Abstract
When Lewis rats were immunized by intradermal injection into the parietal scalp rather than into the footpad with mycobacterial delipidated cells in squalene, arthritis could be produced in the temporomandibular joint (TMJ) with a maximum incidence of 60%, accompanied by systemic polyarthritis. Other methods of immunization including intradermal injection into the tail, posterior cervical region, or intra-inguinal lymph nodes did not induce arthritis in the TMJ. A combination of this inoculation and hemiocclusal loss markedly increased the incidence of arthritis in the TMJ. This arthritis in the TMJ was, however, milder than that in other joints and was apparent only histologically. The group given intradermal injection of adjuvants into the parietal scalp showed definite suppression of body weight gain. Since the method of intradermal injection into the parietal scalp can induce a high incidence of arthritis in the TMJ, our study presents a unique experimental model for study of arthritis in the TMJ.
Full text
PDF
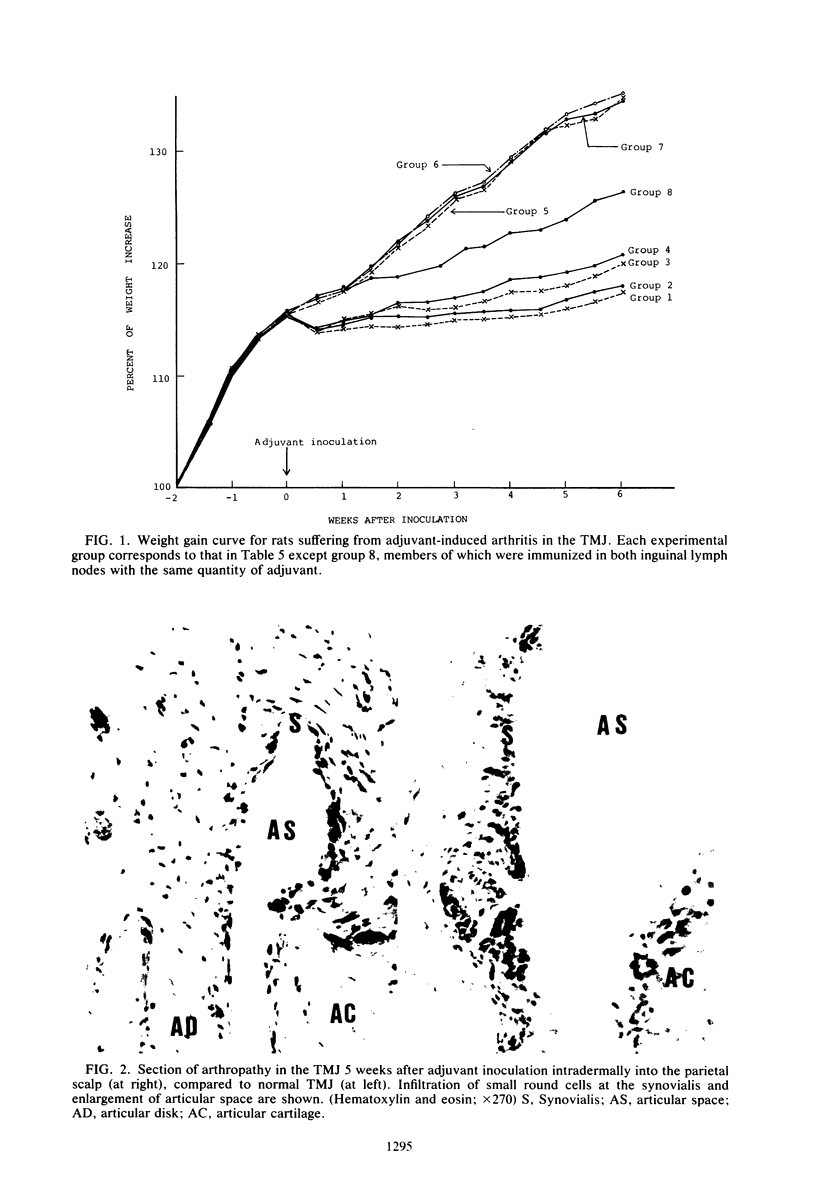
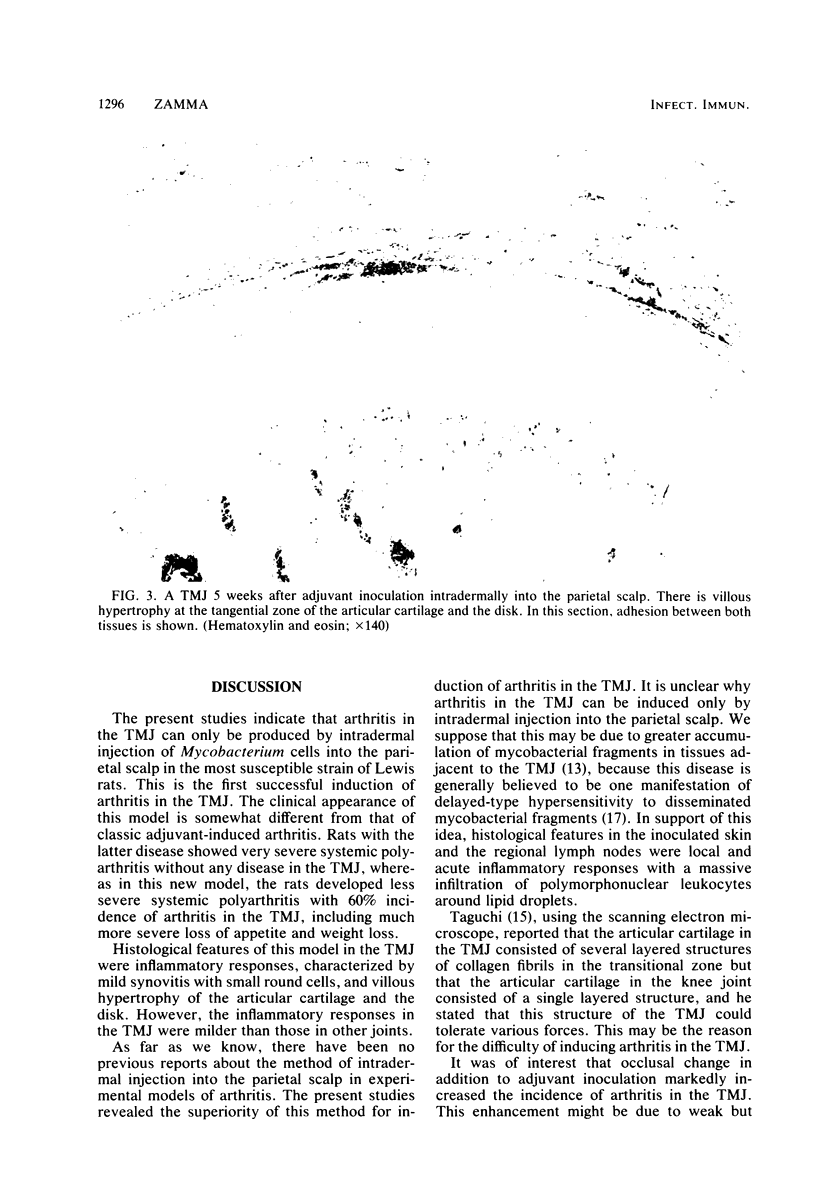
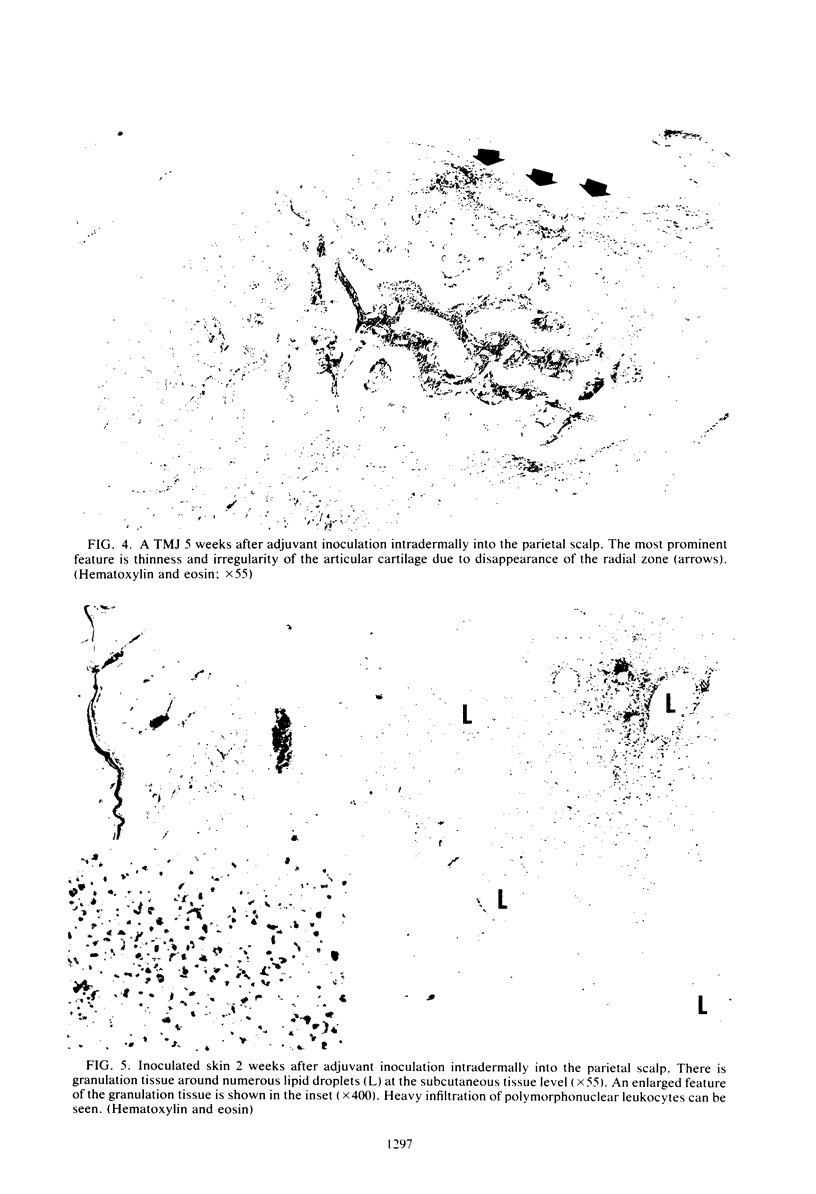
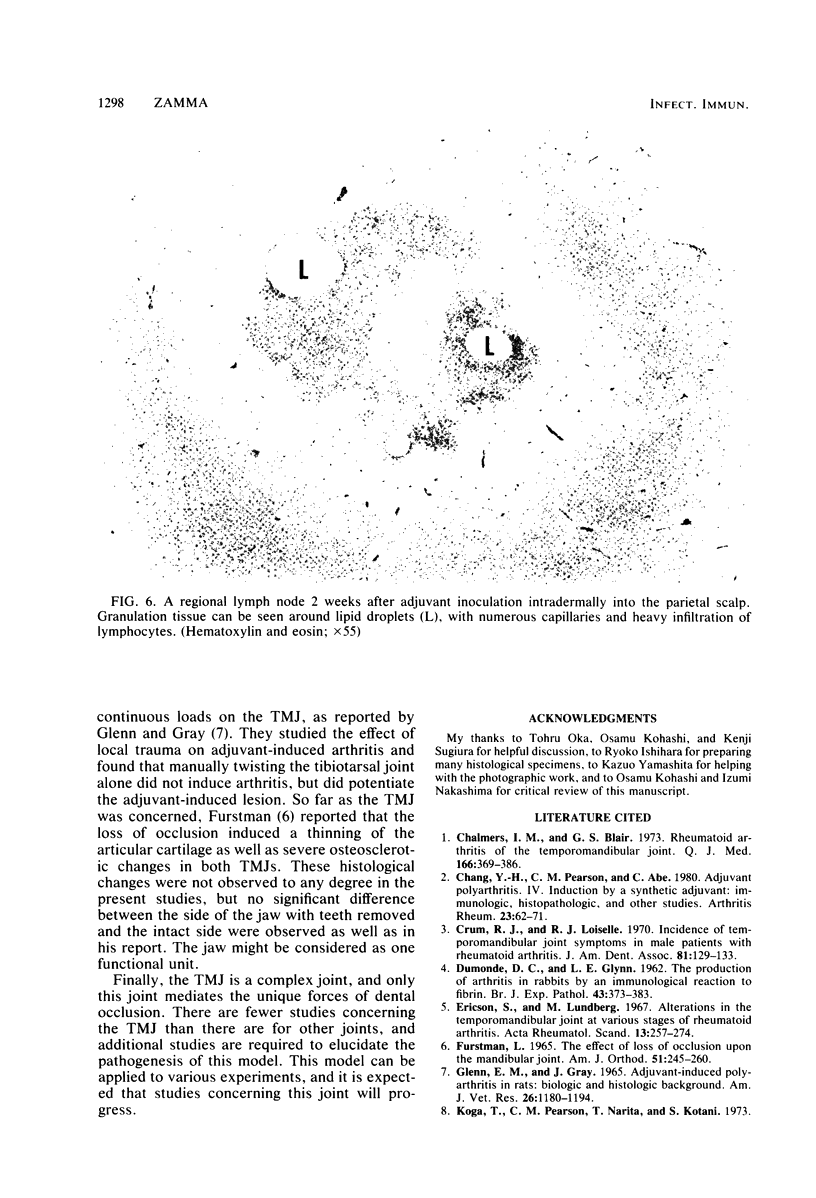

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chalmers I. M., Blair G. S. Rheumatoid arthritis of the temporomandibular joint. A clinical and radiological study using circular tomography. Q J Med. 1973 Apr;42(166):369–386. [PubMed] [Google Scholar]
- Chang Y. H., Pearson C. M., Abe C. Adjuvant polyarthritis. IV. Induction by a synthetic adjuvant: immunologic, histopathologic, and other studies. Arthritis Rheum. 1980 Jan;23(1):62–71. doi: 10.1002/art.1780230111. [DOI] [PubMed] [Google Scholar]
- Crum R. J., Loiselle R. J. Incidence of temporomandibular joint symptoms in male patients with rheumatoid arthritis. J Am Dent Assoc. 1970 Jul;81(1):129–133. doi: 10.14219/jada.archive.1970.0147. [DOI] [PubMed] [Google Scholar]
- DUMONDE D. C., GLYNN L. E. The production of arthritis in rabbits by an immunological reaction to fibrin. Br J Exp Pathol. 1962 Aug;43:373–383. [PMC free article] [PubMed] [Google Scholar]
- Ericson S., Lundberg M. Alterations in the temporomandibular joint at various stages of rheumatoid arthritis. Acta Rheumatol Scand. 1967;13(4):257–274. doi: 10.3109/rhe1.1967.13.issue-1-4.22. [DOI] [PubMed] [Google Scholar]
- FURSTMAN L. THE EFFECT OF LOSS OF OCCLUSION UPON THE MANDIBULAR JOINT. Am J Orthod. 1965 Apr;51:245–261. doi: 10.1016/0002-9416(65)90101-6. [DOI] [PubMed] [Google Scholar]
- Glenn E. M., Gray J. Adjuvant-induced polyarthritis in rats: biologic and histologic background. Am J Vet Res. 1965 Sep;26(114):1180–1194. [PubMed] [Google Scholar]
- Koga T., Pearson C. M., Narita T., Kotani S. Polyarthritis induced in the rat with cell walls from several bacteria and two Streptomyces species. Proc Soc Exp Biol Med. 1973 Jul;143(3):824–827. doi: 10.3181/00379727-143-37421. [DOI] [PubMed] [Google Scholar]
- PEARSON C. M. Development of arthritis, periarthritis and periostitis in rats given adjuvants. Proc Soc Exp Biol Med. 1956 Jan;91(1):95–101. doi: 10.3181/00379727-91-22179. [DOI] [PubMed] [Google Scholar]
- PLANK J., RYCHLO A. Eine Schnellentkalkungsmethode. Zentralbl Allg Pathol. 1952 Dec 10;89(8):252–254. [PubMed] [Google Scholar]
- Senda K., Kaneko M., Sasaki T. [Fundamental study on radioisotopic lymphography of the head and neck (author's transl)]. Kaku Igaku. 1981 Nov;18(9):1199–1205. [PubMed] [Google Scholar]
- Swingle K. F., Jaques L. W., Kvam D. C. Differences in the severity of adjuvant arthritis in four strains of rats. Proc Soc Exp Biol Med. 1969 Nov;132(2):608–612. doi: 10.3181/00379727-132-34270. [DOI] [PubMed] [Google Scholar]
- Trentham D. E., Townes A. S., Kang A. H. Autoimmunity to type II collagen an experimental model of arthritis. J Exp Med. 1977 Sep 1;146(3):857–868. doi: 10.1084/jem.146.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAKSMAN B. H., PEARSON C. M., SHARP J. T. Studies of arthritis and other lesions induced in rats by injection of mycobacterial adjuvant. II. Evidence that the disease is a disseminated immunologic response to exogenous antigen. J Immunol. 1960 Oct;85:403–417. [PubMed] [Google Scholar]
- WARD J. R., JONES R. S. Studies on adjuvant-induced polyarthritis in rats. I. Adjuvant composition, route of injection, and removal of depot site. Arthritis Rheum. 1962 Dec;5:557–564. doi: 10.1002/art.1780050604. [DOI] [PubMed] [Google Scholar]
- Whitehouse M. W., Orr K. J., Beck F. W., Pearson C. M. Freund's adjuvants: relationship of arthritogenicity and adjuvanticity in rats to vehicle composition. Immunology. 1974 Aug;27(2):311–330. [PMC free article] [PubMed] [Google Scholar]
- Wood F. D., Pearson C. M., Tanaka A. Capacity of mycobacterial wax D and its subfractions to induce adjuvant arthritis in rats. Int Arch Allergy Appl Immunol. 1969;35(5):456–467. doi: 10.1159/000230198. [DOI] [PubMed] [Google Scholar]