110000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Hatano H, Delwart EL, Norris PJ, et al. Evidence for persistent low-level viremia in individuals who control human immunodeficiency virus in the absence of antiretroviral therapy. J Virol. 2009 Jan;83(1):329–335. doi: 10.1128/JVI.01763-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee TH, Paglieroni T, Utter GH, et al. High-level long-term white blood cell microchimerism after transfusion of leukoreduced blood components to patients resuscitated after severe traumatic injury. Transfusion. 2005 Aug;45(8):1280–1290. doi: 10.1111/j.1537-2995.2005.00201.x. [DOI] [PubMed] [Google Scholar]
- 26.Lee TH, Chafets DM, Reed W, et al. Enhanced ascertainment of microchimerism with real-time quantitative polymerase chain reaction amplification of insertion-deletion polymorphisms. Transfusion. 2006 Nov;46(11):1870–1878. doi: 10.1111/j.1537-2995.2006.00992.x. [DOI] [PubMed] [Google Scholar]
- 27.Dupuis J, Tardif JC, Cernacek P, Theroux P. Cholesterol reduction rapidly improves endothelial function after acute coronary syndromes. The RECIFE (reduction of cholesterol in ischemia and function of the endothelium) trial. Circulation. 1999 Jun 29;99(25):3227–3233. doi: 10.1161/01.cir.99.25.3227. [DOI] [PubMed] [Google Scholar]
- 28.De Roos NM, Bots ML, Schouten EG, Katan MB. Within-subject variability of flow-mediated vasodilation of the brachial artery in healthy men and women: implications for experimental studies. Ultrasound Med Biol. 2003 Mar;29(3):401–406. doi: 10.1016/s0301-5629(02)00709-3. [DOI] [PubMed] [Google Scholar]
- 29.Hunt PW, Martin JN, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003 May 15;187(10):1534–1543. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 30.Hunt PW, Cao HL, Muzoora C, et al. Impact of CD8+ T cell activation on CD4+ T cell recovery and mortality in HIV-infected Ugandans initiating antiretroviral therapy. AIDS. 2011:25. doi: 10.1097/QAD.0b013e32834c4ac1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hunt PW, Brenchley J, Sinclair E, et al. Relationship between T Cell Activation and CD4(+) T Cell Count in HIV-Seropositive Individuals with Undetectable Plasma HIV RNA Levels in the Absence of Therapy. J Infect Dis. 2008 Jan 1;197(1):126–133. doi: 10.1086/524143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Solages A, Vita JA, Thornton DJ, et al. Endothelial function in HIV-infected persons. Clin Infect Dis. 2006 May 1;42(9):1325–1332. doi: 10.1086/503261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torriani FJ, Komarow L, Parker RA, et al. Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: The ACTG (AIDS Clinical Trials Group) Study 5152s. J Am Coll Cardiol. 2008 Aug 12;52(7):569–576. doi: 10.1016/j.jacc.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006 Nov 30;355(22):2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 35.Emery S, Neuhaus JA, Phillips AN, et al. Major clinical outcomes in antiretroviral therapy (ART)-naive participants and in those not receiving ART at baseline in the SMART study. J Infect Dis. 2008 Apr 15;197(8):1133–1144. doi: 10.1086/586713. [DOI] [PubMed] [Google Scholar]
- 36.Lichtenstein KA, Armon C, Buchacz K, et al. Low CD4+ T cell count is a risk factor for cardiovascular disease events in the HIV outpatient study. Clin Infect Dis. 2010 Aug 15;51(4):435–447. doi: 10.1086/655144. [DOI] [PubMed] [Google Scholar]
- 37.Triant VA, Regan S, Lee H, Sax PE, Meigs JB, Grinspoon SK. Association of Immunologic and Virologic Factors With Myocardial Infarction Rates in a US Healthcare System. J Acquir Immune Defic Syndr. 2010 Sep 8; doi: 10.1097/QAI.0b013e3181f4b752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ho JE, Deeks SG, Hecht FM, et al. Initiation of antiretroviral therapy at higher nadir CD4+ T-cell counts is associated with reduced arterial stiffness in HIV-infected individuals. Aids. 2010 Jul 31;24(12):1897–1905. doi: 10.1097/QAD.0b013e32833bee44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ho JE, Scherzer R, Hecht FM, et al. The association of CD4+ T-cell count on cardiovascular risk in treated HIV disease. Aids. 2012 Feb 29; doi: 10.1097/QAD.0b013e328352ce54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murray JM, Emery S, Kelleher AD, et al. Antiretroviral therapy with the integrase inhibitor raltegravir alters decay kinetics of HIV, significantly reducing the second phase. Aids. 2007 Nov 12;21(17):2315–2321. doi: 10.1097/QAD.0b013e3282f12377. [DOI] [PubMed] [Google Scholar]
- 41.Gandhi RT, Zheng L, Bosch RJ, et al. The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: a randomized controlled trial. PLoS Med. 2010;7(8) doi: 10.1371/journal.pmed.1000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaplan RC, Kingsley LA, Gange SJ, et al. Low CD4+ T-cell count as a major atherosclerosis risk factor in HIV-infected women and men. Aids. 2008 Aug 20;22(13):1615–1624. doi: 10.1097/QAD.0b013e328300581d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Triant VA, Regan S, Lee H, Sax PE, Meigs JB, Grinspoon SK. Association of immunologic and virologic factors with myocardial infarction rates in a US healthcare system. J Acquir Immune Defic Syndr. 2010 Dec 15;55(5):615–619. doi: 10.1097/QAI.0b013e3181f4b752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boulassel MR, Chomont N, Pai NP, Gilmore N, Sekaly RP, Routy JP. CD4 T cell nadir independently predicts the magnitude of the HIV reservoir after prolonged suppressive antiretroviral therapy. J Clin Virol. 2011 Oct 21; doi: 10.1016/j.jcv.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 45.Jain V, Hartogensis W, Bacchetti P, et al. ART initiation during acute/early HIV infection compared to later ART initiation is associated with improved immunologic and virologic parameters during suppressive ART [abstract 517]. Program and abstracts of the 18th Conference on Retroviruses and Opportunistic Infections; Boston. 2011. [Google Scholar]
- 46.Hsue PY, Hunt PW, Wu Y, et al. Association of abacavir and impaired endothelial function in treated and suppressed HIV-infected patients. Aids. 2009 Sep 24;23(15):2021–2027. doi: 10.1097/QAD.0b013e32832e7140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ribaudo HJ, Benson CA, Zheng Y, et al. No risk of myocardial infarction associated with initial antiretroviral treatment containing abacavir: short and long-term results from ACTG A5001/ALLRT. Clin Infect Dis. 2011 Apr 1;52(7):929–940. doi: 10.1093/cid/ciq244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palella FJ, Jr, Gange SJ, Benning L, et al. Inflammatory biomarkers and abacavir use in the Women’s Interagency HIV Study and the Multicenter AIDS Cohort Study. Aids. 2010 Jul 17;24(11):1657–1665. doi: 10.1097/QAD.0b013e3283389dfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baum PD, Sullam PM, Stoddart CA, McCune JM. Abacavir increases platelet reactivity via competitive inhibition of soluble guanylyl cyclase. Aids. 2011 Nov 28;25(18):2243–2248. doi: 10.1097/QAD.0b013e32834d3cc3. [DOI] [PMC free article] [PubMed] [Google Scholar]