Summary
Theory shows that fluctuation of environmental conditions can produce temporal niches for inferior competitors that mitigate effects of inter specific competition and facilitate long-term persistence of poor competitors.
In south Florida the mosquitoes Aedes albopictus and Aedes aegypti often co-occur in water-filled containers despite A. albopictus being competitively superior to A. aegypti. We tested the hypothesis that seasonal fluctuation in environmental conditions reduces or reverses competitive asymmetry between the species, and contributes to persistence of the poorer competitor via stabilizing or equalizing effects.
During the Florida wet and dry seasons we manipulated mosquito egg exposure to desiccation before inducing hatching and allowing surviving larvae to compete for 59 days. The effect of season also incorporated seasonal fluctuations in resource input to experimental containers.
For both species composite index of population performance (λ’) was greater in the dry season than the wet season, indicating strong seasonal effects on population dynamics. Aedes albopictus was not affected by competition in either season. Aedes aegypti was negatively affected by interspecific competition in the wet season.
Aedes aegypti egg survival was unaffected by exposure to the different experimental environments. There was a small reduction in A. albopictus egg survival in the wet season, but this reduction was unrelated to effects on λ’, indicating fluctuation in the egg environment did not contribute to dry season release from competition.
Detritus resource inputs were over three times greater in the dry season than in the wet season. Given the relatively small effect of environment on egg survival these results suggest that seasonal differences in population performance are driven primarily by per-capita food availability.
Large inputs of detritus in the dry season appear to reduce competition, and produce similar responses in both species. This result suggests that seasonal variation contributes to coexistence of A. albopictus and A. aegypti as a fitness equalizing factor.
Keywords: Aedes albopictus, Aedes aegypti, stabilizing mechanisms, equalizing mechanisms, desiccation, detritus, environmental conditions, fluctuation-dependent coexistence, season
Introduction
Mechanisms of coexistence are broadly categorized as being fluctuation-independent or fluctuation-dependent (Chesson 2000). Fluctuation-independent mechanisms such as resource partitioning and predation operate equally in homogenous and heterogeneous environments. Fluctuation-dependent mechanisms, on the other hand, only operate in environments in which conditions vary spatially or temporally. Recognition of the importance of fluctuating conditions as contributors to coexistence of competitors has grown since Hutchinson (1961) proposed it (Chesson & Huntley 1997, Chesson 2000, Chase & Leibold 2003). Fluctuations in environmental conditions that negatively affect species densities can affect the relative strengths of intra- and interspecific competition if species respond differently to fluctuations (Chesson & Huntley 1997, Chesson 2000). Periods in which the negative effects of intraspecific competition increase relative to the negative effects of interspecific competition can have stabilizing effects, which are required to maintain long-term coexistence (Chesson 2000). Fluctuating conditions might also have equalizing effects, which reduce fitness differences between competitors, and thus may reduce the strength of stabilizing effects necessary to maintain coexistence between competitors (Chesson 2000), Adler, HilleRisLambers & Levine 2007). Variation in multiple environmental conditions, including temperature (Holway, Suarez & Case 2002, Descamps-Julien & Gonzalez 2005, Jiang & Morin 2007), resource type (Murrell & Juliano 2008, Juliano 2009), current velocity (Taniguchi & Nakano 2000), and habitat drying (Costanzo, Kesavaraju & Juliano 2005) can alter interspecific competitive interactions and contribute to coexistence. Although diverse tax a could thus exhibit fluctuation-dependent coexistence, we still have a poor understanding of the general importance of fluctuating conditions as drivers of community structure in nature.
Container dwelling Aedes mosquitoes are a desirable model system for field tests of the role of fluctuating conditions in communities. Aedes aegypti invaded North America from Africa between the fifteenth and seventeenth centuries and its range covered much of the Southeast United States (Tabachnick 1991). Aedes albopictus invaded the United States in Texas via shipments of tyres from Japan in the mid-1980s (Hawley et al. 1987). Since then, A. albopictus spread to almost all of the Southeast and portions of the Midwest United States (Lounibos 2002, and at the same time, A. aegypti populations disappeared from much of the southeastern United States, persisting only in parts of Texas, New Orleans, southern Florida (Lounibos 2002, and Georgia (G.F. O’Meara, FMEL, Vero Beach, FL, personal communication). Despite nearly total displacement from sites of A. albopictus invasion in northern Florida, A. aegypti persists, sometimes despite invasion by A. albopictus, in urban areas of southern Florida (O’Meara et al. 1995, Rey et al. 2006).
Aedes aegypti and A. albopictus lay desiccation-resistant eggs near the water surface in containers such as discarded tyres and cemetery vases. Eggs hatch when containers fill with rain water (Christophers 1960, Hawley 1988). Aquatic larvae of both species feed on microorganisms that grow on detritus surfaces and in the water column (Christophers 1960, Hawley 1988). Laboratory (Barrera 1996), Daugherty, Alto & Juliano 2000) and field studies (Juliano 1998, Braks et al. 2004) demonstrated that A. albopictus is the superior competitor when decomposing plant material forms the resource base. Field experiments done in cemetery vases at approximately natural densities indicated that interspecific competition is common in south Florida (Juliano, Lounibos & O’Meara 2004). Those experiments were performed during the wet seasonat sites of Aedes coexistence and sites of A. aegypti exclusion (Juliano, Lounibos & O’Meara 2004), and showed that competitive effects of A. albopictus on A. aegypti were statistically indistinguishable at these site types. These results suggest that differences in wet season larval environments (physical characteristics, resources, etc.) between site types are insufficient to explain the difference in A. aegypti persistence at these sites(Juliano, Lounibos & O’Meara 2004).
The hypothesis that apparently stable coexistence (over about a decade – Lounibos et al. 2010) of these competitors is fluctuation-dependent was suggested by Costanzo, Kesavaraju & Juliano (2005), based on field observations and laboratory experiments. Southern peninsular Florida, where A. aegypti frequently persists, has relatively hot daytime temperatures and relatively high total precipitation from frequent rain showers from May through October (wet season) and warm to cool daytime temperatures and relatively low total precipitation from infrequent rain showers from November through April (dry season) (Duever et al. 1994). Despite seasonal differences in rainfall relative humidity remains relatively high throughout the year. Northern Florida, where A. albopictus appears to have excluded A. aegypti, does not have prolonged periods without precipitation during the winter months (Southeast Regional Climate Center 2007). Aedes aegypti ’s distribution in Florida is positively associated with warm, seasonally dry conditions whereas A. albopictus is negatively associated with these conditions (Juliano et al. 2002). In southern Florida, A. albopictus often occupies fewer containers at the beginning of the wet season than at the end of the wet season, indicating dry season reductions inA. albopictus populations (Juliano et al. 2002). In contrast, A. aegypti container occupancy at the same locations is unrelated to season, suggesting that A. aegypti performs equally well in wet and dry conditions (Juliano et al. 2002). Differences in the geographic distribution of A. albopictus and A. aegypti are associated with differences in egg desiccation tolerance. Aedes aegypti eggs tolerate high temperatures and desiccation better than do A. albopictus eggs (Sota & Mogi 1992, Juliano et al. 2002). In a long-term laboratory cage experiment Costanzo, Kesavaraju & Juliano (2005) produced compelling evidence that interspecific competition between A. albopictus and A. aegypti can be modified by the container drying regimen. In laboratory cages housing water-filled containers that dried fully before being refilled, A. aegypti populations were not affected by competition with A. albopictus, but A. albopictus populations were negatively affected by competition with A. aegypti, suggesting competitive superiority of A. aegypti when containers dry (Costanzo, Kesavaraju & Juliano 2005). In contrast, in laboratory cages housing water-filled containers that never dried, competitive superiority was reversed (Costanzo, Kesavaraju & Juliano 2005). This cage competition experiment suggests that competition is condition-specific relative to container drying, and thus potentially condition-specific between Florida’s wet and dry seasons (Costanzo, Kesavaraju & Juliano 2005). Thus seasonal drying could act as a stabilizing effect, contributing to coexistence of these competitors. This lab experiment leaves unanswered the questions of whether seasonal fluctuation of drying in nature is sufficient to produce such a stabilizing effect, and whether other effects of seasonal variation might also act as equalizing effects, reducing fitness differences between species.
We performed a pair of field experiments to test the hypothesis that seasonal fluctuation of environmental conditions in southern Florida alters the outcomes of competition between A. albopictus and A. aegypti. We manipulated desiccation exposure of Aedes eggs so that conditions for eggs were similar to those for both wet and dry seasons, to test the prediction that season-specific habitat drying is sufficient to modify the effects of competition. We also did an egg mortality experiment to test directly whether A. aegypti and A. albopictus eggs are differentially impacted by wet and dry season field conditions, which would suggest stabilizing effects. Table 1 summarizes egg mortality expectations and predicted outcomes of competition. We also monitored seasonal accumulations of detritus in proxy containers, to test the prediction that seasonal differences in the inputs of detritus resources also contribute to different seasonal outcomes of competition, either as stabilizing or equalizing effects.
Table 1.
The predicted relative levels of egg mortality for A. albopictus based on egg exposure treatment and season. Predictions of competitive superiority are based on the expected levels of A. albopictus egg mortality. Aedes aegypti egg mortality is expected to be low in both seasons and for all egg exposure treatments. Observed temperature and relative humidity values are means ± SE of daily weather data during the egg exposure treatment. Weather data were obtained from Weather Underground, Inc. (2012).
| Egg treatment | Hatch time | Observed Conditions |
Expected A. albopictus egg mortality |
Predicted competitive advantage |
|
|---|---|---|---|---|---|
| Temperature | RH% | ||||
| Lab egg exposure group |
After 2 weeks |
23–26°C | 96% | Wet season: Low | A. albopictus |
| Dry season: Low | A. albopictus | ||||
| Field egg exposure group |
After 2 weeks |
Wet season: 28.29 ± 0.43°C N=14 Starting 6/11/09 |
Wet season: 75.07 ± 1.16% N=14 Starting 6/11/09 |
Wet season: High | A. aegypti |
|
Dry season: 18.93 ± 0.52°C N=14 Starting 3/25/10 |
Dry season: 74.7 ± 1.57% N=14 Starting 3/25/10 |
Dry season: High | A. aegypti | ||
| Natural field egg exposure group |
As rain fills containers |
Wet season: 29.44°C N=1 Starting 6/25/09 |
Wet season: 70.0% N=1 Starting 6/25/09 |
Wet season: Low | A. albopictus |
|
Dry season: 22.96 ± 0.28°C N=12 Starting 4/8/10 |
Dry season: 69.67 ± 1.50% N=12 Starting 4/8/10 |
Dry season: High | A. aegypti | ||
Materials and Methods
We conducted a competition experiment and an egg mortality experiment, each done during the wet season of 2009 and again at the end of the dry season of 2010. The experiments were done under the canopy of a live oak-palmetto hammock on the grounds of the Florida Medical Entomology Laboratory in Vero Beach, Florida. Aedes aegypti occupied natural and artificial containers in the hammock prior to the invasion of A. albopictus (S. A. Juliano, personal observation). At present, A. albopictus is abundant in containers at this site and A. aegypti occurs at other sites in Vero Beach, but adults are sometimes seen at the Laboratory (P. A. O’Neal, personal observation). The persistence of A. aegypti in the surrounding area suggests that this study site is a suitable location to test the hypothesis that seasonal climate differences contribute to the coexistence of A. aegypti and A. albopictus, despite the apparent scarcity of larval A. aegypti at the study site.
Aedes eggs used in the wet season experiments were from established laboratory colonies initiated with individuals obtained from multiple locations in Florida. The colonies consisted of several hundred adults and had been maintained in the lab with overlapping generations for 2–3 years. The eggs used in the wet season were 1–3 months old. In the months between the wet and dry season runs of the experiment, we replaced both colonies. In the dry season we used Aedes eggs from colonies initiated with individuals from cemeteries in Fort Pierce, Florida (A. aegypti) and discarded tyres at FMEL (A. albopictus). Dry season colonies consisted of a few hundred adults and had been maintained in the lab for < 1 year. The eggs used in the dry season were ≤ 2 weeks old.
Competition Experiment
During each season we placed the eggs of both species into dry, 0.946 l plastic cups and we placed the cups under the canopy of the hammock in a small response surface design (Goldberg & Scheiner 2001) with seven A. albopictus: A. aegypti density combinations (40:0, 0:40, 80:0, 0:80, 40:40, 60:20, and 20:60). We exposed eggs in containers of each density combination to one of three environmental conditions (see below) prior to flooding with 500 ml of water: Lab egg exposure - two weeks under optimal conditions in the laboratory without hatching; Field egg exposure - two weeks under ambient field conditions without hatching; Natural field egg exposure - ambient field conditions with container flooding and hatching via naturally occurring precipitation (Table 1).
We affixed experimental containers to 12 acrylic platforms and placed chicken wire around each platform to exclude vertebrates. Each platform had one of each of the seven density combinations and all containers on a platform received the same egg environmental exposure treatment. To implement environmental exposure treatments, we inspected paper towels that had served as the oviposition substrate in colonies (hereafter, “egg papers”) and removed eggs that had partially or completely collapsed (suggesting inviability) from the egg papers. We cut out sections of egg papers with a desired number of eggs and assigned them to an environmental exposure treatment.
We replicated each density × environmental exposure treatment combination four times for a total of 84 containers per season and 168 containers total. Because of limited open area in the hammock, the experiments were done at four sites (≥ 50 m apart). Each combination of density × environmental exposure treatment was replicated once per site. In the dry season we placed platforms within 1 m of the location used in the wet season.
Environmental Egg Exposure Treatments (Table 1). Lab egg exposure
On Day 1 we placed eggs in a sealed plastic container that also housed a vial of super-saturated potassium sulphate solution, which produces an equilibrium relative humidity of approximately 96.3% within the container (Winston & Bates 1960). We kept the egg container at 14 L: 10 D (florescent lighting), 23–26°C in a climate controlled building. These conditions are expected to produce maximal egg survival. On Day 15, we placed the eggs into experimental containers, immediately flooded the containers with 500 ml of water, placed the containers into the field, and allowed larval competition to occur for 59 days.
Field egg exposure
We placed experimental containers in the field on Day 1 to expose eggs to ambient seasonal field conditions for two weeks. We covered containers with mesh to exclude predators and placed a clear acrylic sheet over the chicken wire enclosure to prevent rainwater from inducing premature hatch. On Day 15, we flooded the containers with 500 ml water and allowed larval competition to occur for 59 days.
Natural field egg exposure
We placed experimental containers in the field on Day 15 to expose eggs to ambient conditions with natural container flooding. We covered the containers with mesh and an acrylic sheet, as in the Field egg exposure treatment. Each day we checked a proxy container placed in a location with no overhead canopy and measured the previous day’s total rainfall. We added an amount of water equal to the previous day’s total rainfall to the Natural field egg exposure treatment containers. This procedure was continued until a total of 500 ml had been added to the container or 14 days had elapsed, at which point the difference between the cumulative amount of water added and 500 ml was added to the containers and the acrylic sheet was removed. We assumed some eggs hatched with the first addition of water, so we allowed larval competition to occur for 59 days from the date of the first water addition. This treatment was expected to result in early hatching in the wet season, and later hatching in the dry season. We collected daily precipitation data over 73 days in the wet season and 109 days in the dry season to compare the duration of egg exposure to pre-flooding conditions for the Natural field egg exposure treatment to the seasonal average interval between precipitation events.
Upon flooding, we fitted experimental containers with an adult emergence chamber. We collected adults from containers by aspiration, identified the sex and species, dried them at 50°C, and quantified adult female size by measuring wing length (Christophers 1960. Due to the large number of adults collected and because some of the adults’ wings were damaged, we sampled female adult size for each cohort. We measured the wing lengths for females with earliest, median, and latest emergence times for each cohort (Leisnham & Juliano 2010)or for all females if ≤3 emerged from a cohort. After 59 days of larval competition we collected remaining pupae from experimental containers, allowed them to eclose, and included them in the adult data set.
We calculated Livdahl & Sugihara’s (1984) composite index of performance r’, which estimates instantaneous rate of increase for a cohort by combining in a biologically meaningful way measures of survival, development time, and adult egg production (estimated from adult size). In this way, this index is regarded as a better overall indicator of ecological effects than analysis of individual measures of population performance (Livdahl & Sugihara 1984, Livdahl & Willey 1991, Juliano 1998). We modified Livdahl & Sugihara’s (1984) original method to use the mean wing length of our sample of three females from each container (see above). This simplified the expression for r’ to:
where N0 represents the initial number of females in the container (assumed to be 50% of the total number of eggs), A is the number of females produced from the container over the course of the experiment, f (w) describes a functional relationship between egg production and mean wing length (mm) of our sample of three females from the container, D is the estimated time between female eclosion and reproduction, and x̄ is the mean time to eclosion for all eclosed females. For A. aegypti, D = 12 days (Grill & Juliano 1996), and f (wx) = 2.5(wx)3 − 8.616 (Briegel 1990). For A. albopictus, D = 14 days (Livdahl & Willey 1991), and f (wx) = 78.02wx − 121.24 (Lounibos et al. 2002). For analysis we used λ’ = exp(r’), which estimates the finite rate of increase for a cohort, and has the advantageous property of being estimable even when no females survive to reproductive age (λ’ = 0), a condition that renders r’ inestimable (r’ = − ∞) (Juliano 1998).
We compared the conditions during our experiment to the average conditions from the past 15 years. To do so we downloaded daily averages for temperature and relative humidity from a weather station at the Vero Beach airport (Weather Underground, Inc. 2012) for 14 day periods corresponding to the dates on which eggs were exposed to field conditions during our experiment (6/11 – 6/25 in the wet season; 4/1 – 4/14 in the dry season). We collected data from the same time periods for each year from 1997 to 2011. We calculated a mean temperature and relative humidity for both 14 day periods (wet and dry seasons) for each year. We then calculated from yearly means over this 15-year span overall mean, standard deviation, and 95% confidence interval for temperature and relative humidity in each season.
Analysis
We analyzed λ’ for each species with a randomized complete block ANOVA (PROC MIXED, SAS Institute, Inc. 2008) with environmental exposure, density combination, and season as fixed effects, site as the random block effect, and all possible two- and three-way interactions. We assumed effects of density, treatment, and season were independent of site and thus pooled three-way random effects involving site with the overall error term (Fenster & Dudash 2001).
As a randomized, controlled experiment, our approach constitutes “design based inference”, wherein there is only one statistical model (in our case, a saturated 3 way design) that is dictated by the design (Anderson 2008). We determine the importance of experimental factors using multimodel inference with information theoretic (IT) criteria (Burnham, Anderson, & Huyvaert 2011), evaluating alternative models incorporating or omitting main effects and interactions. A major advantage of this approach is quantification of weight of evidence for different models and avoidance of arbitrary α levels in hypothesis tests (Anderson 2008; Burnham et al. 2011). We did such IT analyses using AICc (corrected for small sample size) and following recommendations of Burnham, Anderson, & Huyvaert (2011) on calculations and conclusions. When multiple models had similar weight of evidence we chose to err on the side of including effects, and chose as our final model the well-supported model that included the greatest number of experimental factors. We report for our final models P values for main effects and interactions, and when necessary multiple pairwise comparisons of means to evaluate the direction of differences produced by our treatments. We also report, for the best models, effect sizes as partial η2 (Sokal & Rohlf 2012, Kromrey & Bell 2010) which estimates the sample effect size in a factorial ANOVA (Kromrey & Bell 2010). As a rule of thumb, η2 = 0.01, 0.06, and 0.14 indicate effect sizes described as small, medium, and large, respectively (Cohen 1988). Effect sizes were calculated using the SAS® Macro by Kromrey & Bell (2010).
Data for λ’ did not meet assumptions of homogeneous variance or normality for either species. We therefore also used randomization ANOVAs (RT 2.1, Manly 1997; SAS® 9.2 Cassell 2002) on the untransformed A. albopictus data and square transformed A. aegypti data. Because RT 2.1 is incapable of analyzing models with more than three main effects, we removed the site effect by expressing each data point as a deviation from the mean of all containers at that site. Results from both the RT and SAS randomization analyses yielded conclusions identical to those of parametric ANOVAs, suggesting that ANOVA results are minimally influenced by departures from normality and homogenous variance. For brevity, we report only results of the parametric multimodel inference approach to ANOVA.
Egg Survival Experiment
Directly assaying the probability of surviving to hatching of the eggs used in the competition experiment was not possible because eggs could not be recovered from containers after inundation. We designed a concurrent experiment to estimate egg survival for each species. During each season we subjected groups of eggs of each species, ranging from 75± 9 eggs, to one of two environmental exposure treatments: 1) two weeks under optimal conditions in the laboratory, and 2) two weeks under ambient field conditions, as described above. We placed groups exposed to field conditions into containers similar to those used in the competition experiment and placed the containers under the hammock canopy on Day 1 so that they would experience the same ambient conditions as eggs in the Field egg exposure treatment of the competition study. We shielded field exposed eggs from rainfall and predatory insects as in the competition experiment. On Day 1 we placed eggs exposed to optimal laboratory conditions into the same sealed, humidified container as the Lab egg exposure treatment of the competition experiment. We replicated the laboratory and field treatments 8 times for each species with one group of A. aegypti in the laboratory treatment destroyed during sampling for a total of 31 and 32 egg groups in the wet and dry seasons, respectively.
On Day 15 we induced eggs to hatch with 0.15 g/L yeast: lactalbumen (1:1) hatching medium. After 48 hours we counted the number of living larvae for each group. We bleached the remaining eggs (Trpis 1970) and examined unhatched eggs for developed embryos, which we assumed to be dead, and undeveloped embryos. We quantified egg survival as: (hatched larvae)/(hatched larvae + unhatched fully embryonated eggs).
Analysis
We used the same multimodel inference approach to analyze egg survival(arcsine square-root transformed), comparing models with or without effects of environmental exposure, species, and season and all interactions (PROC MIXED - SAS Institute 2008), evaluating model weight of evidence using AICc.
Litter Accumulation
Because we covered containers in the competition experiments with an emergence chamber they were incapable of collecting detritus. To quantify seasonal differences in resource input, we placed 84 proxy containers next to experimental containers in the field and allowed them to collect plant and insect detritus, which we added to experimental containers every 6 to 14 days. To establish initial resources, we placed proxy containers into the field 18 and 17 days prior to the start of the competition experiment in the wet and dry seasons, respectively. At the beginning of the experiment, and every week thereafter, we collected litter from the proxy containers, pooled and dried it (50°C for two days), determined its total dry mass, and divided it equally among experimental containers. Thus, in any collection period all containers received the same amount of litter, but that amount varied over time and between seasons.
Analysis
The number of containers collecting litter and the length of collection periods both varied over time from 6 to 14 days. To account for this variation in collection effort and duration, we expressed the amount of litter collected in each period per container-day, using the expression:
where mc is the standardized litter mass (g container−1 day−1), mt is the total litter mass (g) collected in a period, nx is the number of containers collecting litter on day x of the collection period, and y is the number of days in the collection period. Standardized litter mass (log-transformed to meet assumptions of normality and homogeneity of variances) was analyzed by one-way ANOVA with season as the independent variable, and collection periods as replicates (wet season n = 8, dry season n = 6).
Results
Climatic Data
The first addition of water to the Natural field egg exposure treatment occurred one day after eggs had been exposed to field conditions in the wet season and 12 days after eggs had been exposed to field conditions in the dry season. The duration of egg exposure to field conditions in each season was comparable to seasonal intervals between rainfalls that added at least 30 ml of water to a proxy container in the open. The mean (± SE) interval between rainfall events was 3.63 ± 0.64 days in the wet season and 10.9 ± 2.61 days in the dry season. In the wet season no inter-rainfall intervals were greater than 14 days and in the dry season about 30% inter-rainfall intervals were greater than 14 days.
The mean (± SE) daily average temperature for the fortnight eggs were exposed to field conditions was 28.29 ± 0.43°C in the wet season and 21.15 ± 0.68 °C in the dry season. These values were comparable to the 15-year means for temperature (mean ± 95% confidence interval (SD)): 27.07 ± 0.49°C (0.89°C) for the wet season and 21.52 ± 0.53°C (0.96°C) for the dry season. The mean (± SE) daily average relative humidities to which eggs were exposed were similar in both seasons(wet season: 75.07 ± 1.16% RH; dry season 72.57 ± 1.47%RH). Again, these values were comparable to the 15-year means for relative humidity (mean ± 95% confidence intervals (SD)): 78.56 ± 2.59 %RH (4.87%RH) in the wet season and 71.57 ± 1.91%RH (3.45 %RH) in the dry season.
Competition Experiment
For A. aegypti, the best model included only the effect of season (Table 2). However a model including season, density, and interaction fit the data nearly as well (Table 2) and weight of evidence was very nearly as strong as that for the best model (Table 2). The fully saturated model also could not be ruled out, though weight of evidence for it was substantially less than that for the other two models (Table 2). There was, thus, little evidence that egg treatment affected A. aegypti composite index of performance. The model including season, density, and interaction yielded significant effects of season (maximum likelihood χ2=61.20, Df=1, P<0.0001, partial η2 = 0.36), density (χ2=10.34, Df=4, P=0.0351, partial η2 = 0.09), and interaction (χ2=9.52, Df=4, P=0.0493, partial η2 = 0.08).
Table 2.
Multiple model inference for effects of treatments on λ’ for the two Aedes species, and for egg survival. Analyses conducted in SAS 9.2, PROC MIXED. Weight of evidence (wi) expresses exp(ΔAICc/2) as a proportion of the sum of that column, and indicates the evidence for the model (i.e., the probability that the model is the correct one). Following Burnham, Anderson, & Huyvaert (2011), models with ΔAICc < 7 are deemed to have some nontrivial evidence in their favor and are indicated in bold type.
| Effects included | Log likelihood |
Parameters | AICc | ΔAICc | exp(ΔAICc/2) | Weight of evidence wi |
|---|---|---|---|---|---|---|
| Aedes aegypti λ’ | ||||||
| Season | 94.3 | 2 | 96.3 | 0 | 1.000 | 0.4207 |
| Season, Density, S*D | 94.6 | 10 | 96.6 | 0.3 | 0.861 | 0.3621 |
| Season, Density, Treatment, S*D, D*T, S*T, S*D*T | 95.7 | 30 | 97.8 | 1.5 | 0.480 | 0.2017 |
| Season, Density, Treatment, S*D, D*T | 102.0 | 20 | 104.1 | 7.8 | 0.020 | 0.0086 |
| Season, Treatment, S*T | 104.9 | 6 | 106.2 | 9.9 | 0.007 | 0.0030 |
| Season, Density, Treatment, S*D, S*T | 104.8 | 14 | 106.8 | 10.5 | 0.005 | 0.0022 |
| Season, Density, Treatment, S*D, D*T, S*T | 105.8 | 22 | 107.9 | 11.6 | 0.003 | 0.0013 |
| Season, Density, Treatment, D*T, S*T | 108.2 | 18 | 110.2 | 13.9 | 0.001 | 0.0004 |
| Season, Density, Treatment | 103.3 | 9 | 123.1 | 26.8 | 1.515×10−6 | 6.374×10−7 |
| Density | 140.8 | 5 | 142.80 | 46.5 | 7.99×10−11 | 3.362×10−11 |
| Treatment | 142.3 | 3 | 144.3 | 48.0 | 3.775×10−11 | 1.588×10−11 |
| Density, Treatment, D*T | 147.9 | 15 | 149.9 | 53.6 | 2.296×10−12 | 9.658×10−13 |
| Sum | 2.377 | |||||
| Aedes albopictus λ’ | ||||||
| Season | 52.0 | 2 | 56.1 | 0 | 1.000 | 0.7392 |
| Season, Treatment, S*T | 52.0 | 6 | 58.2 | 2.1 | 0.345 | 0.2587 |
| Season, Density, Treatment | 59.7 | 9 | 68.1 | 12 | 0.002 | 0.0018 |
| Treatment | 69.4 | 3 | 73.5 | 17.4 | 1.665×10−4 | 0.0001 |
| Season, Density, S*D | 69.2 | 10 | 75.5 | 19.4 | 6.128×10−5 | 4.5301×10−5 |
| Season, Density, Treatment, S*D, S*T | 69.6 | 14 | 78.0 | 21.9 | 1.756×10−5 | 1.2980×10−5 |
| Season, Density, Treatment, D*T, S*T | 71.0 | 18 | 79.4 | 23.3 | 8.719×10−6 | 6.4458×10−6 |
| Density | 79.8 | 5 | 81.8 | 25.7 | 2.626×10−6 | 1.9414×10−6 |
| Season, Density, Treatment, D*T, D*S | 75.0 | 20 | 83.4 | 27.3 | 1.180×10−6 | 8.7234×10−7 |
| Season, Density, Treatment, D*T, S*D, T*S, D*S*T | 75.3 | 30 | 83.8 | 27.7 | 9.661×10−7 | 7.1421×10−7 |
| Season, Density, Treatment, D*T, S*T, S*D | 77.9 | 22 | 86.3 | 30.2 | 2.768×10−7 | 2.0463×10−7 |
| Density, Treatment, D*T | 87.9 | 15 | 92.1 | 36.0 | 1.523×10−8 | 1.1259×10−8 |
| Sum | 1.353 | |||||
| Egg survival | ||||||
| Season, Species, S*Sp | −90.5 | 4 | −88.4 | 0 | 1.000 | 0.6743 |
| Species | −88.4 | 2 | −86.3 | 2.1 | 0.350 | 0.2360 |
| Season, Species, Treatment, S*Sp, S*T | −84.3 | 6 | −82.2 | 6.2 | 0.045 | 0.0304 |
| Season, Species, Treatment | −83.9 | 4 | −81.9 | 6.5 | 0.039 | 0.0261 |
| Season, Species, Treatment, S*Sp, Sp*T | −83.3 | 6 | −81.3 | 7.1 | 0.029 | 0.0194 |
| Species, Treatment, Sp*T | −81.1 | 4 | −79 | 9.4 | 0.009 | 0.0061 |
| Season, Species Treatment, S*Sp, Sp*T, S*T | −80.2 | 7 | −78.1 | 10.3 | 0.006 | 0.0039 |
| Season, Species, Treatment, S*Sp, Sp*T, S*T, S*Sp*T | −79.7 | 8 | −77.6 | 10.8 | 0.005 | 0.0030 |
| Season, Species, Treatment, Sp*T, S*T | −76.8 | 6 | −74.7 | 13.7 | 0.001 | 0.0007 |
| Season | −67.6 | 2 | −65.6 | 22.8 | 1.120×10−5 | 7.5494×10−6 |
| Treatment | −66.2 | 2 | −64.2 | 24.2 | 5.560×10−6 | 3.7489×10−6 |
| Season, Treatment, S*T | −61.1 | 4 | −59 | 29.4 | 4.129×10−7 | 2.7844×10−7 |
| 1.483 | ||||||
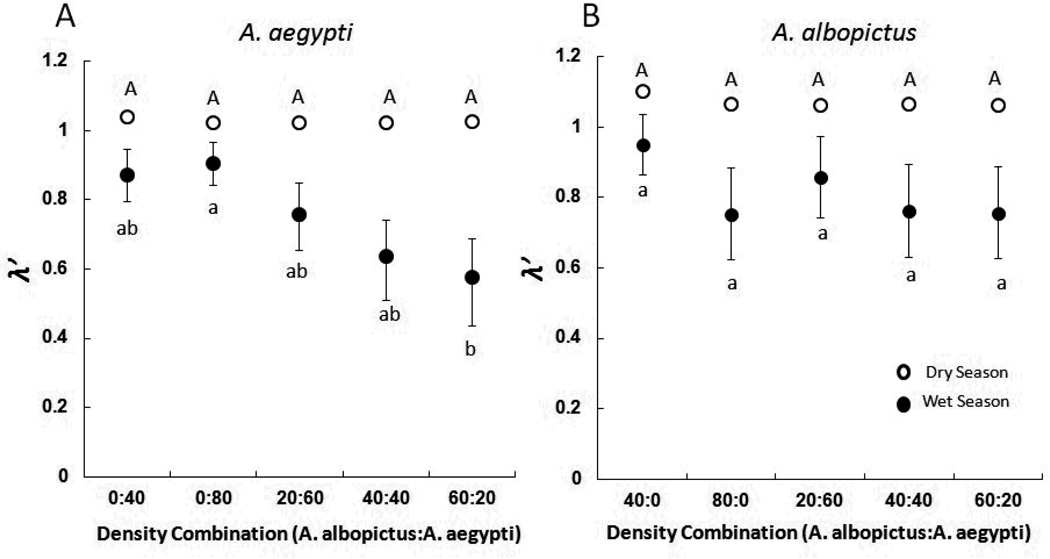
Pairwise differences among density treatment were significant within the wet season (F4,110=4.94, P=0.0001), but not within the dry season (F4,110=0.02, P=0.9989). In the wet season, λ’ was lowest when the greatest proportion of A. albopictus was present (Fig. 1A). Cohorts in low (0:40) and high (0:80) intraspecific density containers were not distinguishable from one another (Fig. 1A). High intraspecific density containers (0:80) yielded greater λ’ than did A. aegypti cohorts in containers where A. albopictus abundance was greater than A. aegypti abundance (60:20; Fig. 1A).
Figure 1.
Mean estimated finite rate of increase (λ’) ± SE of A. aegypti and A. albopictus at five density combinations in two seasons. Means for density treatments for each species within a season associated with the same letter are not significantly different in pairwise comparisons with a Bonferroni correction (experiment wise α = 0.05). Standard error bars for dry season means were small and are obscured by the symbols for means.
For A. albopictus the best model included only season (Table 2), and only a model including season, treatment, and interaction was even slightly supported and its weight of evidence indicated it was only about 1/3 as likely to be correct as the best model (Table 2). Even in this model, only the effect of season was significant (maximum likelihood χ2=22.42, Df=1, P<0.0001). Thus, there was little evidence that egg treatment affected A. albopictus composite index of performance. In the best model, season was significant (maximum likelihood χ2=19.44, Df=1, P<0.0001, partial η2 = 0.17), and λ’ for A. albopictus was substantially greater in the dry season than in the wet season (Fig. 1B).
Egg Survival Experiment
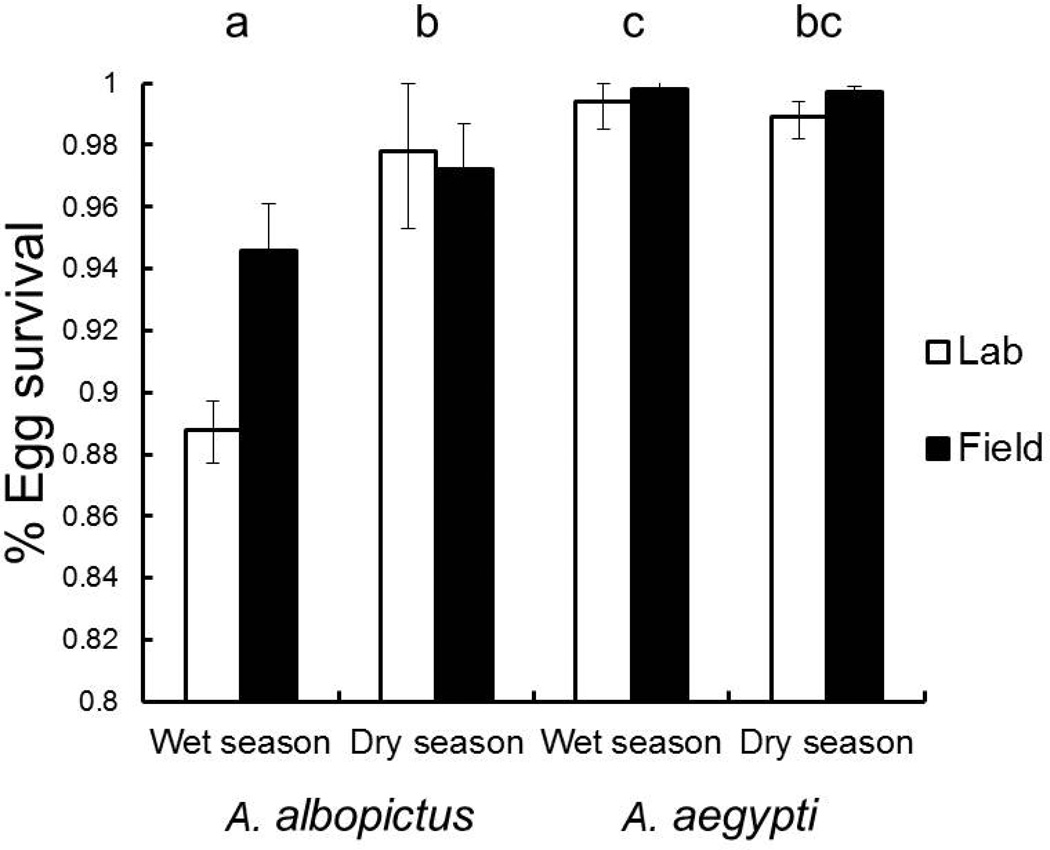
For egg survival the best model included season, species, and interaction (Table 2). A second model with only the effect of species had some support but its weight of evidence was 1/3 that of the best model (Table 2). Two other models had some evidence in their favor but had weights of evidence an order of magnitude less than that of the best model (Table 2). Thus, there was virtually no evidence that egg treatment had any effect on egg survival. In the best model effects of season (maximum likelihood χ2=34.78, Df=1, P<0.0001, partial η2 = 0.07), species (χ2=4.63, Df=1, P=0.0315, partial η2 = 0.38), and interaction (χ2=7.83, DF=1, P=0.0051, partial η2 = 0.12) were all significant. Aedes aegypti egg survival was unaffected by season, but A. albopictus had greater egg survival in the dry season than the wet season (Fig. 2). Egg survival in the dry season did not differ between the species, but in the wet season A. aegypti attained greater survival than did A. albopictus (Fig. 2). Despite differences between species and seasons, exposure to field conditions for intervals of two weeks had no obvious effect on A. albopictus and A. aegypti egg survival (Fig. 2). Both species had egg survival >88% in all cases. Survival of eggs exposed to field conditions was comparable to that of eggs exposed to optimal laboratory conditions (Fig. 2).
Figure 2.
Mean egg survival ± SE of A. albopictus and A. aegypti in the wet and dry seasons. Laboratory exposed, and Field exposed eggs were pooled and pairwise comparisons were performed on species-season means. Groups sharing a letter are not significantly different at Bonferroni experiment wise α = 0.05.
Litter Accumulation
Mean standardized amount of litter (back-transformed) collected in the dry season (0.026 ± 0.007g container−1 d−1) was over three times greater than that collected in the wet season (0.008 ± 0.003g container−1 d−1), and this difference was statistically significant (F 1,12 = 9.71, p = 0.0089) and the standardized effect size Δ was very large (Sokal & Rohlf 2012).
Discussion
Effects deemed important in our multimodel inference approach were also statistically significant and yielded effect sizes interpreted as large or medium. Thus, we are confident that these effects are rather strong, and potentially of biological importance.
In high density containers in the wet season, A. aegypti cohorts reared with > 50% A. albopictus had lower λ’ than did cohorts reared in the absence of A. albopictus (Fig. 1A). This suggests that interspecific competition from A. albopictus had a greater impact on A. aegypti than did intraspecific competition. This effect was largely eliminated in the dry season (Fig. 1A). In contrast, A. albopictus cohorts were unaffected by the presence of A. aegypti in both seasons (Fig. 1B). Such a competitive inequality favouring A. albopictus in the wet season is consistent with previous field competition experiments (Juliano 1998, Braks et al. 2004). Despite evidence of a competitive inequality, manipulation of desiccation exposure of eggs did not alter competitive effects between A. albopictus and A. aegypti. Our prediction of differential effects of competition on A. albopictus or A. aegypti among environmental egg exposure treatments in the dry season was not supported.
The limited effects of environmental exposure treatments were likely associated with low egg mortality for both species across season and treatment (Fig. 2). In the wet season A. albopictus had lower egg survival than A. aegypti. Surprisingly, egg survival was indistinguishable for the two species in the dry season and A. albopictus had greater egg survival in the dry season than in the wet season. These results indicate that seasonal differences in environmental conditions were less severe than expected. Furthermore, temperature and relative humidity observations taken during the experiment were similar to 15-year means suggesting abiotic conditions during the experiment were not unusual for the time of year. Why A. albopictus egg mortality was greater in the wet season is unknown. We speculate that disease is a possibility, but we observed no obvious signs of bacterial or fungal infection of wet season eggs. Another possible explanation is that mortality differences arose due to intrinsic differences in hatching success between the different lab colonies that we were forced to use in the two seasons. Regardless, the seasonal difference in A. albopictus egg survival was very small (< 6%), and by itself does not explain the considerable seasonal differences in λ’ for these Aedes. The egg mortality experiment revealed both species probably had high recruitment to the larval stage in the competition experiment, which we correctly predicted would result in a competitive advantage for A. albopictus (see Table 1).
Despite nearly uniform effects of competition between environmental exposure treatments, we detected strong seasonal differences in λ’ for both species, and a season-density interaction for A. aegypti. The responses of both species to seasonal differences were the same. Mean λ’ were substantially greater for both species in the dry season than in the wet season (Fig. 1A, B). The effects of competition on A. aegypti λ’ were undetectable in the dry season (Fig. 1A). Greater dry season λ’ for both species was associated with a three-fold increase in the mean daily accumulation of detritus in containers. We did not quantify amounts of each detritus type that accumulated in containers, but greater dry season litter biomass resulted primarily from flowers and leaves of live oak trees, Quercus virginiana. Larval mosquitoes reared on live oak flowers grow larger, complete development more quickly, and have greater estimated rates of population growth than those reared on live oak leaves (Lounibos, Nishimura & Escher 1993), indicating that dry season detritus resources were qualitatively, as well as quantitatively more favourable. Combined with the apparent absence of differential egg mortality, the greater λ’ and reduction of competitive effects in the dry season strongly suggests that both species were limited by resource availability in the wet season and were released from density-dependent resource limitation and competitive stress in the dry season due to high quantity and quality of detritus resources.
One of the goals of this investigation was to contribute to a growing body of research investigating coexistence of competing species in temporally variable environments (Pake & Venable 1995, Cáceres 1997, Descamps-Julien & Gonzalez 2005, Jiang & Morin 2007). Multi-year studies with competing species of zooplankton (Cáceres 1997) and desert annuals (Pake & Venable 1995) have shown that species can maintain long-term coexistence through fluctuation-dependent mechanisms. Fluctuation-dependent theory postulates that if, in combination with an environmentally resistant life stage, competing species partition the environment temporally so that each is favoured during different periods (limited more by intraspecific competition than interspecific competition) stable coexistence may occur in fluctuating environments (Chesson 2000). Aedes mosquitoes in south Florida possessing drought-resistant eggs met these criteria, and we hypothesized they could stably coexist due to temporal environmental fluctuations, albeit over a shorter time scale than that for zooplankton (Cáceres 1997) or desert annuals (Pake and Venable 1995).
Despite evidence of differential tolerances to desiccating conditions (Juliano et al. 2002, Costanzo, Kesavaraju & Juliano 2005, Lounibos et al. 2010) we found little evidence that seasonal changes in environmental conditions differentially affected species egg survival. Aedes albopictus and A. aegypti eggs suffered similar mortality levels in the dry season. These results suggest that dry season A. aegypti populations are not more limited by intraspecific than by interspecific competition. Further, these results indicate that A. aegypti coexistence with A. albopictus in Florida is unlikely to be a result of fluctuation-dependent stabilization.
Our data suggest that seasonal differences may contribute to coexistence, not as stabilizing mechanisms, but as fitness equalizing mechanisms (Chesson 2000), Adler, HillRisLambers & Levine 2007). That is, effects that minimize fitness differences between species may make it easier for less robust stabilizing mechanisms to produce species coexistence, or may simply prolong the expected time to competitive exclusion (Adler, HilleRisLambers & Levine 2007). Greater resource accumulation in containers in the dry season is the most likely source of such an equalizing effect on competition between A. albopictus and A. aegypti. Negative effects of interspecific competition on A. aegypti were evident in the wet season when resource inputs were low but not in the dry season when resources were abundant, and both species had greater λ’ during the dry season, indicating a release from resource competition. Unlike stabilizing effects, theory indicates that equalizing effects cannot by themselves maintain coexistence (Adler, HilleRisLambers & Levine 2007), suggesting the seasonal fluctuations in detritus resources in south Florida are unlikely to be the sole factor maintaining coexistence of A. aegypti and A. albopictus. Instead seasonal fluctuations of detritus resources may equalize fitness differences between the species, and this effect might prolong the expected time to competitive exclusion, making exclusion less likely (Chesson 2000).
Live oak trees were major contributors to the large amounts of resources that accumulated in containers in the dry season and accounted for much of the disparity in seasonal detritus accumulations. Live oaks are common throughout Florida and may serve an important role in equalizing competition between A. albopictus and A. aegypti at some sites. However, the results of this study regarding equalizing effects cannot be extrapolated across the diverse site types, with varying coverage of live oak canopy and varying climatic regimes, that support Aedes coexistence in Florida. This further underscores the importance of identifying stabilizing mechanisms that are capable of operating over broad geographic ranges, and simultaneously the potential for local context dependent effects on competitors. The relative importance of stabilizing vs. equalizing effects as contributors to coexistence of competitors are not well studied empirically (but see Adler, Ellner & Levine 2010) and the contributions of environmental fluctuations to stabilization vs. equalization clearly need further empirical study.
Acknowledgements
We thank Florida Medical Entomology Laboratory, L.P. Lounibos, and W.J. Tabachnik for access to facilities while in Florida, J.E. Fader, N. Nishimura, K. Greene, C.J. Westbrook, E. Blosser, and S. Brandt for aid in the field and the laboratory, and S.K. Sakaluk, and R.M. Bowden and three anonymous referees for comments on the manuscript and useful discussion. Supported by a subaward to SAJ from NIAID grant R01-AI-44793, and a Wiegel grant from the Beta Lambda chapter of Phi Sigma Biological Honor Society at Illinois State University to PAO.
Literature Cited
- Adler PB, HilleRisLambers J, Levine JM. A niche for neutrality. Ecology Letters. 2007;10:95–104. doi: 10.1111/j.1461-0248.2006.00996.x. [DOI] [PubMed] [Google Scholar]
- Adler PB, Ellner SP, Levine JM. Coexistence of perennial plants: An embarrassment of niches. Ecology Letters. 2010;13:1019–1029. doi: 10.1111/j.1461-0248.2010.01496.x. [DOI] [PubMed] [Google Scholar]
- Anderson DR. Model based inference in the life sciences: A primer on evidence. New York NY: Springer Science; 2008. [Google Scholar]
- Barrera R. Competition and resistance to starvation in larvae of container-inhabiting Aedes mosquitoes. Ecological Entomology. 1996;21:112–127. [Google Scholar]
- Braks MAH, Honório NA, Lounibos LP, Lourenço-de-Oliveira R, Juliano SA. Interspecific competition between two invasive species of container mosquitoes, Aedes aegypti and Aedes albopictus (Diptera: Culicidae), in Brazil. Annals of the Entomological Society of America. 2004;97:130–139. [Google Scholar]
- Briegel H. Metabolic relationship between female body size, reserves and fecundity of Aedes aegypti. Journal of Insect Physiology. 1990;36:165–172. [Google Scholar]
- Burnham KP, Anderson DR, Huyvaert KP. AIC model selection and multimodel inference in behavioral ecology: Some background, observations, and comparisons. Behavioral Ecology and Sociobiology. 2011;65:23–35. [Google Scholar]
- Cáceres CE. Temporal variation, dormancy, and coexistence: A field test of the storage effect. Proceedings of the National Academy of Sciences. 1997;94:9171–9175. doi: 10.1073/pnas.94.17.9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell DL. A randomization test wrapper for SAS PROCs (paper 251–27) SAS User Group International (SUGI); 2002. p. 27. [Google Scholar]
- Chase JM, Leibold MA. Ecological Niches: Linking Classical and Contemporary Approaches. Chicago, Illinois, USA: University of Chicago Press; 2003. [Google Scholar]
- Chesson PL. Mechanisms and maintenance of species diversity. Annual Review of Ecology and Systematics. 2000;31:343–366. [Google Scholar]
- Chesson PL, Huntly N. The roles of harsh and fluctuating conditions in the dynamics of ecological communities. The American Naturalist. 1997;150:519–553. doi: 10.1086/286080. [DOI] [PubMed] [Google Scholar]
- Christophers R. Aedes aegypti (L.) the Yellow Fever Mosquito. Its Life History, Bionomics and Structure. London, UK: Cambridge University Press; 1960. [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences, 2nd edition. Hillsdale, NJ, USA: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Costanzo KS, Kesavaraju B, Juliano SA. Condition-specific competition in container mosquitoes: the role of noncompeting life-history stages. Ecology. 2005;86:3289–3295. doi: 10.1890/05-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty MP, Alto BW, Juliano SA. Invertebrate carcasses as a resource for competing Aedes albopictus and Aedes aegypti (Diptera: Culicidae) Journal of Medical Entomology. 2000;37:364–372. doi: 10.1093/jmedent/37.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descamps-Julien B, Gonzalez A. Stable coexistence in a fluctuating environment: an experimental demonstration. Ecology. 2005;86:2815–2824. [Google Scholar]
- Duever MJ, Meeder JF, Meeder LC, McCollom JM. The climate of South Florida and its role in shaping the Everglades ecosystem. In: Davis SM, Ogden JC, editors. Everglades: the ecosystem and its restoration. Delray Beach, Florida: St. Lucie Press; 1994. pp. 225–248. [Google Scholar]
- Fenster CB, Dudash MR. Spatiotemporal variation in the role of hummingbirds as pollinators of Silene virginica. Ecology. 2001;82:844–851. [Google Scholar]
- Goldberg DE, Scheiner SM. ANOVA and ANCOVA: Field competition experiments. In: Scheiner SM, Gurevitch J, editors. Design and analysis of ecological experiments. 2nd edn. New York, New York: Chapman and Hall; 1993. pp. 69–93. [Google Scholar]
- Grill CP, Juliano SA. Predicting species interactions based on behaviour: predation and competition in container-dwelling mosquitoes. Journal of Animal Ecology. 1996;65:63–76. [Google Scholar]
- Hawley WA. The biology of Aedes albopictus. Journal of the American Mosquito Control Association. 1988;4(Supplement):1–40. [PubMed] [Google Scholar]
- Hawley WA, Reiter P, Copeland RS, Pumpuni CB, Craig GB., Jr. Aedes albopictus in North America: probable introduction in used tires from Northern Asia. Science. 1987;236:1114–1116. doi: 10.1126/science.3576225. [DOI] [PubMed] [Google Scholar]
- Holway DA, Suarez AV, Case TJ. Role of abiotic factors in governing susceptibility to invasion: a test with Argentine ants. Ecology. 2002;83:1610–1619. [Google Scholar]
- Hutchinson GE. The paradox of the plankton. The American Naturalist. 1961;95:137–145. [Google Scholar]
- Jiang L, Morin PJ. Temperature fluctuation facilitates coexistence of competing species in experimental microbial communities. Journal of Animal Ecology. 2007;76:660–668. doi: 10.1111/j.1365-2656.2007.01252.x. [DOI] [PubMed] [Google Scholar]
- Juliano SA. Species introduction and replacement among mosquitoes: interspecific resource competition or apparent competition? Ecology. 1998;79:255–268. [Google Scholar]
- Juliano SA. Species interactions among larval mosquitoes: context dependence across habitat gradients. Annual Review of Entomology. 2009;54:37–56. doi: 10.1146/annurev.ento.54.110807.090611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SA, Lounibos LP, O’Meara GF. A field test for competitive effects of Aedes albopictus on A. aegypti in South Florida: differences between sites of coexistence and exclusion? Oecologia. 2004;139:583–593. doi: 10.1007/s00442-004-1532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SA, O’Meara GF, Morrill JR, Cutwa MM. Desiccation and thermal tolerance of eggs and the coexistence of competing mosquitoes. Oecologia. 2002;130:458–469. doi: 10.1007/s004420100811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromrey JD, Bell BA. ES_ANOVA: A SAS® Macro for Computing Point and Interval Estimates of Effect Sizes Associated with Analysis of Variance Models. 2010 http://analytics.ncsu.edu/sesug/2010/PO05.Kromrey.pdf.
- Leisnham PT, Juliano SA. Interpopulation differences in competitive effect and response of the mosquito Aedes aegypti and resistance to invasion by a superior competitor. Oecologia. 2010;164:221–230. doi: 10.1007/s00442-010-1624-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livdahl TP, Sugihara G. Non-linear interactions of populations and the importance of estimating per capita rates of change. Journal of Animal Ecology. 1984;53:573–580. [Google Scholar]
- Livdahl TP, Willey MS. Prospects for an invasion: Competition between Aedes albopictus and native Aedes triseriatus. Science. 1991;253:189–191. doi: 10.1126/science.1853204. [DOI] [PubMed] [Google Scholar]
- Lounibos LP. Invasions by insect vectors of human disease. Annual Review of Entomology. 2002;47:233–266. doi: 10.1146/annurev.ento.47.091201.145206. [DOI] [PubMed] [Google Scholar]
- Lounibos LP, Suárez S, Menéndez Z, Nishimura N, Escher RL, O’Connell SM, Rey JR. Does temperature affect the outcome of larval competition between Aedes aegypti and Aedes albopictus? Journal of Vector Ecology. 2002;27:86–95. [PubMed] [Google Scholar]
- Lounibos LP, Nishimura N, Escher RL. Fitness of a treehole mosquito: influences of food type and predation. Oikos. 1993;66:114–118. [Google Scholar]
- Lounibos LP, O’Meara GF, Juliano SA, Nishimura N, Escher RL, Reiskind MH, Cutwa M, Greene K. Differential survivorship of invasive mosquito species in south Florida cemeteries: do site-specific microclimates explain patterns of coexistence and exclusion? Annals of the Entomological Society of America. 2010;103:757–770. doi: 10.1603/AN09142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly BFJ. RT: a program for randomization testing. Version 2.1. University of Otago, New Zealand: Centre for Applications of Statistics and Mathematics; 1997. [Google Scholar]
- Murrell EG, Juliano SA. Detritus type alters the outcome of interspecific competition between Aedes aegypti and Aedes albopictus (Diptera: Culicidae) Journal of Medical Entomology. 2008;45:375–383. doi: 10.1603/0022-2585(2008)45[375:dtatoo]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Meara GF, Evans LF, Jr, Gettman AD, Cuda JP. Spread of Aedes albopictus and decline of Ae. aegypti (Diptera: Culicidae) in Florida. Journal of Medical Entomology. 1995;32:554–562. doi: 10.1093/jmedent/32.4.554. [DOI] [PubMed] [Google Scholar]
- Rey JR, Nishimura N, Wagner B, Braks MAH, O’Connell SM, Lounibos LP. Habitat segregation of mosquito arbovirus vectors in south Florida. Journal of Medical Entomology. 2006;43:1134–1141. doi: 10.1603/0022-2585(2006)43[1134:hsomav]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute. SAS/STAT user’s guide. Cary, North Carolina, USA: SAS Institute; 2008. [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry, 4th edition. New York, NY, USA: WH Freeman; 2012. [Google Scholar]
- Southeast Regional Climate Center, The. The University of North Carolina at Chapel Hill. 2007 Visited March 15, 2012. www.sercc.com/climateinfo/historical/historical_fl.html.
- Sota T, Mogi M. Interspecific variation in desiccation survival time of Aedes (Stegomyia) mosquito eggs is correlated with habitat and egg size. Oecologia. 1992;90:353–358. doi: 10.1007/BF00317691. [DOI] [PubMed] [Google Scholar]
- Tabachnick WJ. Evolutionary genetics and arthropod-borne disease: the yellow fever mosquito. American Entomologist. 1991;37:14–24. [Google Scholar]
- Taniguchi Y, Nakano S. Condition-dependent competition: implications for the altitudinal distribution of stream fishes. Ecology. 2000;81:2027–2039. [Google Scholar]
- Trpis M. A new bleaching and decalcifying method for general use in zoology. Canadian Journal of Zoology. 1970;48:892–893. [Google Scholar]
- Weather Underground, Inc. History for Vero Beach, FL. 2012 Visited March 15, 2012. www.wunderground.com/history/airport/KVRB/2012/3/15/DailyHistory.html?req_city=NA&req_state=NA&req_statename=NA.
- Winston PW, Bates DH. Saturated solutions for the control of humidity in biological research. Ecology. 1960;41:232–237. [Google Scholar]