Abstract
The study aim was to test the hypothesis that motor function undergoes accelerated decline proximate to death. As part of a longitudinal clinical-pathologic study, 124 older Catholic nuns, priests, and monks completed at least 7 annual clinical evaluations, died, and underwent brain autopsy and uniform neuropathologic examination. Each evaluation included administration of 11 motor tests and 19 cognitive tests from which global measures of motor and cognitive function were derived. The global motor measure (baseline mean = 0.82, SD=0.21) declined a mean 0.024-unit per year (95% confidence interval [CI]: −0.032, −0.016) until a mean of 2.46 years (95% CI: −2,870, −2.108) before death when rate of decline increased nearly 5-fold to −0.117-unit per year (95% CI: −0.140, −097). The global cognitive measure (baseline mean =0.07, SD =0.45) declined a mean of 0.027-unit per year (95% CI: −0.041, −0.014) until a mean of 2.76 years (95% CI: −3.157, −2.372) before death when rate of decline increased more than 13-fold to −0.371-unit per year (95% CI: −0.443, −0.306). Onset of terminal motor decline was highly correlated with onset of terminal cognitive decline (r=0.94, 95% CI: 0.81, 0.99), but rates of motor and cognitive change were not strongly correlated (preterminal r = 0.20, 95% CI: −0.05, 0.38; terminal r = 0.34, 95% CI: 0.03, 0.62). Higher level of plaques and tangles was associated with earlier onset of terminal decline in motor function, but no pathologic measures were associated with rate of preterminal or terminal motor decline. The results demonstrate that motor and cognitive function both undergo a period of accelerated decline in the last few years of life.
Keywords: motor decline, cognitive decline, mortality, neuritic plaques, neurofibrillary tangles
Motor functioning declines in old age (Vandervoort, 2002; Bassey, 1998). Lower level of motor function (Al Snih, Markides, Ray,Ostir, & Goodwin, 2002; Fujita et al., 1995; Laukkahen, Heikkinen, & Kauppinen, 1995; Phillips, 1986; Rantanen et al., 2000; Ratanen et al., 2003) and faster rate of motor decline (Buchman, Wilson, Boyle, Bienias, & Bennett, 2007; Onder et al., 2002) are also associated with increased mortality. This suggests that some portion of late life loss of motor function may reflect impending death rather than advancing age. Recent research has established that cognitive function undergoes a period of accelerated decline in the last years of life (Laukka, MacDonald, & Bäckman, 2006; MacDonald, Hultsch, & Dixon, 2011 Sliwinski et al., 2006; Thorvaldsson et al., 2008; Wilson, Beck, Bienias, & Bennett, 2007; Wilson, Beckett, Bienias, Evans, & Bennett, 2003). It is uncertain whether motor function undergoes a similar period of terminal decline, and if so whether common mechanisms underlie terminal cognitive and motor decline.
We examined the relation of mortality to change in motor function using data from the Religious Orders Study, a longitudinal clinical-pathologic investigation of common chronic conditions of old age. Older Catholic nuns, priests, and monks completed annual clinical evaluations for 6 to 15 years before death. The evaluations included detailed performance tests of motor and cognitive function from which composite measures were derived. Level of plaques and tangles and the presence of Lewy bodies and cerebral infarction were ascertained from a uniform neuropathologic examination. Analyses used death as the temporal reference point and allowed rate of decline in motor function to accelerate at some variable time before death to test for terminal motor decline. The models also included cognitive outcome measures, to assess covariation of terminal motor changes with terminal changes in cognitive function that have been previously described in this (Wilson et al., 2003) and other (Laukka et al., 2006; MacDonald et al., 2011; Sliwinski et al., 2006; Thorvaldsson et al., 2008; Wilson et al., 2007) cohorts, and pathologic measures, to identify factors that may be contributing to terminal decline.
Methods
Participants
Subjects are from the Religious Order Study (Wilson, Bienias, Evans, & Bennett, 2004). The study involves annual clinical evaluations, which began in 1994 and are continuing, and brain donation in the event of death. Participants are Catholic nuns, priests, and brothers recruited from about 40 groups across the United States. After a thorough description of the study, written informed consent was obtained from all participants. The study was approved by the institutional review board of Rush University Medical Center.
Eligibility for primary analyses required (i) the absence of dementia at baseline, (ii) a minimum of 7 clinical evaluations before death, to make it possible to observe a shift in rate of cognitive change at some variable point prior to death, and (iii) brain autopsy. A total of 124 participants met these criteria. At baseline, their mean age was 77.7 (SD= 6.3, range: 65–93) and at death it was 88.6 (SD=6.3, range: 75–104). They completed from 7 to 16 annual evaluations (mean=10.9, SD=1.8), with the last one occurring a median of 8.0 months before death (interquartile range: 3.8 – 12.1). They had a mean of 18.0 years of education (SD=3.1) and 62.1% were women. At baseline, 96 had no cognitive impairment and 28 had mild cognitive impairment.
Clinical Classification
Each annual clinical evaluation included a structured medical history, neurological examination, and cognitive and motor testing. On the basis of this evaluation, an experienced clinician diagnosed dementia and mild cognitive impairment, as previously described (Wilson, Schneider, Arnold, Bienias, & Bennett, 2007). Dementia required a history of cognitive decline and evidence of impairment in at least 2 cognitive domains (McKhann et al., 1984). Cognitively impaired individuals who did not meet criteria for dementia were classified as having mild cognitive impairment (Bennett et al., 2002).
Assessment of Motor Function
Motor function was assessed annually with 11 performance tests of familiar motor behaviors. Grip strength and pinch strength in each hand were assessed with the Jamar hydraulic hand and pinch dynamometers (Lafayette Instruments). Manual dexterity was assessed bilaterally with the Purdue Pegboard (Lafayette Instruments; two 30-sec trials per hand) and an electronic finger tapper (Western Psychological Services; two 10-sec trials per hand). Gait and balance were assessed with 7 performance tests: 8 foot walk (time, number of steps), turning 360° (time, number of steps), standing on 1 leg (time), standing on toes (time), 8 foot heel to toe walk (steps off line).
Composite measures of motor function were used in longitudinal analyses to allow for a wide spectrum of motor ability. The primary outcome was a measure of global motor function based on all 11 measures. To ensure that each individual test made an approximately equal contribution to the composite, raw scores were divided by the baseline standard deviation for all study participants, and the standard scores were averaged to yield the global motor measure. Composite measures of manual strength (2 tests), manual dexterity (2 tests), and gait (4 tests), were formed in a similar manner. We did not form a composite balance measure because the balance tests, unlike the other motor tests, were sometimes not attempted. Further information on the individual motor tests is contained in previous publications (Buchman et al., 2011; Buchman Wilson, Bienias, & Bennett, 2005; Buchman et al., 2007).
Assessment of Cognitive Function
Cognitive function was assessed annually with a set of 19 performance tests with minimal motor demands (e.g., no writing, drawing, or assembly required). There were 7 measures of episodic memory (Word List Recall, Memory, and Recognition; immediate and delayed recall of 2 stories); 4 measures of semantic memory (Verbal Fluency; short form of Boston Naming Test; 20-item word recognition test; 15-item Extended Range Vocabulary Test); 4 measures of working memory (Digit Span Forward and Backward; Digit Ordering; Alpha Span); 2 measures of perceptual speed (oral format of Symbol Digit Modalities Test, Number Comparison); and 2 measures of visuospatial ability (short forms of Judgment of Line Orientation and Standard Progressive Matrices).
To minimize measurement error, composite measures of cognitive function were used in analyses. A measure of global cognition based on all 19 tests was the primary outcome. Composite measures of episodic memory (based on 7 tests), semantic memory (4 tests), working memory (4 tests), and perceptual speed (2 tests) were used in secondary analyses. To weight each test approximately equally, raw scores on component tests were converted to z scores, using the baseline mean and standard deviation in the entire cohort, and averaged to yield composite scores. Further information on the individual tests and the derivation of the composite scores is published elsewhere (Wilson et al., 2002; Wilson et al., 2004; Wilson et al., 2002).
Assessment of Other Covariates
Two previously established composite measures of vascular burden were derived from the baseline medical history and clinical evaluation (Wilson, Arnold, Beck, Bienias, & Bennett, 2008). Vascular risk factors was the number of 3 factors present (diabetes, hypertension, smoking). Vascular conditions was the number of 3 conditions present (claudication, stroke, heart attack).
Neuropathologic Examination
Brain removal, tissue sectioning and preservation, and quantification of pathologic results followed a standard protocol that is detailed in previous publications (Schneider, Arvanitakis, Bang, & Bennett, 2007; Schneider, Arvanitakis, Leurgans, & Bennett, 2009; Schneider et al., 2003). Neuritic plaques, diffuse plaques, and neurofibrillary tangles were counted by a neuropathologist or trained technician in 4 brain regions (entorhinal cortex, midfrontal cortex, inferior parietal cortex, middle temporal cortex) using a modified Bielschowsky silver stain. Raw counts of each lesion in each region were standardized and then averaged to yield a composite index of AD pathologic burden (Bennett et al., 2003; Wilson et al., 2003). In previous research, the interrater reliability estimates for the counts of neuritic plaques, diffuse plaques, and neurofibrillary tangles ranged from 0.89 to 0.93, and Cronbach’s coefficient alpha for the 12 components of the AD pathologic index (ie., 3 lesions × 4 regions) was 0.90, suggesting adequate internal consistency reliability (Bennett et al., 2003). This AD pathologic index has previously been associated with likelihood of dementia and rate of cognitive decline (Bennett et al., 2003; Wilson, Krueger, Boyle, & Bennett, 2011).
Lewy bodies were identified with antibodies to alpha-synuclein (Zymed, LB509; 1:100) using the avidin-biotin method as previously described (Schneider et al., 2009; Schneider et al., 2007; Wilson et al., 2011). Six brain regions were examined: substantia nigra, entorhinal cortex, cingulate cortex, midfrontal cortex, inferior parietal cortex, and middle temporal cortex. Lewy bodies were treated as present or absent in analyses. This summary Lewy body index has previously been associated with cognitive, motor, and olfactory impairment (Wilson, Leurgans, Boyle, Schneider & Bennett, 2010; Wilson et al., 2011).
Each brain was visually examined for cerebral infarctions after fixation in 4% paraformaldehyde. For the present analyses, we counted old cortical and subcortical gray and white matter infarctions excluding those thought to have occurred within 6 months of death or confined to the brainstem or cerebellum, as done in previous studies linking chronic cerebral infarcts to dementia, cognitive impairment, and cognitive decline (Schneider et al., 2003; Wilson et al., 2010). Infarction data were summarized in a 3-point index with a score of 0 for no infarctions, 1 for a single infarction, and 2 for multiple infarctions.
Data Analysis
We simultaneously analyzed change in motor and cognitive function in a series of mixed-effects change point models (Laird & Ware, 1982) using a Bayesian Monte Carlo Markov Chain Approach (Gelman, Carli, Stern, & Rubin, 2004) implemented in OpenBUGS software (Lunn, Spiegelhalter, Thomas, & Best, 2009). Each model included random effects for the motor and cognitive intercepts, preterminal slopes, change points, and terminal slopes to allow for individual differences in motor and cognitive trajectories. The initial model used global measures of motor and cognitive function as outcomes. Subsequent analyses included participants with fewer follow-up visits; added terms for age at death or vascular burden; excluded individuals with mild cognitive impairment; used specific motor and cognitive measures as outcomes; and added terms for postmortem pathologic measures.
Results
At baseline, scores on the global motor measure ranged from a low of 0.44 to a high of 1.48 and were approximately normally distributed (mean=0.82, SD=0.21, skewness=0.37). Younger age (r=−.55, p<0.001), male gender (t [116.9] = 2.67, p=0.009), and more education (r=0.22, p=0.013) were associated with higher baseline level of motor function. Baseline motor function was moderately correlated (r=0.40, p<0.001) with baseline score on a global measure of cognitive function (mean = 0.07, SD=0.45, skewness −0.67).
We simultaneously assessed change in the global measures of motor and cognitive function using a mixed-effects model that allowed decline in each function to accelerate at some time before death (first column of Table 1). On average, terminal decline in motor function began 2.464 years before death. The mean rate of motor decline increased from a loss of 0.024-unit per year before the terminal period to a loss of 0.117-unit per year during the terminal period, a 4.9-fold increase. The random effects in the table indicate that there was significant variability in level of motor function proximate to death, rates of preterminal and terminal motor change, and onset of terminal motor decline.
Table 1.
Change in global motor and global cognitive function*
| ≥ 7 Evaluations | ≥ 6 Evaluations | ≥ 5 Evaluations | ≥ 4 Evaluations | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Effects | Model Term | Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI |
| Motor | Fixed | Intercept | 0.363 | 0.317, 0408 | 0.383 | 0.345, 0.423 | 0.384 | 0.348, 0.416 | 0.398 | 0.366, 0.30 |
| Preterminal slope | −0.024 | −0.032, −0.016 | −0.022 | −0.030, −0.015 | −0.022 | −0.028,−0.015 | −0.021 | −0.027, −0.014 | ||
| Terminal slope | −0.117 | −0.140,−0.097 | −0.111 | −0.131, −0.094 | −0.106 | −0.124, −0.092 | −0.104 | −0.118, −0.090 | ||
| Change point | −2.464 | −2.870, −2.108 | −2.727 | −3.129, −2.330 | −2.777 | −3.189, −2.410 | −2.758 | −3.127, −2.429 | ||
| Random | Intercept | 0.038 | 0.025, 0.056 | 0.044 | 0.028, 0.056 | 0.038 | 0.028, 0.051 | 0.039 | 0.031, 0.051 | |
| Preterminal slope | 0.002 | 0.001, 0.002 | 0.001 | 0.001, 0.002 | 0.001 | 0.001, 0.002 | 0.001 | 0.001, 0.002 | ||
| Terminal slope | 0.004 | 0.002, 0.006 | 0.003 | 0.002, 0.005 | 0.003 | 0.002,0.005 | 0.003 | 0.002, 0.004 | ||
| Change point | 1.274 | 0.784, 1.959 | 1.626 | 0.973, 2637 | 1.880 | 1.190, 2.811 | 1.747 | 1.189, 2.594 | ||
| Error | 0.011 | 0.010, 0.012 | 0.010 | 0.009, 0.011 | 0.010 | 0.009, 0.011 | 0.010 | 0.009, 0.011 | ||
| Cognitive | Fixed | Intercept | −1.168 | −1.410, −0.929 | −1.200 | −1.418, −0.987 | −1.259 | −1.444, −1.072 | −1.259 | −1.441, −1.078 |
| Preterminal slope | −0.027 | −0.041, −0.014 | −0.023 | −0.035, −0.011 | −0.026 | −0.036, −0.013 | −0.027 | −0.037, −0.017 | ||
| Terminal slope | −0.371 | −0.443, −0.306 | −0.349 | −0.411, −0.295 | −0.372 | −0.429, −0.320 | −0.381 | −0.435, −0.334 | ||
| Change point | −2.757 | −3.157, −2.372 | −3.032 | −3.450, −2.654 | −3.017 | −3.371, −2.664 | −2.891 | −3.197,−2.609 | ||
| Random | Intercept | 1.674 | 1.267, 2.184 | 1.751 | 1.390, 2.225 | 1.712 | 1.387, 2.134 | 1.721 | 1.400, 2.068 | |
| Preterminal slope | 0.004 | 0.003, 0.005 | 0.004 | 0.003, 0.005 | 0.003 | 0.003, 0.005 | 0.004 | 0.003, 0.005 | ||
| Terminal slope | 0.045 | 0.030, 0066 | 0.041 | 0.029, 0.058 | 0.052 | 0.039, 0.071 | 0.054 | 0.041, 0.070 | ||
| Intercept | 2.017 | 1.433, 2.764 | 2.734 | 2.011, 3.603 | 2.705 | 2.035, 3.531 | 2.371 | 1.865, 3.104 | ||
| Error | 0.058 | 0.053, 0.63 | 0.062 | 0.058, 0.067 | 0.066 | 0.061, 0.071 | 0.065 | 0.061, 0.070 | ||
Estimated from 4 separate mixed-effects change point models. C1, confidence interval.
Terminal decline in cognitive function began a mean of 2.757 years before death (first column of Table 1) which was a mean of 0.293 year (SD = 0.461) before the onset of terminal motor decline. The distribution of these onset differences is shown in Figure 1. In most instances, the terminal motor and terminal cognitive periods began within 6 months of each other. Terminal cognitive changes began before terminal motor changes in most (hatched portion of Figure 1) but not all (shaded portion of Figure 1) individuals. The cognitive score declined a mean of 0.027-unit per year before the terminal period compared to a mean of 0.371-unit per year during it, a 13.7-fold increase in annual rate of cognitive decline. Individual differences in cognitive trajectories were also evident.
Figure 1.
Frequency histogram of the distribution of the differences between predicted onsets of terminal motor decline and terminal cognitive decline; hatched lines, cognitive changes preceded motor changes; dark shading, motor changes preceded cognitive changes.
Figure 2 shows the crude paths of motor and cognitive change of all subjects (colored lines) and the predicted paths of a typical subject with 10 years of follow-up prior to death (black line). Substantial heterogeneity is evident in the crude paths of motor and cognitive change. However, the model-based estimates show that motor decline sharply accelerated about 2.5 years before death at about the same time that comparable changes were occurring in cognitive function.
Figure 2.
Change in global motor and global cognitive function. Crude paths of change (colored lines) and mean paths of change predicted by the model (black lines) in global motor function (top) and global cognitive function (bottom) during the last decade of life.
The mixed-effects model also provided estimates of the correlation between trajectories of motor and cognitive change. The onset of terminal motor decline was highly correlated with the onset of cognitive decline (r=0.94, 95% CI: 081, 0.99). By contrast, rates of motor and cognitive change were not correlated before the terminal period (r=0.20, 95% CI: −0.05, 0.38) and modestly correlated during it (r=0.34, 95% CI: 0.03, 0.62). Because death was the temporal reference point in the analysis, the intercept was the estimated level of function proximate to death. The motor and cognitive intercepts were moderately correlated (r=0.72, 95% CI: 0.56, 0.84).
Eligibility for analyses required completion of at least 7 annual clinical evaluations. To determine whether this criterion affected results, we repeated the analysis requiring a minimum of 6 evaluations (n=158, second column of Table 1), a minimum of 5 evaluations (n=195, third column of Table 1), and a minimum of 4 evaluations (n=235, fourth column of Table 1). The results of each analysis were similar to the original analysis, suggesting that results were not substantially affected by requiring at least 6 years of observation.
Because advancing age is robustly related to motor and cognitive functioning, we repeated the original analysis with a term added for age at death. In this analysis, older age at death was marginally associated with lower motor and cognitive function proximate to death and slower terminal motor decline (first column of Table 2).
Table 2.
Relation of age and vascular disease to change in global motor and global cognitive function*
| Age at Death | V ascular Risk Factors | V ascular Conditions | |||||
|---|---|---|---|---|---|---|---|
| Outcome | Model Term | Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI |
| Motor | Intercept | −0.051 | −0.092,−0.012 | 0.030 | −0.014, 0.073 | −0.014 | −0.057, 0027 |
| Preterminal slope | 0.005 | −0.003, 0.12 | −0.003 | −0.011, 0.005 | 0.002 | −0.005, 0010 | |
| Terminal slope | 0.021 | 0.005, 0.37 | −0.003 | −0.023, 0.14 | 0.007 | −.0.013, 0.22 | |
| Change point | −0.062 | −0.381, 0.255 | 0.236 | −0.070, 0.536 | −0.204 | −0.628, 0167 | |
| Cognitive | Intercept | −0.252 | −0.488, −0.020 | 0.067 | −0.167, 0315 | −0.056 | −0.279, 0181 |
| Preterminal slope | −0.006 | −0.017, 0.008 | 0.004 | −0.009, 0.17 | 0.007 | −0.007, 0.021 | |
| Terminal slope | 0.015 | −0.038, 0071 | −0.044 | −0.100, 0.012 | 0.017 | −0.038, 0.064 | |
| Change point | −0.199 | −0.519, 0.125 | 0.333 | 0.016, 0.638 | −0.352 | −0.708, 0.033 | |
Estimated from 3 separate mixed-effects change point models. Results show the fixed effect of a 1-unit increase in each covariate. CI, confidence interval.
Vascular disease can adversely affect motor and cognitive function. Therefore, we repeated the original analysis first adding a term for the number of 3 vascular risk factors present at baseline (mean = 0.84, SD = 0.74) and then with a term added for the number of 3 vascular conditions present at baseline (mean = 0.26, SD = 0.51). Higher number of vascular risk factors was marginally associated with later onset of terminal cognitive decline (second column in bottom row of Table 2). Vascular conditions were not related to trajectories of motor or cognitive decline (third column of Table 2).
At baseline, 28 participants (22.6%) met criteria for mild cognitive impairment. To examine whether these individuals affected results, we excluded them and repeated the analysis on the remaining 96 participants. In this analysis, the overall trajectories of motor and cognitive change were similar except that the terminal accelerations began slightly later (Table 3).
Table 3.
Change in global motor and global cognitive function in 96 individuals with no cognitive impairment at baseline*
| Outcome | Effects | Model Term | Estimate | 95% CI |
|---|---|---|---|---|
| Global motor function | Fixed | Intercept | 0.362 | 0.303, 0.409 |
| Preterminal slope | −0.027 | −0.037,−0.018 | ||
| Terminal slope | −0.139 | −0.171,−0.112 | ||
| Change point | −1.983 | −2.313, −1.699 | ||
| Random | Intercept | 0.035 | 0.022, 0.057 | |
| Preterminal slope | 0.002 | 0.001, 0.002 | ||
| Terminal slope | 0.005 | 0.003, 0009 | ||
| Change point | 0.570 | 0.326, 1.038 | ||
| Error | 0.011 | 0.010, 0.013 | ||
| Global cognitive function | Fixed | Intercept | −1.034 | −1.273, −0.785 |
| Preterminal slope | −0.031 | −0.047, −0.018 | ||
| Terminal slope | −0.389 | −0.476, −0.312 | ||
| Change point | −2.475 | −2.881, −2.090 | ||
| Random | Intercept | 1.356 | 0.980, 1.873 | |
| Preterminal slope | 0.004 | 0.003, 0.006 | ||
| Terminal slope | 0.049 | 0.030, 0.82 | ||
| Change point | 1.512 | 1.016, 2.208 | ||
| Error | 0.059 | 0.053, 0.065 |
Estimated from a mixed-effects change point model. CI, confidence interval.
Terminal cognitive changes often lead to a diagnosis of dementia (Wilson, Segawa, Hizel, Boyle, & Bennett, in press). In the core group of 124 participants, 53 (42.7%) developed dementia during follow-up. In this subgroup, the initial dementia diagnosis was made approximately one year before the estimated onset of terminal decline in global motor function (median difference = 0.92 year; interquartile range: −2.32–1.85) and at approximately the same time as the estimated onset of terminal decline in global cognitive function (median difference = 0.00 year; interquartile range: −2.98 – 1.31).
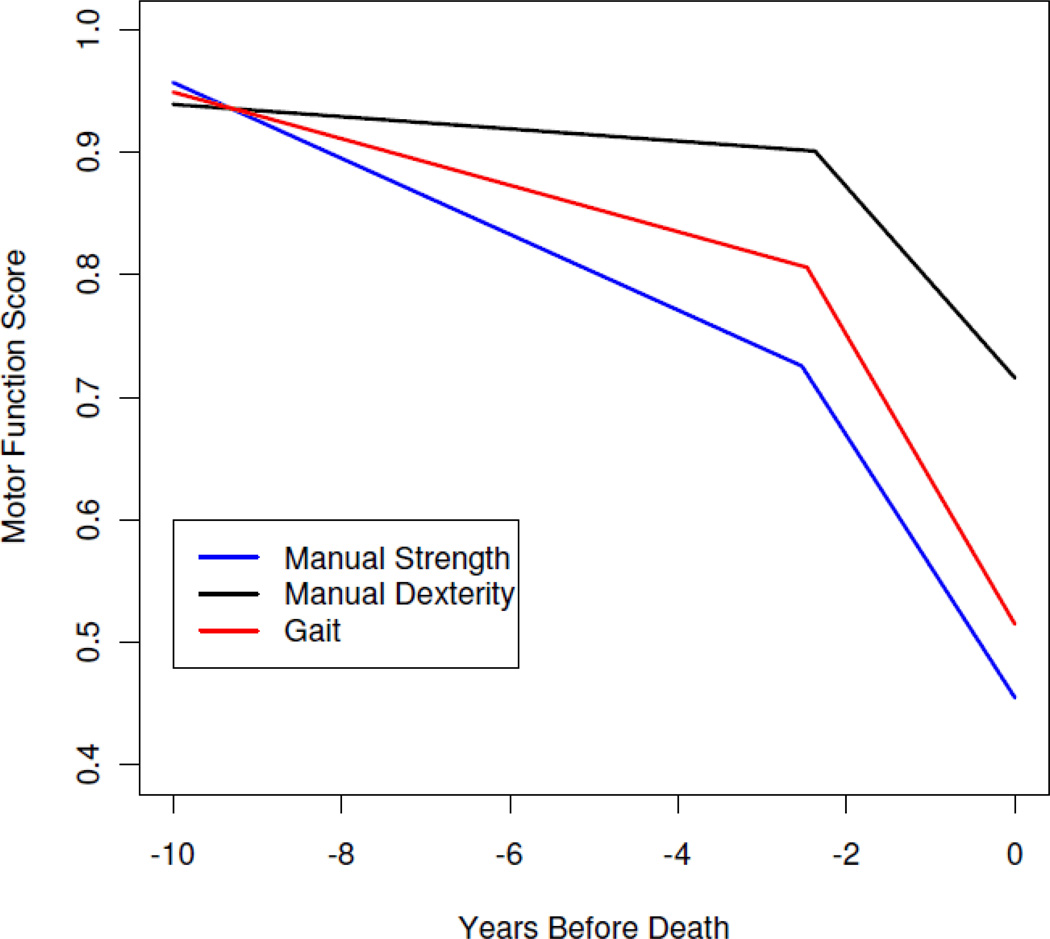
To see if mortality was more related to change in some functions than others, we repeated the original analysis with specific measures of manual strength, manual dexterity, and gait in place of the global motor measure (Table 4). As shown in Figure 3 which is based on these analyses, decline in each motor measure accelerated a mean of about 2.5 years before death. In each case, the onset of terminal cognitive decline preceded and almost perfectly predicted the onset of terminal motor decline. We then repeated the original analysis with the global motor measure and specific measures of cognitive function in place of the global cognitive measure (Table 5). In these analyses, terminal cognitive decline began a mean of 2.7 (semantic memory) to 3.2 (perceptual speed) years before death which was 0.2 (semantic memory) to 0.6 (episodic memory) year before the mean onset of terminal motor decline. The onsets of these cognitive and motor terminal decline periods were highly correlated.
Table 4.
Change in specific motor functions and relation to global cognitive change*
| Fixed Effects | Random Effects | Correlation with Cognition** | |||||
|---|---|---|---|---|---|---|---|
| Outcome | Model Term | Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI |
| Manual strength | Intercept | 0.455 | 0.413, 0.491 | 0.025 | 0.016, 0.037 | 0.49 | 0.27, 0.68 |
| Preterminal slope | −0.031 | −0.039, −0.023 | 0.001 | 0.001, 0.002 | 0.09 | −0.12, 0.29 | |
| Terminal slope | −0.107 | −0.128, −0.089 | 0.003 | 0.002, 0.004 | 0.08 | −.0.23, 0.40 | |
| Change point | −2.530 | 3.009, −2.132 | 1.726 | 0.942, 2.871 | 0.98 | 0.92, 0.996 | |
| Error | 0.010 | 0.009, 0.011 | |||||
| Manual dexterity | Intercept | 0.716 | 0.682, 0.749 | 0.021 | 0.014, 0.030 | 0.69 | 0.51, 0.82 |
| Preterminal slope | −0.005 | −0.011, 0.001 | 0.001 | 0.001, 0.001 | 0.10 | −0.09, 0.30 | |
| Terminal slope | −0.081 | −0.096, −0.062 | 0.003 | 0.002, 0.004 | 0.34 | 0.02, 0.61 | |
| Change point | −2.374 | −2.723, −2.051 | 1.291 | 0.841, 1.896 | 0.96 | 0.88, 0.99 | |
| Error | 0.005 | 0.004, 0.005 | |||||
| Gait | Intercept | 0.515 | 0.459, 0.570 | 0.049 | 0.031, 0.076 | 0.75 | 0.58, 0.87 |
| Preterminal slope | −0.019 | −0.027, −0.011 | 0.002 | 0.001, 0.002 | 0.17 | −0.04, 0.35 | |
| Terminal slope | −0.118 | −0.144, −0.096 | 0.003 | 0.002, 0.005 | 0.32 | −0.02, 0.62 | |
| Change point | −2.468 | −2.847, −2.115 | 1.283 | 0.780, 1.942 | 0.96 | 0.86, 0.995 | |
| Error | 0.019 | 0.017, 0.020 | |||||
Estimated from 3 separate mixed-effects change point models with a single motor measure and global cognition as outcomes. CI, confidence interval.
Model based estimate of correlation with trajectory of change in global cognition.
Figure 3.
Change in specific motor functions. Predicted paths of change in specific motor functions during the last decade of life.
Table 5.
Change in specific cognitive functions and relation to global motor change*
| Fixed Effects | Random Effects | Correlation with Motor Function** | |||||
|---|---|---|---|---|---|---|---|
| Outcome | Model Term | Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI |
| Episodic memory | Intercept | −1.153 | −1.458, −0.879 | 2.396 | 1.821, 3.224 | 0.661 | 0.468, 0.796 |
| Preterminal slope | −0.015 | −0.033, 0.002 | 0.006 | 0.004, 0.009 | 0.145 | −0.065, 0334 | |
| Terminal slope | −0.392 | −0.480, −0.317 | 0.060 | 0.040, 0.090 | 0.331 | −0.004, 0607 | |
| Change point | −3.082 | −3.635, −2.614 | 2.339 | 1.598, 3.498 | 0.904 | 0.694,0.990 | |
| Error | 0.140 | 0.127, 0.153 | |||||
| Semantic memory | Intercept | −1.173 | −1.446, −0.892 | 2.156 | 1.663, 2.844 | 0.676 | 0.490, 0.802 |
| Preterminal slope | −0.023 | −0.035, −0.011 | 0.003 | 0.002, 0.005 | 0.135 | −0.072, 0.318 | |
| Terminal slope | −0.385 | −0.468, −0.315 | 0.066 | 0.046, 0.095 | 0.215 | −0.105, 0.529 | |
| Change point | −2.672 | −3.064, −2.313 | 2.108 | 1.502, 2.947 | 0.970 | 0.894, 0.994 | |
| Error | 0.066 | 0.060, 0.072 | |||||
| Working memory | Intercept | −0.953 | −1.156, −0.771 | 0.971 | 0.726, 1.335 | 0.607 | 0.412, 0.759 |
| Preterminal slope | −0.019 | −0.034, −0.005 | 0.003 | 0.002, 0.005 | 0.164 | −0.035, 0.362 | |
| Terminal slope | −0.281 | −0.343, −0.224 | 0.019 | 0.012,0.030 | 0.129 | −0.153, 0.406 | |
| Change point | −2.841 | −3.462, −2.433 | 1.426 | 0.914,2.209 | 0.983 | 0.928, 0.995 | |
| Error | 0.133 | 0.122,0.144 | |||||
| Perceptual speed | Intercept | −1.774 | −2.030, 1.514 | 1.719 | 1.275, 2.387 | 0.707 | 0.523, 0.829 |
| Preterminal slope | −0.055 | −0.072, −0.037 | 0.004 | 0.003, 0.007 | 0.164 | −0.052, 0.361 | |
| Terminal slope | −0.386 | −0.460, −0.328 | 0.029 | 0.019, 0.046 | 0.238 | −0.052, 0.499 | |
| Change point | −3.186 | −3.763, −2.735 | 2.227 | 1.428, 3.541 | 0.987 | 0.925, 0.996 | |
| Error | 0.146 | 0.133, 0.161 | |||||
Estimated from 4 separate mixed-effects change point models with a single cognitive measure and global motor function as outcomes. CI, confidence interval.
Model based estimate of correlation with trajectory of change in global motor function.
We conducted a final series of analyses to assess the neuropathologic basis of terminal motor decline. The original analysis was repeated with a term added for level of plaques and tangles, then with a term for Lewy bodies, and then with a term for cerebral infarction (Table 6). Higher level of plaques and tangles, but not Lewy bodies or cerebral infarction, was associated with earlier onset of terminal motor decline and terminal cognitive decline. No pathologic measures were related to motor decline, but all 3 were related to preterminal or terminal cognitive decline and to lower levels of motor and cognitive function proximate to death.
Table 6.
Relation of neuropathologic measures to change in global motor and global cognitive function*
| Outcome | Model Term | Plaques and Tangles | Lewy Bodies | Cerebral Infarction | |||
|---|---|---|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | ||
| Global motor function | Intercept | −0.051 | −0.097, −0.007 | −0.097 | −0.188, −0.009 | −0.099 | −0.156, −0.044 |
| Pretermina l slope | 0.001 | −0.007, 0.08 | −0.016 | −0.033, 0.001 | −0.003 | −0.014, 0.009 | |
| Terminal slope | 0.004 | −0.015, 0.22 | −0.003 | −0.043, 0.36 | 0.007 | −0.019, 0.032 | |
| Change point | −0.394 | −0.710, −0.085 | −0.069 | −0.896, 0.678 | −0.123 | −0.731, 0.399 | |
| Global cognitive function | Intercept | −0.554 | −0.780, −0.332 | −0.666 | −1.201, −0.172 | −0.431 | −0.755, −0.107 |
| Preterminal slope | −0.015 | −0.027,−0.002 | −0.032 | −0.059, −0.005 | −0.019 | −0.037, 0.000 | |
| Terminal slope | −0.035 | −0.089, 0.21 | −0.129 | −0.258, −0.009 | −0.046 | −0.118, 0.027 | |
| Change point | −0.641 | −0.945, −0.341 | −0.064 | −0.800, 0.647 | −0.306 | −0.779, 0.174 | |
Estimated from 3 separate mixed-effects change point models. Results show the fixed effect of a 1-unit increase in the pathologic measure. CI, confidence interval.
Discussion
A group of 124 older persons completed annual assessments of motor function for 6 to 15 years prior to death. Rate of motor decline was minimal until the last 2 to 3 years of life when it increased more than 4-fold. The results demonstrate that motor function undergoes a period of accelerated terminal decline.
We are not aware of prior research on terminal decline in motor function. Previous studies have shown, however, that lower level of motor function (Al Snih et al., 2002; Fujita et al., 1995; Laukkahen et al., 1995; Phillips, 1986; Rantanen et al., 2000; Ratanen et al., 2003) and more rapid motor decline (Buchman et al., 2007; Onder et al., 2002) predict subsequent mortality. The present study builds on this knowledge by showing that person-specific paths of change in motor function undergo a period of accelerated decline beginning about 2.5 years before death with more gradual decline in motor function prior to that point. This suggests that a substantial part of late life decline in motor function is due to processes related to impending death rather than to processes related to growing older.
Terminal decline in cognitive function was also evident in these analyses, consistent with previous studies of this (Wilson et al., 2003) and other (Laukka et al., 2006; MacDonald et al., 2011; Sliwinski et al., 2006; Thorvaldsson et al., 2008; Wilson et al., 2007) cohorts. Although the existence of terminal cognitive decline is well established, estimates of its duration have varied from about 3 years to more than a decade. Some of this variability reflects the challenges of modeling the evolving behavioral manifestations of chronic disease processes. Further variability reflects differences in the data available for modeling due to differences in study design and operations including the number and spacing of cognitive assessments; length of the study period and its proximity to death; rate of follow-up participation; and the age of participants and whether or not those with mild cognitive impairment or incipient dementia are included. That terminal cognitive decline has been consistently observed in epidemiologic research despite these differences in study designs and operations attests to the robustness of the phenomenon.
The relationship between late life trajectories of change in motor function and cognitive function is not well understood. In this study, rates of motor decline and cognitive decline were not correlated before or during the terminal period, suggesting that the underlying abilities being measured were relatively independent. Damage to the brain can impair both motor and cognitive function and might thereby increase their intercorrelation. However, the physiologic systems underlying motor function are more widely distributed than the systems underlying cognition, extending to spinal cord, peripheral nervous system, and muscle. Because the peripheral nervous system and muscle are outside the blood brain barrier, they are vulnerable to catabolic, inflammatory, immune, endocrine, and metabolic processes from which the central nervous system is protected. In addition, recent studies indicate that the blood barriers in the brain and spinal cord may differ (Bartanusz, Jezova, Alajajian, & Digicaylioglu, 2011), suggesting differential vulnerability to disease in spinal motor networks compared to brain motor networks and brain cognitive networks. These anatomic and physiologic considerations are consistent with the relative independence observed in late life trajectories of change in motor and cognitive function.
By contrast, the onsets of terminal motor decline and terminal cognitive decline occurred an average of less than 4 months apart, were strongly intercorrelated, and were robustly related to pathologic level of AD. This suggests that the accumulation of plaques and tangles, or something related to their accumulation, plays a key role in the transition from a period of relatively slow decline in function to a period of rapid functional decline in the last few years of life. However, neither AD pathology nor the other neuropathologic measures (i.e., Lewy bodies, cerebral infarction) were related to rate of motor decline during the terminal period, and only Lewy bodies were related to terminal cognitive decline. It may be, therefore, that dementia- related pathology indirectly contributes to terminal motor and cognitive decline by initiating a cascade of biologic changes which eventually lead to rapid functional decline and death. Terminal changes might be due to unmeasured pathologic processes such TDP-43, a DNA-binding protein that is the main constituent of inclusion bodies associated with a range neurodegenerative disorders including frontotemporal lobar degeneration, amyotrophic lateral sclerosis, and AD (Wilson, Dugger, Dickson, & Wang, 2011). It is also possible that factors that enhance resilience to progressive neuropathologic changes may at some point prove insufficient (Wilson et al., 2010).
Strengths and limitations of these data should be noted. Motor and cognitive functions were assessed with composite measures based on multiple performance tests, minimizing floor and ceiling artifacts and other forms of measurement error. There was a mean of about 10 years of observation with a high rate of follow-up participation among survivors, allowing us to reliably characterize individual trajectories of change. Consistent results were obtained with diverse measures of motor and cognitive function. Clinical data were collected up to the last year of life, enhancing our ability to capture mortality effects. An important limitation is that the cohort is selected. So the generalizability of the findings is uncertain though it should be noted that the terminal cognitive changes described in this cohort in 2003 (Wilson et al., 2003) have been consistently replicated (Sliwinski et al., 2006, Laukka et al., 2006, Wilson et al., 2007; Thorvaldsson et al., 2008; MacDonald et al., 2011).
In summary, these results indicate that in the last few years of life motor function undergoes a period of rapid terminal decline similar to the terminal changes known to occur in cognitive function. This suggests that much late life loss of motor and cognitive function reflects impending death rather than aging. The pathologic burden of AD was related to the onsets of the terminal periods but not to rates of motor and cognitive decline during them. This suggests that Alzheimer’s disease may be a necessary condition for terminal motor and cognitive decline but that other pathologic processes are primarily responsible for individual differences in rate of terminal motor and cognitive changes. Understanding these additional pathologic factors may suggest novel approaches to preserving motor and cognitive function in old age.
Acknowledgments
The authors thank the hundreds of Catholic nuns, priests, and brothers who have participated in the Religious Orders Study; Traci Colvin, MPH, for study coordination; John Gibbons, MS, and Greg Klein, MS, for data management; and Woojeong Bang, MS, for statistical programming. This research was supported by National Institute on Aging grants P30AG10161, R01AG15819, R01AG33678, R01AG34374, and R01AG24480 and by the Illinois Department of Public Health. The funding organizations had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
References
- Al Snih S, Markides KS, Ray L, Ostir G, Goodwin J. Handgrip strength and mortality in older Mexican Americans. Journal of the American Geriatrics Society. 2002;50:1250–1256. doi: 10.1046/j.1532-5415.2002.50312.x. [DOI] [PubMed] [Google Scholar]
- Bartanusz V, Jezova D, Alajajian B, Digicaylioglu M. The blood-spinal cord barrier: morphology and clinical implications. Annals of Neurology. 2011;70:194–206. doi: 10.1002/ana.22421. [DOI] [PubMed] [Google Scholar]
- Bassey EJ. Longitudinal changes in selected physical capabilities: muscle strength, flexibility, and body size. Age and Ageing. 1998;3:12–16. doi: 10.1093/ageing/27.suppl_3.12. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Aggarwal NT, Arnold SE, Cochran EJ, Berry-Kravis E, Bienias JL. Apolipoprotein E ε4 allele, AD pathology, and the clinical expression of Alzheimer’s disease. Neurology. 2003;60:245–252. doi: 10.1212/01.wnl.0000042478.08543.f7. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, Barnes LL, Fox JH, Bach J. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- Buchman AS, Leurgans SE, Boyle PA, Schneider JA, Arnold SE, Bennett DA. Combinations of motor measures more strongly predict adverse health outcomes in old age: the rush memory and aging project, a community-based cohort study. BMC Medicine. 2011;9:42. doi: 10.1186/1741-7015-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman AS, Wilson RS, Bienias JL, Bennett DA. Gender difference in motor performance of older persons. Geriatrics & Gerontology International. 2005;5:59–65. doi: 10.1111/j.1447-0594.2005.00266.x. [DOI] [PubMed] [Google Scholar]
- Buchman AS, Wilson RS, Boyle PA, Bienias JL, Bennett DA. Change in motor function and risk of mortality in older persons. Journal of the American Geriatrics Society. 2007;55:11–19. doi: 10.1111/j.1532-5415.2006.01032.x. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Nakamura Y, Hiraoka J, Kobavashi K, Sakata K, Nagai M, Yanagawa H. Physical-strength tests and mortality among visitors to health-promotion centers in Japan. Journal of Clinical Epidemiology. 1995;48:1349–1359. doi: 10.1016/0895-4356(95)00533-1. [DOI] [PubMed] [Google Scholar]
- Gelman A, Carli JB, Stern HS, Rubin DB. Bayesian data analysis. New York: Chapman and Hall; 2004. [Google Scholar]
- Laird N, Ware J. Random effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Laukka EJ, MacDonald SW, Bäckman L. Contrasting cognitive trajectories of impending death and preclinical dementia in the very old. Neurology. 2006;66:833–838. doi: 10.1212/01.wnl.0000203112.12554.f4. [DOI] [PubMed] [Google Scholar]
- Laukkanen P, Heikkinen E, Kauppinen M. Muscle strength and mobility as predictors of survival in 75–84-year-old people. Age and Ageing. 1995;24:468–473. doi: 10.1093/ageing/24.6.468. [DOI] [PubMed] [Google Scholar]
- Lunn D, Spiegelhalter D, Thomas A, Best N. The BUGS project: evolution, critique and future direction (with discussion) Statistics in Medicine. 2009;28:3049–3082. doi: 10.1002/sim.3680. [DOI] [PubMed] [Google Scholar]
- MacDonald SWS, Hultsch DF, Dixon RA. Aging and the shape of cognitive change before death: terminal decline or terminal drop? Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2011;66:292–301. doi: 10.1093/geronb/gbr001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Onder G, Penninx BW, Lapuerta P, Fried LP, Ostir GV, Guralnik JM, Pahor M. Change in physical performance over time in older women: the women’s health and aging study. Journals of Gerontology, Series A: Biological Sciences and Medical Sciences. 2002;57:289–293. doi: 10.1093/gerona/57.5.m289. [DOI] [PubMed] [Google Scholar]
- Phillips P. Grip strength, mental performance and nutritional status as indicators of mortality risk among female geriatric patients. Age and Ageing. 1986;15:53–56. doi: 10.1093/ageing/15.1.53. [DOI] [PubMed] [Google Scholar]
- Rantanen R, Harris T, Leveille SE, Visser M, Foley D, Masaki K, Guralnik JM. Muscle strength and body mass index as long-term predictors of mortality in initially healthy men. Journals of Gerontology, Series A: Biological Sciences and Medical Sciences. 2000;55:168–173. doi: 10.1093/gerona/55.3.m168. [DOI] [PubMed] [Google Scholar]
- Rantanen T, Volpato S, Ferrucci L, Heikkinen E, Fried LP, Guralnik JM. Handgrip strength and total mortality in older disabled women: exploring the mechanism. Journal of the American Geriatrics Society. 2003;51:636–641. doi: 10.1034/j.1600-0579.2003.00207.x. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvantakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Annals of Neurology. 2009;66:200–208. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JA, Wilson RS, Cochran EJ, Bienias JL, Arnold SE, Evans DA, Bennett DA. Relation of cerebral infarctions to dementia and cognitive function in older persons. Neurology. 2003;60:1082–1088. doi: 10.1212/01.wnl.0000055863.87435.b2. [DOI] [PubMed] [Google Scholar]
- Sliwinski MJ, Stawski RS, Hall CB, Katz M, Verghese J, Lipton R. Distinguishing preterminal and terminal cognitive decline. European Psychologist. 2006;11:172–181. [Google Scholar]
- Thorvaldsson V, Hofer SM, Berg S, Skoog I, Sacuiu S, Johansson B. Onset of terminal decline in cognitive abilities in individuals without dementia. Neurology. 2008;71:882–887. doi: 10.1212/01.wnl.0000312379.02302.ba. [DOI] [PubMed] [Google Scholar]
- Vandervoort AA. Aging of the human neuromuscular system. Muscle & Nerve. 2005;25:17–25. doi: 10.1002/mus.1215. [DOI] [PubMed] [Google Scholar]
- Wilson AC, Dugger BN, Dickson DW, Wang DS. TDP-43 in aging and Alzheimer’s disease – a review. Int J Clin Exp Pathol. 2011;4:147–155. [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Arnold SE, Beck TL, Bienias JL, Bennett DA. Change in depressive symptoms during the prodromal phase of Alzheimer disease. Archives of General Psychiatry. 2008;65:439–446. doi: 10.1001/archpsyc.65.4.439. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Aggarwal NT, Boyle PA, Hebert LE, Mendes de Leon CF, Evans DA. Cognitive activity and the cognitive morbidity of Alzheimer disease. Neurology. 2010;75:990–996. doi: 10.1212/WNL.0b013e3181f25b5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Beck TL, Bienias JL, Bennett DA. Terminal cognitive decline: accelerated loss of cognition in the last years of life. Psychosomatic Medicine. 2007;69:131–137. doi: 10.1097/PSY.0b013e31803130ae. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, Bennett DA. Individual differences in rates of change in cognitive abilities of older persons. Psychology and Aging. 2002;17:179–193. [PubMed] [Google Scholar]
- Wilson RS, Beckett LA, Bienias JL, Evans DA, Bennett DA. Terminal decline in cognitive function. Neurology. 2003;60:1782–1787. doi: 10.1212/01.wnl.0000068019.60901.c1. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Bienias JL, Evans DA, Bennett DA. Religious orders study: Overview and change in cognitive and motor speed. Aging, Neuropsychology, & Cognition. 2004;11:280–303. [Google Scholar]
- Wilson RS, Evans DA, Bienias JL, Mendes de Leon CF, Schneider JA, Bennett DA. Proneness to psychological distress is associated with risk of Alzheimer’s disease. Neurology. 2003;61:1479–1485. doi: 10.1212/01.wnl.0000096167.56734.59. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Krueger KR, Boyle PA, Bennett DA. Loss of basic lexical knowledge in old age. Journal of Neurology, Neurosurgery, and Psychiatry. 2011;82:369–372. doi: 10.1136/jnnp.2010.212589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Leurgans SE, Boyle PA, Schneider JA, Bennett DA. Neurodegenerative basis of age-related cognitive decline. Neurology. 2010;75:1070–1078. doi: 10.1212/WNL.0b013e3181f39adc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Mendes de Leon CF, Barnes LL, Schneider JA, Bienias JL, Evans DA, Bennett DA. Participation in cognitively stimulating activities and risk of incident Alzheimer’s disease. Journal of the American Medical Association. 2002;287:742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Arnold SE, Bienias JL, Bennett DA. Conscientiousness and the incidence of Alzheimer disease and mild cognitive impairment. Archives of General Psychiatry. 2007;64:1204–1212. doi: 10.1001/archpsyc.64.10.1204. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Segawa E, Hizel LP, Boyle PA, Bennett DA. Terminal dedifferentiation of cognitive abilities. Neurology. doi: 10.1212/WNL.0b013e31824f7ff2. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Yu L, Schneider JA, Arnold SE, Buchman AS, Bennett DA. Lewy bodies and olfactory dysfunction in old age. Chemical Senses. 2011;36:367–373. doi: 10.1093/chemse/bjq139. [DOI] [PMC free article] [PubMed] [Google Scholar]