Abstract
Total glucocorticoid hormone levels in plasma of various species, including humans, follow a circadian rhythm that is made up from an underlying series of hormone pulses. In blood most of the glucocorticoid is bound to corticosteroid-binding globulin and albumin, resulting in low levels of free hormone. Although only the free fraction is biologically active, surprisingly little is known about the rhythms of free glucocorticoid hormones. We used single-probe microdialysis to measure directly the free corticosterone levels in the blood of freely behaving rats. Free corticosterone in the blood shows a distinct circadian and ultradian rhythm with a pulse frequency of approximately one pulse per hour together with an increase in hormone levels and pulse height toward the active phase of the light/dark cycle. Similar rhythms were also evident in the subcutaneous tissue, demonstrating that free corticosterone rhythms are transferred from the blood into peripheral target tissues. Furthermore, in a dual-probe microdialysis study, we demonstrated that the circadian and ultradian rhythms of free corticosterone in the blood and the subcutaneous tissue were highly synchronized. Moreover, free corticosterone rhythms were also synchronous between the blood and the hippocampus. These data demonstrate for the first time an ultradian rhythm of free corticosterone in the blood that translates into synchronized rhythms of free glucocorticoid hormone in peripheral and central tissues. The maintenance of ultradian rhythms across tissue barriers in both the periphery and the brain has important implications for research into aberrant biological rhythms in disease and for the development of improved protocols for glucocorticoid therapy.
Glucocorticoid hormones (cortisol in humans, corticosterone in rats and mice) are secreted by the adrenal gland and act on corticosteroid receptors found throughout the body, including the brain. They play an essential role in the regulation of homeostatic processes and in safeguarding health. Circulating levels of glucocorticoids demonstrate a circadian pattern which consists of a series of pulses of varying amplitude with a frequency of approximately one pulse per hour (1). Recent work has shown that this pulsatile profile translates into a fundamentally different transcription program as compared with the program generated by application of constant glucocorticoid hormone levels (2).
Glucocorticoids in the circulation are bound to plasma proteins, in particular with high affinity to corticosteroid-binding globulin, but also to albumin with low affinity (3–5). Consequently, only a relatively small percentage of glucocorticoids is free and is able to pass through cell membranes and bind to corticosteroid receptors (4, 6). Given its lipophilicity, free corticosterone levels and rhythms should be similar in all compartments of the body. However, this may not necessarily be the case because we have recently found that a rapid release of corticosteroid-binding globulin from the liver after stress restrains free corticosterone for approximately 20–30 min in the face of fast rising total hormone levels (7). This finding, together with the notion that only the free glucocorticoid hormone fraction is biologically active, clearly underscores the need for a detailed understanding of the regulation of free glucocorticoid levels. We have therefore used a single- and dual-probe microdialysis strategy in freely behaving rats to elucidate the following: 1) whether an ultradian rhythm of free corticosterone could be identified directly in the blood, 2) whether the putative rhythm in the blood would translate in an ultradian rhythm in peripheral target tissues such as the subcutaneous tissue, and 3) whether a delay would exist between the rhythms found in the blood and in peripheral and/or central target tissues. We demonstrate that peripheral in vivo microdialysis is a powerful and stress-free method to investigate free glucocorticoid hormone rhythms over extended time periods. Furthermore, this study provides the first evidence that free corticosterone levels in the blood and in target tissues show highly synchronized circadian and ultradian rhythms without an apparent delay between the blood and the target compartments.
Materials and Methods
Microdialysis
Procedures were performed on male Wistar rats (Harlan, Loughborough, UK) and executed in accordance with the Animals (Scientific Procedures) Act 1986 (United Kingdom). For a full description of the surgeries and experimental designs, see the Supplemental Information, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org.
Protocol 1: single-probe microdialysis
To investigate the rhythms of free corticosterone in peripheral compartments on 2 consecutive days, we performed single-probe microdialysis in freely behaving rats implanted with either a microdialysis probe in the blood (jugular vein) or the subcutaneous tissue (neck). Two days after insertion of the probe, sampling started at 0900 h and continued for 47 h. Samples were automatically collected in 10-min (between 0900 and 2100 h) or 30-min (between 2100 and 0800 h) intervals.
Protocol 2: dual-probe microdialysis of blood and subcutaneous tissue
To directly compare the rhythms of free corticosterone in the blood and in a peripheral target compartment, we collected microdialysis samples from both the blood and the subcutaneous tissue simultaneously by dual-probe microdialysis. Two days after insertion of the probes, samples were collected for 20 h as described under protocol 1.
Protocol 3: dual-probe microdialysis of blood and hippocampus
To directly compare the rhythms of free corticosterone in the blood and the brain, we collected microdialysis samples from the blood and the hippocampus simultaneously. Rats were equipped with a hippocampal guide cannula (7). Seven days later, a microdialysis probe was inserted through the guide cannula into the hippocampus, and a peripheral microdialysis probe was inserted into the jugular vein. After 2 d, samples were collected as described under protocol 2. The placement of the microdialysis probe was histologically verified (8). No misplacements were found.
Measurement of corticosterone
Dialysate corticosterone concentrations were measured using a 125I-corticosterone RIA (MP Biomedicals, Solon, OH) (8) (see the Supplemental Information for details).
Calculations and statistics
The PULSAR algorithm (9) was applied using data from a 12-h period (0900–2100 h) to calculate the following: 1) pulse frequency (i.e. number of pulses per hour); 2) mean pulse amplitude [i.e. mean height of pulses with respect to a circadian rhythm baseline as calculated by PULSAR (micrograms per deciliter)]; 3) mean pulse height [i.e. mean height of pulses above zero (micrograms per deciliter)]; 4) mean corticosterone concentration (micrograms per deciliter); and 5) area under the curve (AUC; arbitrary units) (10). Because we had previously found that the ultradian parameters of free corticosterone in the brain are different between the morning/early afternoon (i.e. 0900–1500 h) and the late afternoon/early night (i.e. 1500–2100 h) time period (10), separate PULSAR analyses were performed for these time periods. The pulse parameters were statistically analyzed by repeated-measures ANOVA with day (protocol 1), compartment (protocol 2/3) and time period (protocol 1/2/3) as within-subject factors (results summarized in Supplemental Table 1; SPSS version 16.0; SPSS Inc., IBM Corporation, Armonk, New York; level of significance P < 0.05). ANOVA were followed by post hoc paired t tests with Bonferroni correction when appropriate.
Cross-correlation analysis was performed to assess whether the ultradian rhythms of free corticosterone in the blood and in the target compartments were synchronous. For each rat, the cross-correlation coefficient for different lag times (between −6 and 6) was calculated after prewhitening of the data by first order differencing (11) to compensate for the high level of autocorrelation (SPSS). The mean cross-correlation at each lag time was statistically tested using a one-sample t test (against the null hypothesis that the cross-correlation coefficient is 0). Because corticosterone is secreted from the adrenal gland into the blood and from there distributed to target tissues, only positive cross-correlation coefficients were considered to be of interest.
Results
Free corticosterone rhythms in peripheral compartments on 2 consecutive days (protocol 1)
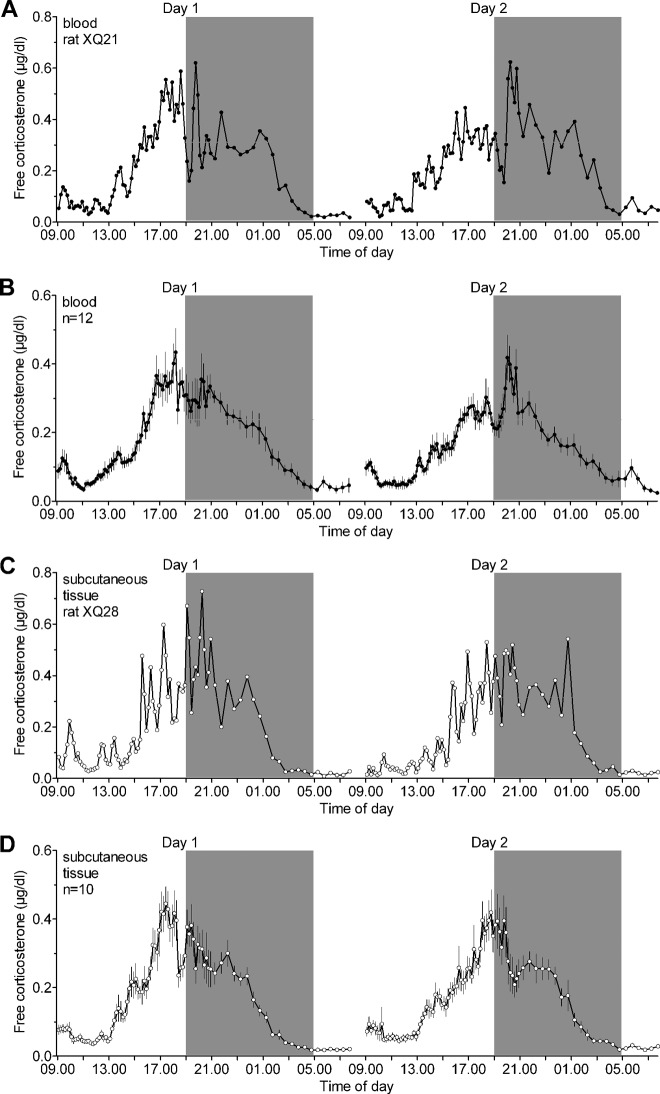
Free corticosterone levels were measured in the blood or the subcutaneous tissue over two consecutive 24-h periods. A clear circadian and ultradian rhythm in free corticosterone was found in the blood (Fig. 1, A and B) and the subcutaneous tissue (Fig. 1, C and D). Free corticosterone levels were low during the early morning and started to rise from approximately 1300 h reaching maximum levels about 1–2 h before the dark period. Free corticosterone levels gradually declined during the night reaching trough levels just before the light period. The pulse frequency was approximately one pulse per hour in both compartments and was stable over the light/dark cycle. In contrast, both the mean pulse amplitude and height, and consequently the mean free corticosterone level and AUC, were significantly higher in the late afternoon/early night compared with the morning/early afternoon period (Table 1 and Supplemental Table 1). Importantly, the circadian and ultradian rhythms of free corticosterone were remarkably stable over the 2 consecutive days. Except for a slightly lower pulse frequency in the blood on day 2 [Fday (1,11) = 8.80, P < 0.02], all other parameters were not statistically different between the 2 d in both compartments (Table 1 and Supplemental Table 1).
Fig. 1.
Free corticosterone in the blood and subcutaneous tissue of freely behaving rats shows a distinct circadian and ultradian rhythm with similar pulse characteristics on two consecutive 24-h periods (day 1 and 2). Free corticosterone (micrograms per deciliter) was assessed by single-probe microdialysis in the jugular vein (A and B) or the subcutaneous tissue of the neck region (C and D). A, Representative example of a 48-h profile of free corticosterone in the blood of rat XQ21. B, Mean (±sem) free corticosterone concentrations in the blood (n = 12). C, Representative example of a 48-h profile of free corticosterone in the subcutaneous tissue of rat XQ28. D, Mean (±sem) free corticosterone concentrations in the subcutaneous tissue (n = 10). A–D, Samples were collected in 10-min (0900–2100 h) and 30-min (2100–0800 h) intervals. Between 0800 and 0900 h, perfusion syringes were refilled with Ringer solution and empty vials were inserted in the automated sample collectors. Symbols are placed at the midpoint of the sample duration. The gray area indicates the dark period of the light-dark cycle. For pulse characteristics and statistical analyses, see Table 1 and Supplemental Table 1 in the Supplemental Information.
Table 1.
Characterization of the circadian rhythm and pulsatile pattern of free corticosterone levels in the blood and the subcutaneous tissue on 2 consecutive days as calculated using the PULSAR algorithm (protocol 1, single-probe microdialysis)
| Compartment | Time period (time of day, h) | Day | Pulse frequency (pulse per hour) | Mean pulse amplitude (μg/dl) | Mean pulse height (μg/dl) | Mean free corticosterone (μg/dl) | AUC (arbitrary units) |
|---|---|---|---|---|---|---|---|
| Blood (n = 12) | 0900–1500 | 1 | 1.10 ± 0.10 | 0.07 ± 0.01 | 0.15 ± 0.01 | 0.09 ± 0.01 | 0.58 ± 0.03 |
| 2 | 0.90 ± 0.09 | 0.07 ± 0.01 | 0.14 ± 0.01 | 0.08 ± 0.01 | 0.56 ± 0.06 | ||
| 1500–2100 | 1 | 1.10 ± 0.07 | 0.20 ± 0.03a | 0.40 ± 0.04a | 0.29 ± 0.03a | 1.72 ± 0.18a | |
| 2 | 0.94 ± 0.07 | 0.17 ± 0.02a | 0.35 ± 0.03a | 0.25 ± 0.02a | 1.45 ± 0.10a | ||
| Subcutaneous tissue (n = 10) | 0900–1500 | 1 | 1.13 ± 0.12 | 0.07 ± 0.01 | 0.14 ± 0.01 | 0.08 ± 0.005 | 0.54 ± 0.03 |
| 2 | 0.89 ± 0.07 | 0.06 ± 0.01 | 0.15 ± 0.02 | 0.09 ± 0.01 | 0.57 ± 0.04 | ||
| 1500–2100 | 1 | 1.17 ± 0.06 | 0.26 ± 0.04a | 0.45 ± 0.04a | 0.30 ± 0.02a | 1.78 ± 0.13a | |
| 2 | 0.98 ± 0.08 | 0.23 ± 0.05b | 0.39 ± 0.05a | 0.26 ± 0.02a | 1.53 ± 0.12a |
Free corticosterone levels were monitored in either the blood or the subcutaneous tissue by single-probe microdialysis in separate animals. Pulse characteristics were calculated using the PULSAR algorithm on data depicted in Fig. 1. All rats (blood, n = 12; subcutaneous tissue, n = 10) showed significant circadian and ultradian rhythms during the analyzed 12-h sampling period between 0900 and 2100 h (10 min interval samples) on the 2 consecutive days (d 1 and d 2). Post hoc statistical analysis of the morning/early afternoon period (0900–1500 h) vs. the late afternoon/early night period (1500–2100 h) showed significant circadian variations in the pulse amplitude, pulse height, mean free corticosterone level, and AUC with higher values attained during the later time period. No significant circadian variations were found for the pulse frequency in either compartment. Notably, post hoc statistical analysis revealed no significant differences between the parameters calculated for the rhythms on d 1 and d 2 in either compartment, demonstrating the tight regulation of these biological rhythms within individual rats. See Supplemental Table 1 for ANOVA results. Values shown are mean ± sem.
Time period 1500–2100 h vs. time period 0900–1500 h (P ≤ 0.002). Paired Student's t tests with Bonferroni correction.
Time period 1500–2100 h vs. time period 0900–1500 h (P = 0.01). Paired Student's t tests with Bonferroni correction.
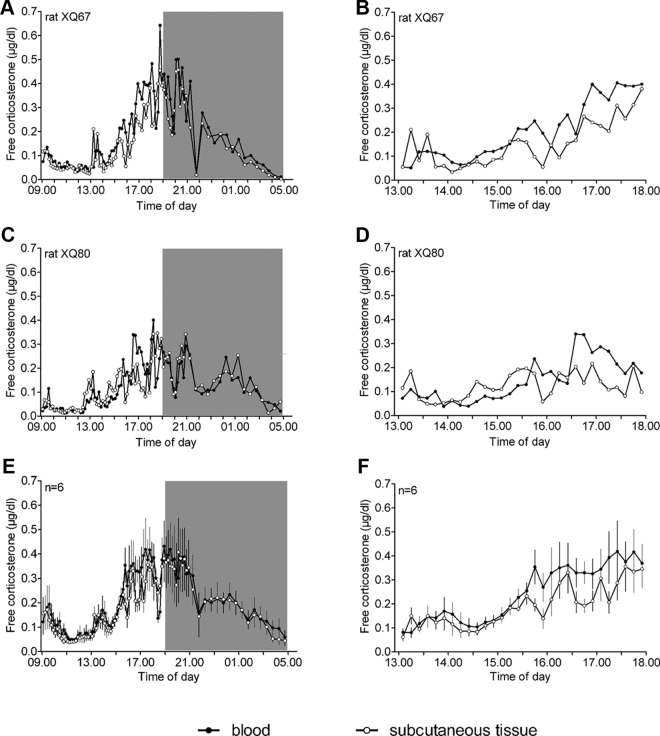
Synchronous rhythms of free corticosterone in the blood and the subcutaneous tissue (protocol 2)
To directly investigate the degree of similarity and synchronicity between the rhythms of free corticosterone in the blood and in target tissues, it is essential to collect data from both compartments within the same animal. Therefore, rats were implanted with a probe in both the jugular vein and the subcutaneous tissue. Figure 2 shows the high degree of similarity between the circadian and ultradian rhythms of free corticosterone in the two peripheral compartments. No major differences in pulse parameters between the blood and the subcutaneous tissue were found as indicated by the absence of significant main and post hoc effects (Supplemental Tables 1 and 2). For mean pulse height, corticosterone concentration, and AUC, significant interactions between compartment and time period [pulse height, Fcompartment × time period (1,5) = 7.35, P < 0.05; mean corticosterone, Fcompartment × time period (1,5) = 13.33, P < 0.02; and AUC, Fcompartment × time period (1,5) = 7.40, P < 0.05], but no significant post hoc group differences between the two compartments, were found (Supplemental Table 2). The significant interactions reflect the slightly lower overall levels of free corticosterone in the subcutaneous tissue (−13 to 15%) compared with the blood during the later time period (1500–2100 h).
Fig. 2.
Simultaneous assessment of the circadian and ultradian rhythm of free corticosterone (micrograms per deciliter) in the blood and the subcutaneous tissue using dual-probe microdialysis in the same freely behaving rats. Free corticosterone shows a clear circadian and ultradian rhythm, which is highly synchronized between the two peripheral compartments. A and C, Representative examples of simultaneous free corticosterone rhythms in the blood and subcutaneous tissue of rats XQ67 and XQ80. E, Mean (±sem) free corticosterone concentrations in the blood and the subcutaneous tissue (n = 6). B, D, and F, Expanded views of the data presented in A, C, and E (time period 1300–1800 h), respectively, showing the synchronization between free corticosterone levels in the two compartments. A–F, Samples were collected in 10-min (0900–2100 h) and 30-min (2100–0500 h) intervals. Symbols are placed at the midpoint of the sample duration. The gray area indicates the dark period of the light-dark cycle. For pulse characteristics and statistical analyses, see Supplemental Tables 1 and 2.
Cross-correlation analysis showed that the highest and only significant cross-correlation was found using a lag time of 0 (mean cross-correlation coefficientlag0 = 0.30 ± 0.06 (sem); P < 0.007), clearly demonstrating the high degree of synchronicity of free corticosterone rhythms between the peripheral compartments.
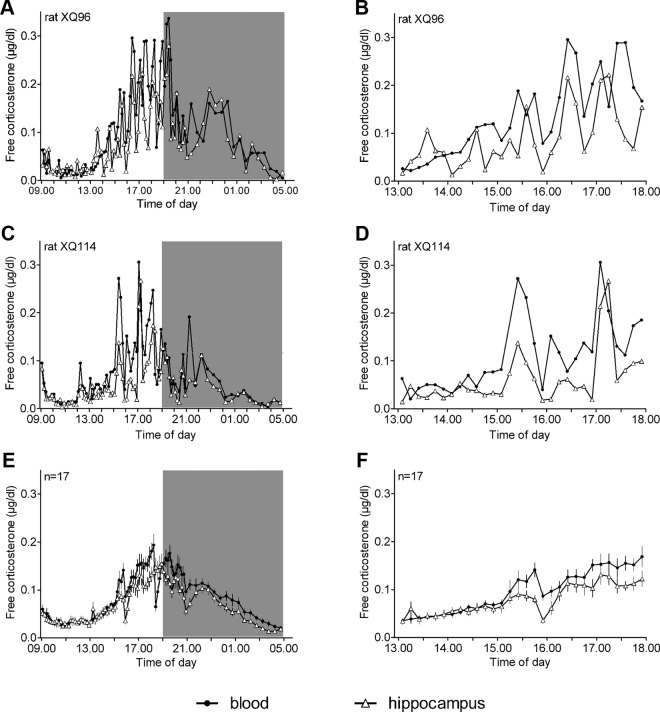
Synchronous rhythms of free corticosterone in the blood and the brain (protocol 3)
Recently we found that the stress-induced free corticosterone response in the brain is delayed by about 20 min compared with the response of total corticosterone in the blood (10). This delay was not present when comparing the stress-induced free corticosterone responses in the brain and the blood (7). It was therefore important to elucidate whether the rhythms of free corticosterone in the blood and the brain are similar. We performed dual-probe microdialysis by insertion of a microdialysis probe in both the jugular vein and the hippocampus and found very similar rhythms of free corticosterone in both compartments (Fig. 3). Similar to the blood/subcutaneous tissue comparison, no major differences in the pulse parameters frequency, amplitude, and height between the blood and the brain were found as indicated by the absence of significant main and post hoc effects (Supplemental Tables 1 and 3). However, there was a significant interaction between compartment and time period for pulse height [Fcompartment × time period (1,16) = 7.88, P < 0.02]. The slightly lower pulse height in the hippocampus between 1500 and 2100 h (−17%) may underlie the significantly lower mean free corticosterone levels (−15%) and AUC (−16%) in this brain structure as compared with the blood during the later time period (Supplemental Tables 1 and 3).
Fig. 3.
Simultaneous assessment of the circadian and ultradian rhythm of free corticosterone (micrograms per deciliter) in the blood and the hippocampus using dual-probe microdialysis in the same freely behaving rats. Free corticosterone shows a clear circadian and ultradian rhythm, which is highly synchronized between the peripheral and the central compartment. A and C, Representative examples of simultaneous free corticosterone rhythms in the blood and hippocampus of rats XQ96 and XQ114. E, Mean (±sem) free corticosterone concentrations in the blood and the hippocampus (n = 17). B, D, and F, Expanded views of the data presented in panels A, C, and E (time period 1300–1800 h), respectively, showing the synchronization between free corticosterone levels in the two compartments. A–F, Samples were collected in 10-min (0900–2100 h) and 30-min (2100–0500 h) intervals. Because of the size of the brain structure, 4-mm probes (instead of 10 mm probes as for protocols 1 and 2) were used in both the jugular vein and the hippocampus. Consequently, the levels of free corticosterone depicted in Fig. 3 and in Supplemental Table 3 are lower than those in Figs. 1 and 2 and Table 1 and Supplemental Table 2. Symbols are placed at the midpoint of the sample duration. The gray area indicates the dark period of the light-dark cycle. For pulse characteristics and statistical analyses, see Supplemental Tables 1 and 3.
Cross-correlation analysis revealed significant cross-correlations between the blood and the hippocampus only at lag time 0 and −1 [meanlag0 = 0.17 ± 0.04 (sem); P < 0.007; meanlag-1 = 0.19 ± 0.05 (sem); P < 0.007], demonstrating the high degree of synchronization between the peripheral and central compartments.
Discussion
The circadian rhythm of plasma total corticosterone consists of a series of pulses of hormone release from the adrenal gland (12), which are generated by the pulsatile release of ACTH from the anterior pituitary (13–16). It is, however, unknown whether the plasma free fraction of corticosterone shows a similar circadian and ultradian rhythm in the circulation to that described for total levels of corticosterone. Furthermore, the exact relationship between free corticosterone rhythms in the blood and in peripheral and central target tissues has never been investigated. These are essential questions because only free corticosterone is biologically active. Observations that the total and free fractions of corticosterone may respond differentially under certain (stress) circumstances (7, 10) further underscore the need to understand the regulation of free glucocorticoid hormone levels.
We used rapid-sampling microdialysis to study free corticosterone levels, over extended time periods, in the blood, the subcutaneous tissue, and the brain of freely behaving rats (7, 17). We found that free corticosterone levels in the blood show distinct circadian and ultradian rhythms, with a pulse frequency of approximately one pulse per hour. These data are in accordance with reports showing ultradian rhythms (approximately one pulse per hour) of total glucocorticoid hormone in plasma of different species, including rat and human (18–20). The stability of the rhythms over 48 h shows their tight regulation within individual rats and emphasizes the robustness of the microdialysis method for free corticosterone assessment. The higher levels of free corticosterone during the late afternoon/early night are caused by an increased pulse height. This observation agrees with a mathematical model of hypothalamic-pituitary-adrenal axis oscillations, which was recently tested in vivo and predicts that changes in hypothalamic drive will not alter ACTH and corticosterone pulse frequency but will increase secreted hormone pulse mass (15, 16). The increased pulse height is most likely caused by a combination of increased CRH drive and a splanchic nerve-mediated augmentation of the sensitivity of the adrenal gland for ACTH (21, 22). To our knowledge there are no other studies that have investigated the ultradian rhythms of free corticosterone in the blood of rodents. There is some evidence in humans that saliva free cortisol demonstrates an ultradian rhythm. Unfortunately, because these studies took samples at long sample intervals (≥30 min) and were performed using depressed subjects (23, 24), further studies are needed to define the precise characteristics of the ultradian rhythmicity.
Our next question was whether the rhythms of free corticosterone we found in the blood would translate into identical rhythms in peripheral and central target tissues, both with respect to their timing and magnitude. Recently we found that free glucocorticoid responses to stress are very similar in the blood and target tissues, albeit with a slightly faster return to baseline levels in the brain (7). We therefore performed dual-probe microdialysis to directly compare hormone rhythms both between the blood and the subcutaneous tissue, and between the blood and the hippocampus, within the same animals. We did indeed find that the circadian and ultradian rhythms of free corticosterone are highly synchronized between the blood and the target tissues. For the subcutaneous tissue, a significant cross-correlation with the blood was found when analyzing the data with a lag time of zero but not with other lag times, indicating highly synchronized patterns between the two compartments. Also, for the brain, a significant cross-correlation was found at a lag time of 0, again indicating a high level of synchronicity between this central compartment and the blood. The levels in the blood and in the brain also showed cross-correlation at a lag time of −1, which is most likely caused by the fact that in a few, but not all, animals, changes in hippocampal free corticosterone levels could occasionally be detected one 10-min sample earlier than in the blood. The exact reason for this is not known, but the occurrence of random variation or differences in the contact of the membrane with the blood or the tissue in these animals may in part explain this observation.
No differences in the pulse amplitude between the blood, the subcutaneous tissue, and the brain during the early period of the light-dark cycle were found. However, free corticosterone levels were slightly lower (−15 to 20%) in target compartments compared with the blood during the late afternoon/early night period. The cause of this is unknown, but it may be that corticosterone is cleared at a higher rate from tissue compartments than from the blood during periods of enhanced behavioral activity and metabolism. Processes at blood-tissue barriers may also play a role. However, a role for P-glycoprotein at the blood-brain barrier is unlikely, given reports that corticosterone is not a P-glycoprotein ligand (25, 26) and our recent observation that the timing and magnitude of free corticosterone responses after stress are identical in the blood and the brain (7).
We conclude that our data reveals for the first time that there are distinct circadian and ultradian rhythms of free corticosterone in the blood and in peripheral and central target tissues, which are highly synchronized between body compartments. The observation that ultradian rhythms in free glucocorticoid hormone are translated from the blood into the target tissues, in which they may subsequently result in gene pulsing effects (2), further underscores the importance of optimizing steroid treatment, in particular with respect to timing, in patients with glucocorticoid sensitive disease.
Supplementary Material
Acknowledgments
This work was supported by the Biotechnology and Biological Sciences Research Council, United Kingdom, Grant BB/F006802/1 and The Wellcome Trust, Grant 092947/Z/10/Z.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUC
- Area under the curve.
References
- 1. Lightman SL, Conway-Campbell BL. 2010. The crucial role of pulsatile activity of the HPA axis for continuous dynamic equilibration. Nat Rev Neurosci 11:710–718 [DOI] [PubMed] [Google Scholar]
- 2. Stavreva DA, Wiench M, John S, Conway-Campbell BL, McKenna MA, Pooley JR, Johnson TA, Voss TC, Lightman SL, Hager GL. 2009. Ultradian hormone stimulation induces glucocorticoid receptor-mediated pulses of gene transcription. Nat Cell Biol 11:1093–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Westphal U. 1971. Steroid-protein interactions. Monogr Endocrinol 4:1–567 [PubMed] [Google Scholar]
- 4. Hammond GL. 1990. Molecular properties of corticosteroid binding globulin and the sex-steroid binding proteins. Endocr Rev 11:65–79 [DOI] [PubMed] [Google Scholar]
- 5. Breuner CW, Orchinik M. 2002. Plasma binding proteins as mediators of corticosteroid action in vertebrates. J Endocrinol 175:99–112 [DOI] [PubMed] [Google Scholar]
- 6. Lewis JG, Bagley CJ, Elder PA, Bachmann AW, Torpy DJ. 2005. Plasma free cortisol fraction reflects levels of functioning corticosteroid-binding globulin. Clin Chim Acta 359:189–194 [DOI] [PubMed] [Google Scholar]
- 7. Qian X, Droste SK, Gutièrrez-Mecinas M, Collins A, Kersanté F, Reul JMHM, Linthorst ACE. 2011. A rapid release of corticosteroid-binding globulin from the liver restrains the glucocorticoid hormone response to acute stress. Endocrinology 152:3738–3748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Linthorst ACE, Flachskamm C, Holsboer F, Reul JMHM. 1994. Local administration of recombinant human interleukin-1β in the rat hippocampus increases serotonergic neurotransmission, hypothalamic-pituitary-adrenocortical axis activity, and body temperature. Endocrinology 135:520–532 [DOI] [PubMed] [Google Scholar]
- 9. Merriam GR, Wachter KW. 1982. Algorithms for the study of episodic hormone secretion. Am J Physiol 243:E310–E318 [DOI] [PubMed] [Google Scholar]
- 10. Droste SK, de Groote L, Atkinson HC, Lightman SL, Reul JMHM, Linthorst ACE. 2008. Corticosterone levels in the brain show a distinct ultradian rhythm but a delayed response to forced swim stress. Endocrinology 149:3244–3253 [DOI] [PubMed] [Google Scholar]
- 11. Box GEP, Jenkins GM. 1976. Time series analysis: forecasting and control. Oakland, CA: Holden-Day [Google Scholar]
- 12. Jasper MS, Engeland WC. 1991. Synchronous ultradian rhythms in adrenocortical secretion detected by microdialysis in awake rats. Am J Physiol 261:R1257–R1268 [DOI] [PubMed] [Google Scholar]
- 13. Carnes M, Lent S, Feyzi J, Hazel D. 1989. Plasma adrenocorticotropic hormone in the rat demonstrates three different rhythms within 24 h. Neuroendocrinology 50:17–25 [DOI] [PubMed] [Google Scholar]
- 14. Gudmundsson A, Carnes M. 1997. Pulsatile adrenocorticotropic hormone: an overview. Biol Psychiatry 41:342–365 [DOI] [PubMed] [Google Scholar]
- 15. Walker JJ, Terry JR, Lightman SL. 2010. Origin of ultradian pulsatility in the hypothalamic-pituitary-adrenal axis. Proc Biol Sci 277:1627–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Walker JJ, Spiga F, Waite E, Zhao Z, Kershaw Y, Terry JR, Lightman SL. 2012. The origin of glucocorticoid hormone oscillations. PLoS Biol 10:e1001341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Linthorst ACE, Reul JMHM. 2008. Stress and the brain: solving the puzzle using microdialysis. Pharmacol Biochem Behav 90:163–173 [DOI] [PubMed] [Google Scholar]
- 18. Tapp WN, Holaday JW, Natelson BH. 1984. Ultradian glucocorticoid rhythms in monkeys and rats continue during stress. Am J Physiol Regul Integr Comp Physiol 247:R866–R871 [DOI] [PubMed] [Google Scholar]
- 19. Windle RJ, Wood SA, Shanks N, Lightman SL, Ingram CD. 1998. Ultradian rhythm of basal corticosterone release in the female rat—dynamic interaction with the response to acute stress. Endocrinology 139:443–450 [DOI] [PubMed] [Google Scholar]
- 20. Henley DE, Leendertz JA, Russell GM, Wood SA, Taheri S, Woltersdorf WW, Lightman SL. 2009. Development of an automated blood sampling system for use in humans. J Med Eng Technol 33:199–208 [DOI] [PubMed] [Google Scholar]
- 21. Kaneko M, Kaneko K, Shinsako J, Dallman MF. 1981. Adrenal sensitivity to adrenocorticotropin varies diurnally. Endocrinology 109:70–75 [DOI] [PubMed] [Google Scholar]
- 22. Engeland WC, Arnhold MM. 2005. Neural circuitry in the regulation of adrenal corticosterone rhythmicity. Endocrine 28:325–332 [DOI] [PubMed] [Google Scholar]
- 23. Kahn JP, Rubinow DR, Davis CL, Kling M, Post RM. 1988. Salivary cortisol: a practical method for evaluation of adrenal function. Biol Psychiatry 23:335–349 [DOI] [PubMed] [Google Scholar]
- 24. Bao AM, Ji YF, Van Someren EJ, Hofman MA, Liu RY, Zhou JN. 2004. Diurnal rhythms of free estradiol and cortisol during the normal menstrual cycle in women with major depression. Horm Behav 45:93–102 [DOI] [PubMed] [Google Scholar]
- 25. Karssen AM, Meijer OC, van der Sandt IC, Lucassen PJ, de Lange EC, de Boer AG, de Kloet ER. 2001. Multidrug resistance P-glycoprotein hampers the access of cortisol but not of corticosterone to mouse and human brain. Endocrinology 142:2686–2694 [DOI] [PubMed] [Google Scholar]
- 26. Mason BL, Pariante CM, Thomas SA. 2008. A revised role for P-glycoprotein in the brain distribution of dexamethasone, cortisol, and corticosterone in wild-type and ABCB1A/B-deficient mice. Endocrinology 149:5244–5253 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.