Abstract
The lysophosphatidic acid (LPA) receptor LPA1/Edg2 is the first identified LPA receptor. Although its wide tissue distribution and biological functions have been well studied, little is known about how LPA1 is transcriptionally regulated. In the current study, we showed that LPA1 is a physiological target of transforming growth factor beta (TGFβ)-mediated repression. In both normal and neoplastic cells, TGFβ inhibits LPA1 promoter activity, LPA1 mRNA expression, and LPA1-dependent chemotaxis and tumor cell invasion. Knockdown of the TGFβ intracellular effector Smad3 or Smad4 with lentivirally transduced shRNA relieved these inhibitory effects of TGFβ. Interestingly, the LPA1 promoter contains two potential TGFβ inhibitory elements (TIEs), each consisting of a Smad binding site and an adjacent E2F4/5 element, structurally similar to the TIE found on the promoter of the well-defined TGFβ target gene c-myc. Deletion and point mutation analyses indicate that the distal TIE located at 401 bp from the transcription initiation site, is required for TGFβ repression of the LPA1 promoter. A DNA pull-down assay showed that the -401 TIE was capable of binding Samd3 and E2F4 in TGFβ-treated cells. TGFβ-induced binding of the Smad complex to the native -401 TIE sequence of the LPA1 gene promoter was further verified by chromatin immunoprecipitation assays. We therefore identified a novel role of TGFβ in the control of LPA1 expression and LPA1-coupled biological functions, adding LPA1 to the list of TGFβ-repressed target genes.
Keywords: cancer, LPA, TGFβ, LPA1, migration, invasion
INTRODUCTION
Lysophosphatidic acid (1-acyl-sn-glycerol-3-phosphate) is a naturally occurring intercellular mediator of diverse biological processes including neurogenesis, angiogenesis, wound healing, immunity, and carcinogenesis (1). LPA is produced by activated platelets during coagulation and thus is a normal constituent of serum (2). LPA is a ligand of multiple G protein-coupled receptors (GPCRs) (3). The LPA1/Edg2, LPA2/Edg4 and LPA3/Edg7 receptors are members of the endothelial differentiation gene (Edg) family, sharing 50-57% homology in their amino acid sequences (3). In addition to the Edg LPA1-3 receptors, GPR23/P2Y9/LPA4 of the purinergic receptor family, and the related GPR92/LPA5 and P2Y5/LPA6 have been reported to be additional LPA receptors (4-6).
LPA1 is expressed in most adult tissues and in embryonic cells (3). Only minor abnormalities such as craniofacial dysmorphism and defective sucking behavior were found in lpa1-deficient mice (7). However, more recent studies of lpa -/-1 mice subjected to various pathophysiological conditions revealed that LPA1 is involved in initiation of neuropathic pain (8), embryonic and adult neurogenesis, and promotion of pulmonary and renal fibrosis (9, 10). Some of these biological functions of LPA1 are attributed to the motility-stimulating activity of LPA in mammalian cells. Substantial evidence indicates that LPA1 is the primary LPA receptor subtype to mediate LPA-dependent chemotaxis and tumor cell invasion (11). In contrast to the LPA2 receptor that is commonly overexpressed in various cancers (12-14), gene expression profiling studies failed to show any consensus increase in LPA1 expression between normal and malignant cells (15-19). Instead, some expression profiling or array analyses suggest decreases in LPA1 mRNA expression in various malignancies (15, 17-19).
Several groups recently reported that LPA1 expression is repressed by Nm23 (20, 21). Nm23 is the first identified metastasis suppressor gene that, by definition, inhibits the process of metastasis but not growth of primary tumors (20). In human breast carcinomas, LPA1 expression inversely correlated with that of Nm23 (21). However, little is known about how Nm23 represses LPA1. Furthermore, there is no evidence that LPA1 expression is elevated in metastatic cancer compared to primary tumors. Thus, LPA1 expression is apparently controlled by complex regulatory mechanisms involving other unrecognized activators or repressors. In the present study, we showed for the first time that that transforming growth factor beta (TGFβ), a platelet-derived cytokine co-present with LPA in the circulation and tumor microenvironments, represses LPA1 gene transcription and LPA1-dependent motility-stimulating activity via a TGFβ inhibitory element (TIE) containing both Smad and E2F4/5 binding sites on the LPA1 gene promoter. These results represent a novel form of crosstalk between TGFβ and LPA signaling.
RESULTS
TGFβ inhibits expression of LPA1
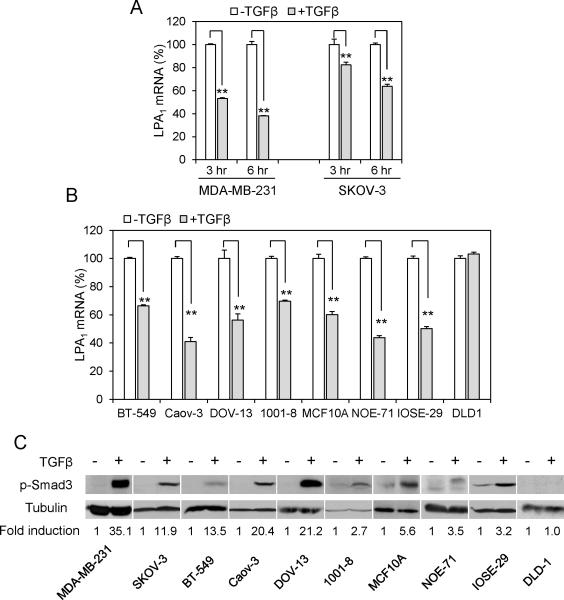
Previous studies showed that LPA stimulates production and release of TGFβ (22, 23), trans-activates the intracellular effectors of TGFβ (24) or cooperates with TGFβ to regulate gene expression (25). However, little is known about whether TGFβ communicates with LPA signal transduction to modify cellular responses to the multi-functional LPA. To explore this possibility, we treated the MDA-MB-231 breast carcinoma and the SKOV-3 ovarian carcinoma cell lines with TGFβ for 3 or 6 hours, and monitored changes in mRNA expression of LPA signaling molecules including various LPA receptors. MDA-MB-231 and SKOV-3 cells expressed LPA1, LPA2, LPA3, LPA4 and LPA6 mRNAs as shown by quantitative PCR (qPCR) (Fig. S1A). The treatment with TGFβ for 6 hours resulted in 62% and 37% decreases in LPA1 mRNA levels in MDA-MB-231 and SKOV-3, respectively (Fig. 1A). TGFβ did not downregulate mRNA levels of other LPA receptors present in these cells (Fig. S2). To generalize the observation of the specific inhibitory effect of TGFβ on LPA1, we examined a panel of breast, ovarian and other cancer cell lines, including BT-549, Caov-3 and DOV-13. Treatment with TGFβ induced 30-67% decreases in LPA1 mRNA levels in these cell lines (Fig. 1B). Most of the cancer cell lines such as BT-549, SKOV-3 and DOV-13 were resistant to the growth inhibitory effect of TGFβ as we reported recently (25). Thus, the inhibition of LPA1 expression by TGFβ was independent of the cytostatic program of TGFβ. In addition, TGFβ also downregulated expression of LPA1 in normal primary and immortalized epithelial cells such as primary mammary epithelial cells (1001-8), primary ovarian epithelial cells (NOE-71), immortalized breast epithelial cell line MCF-10A, and immortalized ovarian surface epithelial cell line IOSE-29 (Fig. 1B). In an independent project to profile transcriptional effects of TGFβ in the OE33 esophageal cancer cell line, we also observed TGFβ-induced decrease in LPA1 mRNA by 45% (data not shown). The only exception to the negative regulation by TGFβ was the DLD1 colon cancer cell line. DLD1 was deficient in TβRII as reported previously (26) and as evidenced by the inability of TGFβ to induce Smad3 phosphorylation in this particular line (Fig. 1C). It is also worth noting that LPA1 was highly expressed in DLD1 cells (27, 28), likely as a result of the absence of TGFβ-mediated repression.
Figure 1. TGFβ inhibits expression of LPA1 mRNA.
A. MDA-MB-231 and SKOV-3 cells were cultured with TGFβ (2.5 ng/ml) or vehicle for 3 and 6 hours. LPA1 mRNA levels were determined by RT and qPCR. The mRNA levels of LPA1 in TGFβ treated cells were presented as percentages relative to those in control cells (defined as 100%). B. Multiple cancer cell lines, immortalized breast (MCF-10A) and ovarian (IOSE-29) epithelial cell lines, primary mammary (1001-8) and ovarian (NOE71) epithelial cells were treated for 6 hours with TGFβ (2.5 ng/ml) and analyzed for LPA1 mRNA expression as in A. C. Cancer cell lines and primary cells were treated with TGFβ (2.5 ng/ml) or vehicle for 1 hour before lysis with SDS sample buffer and immunoblotting analysis of Smad3 phosphorylated at Ser423/425. The intensity of phospho-Smad3 in each cell line was quantified by densitometry and presented as fold of that in control cells (arbitrary 1.0).
TGFβ attenuates LPA1-dependent cell migration and invasion
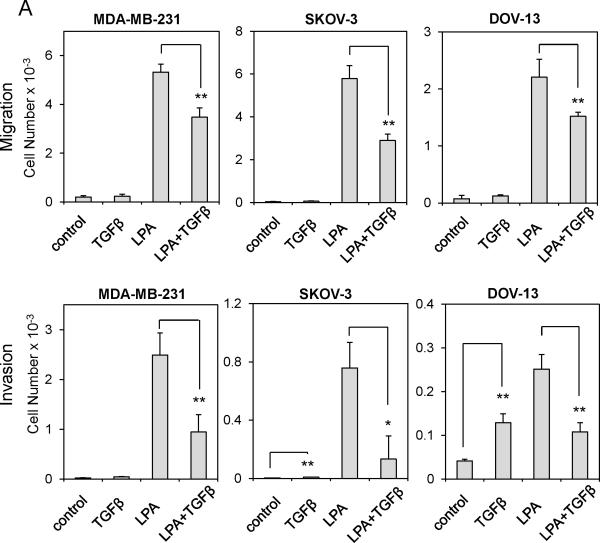
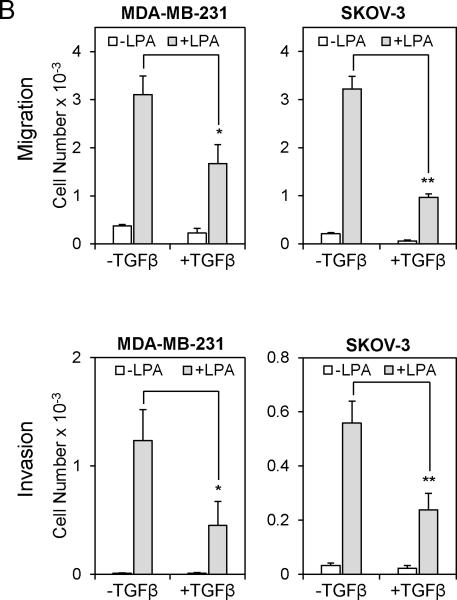
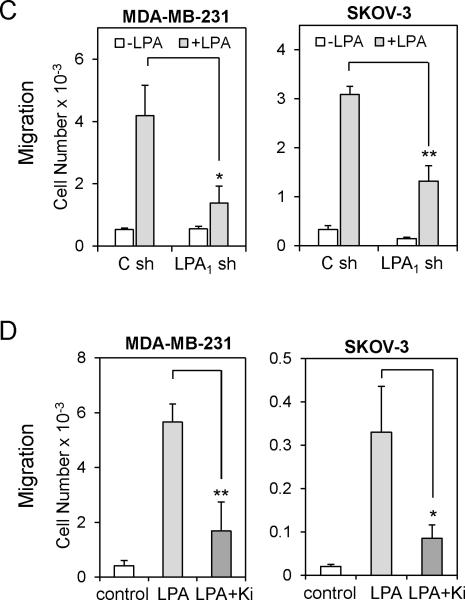
Since TGFβ represses LPA1 mRNA expression, we anticipated that TGFβ would attenuate LPA1-dependent actions of LPA. Although each of the Edg-family LPA receptors may contribute to cell motility in certain cellular contexts, substantial evidence supports an essential and probably sufficient role for LPA1 in driving random migration, chemotaxis and tumor cell invasion (27, 29, 30). In breast and ovarian cancer cell lines we examined, LPA stimulated robust chemotactic responses as analyzed by the transwell assay (Fig. 2A upper). LPA also drastically promoted invasion of these cells through Matrigel (Fig. 2A lower). In agreement with the crucial role of LPA1 in stimulation of cell motility, shRNA knockdown of LPA1 expression (Fig. S3) or pharmacological inhibition of LPA1 with Ki16425 decreased LPA-induced chemotaxis (Fig. 2C & 2D). On the other hand, TGFβ only weakly increased migration of MDAMB-231, SKOV-3 and DOV-13 cells (Fig. 2A upper). This trend of increase in chemotactic migration towards TGFβ was not statistically significant. Nevertheless, TGFβ was capable of stimulating modest but significant increases in invasion of SKOV-3 and DOV-13 cells (Fig. 2A lower). However, the ability of TGFβ to stimulate invasion of breast and ovarian cancer cell lines was much weaker than that of the potent motogen LPA. Levels of TGFβ receptors did not seem to correlate with the responsiveness of these cells to TGFβ (Fig. S1B.)
Figure 2. TGFβ attenuates LPA1-dependent cell migration and invasion.
A. The chemotactic responses to TGFβ (2.5 ng/ml), LPA (5 μM), or LPA+TGFβ in the breast cancer cell line MDA-MB-231, and the ovarian cancer cell lines SKOV-3 and DOV-13 were measured by transwell chambers (upper). The cells (2 × 104 cells/well) were loaded to the upper wells and allowed to migrate for 6 hours. The migrated cells on the underside of the Transwell were stained with crystal violet, counted under a microscope and presented as numbers of cells/well. Cell invasion induced by TGFβ (2.5 ng/ml), LPA (5 μM), or LPA+TGFβ in MDA-MB-231, SKOV-3 and DOV-13 cells was measured with the growth factor–reduced Matrigel invasion chambers (lower). The experiment was performed as the migration assay except that the cells were allowed to invade for 20 hours. B. MDA-MB-231 and SKOV-3 cells were pre-treated with TGFβ (2.5 ng/ml) for 6 hours before analysis of LPA-induced migration (upper) and invasion (lower) as described in A. C. Expression of LPA1 in MDA-MB-231 and SKOV-3 cells was silenced with lentivirally transduced shRNA. The chemotactic migration of these knockdown and control cells induced by LPA was analyzed as described in A. D. LPA-induced migration of MDA-MB-231 and SKOV-3 cells was analyzed in the presence or absence of Ki16425 (Ki) (10 μM).
When MDA-MB-231, SKOV-3 and Dov-13 cells were co-stimulated with both LPA and TGFβ, TGFβ significantly inhibited LPA stimulation of migration and invasion. We observed 30-50% decreases in migration and 60-80% decreases in invasion in the presence of LPA and TGFβ compared to the effects of LPA alone (Fig. 2A). Similarly, pretreatment of MDA-MB-231 and SKOV-3 cells with TGFβ also significantly inhibited LPA-mediated cell migration and invasion (Fig. 2B). In all cell lines we examined, the TGFβ mediated inhibition of invasion was more prominent than the effect of TGFβ on migration. This was likely due to the longer incubation of the cells with TGFβ during the invasion experiments. These data demonstrated that TGFβ repression of LPA1 expression was sufficient to impair LPA1-dependent cell migration and invasion.
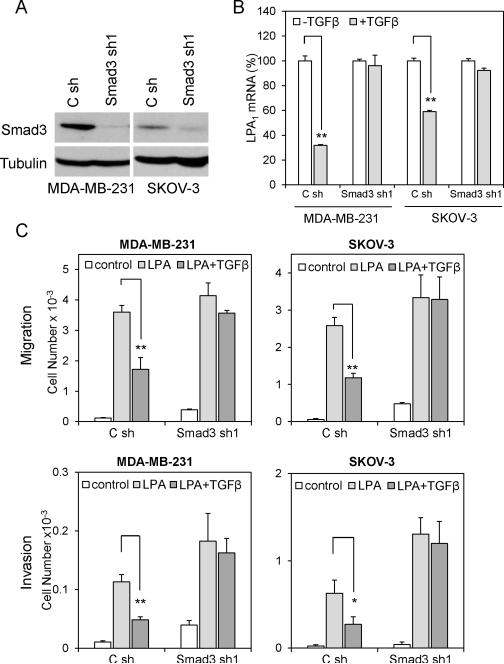
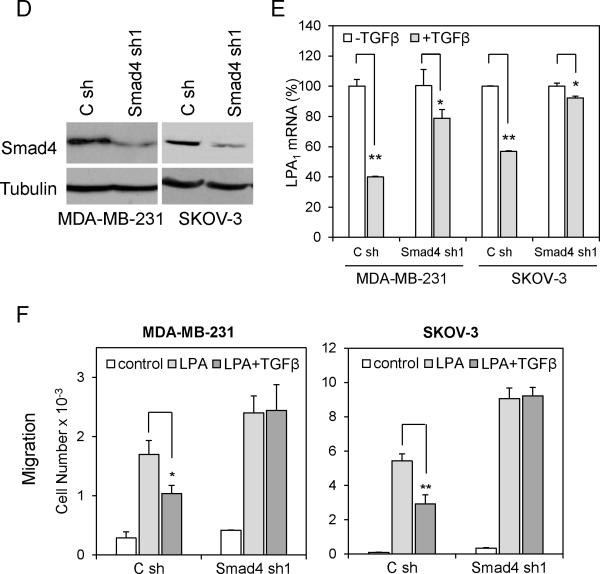
TGFβ represses LPA1 expression and LPA1-dependent cell migration and invasion in a Smad-dependent manner
Upon binding of TGFβ to its receptors, both Smad-dependent and Smad-independent pathways are activated by the kinase activity of TGFβ receptors (TβRs) (31). Regulatory Smads (R-Smads), such as Smad2 and Smad3, are phosphorylated by TβRs, and form heterodimer with Smad4 to translocate to the nucleus where the Smad complex regulates transcription of target genes (32). In addition, TGFβ activates TβR-associated proteins and other intracellular signaling pathways such as MAPK, PP2A/p70S6K, RhoA and TAK1/MEKK1 to elicit Smad-independent responses to TGFβ (33). To elucidate the mechanism underlying TGFβ repression of LPA1, we examined the possibility for the participation of the Smad-dependent pathways in the process. Smad3, but not Smad2, was reported to be the R-Smad involved in binding to TIE to downregulate TGFβ target genes, most notably c-myc (34). We therefore knocked-down Smad3 expression in MDA-MB-231 and SKOV-3 cells using lentivirally transduced shRNA. Expression of Smad3 protein was efficiently silenced by Smad3 shRNA (Fig. 3A). The silencing of Smad3 reduced the inhibitory effect of TGFβ on expression of LPA1 (Fig. 3B). Furthermore, in Smad3 knockdown cells, TGFβ no longer inhibited LPA-driven cell migration (Fig. 3C upper) and invasion (Fig. 3C lower). These results suggest a Smad3-dependent mechanism to repress LPA1 expression and LPA1-linked migration and invasion by TGFβ. In further support of this, Smad3 knockdown was accompanied by considerable increases in basal and LPA-induced cell migration and invasion (Fig. 3C). Likewise, shRNA knockdown of Smad4, the co-Smad in these cells, also inhibited the effects of TGFβ on LPA1 expression and LPA1-dependent cell migration (Fig. 3D, 3E & 3F).
Figure 3. TGFβ represses LPA1 in a Smad-dependent manner.
A. Expression of Smad3 in MDA-MB-231 and SKOV-3 cells was silenced with lentivirally transduced shRNA and confirmed by immunoblotting. B. Smad3 shRNA and control shRNA-transduced MDA-MB-231 and SKOV-3 cells were treated with TGFβ (2.5 ng/ml) or vehicle for 6 hours followed by RT and qPCR analysis of LPA1 mRNA. C. LPA-induced chemotactic migration (upper) and invasion (lower) of control and Smad3 knockdown cells were analyzed in the absence or presence of TGFβ (2.5 ng/ml). D, E & F. The effects of TGFβ on LPA1 expression and LPA-induced migration were analyzed in Smad4 shRNA knockdown cells as detailed for the experiments in Smad3-silenced cells in A, B & C.
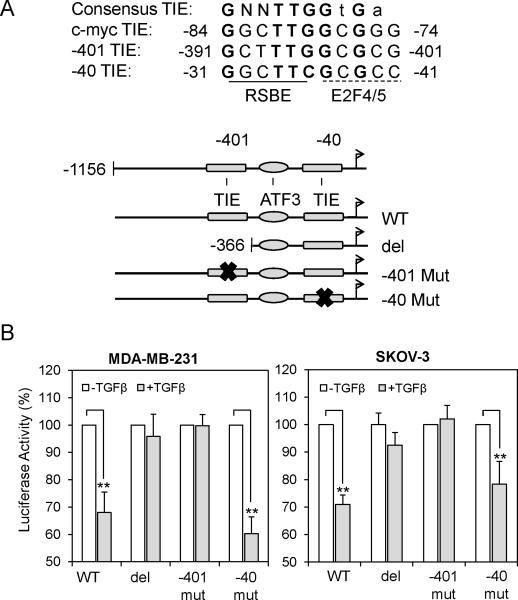
TGFβ represses the transcriptional activity of the LPA1 gene promoter which contains two potential TIEs
The TGFβ-Smad pathway both activates and represses gene transcription. There is a long list of TGFβ activated targets such as type I collagen and cyclin-dependent kinase inhibitors p21Cip1 and p15Ink4b. Conversely, only a few TGFβ-repressed genes have been well defined with the c-myc and Id1 being the best characterized. Downregulation of c-myc by TGFβ is mediated by the Smad3-Smad4-E2F4/5-p107 complex that binds to the consensus TGFβ inhibitory element (TIE, GGCTTGGCGGGAAA) which consists of a repressive Smad binding element (RSBE) (35) and an E2F binding site on the c-myc gene promoter. Different from cmyc, Id1 is inhibited by TGFβ through combined effects of a Smad binding element (SBE) and a separate CREB binding site that recruits the ATF3 repressor to the Id1 gene promoter (36). Interestingly, analysis of the human LPA1 gene promoter sequences revealed the presence of two potential TIEs, one located at -401 (designated -401 TIE) and the other at -40 (designated -40 TIE) from the transcription initiation site (Fig. 4A). The composite TIE consisting of the Smad and E2F4/5 binding sites is present only in the LPA1 gene promoter but not in the promoters of other LPA receptors (LPA2-6) (Fig. S4). Between these two TIEs, there is also an SBE (-324 GTCT -321) and a probable ATF site (-348 TGACGCTC -341) with 5 out of 8 nucleotides matching with the ATF consensus sequence (TGACGTCA).
Figure 4. TGFβ represses the transcriptional activity of the LPA1 gene promoter containing TIEs.
A. DNA sequences of two potential TIEs of the human LPA1 promoter were compared with that of the c-myc TIE (upper). The potential Smad, E2F4/5 and ATF3 binding sites were indicated. The LPA1 promoter (-1156 to +86) was cloned into pGL2-Basic-Luc to constructed the pGL2-LPA1-Luc luciferase reporter (WT) (lower). The deletion (del) and point mutations of each TIE (-401 Mut and -40 Mut) were made as described in Materials and Methods. B. MDA-MB-231 and SKOV-3 cells were transfected with the indicated plasmids and cultured with TGFβ or vehicle for 16 hours before luciferase activities were determined. The results were presented as percentages relative to the values of the vehicle control cells (defined as 100%).
We therefore cloned a 1242-bp fragment of the LPA1 gene promoter (-1156 to +86) into the pGL2-Basic-Luc vector to construct pGL2-LPA1-Luc. MDA-MB-231 and SKOV-3 cells were transfected with pGL2-LPA1-Luc and cultured with TGFβ or vehicle for 16 hours before measurement of luciferase activity in cell lysates. TGFβ treatment resulted in modest but consistent decrease in luciferase activity (Fig. 4B). Deletion of the proximal -401 TIE (named del in Fig. 4) at -366 abolished the negative effect of TGFβ on the LPA1 promoter-driven luciferase activity (Fig. 4B), suggesting that the deleted sequence containing the -401 TIE, rather than the potential SBE-ATF3 or the further downstream -40 TIE, is the major site for TGFβ repression of LPA1 transcription. Indeed, similar to the deletion mutant, point mutation of the -401 TIE (GGCTTTGGCGCG to GGCTAATTCGCGC) also eliminated the repressive effect of TGFβ on the LPA1 promoter activity. However, mutation of the -40 TIE (GGCTTCGCGCC to GGCAATTCGCC) only slightly reduced the effect of TGFβ, which was statistically insignificant. Taken together, these experiments indicate that the -401 TIE site is required for TGFβ-Smad mediated repression of the LPA1 gene.
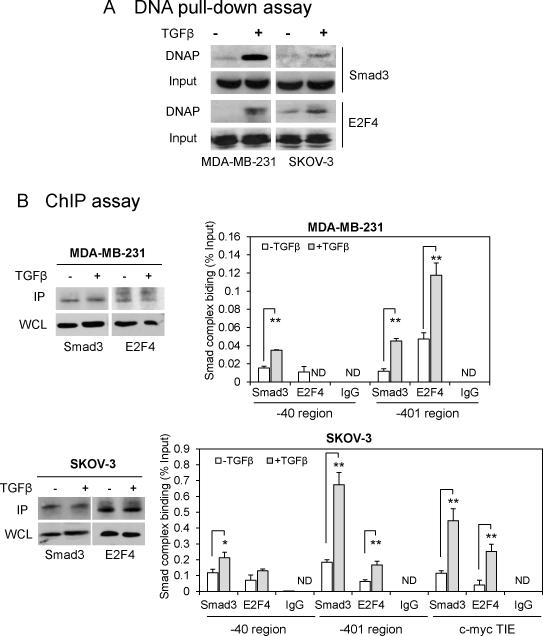
Smad complex binds to the -401 TIE of the LPA1 promoter
To gain evidence that the Smad complex binds to the LPA1 promoter at the -401 TIE, we performed DNA pull-down assay using biotinylated double-stranded oligonucleotides corresponding to the sequences between -413 and -378 that included the -401 TIE of the LPA1 promoter. MDA-MB-231 and SKOV-3 cells were treated for 1 hour with TGFβ or vehicle. The 36-bp DNA fragment was incubated with cell lysates to allow binding and precipitating Smad3 and E2F4 as detailed in Materials and Methods. As demonstrated in Fig. 5A, co-precipitated Smad3 and E2F4 were detected from TGFβ-treated cells but not from vehicle-treated control cells, suggesting that the 36-bp DNA fragment is capable of binding active Smad3 and E2F4.
Figure 5. TGFβ induces occupancy of the Smad complex to the LPA1 gene promoter.
A. Cell extracts from MDA-MB-231 and SKOV-3 cells treated with TGFβ (2.5 ng/ml) or vehicle for 1 h our were incubated with biotinylated DNA fragment containing the -401 TIE and strepatavidin beads. The DNA precipitates (DNAP) were subjected to western blot analysis for Smad3 and E2F4. Whole cell lysates were included as input. B. ChIP assays were performed to examine the binding of Smad3 and E2F4 to the -40 and -401 TIEs of the LPA1 promoter and to the c-myc TIE (positive controls). The immunoprecipitation of Smad3 and E2F4 was verified by western blotting analysis of immunoprecipitates (IP) and whole cell lysates (WCL). The binding was quantitated by qPCR using SYBR Green and the specific primers listed in Table 1. The results were normalized to the Ct values of inputs and presented as percentages of inputs. ND: not detectable.
To determine if TGFβ induces Smad3 and E2F4 binding to the native -401 TIE region of the LPA1 promoter, we performed chromatin immunoprecipitation (ChIP) assays in MDA-MB-231 and SKOV-3 cells. The qPCR analysis of Smad3 immunoprecipitates from MDA-MB-231 and SKOV-3 cells showed 3.8- and 3.7-fold induction of Smad3 binding to the -401 TIE (Fig. 5B). We also observed 2.0- and 1.8-fold increases in Smad3 binding to the -40 TIE in MDA-MB-231 and SKOV-3, respectively. Thus TGFβ induced physical binding of activated Smad3 to the -401 TIE and to a lesser extent, to the -40 TIE of the LPA1 promoter. The binding of E2F4, another partner of the Smad complex, to the -401 TIE also increased by 2.5 and 2.7 fold following TGFβ treatment of MDA-MB-231 and SKOV-3 cells. However, no significant increase in binding of E2F4 to the -40 TIE in TGFβ-treated MDA-MB-231 cells was observed. In these ChIP experiments, we included the c-myc TIE as positive controls and confirmed the binding of Smad3 and E2F4 to the c-myc TIE in SKOV-3 cells treated with TGFβ. In sum, these experiments provide mechanistic insight into the TGFβ-mediated repression of LPA1 transcription and LPA1-linked biological activities.
DISCUSSION
In the present study, we showed that the LPA1 gene is a direct target of TGFβ-mediated repression. This inhibitory effect of TGFβ on LPA1 expression is detected in both normal and neoplastic cells with intact TβR and Smad signaling. Importantly, the inhibition of LPA1 by TGFβ is sufficient to suppress the LPA1-dependent migratory responses to LPA. The detailed analysis of the underlying mechanism indicates that TGFβ triggers downregulation of LPA1 through activation of Smad and binding of the Smad-E2F4 complex to the -401 TIE of the LPA1 gene promoter, a process analogous to the well-defined mode of repression of c-myc (34).
Among the multiple LPA receptors, LPA1 is the only receptor subtype transcriptionally repressed by the TGFβ-Smad signaling. In TGFβ-challenged cells, Smad3 forms a large complex with E2F4/5-p107 and Smad4 in the cytoplasm, translocates to the nucleus and binds to the TIE motif where the complex recruits other co-repressors and silences gene expression (37). Hence both Smad binding site and the conjugated E2F4/5 element are instrumental to TGFβ repression of target genes (34). Extensive analysis of the promoter sequences of other LPA receptors does not identify any TIE consensus sequence in the LPA4, LPA5 and LPA6 promoters (Fig. S4). There are putative RSBE in the LPA2, and LPA3 promoter sequences. However, none of these RSBEs is closely linked to a nearby E2F4/5 binding site. It is intriguing that the two TIE sites of the LPA1 gene promoter do not function equally. The -401 TIE was identified to be the major one for Smad-mediated repression of LPA1 while the contribution of the -40 TIE was negligible. This difference could be attributed to the fact that only 4 out of 11 nucleotides match with the consensus E2F4/5 sequence at the -40 TIE while the -401 TIE matches the consensus at 9 out of 11 nucleotides. Alternatively, the TIE location relative to the transcriptional initiation site or other regulatory sequences beyond the TIE sites could influence the interaction with the Smad complex and the transcriptional repression..
The biological function of LPA1 has been a subject of extensive studies in both in vitro cell culture and genetic animal models (8-10, 27). Compared to other LPA receptors, LPA1 is most widely expressed (3). The nearly ubiquitous distribution of LPA1 has led to the assumption that LPA1 is constitutively expressed. However, a few recent studies have hinted at the regulation of LPA1 by intracellular and extracellular cues (38, 39). The most exciting observation is that LPA1 is one of the target genes repressed by the metastatic tumor suppressor Nm23 (20). Another study showed that germline polymorphism of fibroblast growth factor receptor 4 (FGFR4) at residue 388 (G388R) correlates with enhancement of LPA1 expression and more aggressive migratory and invasive responses to LPA in tumors carrying R388 FGFR4 (40). Although LPA1 expression may be indeed regulated by Nm23 and FGFR4, it is not known whether or how these modulators affect transcription, stability or translation of LPA1. The results from the current study represent the first example that an endogenous factor could transcriptionally restrain expression of LPA1 and LPA1-dependent cellular effects.
The roles of LPA and LPA receptors in cancer have drawn considerable attention in recent years. The LPA2 receptor is overexpressed in ovarian, breast, thyroid and rectal colon cancers (15-19). The transgenic and knockout mouse models further support an oncogenic role of LPA2 (14, 41). Expression of LPA1, on the other hand, does not show consensus increases from normal to malignant phenotypes. Instead, several independent groups have reported a tendency of downregulation of LPA1 in diverse cancers (15, 17-19, 42, 43) in sharp contrast to the upregulation of LPA2 in malignant diseases. The findings of the current study offer a plausible explanation to this phenomenon. The enhanced TGFβ signaling during cancer development and progression may serve as a repressor of expression of LPA1 but not other LPA receptors.
TGFβ controls a multitude of biological activities in mammalian cells. It inhibits proliferation of epithelial cells and thus plays a part in early tumor suppression. However, TGFβ frequently fails to induce growth arrest in transformed epithelial cells. Instead, TGFβ stimulates migration and invasion of cancer cells, thereby promoting the metastatic potential in advanced cancer (44). This presumed effect of TGFβ on tumor cell invasion and metastasis is largely based on in vitro assays involving only TGFβ as a motogen (45). The conclusion may not truly reflect the physiological role of TGFβ in in vivo conditions where tumor cells are exposed to a complex mix of multiple chemokines, cytokines, nutrients and growth factors. We found in the current study that the effects of TGFβ on cell motility could be opposite under different conditions. In the cancer cell lines we tested, TGFβ itself was a weak stimulus of tumor cell invasion. In the presence of LPA, however, the role of TGFβ was reversed, counteracting the strong motogenic activity of LPA. Since both TGFβ and LPA are present in the circulation and malignant effusions, TGFβ probably acts as a negative regulator of cell motility in physiological and pathophysiological conditions. By extension, the findings of the current work underscore the importance of crosstalk between LPA and other coexisting factors in coordination of the overall cellular responses.
MATERIALS AND METHODS
Materials
LPA (1-oleoly, 18:1) was obtained from Avanti Polar Lipids, Inc. (Alabaster, AL). Prior to use, LPA was dissolved in PBS containing 0.5% fatty acid-free bovine serum albumin (BSA) obtained from Roche (Indianapolis, IN). TGFβ was obtained from PeproTech Inc. (Rocky Hill, NJ). Anti-Smad3 and Smad4 antibodies were from Abcam (Cambridge, MA). Tubulin α/β antibody was obtained from Cell Signaling (Danvers, MA). Anti-E2F4 antibody was from Santa Cruz Biotech (Santa Cruz, CA). FBS was obtained from Atlanta Biological (Atlanta, GA). All primers were synthesized by Operon Biotechnologies, Inc. (Huntsville, AL). Biotinylated dsDNA were synthesized by IDT (Coralville, IA). TRIzol and cell culture reagents were obtained from Invitrogen Inc. (Carlsbad, CA). The RT kit, TaqMan gene expression assays, SYBR Green PCR mix and QPCR master mix were obtained from Applied Biosystems (Carlsbad, CA). The transfection reagent LT1 was obtained from Mirus (Madison, WI). Plasmid DNA was purified using the endo-free purification kit from Qiagen (Valencia, CA).
Cell Culture
MDA-MB-231 was provided by Dr. S. Spiegel (Virginia Commonwealth University) and was maintained in Dulbecco modified Eagle medium (DMEM) with 10% FBS and 100 U/ml penicillin and 100 μg/ml streptomycin. IOSE-29 was originally obtained from Dr. N. Auersperg (University of British Columbia, Canada) and cultured as described previously (46). Primary Mammary epithelial cells (1001-8) and primary ovarian epithelial cells (NOE-71) were provided by Dr. Y. Yu (MD Anderson Cancer Center) and were cultured in HuMEC Ready Medium (Invitrogen) and 50:50 M199/F12 medium with 10% FBS, 20 ng/ml EGF and gentamicin (10 μg/ml), respectively. MCF-10A was provided by Dr. D. Gewirtz (Virginia Commonwealth University) and cultured in DMEM/F12 medium with 5% horse serum, 10 μg/ml insulin, 20 ng/ml EGF, 100 ng/ml cholera toxin and 0.5 μg/ml hydrocortisone. Other cancer cell lines used in the study were cultured in RPMI 1640 supplemented with 10% FBS and antibiotics as we described previously (47).
Migration and invasion assays
Cell migration was measured using the Transwell chambers (Costar, Corning, NY). Transwells were coated with 10 μg/ml type I collagen and placed in the lower chamber containing serum-free medium supplemented with vehicle, TGFβ, LPA or LPA+TGFβ. Cells suspended in serum-free medium containing 0.01% fatty acid-free BSA were added to the upper chamber at 2 × 104 cells/well. Cells were allowed to migrate for 6 hours at 37°C. Non-migrated cells were removed from the top filter surface with a cotton swab. Migrated cells attached to the underside of the Transwell were washed with PBS and stained with crystal violet and counted under a microscope. The invasion of tumor cell lines was measured using the growth factor–reduced Matrigel invasion chambers (BD Biosciences, San Jose, CA). The assays were performed as migration assays except that the cells were incubated for 20 hours. All migration and invasion assays were repeated three times with consistent results.
shRNA
short hairpin RNA (shRNA)-expressing lentivirus constructs were generated using pLV-RNAi vector (Biosettia, San Diego, CA). The Smad3 target sequences Smad3sh1 GTGACCACCAGATGAACCA (48), Smad3sh2 GGATTGAGCTGCACCTGAATG (49) and Smad4 target sequences Smad4sh1 GCAGGTGGCTGGTCGGAAA (50), Smad4sh2 GCCAGCTACTTACCATCATA (51) were inserted to the pLV-RNAi plasmid following the manufacturer's protocol. The LPA1 shRNA lentiviral vectors were obtained from Dr. S. Huang (Medical College of Georgia) (11). The shRNA lentiviruses were propagated in 293FT cells. The culture supernatants were used to infect cancer cell lines. The GFP-positive cells were sorted out using flow cytometer 96 hours post virus infection.
qPCR
Total cellular RNA was isolated using Trizol (Invitrogen). Complementary DNA (cDNA) was synthesized from RNA (1 μg, random primers) using the High-Capacity cDNA Reverse Transcription Kit from Applied Biosystems. The mRNA levels of individual LPA receptors were determined using gene specific probes, the TaqMan Universal PCR Master Mix and the 7900HT Prism Real-Time PCR System.
Luciferase vectors, deletion, and site-directed mutagenesis
The luciferase reporter vector pGL2-LPA1-Luc containing -1156 to +86 was generated by PCR amplification of the LPA1 promoter sequence (forward 5’-GCACTCGAGTGCAAAGCTACACTGGGAAA-3’, reverse 5’-GCAAAGCTTCACACTCTCACTGGCACTCG-3’). The PCR product was inserted into pGL2-Basic-Luc at XhoI and HindIII sites. The deletion mutant (-366 to +86) was made by PCR amplification of the fragment from pGL2-LPA1-Luc (forward 5’-GCACTCGAGCTGACGCTCCCTGAGTGG-3’, reverse 5’-GCAAAGCTTCACACTCTCACTGGCACTCG-3’) and re-inserted into the pGL2-Basic-Luc at the XhoI and HindIII sites. The promoter sequences in these plasmids were verified by automatic sequencing. The -401 and -40 TIE consensus sites within pGL-LPA1-Luc were converted into inactive sequences by site-directed mutagenesis. The wild type -401 TIE 5’-GGCTTTGGCGCG and wide type -40 TIE 5’-GGCTTCGCGC were converted into 5’-GGCTAATTCGCGC and 5’-GGCAATTCGCC, respectively. For luciferase assays, MDA-MB-231 and SKOV-3 were transfected with luciferase vectors along with β-gal plasmid using TransIT-LT1 (Mirus Bio). About 48 to 60 hours after transfection, the cells were treated with TGFβ or vehicle for 16-20 hours. Cell extracts were prepared and assayed for luciferase activity using the luciferase assay kit from Promega.
DNA pull-down assay
Lysates of MDA-MB-231 and SKOV-3 cells were prepared by brief sonication in the HKMG buffer (10 mM, Tris-HCl, pH 7.9, 100 mM KCl, 5 mM MgCl2, 10% glycerol, 1 mM DTT, 0.1% NP40 and protease inhibitors) using the Fisher Scientific Sonic Dismembrator Model 100, followed by 10 minutes of centrifugation at 12,000×g at 4 °C. Cellular proteins (400 μg) were incubated with 4 μg of biotinylated double-stranded oligonucleotides (5’-CCCTACTGCCCGGCTTTGGCGCGCTGGCAGGAGGAG–biotin) for 16 hours at 4 °C. The M-280 Streptavidin Dynabeads (Invitrogen) (30 μl) were added to each sample and incubated for another hour at 4 °C. The Dynabeads were washed three times with PBS before western analysis of Smad3 or E2F4.
ChIP assay
TGFβ or vehicle-treated MDA-MB-231 and SKOV-3 cells were cross-linked with 1% formaldehyde for 10 minutes at room temperature. The cells were lysed for 10 minutes in ice-cold lysis buffer (5 mM HEPES, pH 8.0, 80 mM KCl, 1% NP40 and protease inhibitors). The nuclear fraction that was recovered by centrifugation (5 minutes at 5000×g) was resuspended in a ChIP assay buffer (50 mM HEPES, pH 8.0, 10 mM EDTA, 1% SDS, and protease inhibitors) and sonicated on ice to achieve an average chromatin length of 200-1000 bp. The sonicated samples were pre-cleared by incubation with Protein G Dynabeads (Invitrogen). The material recovered from the equivalent of 106 cells was incubated for 16 hours at 4 °C with 2 μg of either normal rabbit IgG (Santa Cruz) or anti-Smad or anti-E2F4 antibodies. Protein G Dynabeads were added and incubated for 2 hours. The DNA-protein-beads mixes were washed sequentially once with a low salt buffer (20 mM Tris, pH 8.0, 150 mM NaCl, 2 mM EDTA, 0.1% SDS, 1% Triton 100), once with a high salt buffer (20 mM Tris, pH 8.0, 500 mM NaCl, 2 mM EDTA, 0.1% SDS, 1% Triton 100), once with LiCl buffer (10 mM Tris-HCl, pH 8.0, 0,25 M LiCl, 1 mM EDTA, 1% deoxycholate, 1% NP-40), and finally twice with TE buffer (10 mM Tris-HCl, pH 8, 1 mM EDTA). The specifically bound complexes were eluted from the Protein G Dynabeads by incubation twice for 15 minutes at 65 °C with TE elution buffer (10 mM Tris-HCl, pH 8, 1 mM EDTA, 1% SDS). The immunoprecipitated complexes and the starting material (input) were incubated overnight at 65 °C to reverse cross linking, then treated with RNase A followed by proteinase K and purified using the QIAquick Spin Columns (Qiagen, Valencia, CA). The DNA samples were recovered in 100 μL H2O, and analyzed by qPCR using SYBR Green. Details of the primer used for qPCR were listed in Table 1.
Table 1.
Primers used in ChIP assays
| -401 Forward | 5’-GTGCTACGTGGAACAAGCAG-3’ |
| -401 Reverse | 5’-GGCGGGACAGTGTGAGC-3’ |
| -40 Forward | 5’-AGCGAGCGCAGGTAAGG-3’ |
| -40 Reverse | 5’-GCACCCACACTCTCACTGG-3’ |
| c-myc TIE Forward | 5’-TTATAATGCGAGGGTCTGGA-3’ |
| c-myc TIE Reverse | 5’-TGCCTCTCGCTGGAATTACT-3’ |
Statistics
All numerical data were presented as mean ± SD of triplicate assays, representative of three independent experiments. The statistical significances were analyzed using Student's t test where p<0.05 was considered statistically significant. In all figures, the statistical significances were indicated with * if p<0.05 or ** if p<0.01.
Supplementary Material
ACKNOWLEDGEMENTS
The work was supported in part by the NIH/NCI grant 2R01CA102196 (XF), the Department of Defense ovarian cancer research program grant W81XWH-11-1-0541 (XF), the Jeffress Memorial Fund award (XF), and the NIH grant P30 CA16059 to Massey Cancer Center of Virginia Commonwealth University School of Medicine.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
REFERENCES
- 1.Panupinthu N, Lee HY, Mills GB. Lysophosphatidic acid production and action: critical new players in breast cancer initiation and progression. Br J Cancer. 2010 Mar 16;102(6):941–6. doi: 10.1038/sj.bjc.6605588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sano T, Baker D, Virag T, Wada A, Yatomi Y, Kobayashi T, et al. Multiple mechanisms linked to platelet activation result in lysophosphatidic acid and sphingosine 1-phosphate generation in blood. J Biol Chem. 2002 Jun 14;277(24):21197–206. doi: 10.1074/jbc.M201289200. [DOI] [PubMed] [Google Scholar]
- 3.Choi JW, Herr DR, Noguchi K, Yung YC, Lee CW, Mutoh T, et al. LPA receptors: subtypes and biological actions. Annu Rev Pharmacol Toxicol. 2010;50:157–86. doi: 10.1146/annurev.pharmtox.010909.105753. [DOI] [PubMed] [Google Scholar]
- 4.Noguchi K, Ishii S, Shimizu T. Identification of p2y9/GPR23 as a novel G protein-coupled receptor for lysophosphatidic acid, structurally distant from the Edg family. J Biol Chem. 2003 Jul 11;278(28):25600–6. doi: 10.1074/jbc.M302648200. [DOI] [PubMed] [Google Scholar]
- 5.Lee CW, Rivera R, Gardell S, Dubin AE, Chun J. GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. J Biol Chem. 2006 Aug 18;281(33):23589–97. doi: 10.1074/jbc.M603670200. [DOI] [PubMed] [Google Scholar]
- 6.Pasternack SM, von Kugelgen I, Aboud KA, Lee YA, Ruschendorf F, Voss K, et al. G protein-coupled receptor P2Y5 and its ligand LPA are involved in maintenance of human hair growth. Nat Genet. 2008 Mar;40(3):329–34. doi: 10.1038/ng.84. [DOI] [PubMed] [Google Scholar]
- 7.Contos JJ, Fukushima N, Weiner JA, Kaushal D, Chun J. Requirement for the lpA1 lysophosphatidic acid receptor gene in normal suckling behavior. Proc Natl Acad Sci U S A. 2000 Nov 21;97(24):13384–9. doi: 10.1073/pnas.97.24.13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inoue M, Rashid MH, Fujita R, Contos JJ, Chun J, Ueda H. Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nat Med. 2004 Jul;10(7):712–8. doi: 10.1038/nm1060. [DOI] [PubMed] [Google Scholar]
- 9.Pradere JP, Klein J, Gres S, Guigne C, Neau E, Valet P, et al. LPA1 receptor activation promotes renal interstitial fibrosis. J Am Soc Nephrol. 2007 Dec;18(12):3110–8. doi: 10.1681/ASN.2007020196. [DOI] [PubMed] [Google Scholar]
- 10.Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z, et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008 Jan;14(1):45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- 11.Chen H, Wu X, Pan ZK, Huang S. Integrity of SOS1/EPS8/ABI1 tri-complex determines ovarian cancer metastasis. Cancer Res. 2010 Dec 1;70(23):9979–90. doi: 10.1158/0008-5472.CAN-10-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu S, Murph MM, Lu Y, Liu S, Hall HS, Liu J, et al. Lysophosphatidic acid receptors determine tumorigenicity and aggressiveness of ovarian cancer cells. J Natl Cancer Inst. 2008 Nov 19;100(22):1630–42. doi: 10.1093/jnci/djn378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang X, Schummer M, Mao M, Yu S, Tabassam FH, Swaby R, et al. Lysophosphatidic acid is a bioactive mediator in ovarian cancer. Biochim Biophys Acta. 2002 May 23;1582(1-3):257–64. doi: 10.1016/s1388-1981(02)00179-8. [DOI] [PubMed] [Google Scholar]
- 14.Lin S, Wang D, Iyer S, Ghaleb AM, Shim H, Yang VW, et al. The absence of LPA2 attenuates tumor formation in an experimental model of colitis-associated cancer. Gastroenterology. 2009 May;136(5):1711–20. doi: 10.1053/j.gastro.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su LJ, Chang CW, Wu YC, Chen KC, Lin CJ, Liang SC, et al. Selection of DDX5 as a novel internal control for Q-RT-PCR from microarray data using a block bootstrap re-sampling scheme. BMC Genomics. 2007 Jun 1;8:140. doi: 10.1186/1471-2164-8-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radvanyi L, Singh-Sandhu D, Gallichan S, Lovitt C, Pedyczak A, Mallo G, et al. The gene associated with trichorhinophalangeal syndrome in humans is overexpressed in breast cancer. Proc Natl Acad Sci U S A. 2005 Aug 2;102(31):11005–10. doi: 10.1073/pnas.0500904102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hendrix ND, Wu R, Kuick R, Schwartz DR, Fearon ER, Cho KR. Fibroblast growth factor 9 has oncogenic activity and is a downstream target of Wnt signaling in ovarian endometrioid adenocarcinomas. Cancer Res. 2006 Feb 1;66(3):1354–62. doi: 10.1158/0008-5472.CAN-05-3694. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez-Carbayo M, Socci ND, Lozano J, Saint F, Cordon-Cardo C. Defining molecular profiles of poor outcome in patients with invasive bladder cancer using oligonucleotide microarrays. J Clin Oncol. 2006 Feb 10;24(5):778–89. doi: 10.1200/JCO.2005.03.2375. [DOI] [PubMed] [Google Scholar]
- 19.Skrzypczak M, Goryca K, Rubel T, Paziewska A, Mikula M, Jarosz D, et al. Modeling oncogenic signaling in colon tumors by multidirectional analyses of microarray data directed for maximization of analytical reliability. PLoS One. 2010;5(10) doi: 10.1371/journal.pone.0013091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marshall JC, Collins J, Marino N, Steeg P. The Nm23-H1 metastasis suppressor as a translational target. Eur J Cancer. 2010 May;46(7):1278–82. doi: 10.1016/j.ejca.2010.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horak CE, Mendoza A, Vega-Valle E, Albaugh M, Graff-Cherry C, McDermott WG, et al. Nm23-H1 suppresses metastasis by inhibiting expression of the lysophosphatidic acid receptor EDG2. Cancer Res. 2007 Dec 15;67(24):11751–9. doi: 10.1158/0008-5472.CAN-07-3175. [DOI] [PubMed] [Google Scholar]
- 22.Jeon ES, Moon HJ, Lee MJ, Song HY, Kim YM, Cho M, et al. Cancer-derived lysophosphatidic acid stimulates differentiation of human mesenchymal stem cells to myofibroblast-like cells. Stem Cells. 2008 Mar;26(3):789–97. doi: 10.1634/stemcells.2007-0742. [DOI] [PubMed] [Google Scholar]
- 23.Xu MY, Porte J, Knox AJ, Weinreb PH, Maher TM, Violette SM, et al. Lysophosphatidic acid induces alphavbeta6 integrin-mediated TGF-beta activation via the LPA2 receptor and the small G protein G alpha(q). Am J Pathol. 2009 Apr;174(4):1264–79. doi: 10.2353/ajpath.2009.080160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sauer B, Vogler R, Zimmermann K, Fujii M, Anzano MB, Schafer-Korting M, et al. Lysophosphatidic acid interacts with transforming growth factor-beta signaling to mediate keratinocyte growth arrest and chemotaxis. J Invest Dermatol. 2004 Nov;123(5):840–9. doi: 10.1111/j.0022-202X.2004.23458.x. [DOI] [PubMed] [Google Scholar]
- 25.Wu J, Mukherjee A, Lebman DA, Fang X. Lysophosphatidic Acid-Induced p21Waf1 Expression Mediates the Cytostatic Response of Breast and Ovarian Cancer Cells to TGFbeta. Mol Cancer Res. 2011 Nov;9(11):1562–70. doi: 10.1158/1541-7786.MCR-11-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Markowitz S, Wang J, Myeroff L, Parsons R, Sun L, Lutterbaugh J, et al. Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability. Science. 1995 Jun 2;268(5215):1336–8. doi: 10.1126/science.7761852. [DOI] [PubMed] [Google Scholar]
- 27.Shida D, Kitayama J, Yamaguchi H, Okaji Y, Tsuno NH, Watanabe T, et al. Lysophosphatidic acid (LPA) enhances the metastatic potential of human colon carcinoma DLD1 cells through LPA1. Cancer Res. 2003 Apr 1;63(7):1706–11. [PubMed] [Google Scholar]
- 28.Lee Z, Cheng CT, Zhang H, Subler MA, Wu J, Mukherjee A, et al. Role of LPA4/p2y9/GPR23 in negative regulation of cell motility. Mol Biol Cell. 2008 Dec;19(12):5435–45. doi: 10.1091/mbc.E08-03-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Leeuwen FN, Olivo C, Grivell S, Giepmans BN, Collard JG, Moolenaar WH. Rac activation by lysophosphatidic acid LPA1 receptors through the guanine nucleotide exchange factor Tiam1. J Biol Chem. 2003 Jan 3;278(1):400–6. doi: 10.1074/jbc.M210151200. [DOI] [PubMed] [Google Scholar]
- 30.Hama K, Aoki J, Fukaya M, Kishi Y, Sakai T, Suzuki R, et al. Lysophosphatidic acid and autotaxin stimulate cell motility of neoplastic and non-neoplastic cells through LPA1. J Biol Chem. 2004 Apr 23;279(17):17634–9. doi: 10.1074/jbc.M313927200. [DOI] [PubMed] [Google Scholar]
- 31.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003 Oct 9;425(6958):577–84. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 32.Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005 Dec 1;19(23):2783–810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 33.Seoane J. Escaping from the TGFbeta anti-proliferative control. Carcinogenesis. 2006 Nov;27(11):2148–56. doi: 10.1093/carcin/bgl068. [DOI] [PubMed] [Google Scholar]
- 34.Chen CR, Kang Y, Siegel PM, Massague J. E2F4/5 and p107 as Smad cofactors linking the TGFbeta receptor to c-myc repression. Cell. 2002 Jul 12;110(1):19–32. doi: 10.1016/s0092-8674(02)00801-2. [DOI] [PubMed] [Google Scholar]
- 35.Frederick JP, Liberati NT, Waddell DS, Shi Y, Wang XF. Transforming growth factor beta-mediated transcriptional repression of c-myc is dependent on direct binding of Smad3 to a novel repressive Smad binding element. Mol Cell Biol. 2004 Mar;24(6):2546–59. doi: 10.1128/MCB.24.6.2546-2559.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang Y, Chen CR, Massague J. A self-enabling TGFbeta response coupled to stress signaling: Smad engages stress response factor ATF3 for Id1 repression in epithelial cells. Mol Cell. 2003 Apr;11(4):915–26. doi: 10.1016/s1097-2765(03)00109-6. [DOI] [PubMed] [Google Scholar]
- 37.Li JM, Hu PP, Shen X, Yu Y, Wang XF. E2F4-RB and E2F4-p107 complexes suppress gene expression by transforming growth factor beta through E2F binding sites. Proc Natl Acad Sci U S A. 1997 May 13;94(10):4948–53. doi: 10.1073/pnas.94.10.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horak CE, Lee JH, Elkahloun AG, Boissan M, Dumont S, Maga TK, et al. Nm23-H1 suppresses tumor cell motility by down-regulating the lysophosphatidic acid receptor EDG2. Cancer Res. 2007 Aug 1;67(15):7238–46. doi: 10.1158/0008-5472.CAN-07-0962. [DOI] [PubMed] [Google Scholar]
- 39.Stadler CR, Knyazev P, Bange J, Ullrich A. FGFR4 GLY388 isotype suppresses motility of MDA-MB-231 breast cancer cells by EDG-2 gene repression. Cell Signal. 2006 Jun;18(6):783–94. doi: 10.1016/j.cellsig.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 40.Sugiyama N, Varjosalo M, Meller P, Lohi J, Chan KM, Zhou Z, et al. FGF receptor-4 (FGFR4) polymorphism acts as an activity switch of a membrane type 1 matrix metalloproteinase-FGFR4 complex. Proc Natl Acad Sci U S A. 2010 Sep 7;107(36):15786–91. doi: 10.1073/pnas.0914459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu S, Umezu-Goto M, Murph M, Lu Y, Liu W, Zhang F, et al. Expression of autotaxin and lysophosphatidic acid receptors increases mammary tumorigenesis, invasion, and metastases. Cancer Cell. 2009 Jun 2;15(6):539–50. doi: 10.1016/j.ccr.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murph MM, Nguyen GH, Radhakrishna H, Mills GB. Sharpening the edges of understanding the structure/function of the LPA1 receptor: expression in cancer and mechanisms of regulation. Biochim Biophys Acta. 2008 Sep;1781(9):547–57. doi: 10.1016/j.bbalip.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shida D, Watanabe T, Aoki J, Hama K, Kitayama J, Sonoda H, et al. Aberrant expression of lysophosphatidic acid (LPA) receptors in human colorectal cancer. Lab Invest. 2004 Oct;84(10):1352–62. doi: 10.1038/labinvest.3700146. [DOI] [PubMed] [Google Scholar]
- 44.Bierie B, Moses HL. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006 Jul;6(7):506–20. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- 45.Seton-Rogers SE, Lu Y, Hines LM, Koundinya M, LaBaer J, Muthuswamy SK, et al. Cooperation of the ErbB2 receptor and transforming growth factor beta in induction of migration and invasion in mammary epithelial cells. Proc Natl Acad Sci U S A. 2004 Feb 3;101(5):1257–62. doi: 10.1073/pnas.0308090100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Auersperg N, Maines-Bandiera SL, Dyck HG, Kruk PA. Characterization of cultured human ovarian surface epithelial cells: phenotypic plasticity and premalignant changes. Lab Invest. 1994 Oct;71(4):510–8. [PubMed] [Google Scholar]
- 47.Lee Z, Swaby RF, Liang Y, Yu S, Liu S, Lu KH, et al. Lysophosphatidic acid is a major regulator of growth-regulated oncogene alpha in ovarian cancer. Cancer Res. 2006 Mar 1;66(5):2740–8. doi: 10.1158/0008-5472.CAN-05-2947. [DOI] [PubMed] [Google Scholar]
- 48.Blount AL, Vaughan JM, Vale WW, Bilezikjian LM. A Smad-binding element in intron 1 participates in activin-dependent regulation of the follistatin gene. J Biol Chem. 2008 Mar 14;283(11):7016–26. doi: 10.1074/jbc.M709502200. [DOI] [PubMed] [Google Scholar]
- 49.Jazag A, Ijichi H, Kanai F, Imamura T, Guleng B, Ohta M, et al. Smad4 silencing in pancreatic cancer cell lines using stable RNA interference and gene expression profiles induced by transforming growth factor-beta. Oncogene. 2005 Jan 20;24(4):662–71. doi: 10.1038/sj.onc.1208102. [DOI] [PubMed] [Google Scholar]
- 50.Giampieri S, Manning C, Hooper S, Jones L, Hill CS, Sahai E. Localized and reversible TGFbeta signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol. 2009 Nov;11(11):1287–96. doi: 10.1038/ncb1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deckers M, van Dinther M, Buijs J, Que I, Lowik C, van der Pluijm G, et al. The tumor suppressor Smad4 is required for transforming growth factor beta-induced epithelial to mesenchymal transition and bone metastasis of breast cancer cells. Cancer Res. 2006 Feb 15;66(4):2202–9. doi: 10.1158/0008-5472.CAN-05-3560. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.