Abstract
Objective
To examine the relative effects of high blood pressure (BP) and obesity on left ventricular mass (LVM) among African-American adolescents; and if metabolic or inflammatory factors contribute to LVM.
Study design
Using a 2×2 design, AA adolescents, were stratified by body mass index (BMI) percentile (BMI <95th %=non-obese; ≥95th %=obese) and average BP (normal <120/80 mm Hg; high BP ≥120/80). Glucose, insulin, insulin resistance, lipids, and inflammatory cytokines were measured. From echocardiography measures of LVM, calculated LVM index (LVMI) ≥95th % defined left ventricular hypertrophy (LVH).
Results
Data included 301 adolescents (48% female), mean age 16.2 years, 51% obese, and 29% high BP. LVMI was highest among adolescents with both obesity and high BP. The multiplicative interaction of obesity and high BP on LVH was not significant (OR= 2.35, p=0.20) but the independent additive associations of obesity and high BP with log-odds of LVH were significant; obesity OR = 3.26, p<0.001; high BP OR = 2.92, p<0.001. Metabolic and inflammatory risk factors were associated with obesity, but had no independent association with LVMI. Compared with those with average systolic BP <75th %, adolescents with systolic BP from the 75th to 90th % had higher LVMI (33.2 vs 38.7 gm/m2.7, p<0.001) and greater LVH (18% vs 43%, p<0.001), independent of obesity.
Conclusions
Prevalence of LVH is highest among AA adolescents with average BP ≥120/80 mm Hg and obesity. There also is an independent association of LVMI with BP, beginning at the 75th systolic BP percentile.
Keywords: Adolescents, Blood Pressure, Obesity, Cardiac Hypertrophy, Minority Children
The prevalence of high BP among adolescents is increasing, in concert with the childhood obesity epidemic, and the increases of both high BP and obesity are greater among minority children.(1) Although long term outcome data following onset of prehypertension or hypertension in childhood are limited, a few recent reports, based on longitudinal data extending from childhood into adulthood, provide some evidence that high BP in the young can be linked to premature cardiovascular events in adulthood, particularly among minority groups. Childhood hypertension is associated with an increase in premature death among Native Americans.(2) The CARDIA study reported that high BP with high body mass index (BMI) in young African American adults is associated with subsequent premature heart failure.(3) High BP in childhood has also been associated with increased risk of coronary artery disease in adult life.(4)
Measures of target organ damage, especially cardiac hypertrophy indicate a significant increase in risk for cardiovascular events among adult patients with hypertension. Longitudinal data, although limited, indicate that both childhood obesity and high BP are associated with higher left ventricular mass in young adulthood.(5) Several reports describe left ventricular hypertrophy (LVH), based on echocardiographic measurements, in some adolescents with untreated primary hypertension. In these reports, obesity is commonly present among adolescents with hypertension and LVH.(6-8) Metabolic factors associated with obesity such as insulin resistance(9) and inflammation(10) are components of the metabolic syndrome and could have an effect on cardiovascular growth or injury in the young. Dietary patterns expressed as a high ratio of sodium to potassium is also associated with greater LVM even among healthy young adults.(11)
The excess risks related to hypertension among African Americans begin in the young. The prevalence of prehypertension in boys is higher in non-whites compared with Whites.(12) The progression from prehypertension to hypertension is accelerated in adult African Americans,(13) and among adults, both high BP and high BMI contribute to LVH.(14) Considering these overall observations, we conducted a study to examine the relationships of BP and BMI with cardiac mass at a young age in African-Americans. The purpose of our study was to determine if the associations of obesity and high BP with left ventribular mass (LVM) are additive or if there is a synergistic relationship between obesity and high BP that results in an effect on LVM that is greater than additive. Our study was designed to test the hypothesis: adolescents with the clinical phenotype of obesity plus high BP have greater left ventricular mass index (LVMI) compared with adolescents without obesity or without high BP. Our study was also designed to examine the association of other metabolic risk factors, including insulin resistance, and biomarkers of inflammation with LVM independent of obesity.
METHODS
Healthy African-American adolescents (ages 13-18 years) were recruited in Philadelphia, PA and Wilmington DE, between 2009 and 2011, through primary care practices in the Departments of Family Medicine and Pediatrics at Thomas Jefferson University and from community primary care practices. Non-obese and obese (body mass index ≥95th percentile) adolescents were enrolled, as were adolescents with normal BP (average BP <120/80 mm Hg) and adolescents with high BP (average systolic BP ≥120 mm Hg or average diastolic BP ≥80 mm Hg). Using a 2 × 2 factorial design participants were stratified into four different groups predetermined on the basis of BMI and BP status: non-obese normal BP (N-NBP), non-obese high BP (N-HBP), obese normal BP (O-NBP), and obese high BP (O-HBP). Electronic records in the primary care practices were utilized in screening for potential participants with high BP. Exclusion criteria included known secondary hypertension, diabetes, renal disease, cardiovascular disease, autoimmune disease, thyroid disease, sickle cell disease, eating disorders, and use of steroids. Adolescents with stage 2 hypertension and adolescents with suspected secondary hypertension, based on medical history and urinalysis were also excluded. The study protocol was approved by the Institutional Review Board of Thomas Jefferson University and the A. I. DuPont Hospital for Children. Written informed consent was obtained from 18-year-old participants, while for adolescents age <18 years, consent was obtained from the parent or guardian at enrollment and assent was obtained from the child.
Data on health status, medication use, and health related behaviors were obtained by self report of each participant or guardian (for younger adolescents). Clinical assessment consisted of BP and anthropometric measurements (height, weight, and waist circumference). Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2), and obesity was defined as BMI ≥95th percentile according to the CDC criteria for children (http://www.cdc.gov/obesity/childhood/defining.html), which are derived from population-standardized BMI Z-scores based on age, sex, and BMI. All BP measurements in this study were obtained by research staff trained in child BP measurement methodology. On each adolescent participant, BP measurements were obtained, by auscultation with an aneroid device, following a 10-minute rest period. During both rest period and BP measurement the adolescent remained in a seated position with his/her back supported and feet flat on the floor. Measurements were performed on the right arm, supported at heart level, using a cuff with a width that was at least 40% of the measured arm circumference and was large enough to encircle 80% of the subject’s upper arm.(15) The average of three successive measurements of systolic BP (SBP) and diastolic BP (DBP) on two separate visits was used as the BP value for each participant. For adolescents with high BP, a third separate set of BP measurements were obtained to ensure that the average of all BP measurements were ≥120 systolic or ≥80 diastolic mm Hg. BP percentiles were also calculated based on population-standardized BP Z-scores.(15) Adolescents invited for screening, based on a record of recent elevated BP in their medical record, but during BP screening by study staff had an average BP <120/80 mm Hg were not enrolled.
Echocardiography was performed for determination of LVM, which was measured by 2-dimensional guided M-mode echocardiography. A trained technician obtained measurements of the left ventricular internal dimension, interventricular septal thickness, and posterior wall thickness during diastole. LVM was calculated from measurement of the left ventricle (LV) using the equation LVM (g) = 0.81 [1.04 (interventricular septal thickness + posterior wall thickness + LV end diastolic internal dimension)3 − (LV end diastolic internal dimension)3 +0.06.(16) According to the methods of diSimone et al,(17) LVM was corrected for height by dividing LVM by height in meters2.7 to calculate a LVM index (LVMI). LV hypertrophy (LVH) in children and adolescents, is defined as LVMI ≥95th percentile on sex specific normative LVMI data published by Khoury et al.(18) A single echo technician performed the echocardiographic measurements and an investigator (SG) blinded to the adolescent’s blood pressure measurements interpreted the data. Intra-reader reproducibility of LVM measurement showed a correlation of 0.97 for repeat samples with a mean difference in LVM of 2 g between readings.
On a separate visit, participants returned to the clinical research unit following an overnight fast for an oral glucose tolerance test. Each participant saved the first morning voided urine sample (with the time interval from the previous void) and brought the sample to the visit. An indwelling venous catheter was placed and a fasting blood sample was obtained for glucose, insulin, and lipid profile. Following the ingestion of 75 g of glucose solution (Glucola; Ames Diagnostics, Elkhart, IN), blood samples were then obtained at 30, 60 and 120 minutes post-ingestion and assayed for plasma glucose and insulin concentrations. Plasma glucose concentration was analyzed with the glucose oxidase technique (YS Model 27; Glucostat, Yellow Springs, OH). Plasma insulin concentration was determined with a solid phase radioimmunoassay (RIA) (Coat-a-Count; Diagnostic Products Corp, Los Angeles, CA). Coefficients of variation for intra- and inter-assay variability for glucose and insulin assays were <5%. Insulin resistance was estimated using the homeostasis model assessment of insulin resistance (HOMA).(19) Fasting lipids including total cholesterol (TC), low density lipoprotein (LDL), high density lipoprotein (HDL), and triglycerides (TG) were measured using the Hitachi 704 standard enzymatic method in the Lipid Laboratory of Thomas Jefferson University.
Urine albumin excretion (UAE) was measured by ELISA (Exocell, Philadelphia, PA) and corrected for creatinine (cr) (mg/g cr). These assays were highly reliable with consistently low coefficients of variation (<5%). In addition, plasma was saved from the fasting blood samples and stored at −80 degrees centigrade for later assay of adiponectin and the inflammatory cytokines high sensitivity C Reactive Protein (hsCRP), interlukin 6 (IL-6), Plasminogen Activator Inhibitor-1 (PAI-1), Tumor Necrosis Factor-alpha (TNF-α), and Tumor Necrosis Factor-alpha receptor (TNF-αR). All assays for the cytokines were performed by ELISA in duplicate using commercially available kits. Kits for Adiponectin, IL-6, TNF-α, TNF-αR and hsCRP were obtained from R&D Systems (Minneapolis, MN). The kits for PAI-1 were obtained from Aniara (Mason, OH). The coefficient of variation for these assays was consistently <10% and most <6%.
Statistical analyses
Study variables based on approximately normally distributed continuous measurements were summarized by means and standard deviations. When the data were substantially skewed, they were naturallog transformed. Central tendency, in these cases, was summarized by geometric means and, as an alternative to standard deviation; the variability was summarized by the first and third quartiles of the data. Univariate comparisons were made for study variables across the four study groups. Differences in proportions were evaluated by chi-square or Fisher’s exact tests when comparing frequencies. Differences in means were evaluated by analysis of variance (ANOVA) F-test with Dunnett’s adjustment planned pairwise comparisons when comparing continuous data. Overall false discovery rate adjustments(20) were made to all p-values in these analyses. The significance level for all test was set in advance at α = 0.05.
In multivariate analyses, we used logistic regression to investigate associations LVH, as a binary dependent variable, might have with obesity and HBP after adjusting for age and sex. A term for the statistical interaction between obesity and HBP was tested and subsequently removed if not significant to the model. The same approach was taken to evaluate how LVH might relate to moderate SBP elevation (75th percentile ≤ SPB < 90th percentile) and high SBP (SBP ≥ 90th percentile) in comparison with SBP < 75th percentile, after adjusting for obesity, age, and sex. Results from these models are summarized in terms of odds ratios, p-values, and 95% confidence intervals (CIs). All statistical analyses were conducted using SAS version 9.2 (SAS Institute, Inc., Cary, NC).
RESULTS
Data were obtained on 301 adolescents enrolled in this study. Table I provides the age of the study cohort and percent of females enrolled in each of the four study groups. There were no group differences in self-reported smoking, or alcohol use (data not shown). Also provided in Table I are the anthropometric and BP data for each study group. To fully represent the BP data, both SBP and DBP are presented using geometric mean, interquartile range, and BP Z-score and BP percentile, as derived from the normative data.(15) High BP designation for both N-HBP and O-HBP was largely based on elevated SBP. SBP as well as SBP Z-score and SBP percentile were somewhat higher in the O-HBP compared with N-HBP, but this difference was not significant. The geometric mean for SBP in N-HBP was at the 75th percentile; and the geometric mean for SBP in O-HBP was at the 81st percentile (Table I). Urinary sodium excretion is represented by the ratio of sodium: potassium concentration in the overnight urine sample. The ratio indicates a high dietary sodium intake that was consistent across all study groups.
Table 1.
Adolescent demographics, anthropometrics, blood pressure, and LV mass by BMI and blood pressure groups - categorical variables: frequencies (percents); continuous variables: mean (SD) or geometric mean [1st quartile, 3rd quartile].
| N-NBP (N = 111) |
N-HBP (N = 35) |
O-NBP (N = 103) |
O-HBP (N = 52) |
|
|---|---|---|---|---|
| Age (yrs) | 16.2 (1.7) | 16.7 (1.4) | 15.8 (1.7) | 16.5 (1.6) |
| Sex (Female) | 51 (46%) | 10 (29%) | 63 (61%) | 19 (37%) |
| BMI (kg/m2) ‡ | 22.4 [20.0, 25.0] | 23.8 [22.5, 25.7] | 34.2 [30.2, 36.7] | 37.1 [32.4, 42.2] |
| BMI Z-score | 0.46 (0.82) | 0.76 (0.67) | 2.15 (0.33) | 2.36 (0.38) |
|
Waist Circumference
(cm)‡ |
73.2 [67.5, 79.5] | 76.1 [74.0, 81.0] | 98.7 [90.0, 107] | 101.1 [91.0, 118] |
| SBP (mmHg)‡ | 107.3 [103, 114] | 125.4 [121, 128] | 109.4 [105, 116] | 128.1 [124, 130] |
| SBP Z-score | −0.55 (0.71) | 0.87 (0.63) | −0.28 (0.68) | 1.14 (0.71) |
| SBP Percentile‡ | 24.2 [14.6, 50.9] | 75.3 [67.6, 87.8] | 33.2 [21.3, 57.7] | 81.1 [76.7, 92.4] |
| DBP (mmHg)‡ | 61 . 2 [56.3, 66.3] | 67.5 [60.0, 74.7] | 60.8 [57.3, 65.7] | 65.5 [61.4, 72.9] |
| DBP Z-score | −0.40 (0.60) | 0.05 (0.73) | −0.42 (0.57) | −0.13 (0.77) |
| DBP Percentile‡ | 3 0.5 [20.2, 52.3] | 44.3 [27.0, 74.4] | 30.0 [20.9, 48.6] | 37.3 [26.9, 62.8] |
| Urinary Na/K Ratio‡ | 4.20 [2.93, 6.25] | 4.12 [2.70, 6.21] | 4.52 [3.10, 7.30] | 3.93 [2.44, 6.73] |
Data natural log transformed: geometric means with [first quartile, third quartile] presented Pctl: Percentile; N-NBP: non-obese normal BP; N-HBP: non-obese high BP; O-NBP: obese normal BP; O-HBP: obese normal BP; SBP: systolic blood pressure; DBP: diastolic blood pressure.
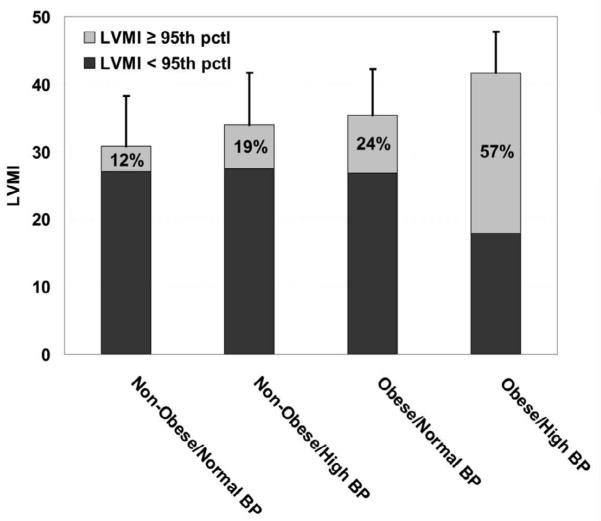
The cardiac data for each study group are provided in the Figure. Mean LVMI in each group is depicted by the height of each column. Compared with non-obese patients with normal BP, the LVMI was greater in non-obese patients with high BP and in obese patients with normal BP (30.8+7.5 vs 34.0+7.7 vs 35.4+6.9) and LVMI was highest in obese patients with high BP (41.7+6.1, p<0.001). The light gray portion of the column depicts the portion of each group that has LVH (LVMI ≥95th percentile(18)). Compared with non-obese patients with normal BP, the highest portion of LVH was in the obese patients with high BP (p<0.001) and intermediate portions of LVH were found in the non-obese patients with high BP and obese patients with normal BP. These data were analyzed to determine if there was a synergistic association between obesity and high BP that resulted in an amplification of the risk for LVH. In a logistic regression model adjusted for age and sex, the interaction between obesity and HBP had an odds ratio (OR) of 2.34 (p = 0.20; 95% CI 0.64, 8.58). Although this OR is of an important magnitude, suggesting that the combination of obesity and HBP relate synergistically with elevated likelihood of LVH, it was not statistically significant. After removing the interaction from the model, HBP was associated with an approximate 3-fold increase in odds of LVH (OR = 2.92; P < 0.001; 95% CI 1.57, 5.43) and similarly was obesity (OR = 3.26; P < 0.001; 95% CI 1.76, 6.02).
Figure. LVMI means (with standard deviations) and proportions of each group having left ventricular hypertrophy (indicated by the grey proportion in each bar).
Table II provides the data on metabolic risk factors and inflammatory cytokines for each of the four study groups. The O-HBP group had significantly higher fasting and 2 hour insulin; and also greater insulin resistance, estimated by HOMA, compared with the other groups (p<0.001). HDL-cholesterol was significantly lower (p<0.001) and triglycerides were significantly higher (p<0.001) in the O-HBP group compared with the other groups. There were no significant differences between the groups in LDL-cholesterol or total cholesterol. There were also no significant differences between the groups in urinary albumin excretion (UAE). Adiponectin, the anti-inflammatory adipocyte cytokine, was significantly lower and hsCRP was significantly higher in the obese groups compared with the non-obese groups. PAI-1 was significantly higher in O-HBP compared with other groups. Interestingly, Il-6 and TNF-αR were both significantly lower in the N-NBP group compared with the other groups. The metabolic variables indicating heightened risk tended to co-segregate in the two obese groups (Table III). Similarly the distribution for adiponectin was lowest in the two obese groups and the distribution of hsCRP was highest in the two obese groups. Following adjustment for obesity, there were no statistically significant associations of any metabolic or cytokine variables with LVMI.
Table 2.
Blood level measurements by BMI and blood pressure groups - categorical variables: frequencies (percents); continuous variables: mean (SD) or geometric mean [1st quartile, 3rd quartile]
| N-NBP (N = 111) |
N-HBP (N = 35) |
O-NBP (N = 103) |
O-HBP (N = 52) |
4- Group p-value† |
2- Group p-value† |
|
|---|---|---|---|---|---|---|
|
Fasting Glucose
(mg/dl) |
95.5 ( 8.5) | 96.4 ( 7.8) | 98.4 (13.5) | 97.4 ( 8.3) | 0.402 | 0.774 |
| Glucose 2 hr (mg/dl) | 1 12.3 (21.7) | 115.4 (22.9) | 123.1 (41.8) | 121.3 (23.1) | 0.090 | 0.410 |
|
Fasting Insulin
(uU/ml) ‡ |
5.1 [3.6, 7.2] | 5.7 [4.0, 7.3] | 10.6 [6.6, 16.9] | 13.2 [9.7, 17.4] | <.001 | <.001 |
| Insulin 2 hr (uU/ml) ‡ | 39.7 [26.4, 62.2] | 33.8 [19.5, 65.1] | 55.4 [34.9, 103] |
69.1 [42.8, 110] |
<.001 | 0.006 |
| HOMA (mg/dl) ‡ | 1.19 [0.84, 1.71] | 1.33 [0.91, 1.86] | 2.56 [1.57, | 3.17 [2.30, | <.001 | <.001 |
| 4.09] | 4.45] | |||||
| HDL (mg/dl) | 58.1 (12.0) | 57.0 (13.4) | 48.8 (11.3) | 45.3 ( 9.6) | <.001 | <.001 |
| LDL (mg/dl) | 85.0 (23.5) | 83.2 (25.1) | 91.2 (29.6) | 94.8 (27.1) | 0.151 | 0.166 |
| Triglycerides (mg/dl) ‡ | 5 4 . 4 [43.0, 69.0] | 60.7 [48.0, 76.0] | 66.2 [52.0, | 71.7 [56.5, | <.001 | 0.016 |
| 88.0] | 87.5] | |||||
|
Total Cholesterol
(mg/dl) |
155.0 (27.6) | 154.9 (31.2) | 154.7 (31.7) | 156.4 (31.1) | 1.000 | 0.818 |
| UAE (mg/gm creat) ‡ | 2.79 [1.21, 6.11] | 2.61 [1.13, 5.20] | 2.30 [0.93, | 2.27 [1.15, | 0.746 | 0.599 |
| 4.61] | 3.54] | |||||
| Adiponectin (ug/ml) ‡ | 6.8 [4.7, 11.1] | 6.4 [3.8, 12.2] | 4.6 [3.4, 7.1] | 4.8 [3.3, 7.2] | <.001 | 0.205 |
| IL6 (pg/ml) ‡ | 2.3 [1.7, 2.8] | 3.0 [2.1, 4.1] | 3.0 [2.2, 4.0] | 2.9 [2.1, 4.1] | 0.004 | 0.410 |
| PAI1 (ng/ml) | 44.5 (19.9) | 47.2 (23.6) | 71.7 (23.4) | 78.8 (31.2) | <.001 | <.001 |
| TNFα (pg/ml) ‡ | 8.2 [7.0, 9.4] | 8.3 [7.4, 8.9] | 8.0 [6.8, 9.5] | 8.2 [6.6, 9.0] | 0.979 | 0.937 |
|
TNFα Receptor
(Pg/ml) ‡ |
0.8 [0.6, 1.0] | 0.9 [0.7, 1.1] | 1.0 [0.8, 1.2] | 1.0 [0.8, 1.2] | 0.010 | 0.022 |
| hsCRP (mg/dl) ‡ | 0.4 [0.2, 0.7] | 0.4 [0.2, 1.0] | 1.4 [0.6, 3.5] | 1.4 [0.6, 3.1] | <.001 | <.001 |
| Metabolic Syndrome | 0 (0%) | 1 ( 3%) | 15( 15%) | 31 (60%) | <.001 | <.001 |
Fishers exact test (categorical) or ANOVA F-test (continuous): any groups different (4-group) and O-HBP vs. others combined (2-group). Adjustments made for multiple comparisons and false discovery rate.
Data natural log transformed: geometric means with [first quartile, third quartile] presented
UAE: creatinine-corrected urinary albumin excretion; N-NBP: non-obese normal BP; N-HBP: non-obese high BP; O-NBP: obese normal BP; O-HBP: obese normal BP;
Metabolic Syndrome: 3 or more of following: BMI Percentile > 95; SBP >120 or DBP >80; HDL <40 (males) or <50 (females); Triglycerides >110; Fasting glucose >110
Table 3.
Anthropometrics, blood pressure, and LV mass by SBP percentile groups - categorical variables: frequencies (percents); continuous variables: mean (SD) or geometric mean [1st quartile, 3rd quartile]
| Low SBP Pctl < 75th (N = 230) |
Mid 75th ≤ SBP Pctl < 90th (N = 48) |
High SBP Pctl ≥ 90th (N = 23) |
3-way comparis on p-value† |
Low vs. Mid p-value† |
Low- High p-value† |
|
|---|---|---|---|---|---|---|
| BMI (kg/m2) ‡ | 27.4 [22.1, 33.1] | 31.8 [26.6, 37.8] | 32.4 [25.7, 41.2] | 0.001 | 0.001 | 0.011 |
| BMI Z-score | 1.27 (1.07) | 1.87 (0.82) | 1.80 (0.82) | <.001 | <.001 | 0.011 |
|
Waist Circumference
(cm)‡ |
85.0 [72.0, 98.0] | 87.1 [80.0, 107] | 95.3 [80.0,112] | 0.085 | ||
| SBP (mmHg)‡ | 109.6 [104, 116] | 123.0 [120, 126] | 134.8 [129, 143] | |||
| SBP Z-score | −0.39 (0.68) | 0.95 (0.17) | 1.93 (0.51) | |||
| SBP Percentile‡ | 29.4 [18.2, 56.6] | 82.3 [78.7, 85.9] | 95.9 [93.2, 99.0] | |||
| DBP (mmHg)‡ | 61.5 [57.3, 67.0] | 63.9 [58.5, 67.7] | 69.8 [65.1, 73.7] | <.001 | 0.097 | <.001 |
| DBP Z-score | −0.41 (0.61) | −0.12 (0.69) | 0.29 (0.66) | <.001 | 0.022 | <.001 |
| DBP Percentile‡ | 3 0.1 [20.5, 50.1] | 38.9 [27.6, 57.1] | 54.2 [42.9, 81.4] | <.001 | 0.024 | <.001 |
|
Urinary Na (mEq/gm Cr)‡ |
80.1 [55.1, 128] | 77.4 [64.3, 118] | 82.3 [44.1,141] | 0.922 | ||
| Urinary Na/K Ratio‡ | 4.29 [2.97, 6.90] | 3.85 [2.47, 5.89] | 4.79 [3.00, 7.99] | 0.480 | ||
|
LVMI (g/[height in m]^2.7) |
33.20 (7.69) | 38.68 (8.24) | 40.58 (4.65) | <.001 | <.001 | <.001 |
| LVMI ≥ 95th pctl | 39( 18%) | 19 ( 43%) | 12(60%) | <.001 | <.001 | <.001 |
Fishers exact test (categorical) or ANOVA F-test (continuous): any groups different (3-way comparison test); adjustments made for multiple comparisons and false discovery rate
Data natural log transformed: geometric means with [first quartile, third quartile] presented
Pctl: Percentile; HBP: hi blood pressure; SBP: systolic blood pressure; DBP: diastolic blood pressure; LVMI: left ventricular mass index
The geometric mean for SBP in N-HBP was at the 75th percentile; and the geometric mean for SBP in O-HBP was at the 81st percentile (Table I). To further explore the possibility that evidence of LVH may be detectable below the 95th percentile for SBP, a BP level that represents the current definition of hypertension in children and adolescents, we conducted additional analyses to determine if an association of LVMI with BP is detectable in adolescents with systolic BP below the 95th percentile. All cohort participants were stratified as low, moderate, or high according to SBP <75th percentile, 75th to <90th percentile, and ≥90th percentile, respectively. The cardiac measures and also the metabolic and inflammatory biomarkers were compared across the three SBP percentile groups. Table III provides the BP, anthropometric, and cardiac data according to SBP percentile group. LVMI was significantly higher among those having moderate SBP elevation (p<0.001) compared with low SBP (<75th percentile); and LVMI was highest among those having SBP ≥90th percentile. In a logistic regression model adjusted for age, sex, and obesity, moderate SBP elevation (75 to <90th SBP percentile) was associated with approximately a 3-fold increase in odds of LVH (OR = 2.94; p = 0.003; 95% CI 1.44, 6.02) while high SBP (≥90th SBP percentile) was associated with more than a 5-fold increase in odds of LVH (OR = 5.30; p = 0.001; 95% CI 1.95, 14.40). The metabolic risk factors and inflammatory cytokines were also compared between the three SBP percentile groups. There were no significant differences between the SBP groups in the inflammatory cytokines or in adiponectin. The only metabolic variables that were significantly different between the SBP groups were the HOMA index for insulin resistance (p = 0.008) and fasting insulin (p = 0.049). In similar logistic regression models adjusted for age, sex, and obesity the geometric mean ratio (GMR) for the moderate SBP percentile group compared with low SBP percentile group was 1.13 (p = 0.26). The difference between the high SBP percentile group versus the low SBP percentile group remained marginally significant (GMR = 1.38, p = 0.04).
DISCUSSION
Our data on African-American adolescents demonstrate that high BP and obesity each have a significant independent association with LVH. LVMI is highest among adolescents with both obesity and high BP. Although the prevalence of LVH among African American adolescents with both obesity and high BP (57%) appeared to be greater than the sum of LVH in Non-Obese/High BP (19%) plus LVH in Obese/Normal BP (24%), the logistic regression model failed to detect a synergistic association of high BP with obesity that markedly increased LVH prevalence. The average BP among participants with high BP was modestly elevated and tended to cluster in the prehypertensive range. Our data also demonstrate that, compared with adolescents with average SBP <75th percentile, those with average SBP from the 75th to 90th percentile had significantly greater LVMI and significantly more LVH. This difference remains significant even after adjusting for adiposity.
LVH is well described and highly prevalent among children and adolescents with significant hypertension.(6-8) In a study that used ambulatory blood pressure to quantify the severity of hypertension Sorof et al(6) reported a 27% prevalence of LVH, using adult criteria of LVMI >51 gm/m2.7. Another study that analyzed pooled data from several different clinical sites on 129 children with hypertension, verified by BP repeatedly >95th percentile, found a prevalence of LVH at 15.5% using the adult criteria, and a LVH prevalence of 41.1% using the pediatric criteria of ≥95th percentile (LVMI percentile).(7) The association of obesity with LVH in children with hypertension is consistently described in these reports and the LVH prevalence was greatest among the more severely obese children with hypertension.(21) Brady et al(8) examined the prevalence of LVH in children with confirmed primary hypertension from three separate centers. These investigators reported that the highest prevalence of LVH (60%) was detected in children who were both non-white and obese. The prevalence of LVH among African American adolescents with both high BP and obesity (57%) in our study is consistent with that reported by Brady et al in obese non-white children, although there are some differences in characteristics of the study participants. Participants in the earlier reports were drawn from specialty clinics and uniformly met criteria for a clinical diagnosis of hypertension. In contrast, the participants with high BP in our study were not recruited from a hypertension clinic and were not previously noted to have high BP. Moreover, the average BP levels among those designated with high BP were lower than the BP levels described for children with hypertension in the previous reports. Despite this difference, our data show a significant association between high BP and elevated LVMI in otherwise healthy African American adolescents.
Detection of LVH in children with less severe BP elevation was first described by Daniels et al,(22) who reported a 38.5% prevalence of LVH in a sample of children with average BP above the 90th percentile, and those with LVH were also obese. Reports on data from the Bogalusa Heart Study identify predictors of LVMI in young adults to be adiposity in childhood, systolic BP in adulthood, and the cumulative burden of adiposity and systolic BP from childhood to adulthood.(5, 23) An association of obesity with cardiac mass was recently reported in very young children. The Generation R Study examined growth and cardiac structure prospectively in a population of Dutch children during the first two years of life.(24) At 24 months of age, obese children had significantly greater LVM compared with normal weight children. No association of BP with cardiac mass was detected indicating that association of obesity with increased cardiac mass is detectable at an early age and appears to precede the association of BP with cardiac mass. To determine if these findings in early childhood represent physiologic cardiac adaptations to obesity or if they reflect an early phase of cardiovascular injury requires study with longitudinal observations.
There are some limitations in our study. Although our data suggested that there could be a synergetic relationship between obesity and high BP in relation to LVMI resulting in greater than additive effect on LVM, the interaction test was not statistically significant. It is possible that we did not have sufficient power from the size of our study sample. Another limitation to be considered in our study is the lack of ambulatory blood pressure measurements (ABPM). ABPM provides more extensive BP data, overcomes problems with BP variability, eliminates the “white coat” effect, and is now considered the most useful method to characterize and diagnosis hypertension in the young.(28) However, in the study on LVH in children with newly diagnosed hypertension reported by Brady et al,(8) no ABPM variables were found to be predictive of LVH. Nevertheless, it is possible that ABPM could have provided additional insights in this study on African American adolescents with mild BP elevations. Although our data show no association of inflammatory cytokines with LVMI or BP, it cannot be determined from our cross-sectional study if inflammation contributes to a subsequent increase in BP or incident hypertension. Finally, our study sample was composed entirely of African American adolescents, which limit generalizability to other ethnic groups.
Recent data indicate the prevalence of obesity in children and adolescents is 16.9% and the prevalence of children with both overweight and obesity is 31.8%.(29) The probability of BP levels reaching prehypertension and hypertension levels increases markedly among children with BMI above the overweight threshold of the 85th BMI percentile.(30) While childhood obesity heralds adult obesity with obesity-associated morbidities, a recent analysis of combined data from four prospective cohort studies found evidence that consequences of childhood obesity may be reversible. From longitudinal data on over 6,000 subjects who were examined from childhood into adulthood the authors found that subjects who were overweight or obese as children and also as adults had greater risk for metabolic and cardiovascular disease including hypertension. However, subjects who were overweight or obese as children and became non-obese by adulthood had risks for these outcomes that were similar to subjects who were never obese.(31) These findings lend considerable support to the cardiovascular benefits of interventions to reverse childhood obesity as well as obesity prevention.
Acknowledgments
Supported by the National Institutes of Health-National Heart Lung and Blood Institute (RO1HL092030).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
REFERENCES
- 1.Munter P, He J, Cutler JA, Wildman RP, Welton PK. Trends in blood pressure among children and adolescents. Jama. 2004;291:2107–13. doi: 10.1001/jama.291.17.2107. [DOI] [PubMed] [Google Scholar]
- 2.Franks PW, Hanson RL, Knowler WC, Sievers ML, Bennett PH, Looker HC. Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med. 2010;362:485–93. doi: 10.1056/NEJMoa0904130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bibbins-Domingo K, Pletcher MJ, Lin F, Vittinghoff E, Gardin JM, Arynchyn A, et al. Racial differences in incident heart failure among young adults. N Engl J Med. 2009;360:1179–90. doi: 10.1056/NEJMoa0807265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erlingsdottir A, Indridason OS, Thorvaldsson O, Edvardsson VO. Blood pressure in children and target-organ damage later in life. Pediatr Nephrol. 2010;25:323–8. doi: 10.1007/s00467-009-1350-3. [DOI] [PubMed] [Google Scholar]
- 5.Urbina EM, Gidding SS, Bao W, Pickoff AS, Berdusis K, Berenson GS. Effect of body size, ponderosity, and blood pressure on left ventricular growth in children and young adults in the Bogalusa Heart Study. Circulation. 1995;91:2400–6. doi: 10.1161/01.cir.91.9.2400. [DOI] [PubMed] [Google Scholar]
- 6.Sorof JM, Cardwell G, Franco K, Portman RJ. Ambulatory blood pressure and left ventricular mass index in hypertensive children. Hypertension. 2002;39:903–8. doi: 10.1161/01.hyp.0000013266.40320.3b. [DOI] [PubMed] [Google Scholar]
- 7.Hanevold C, Waller J, Daniels S, Portman R, Sorof J. The effects of obesity, gender, and ethnic group on left ventricular hypertrophy and geometry in hypertensive children: a collaborative study of the International Pediatric Hypertension Association. Pediatrics. 2004;113:328–33. doi: 10.1542/peds.113.2.328. [DOI] [PubMed] [Google Scholar]
- 8.Brady TM, Fivush B, Flynn JT, Parekh R. Ability of blood pressure to predict left ventricular hypertrophy in children with primary hypertension. J Pediatr. 2008;152:73–8. 8 e1. doi: 10.1016/j.jpeds.2007.05.053. [DOI] [PubMed] [Google Scholar]
- 9.Falkner B, Hulman S, Tannenbaum J, Kushner H. Insulin resistance and blood pressure in young black men. Hypertension. 1990;16:706–11. doi: 10.1161/01.hyp.16.6.706. [DOI] [PubMed] [Google Scholar]
- 10.Sinaiko AR, Steinberger J, Moran A, Prineas RJ, Vessby B, Basu S, et al. Relation of body mass index and insulin resistance to cardiovascular risk factors, inflammatory factors, and oxidative stress during adolescence. Circulation. 2005;111:1985–91. doi: 10.1161/01.CIR.0000161837.23846.57. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez CJ, Bibbins-Domingo K, Jin Z, Daviglus ML, Goff DC, Jr., Jacobs DR., Jr. Association of sodium and potassium intake with left ventricular mass: coronary artery risk development in young adults. Hypertension. 2011;58:410–6. doi: 10.1161/HYPERTENSIONAHA.110.168054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosner B, Cook N, Portman R, Daniels S, Falkner B. Blood pressure differences by ethnic group among United States children and adolescents. Hypertension. 2009;54:502–8. doi: 10.1161/HYPERTENSIONAHA.109.134049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Selassie A, Wagner CS, Laken ML, Ferguson ML, Ferdinand KC, Egan BM. Progression is accelerated from prehypertension to hypertension in blacks. Hypertension. 2011;58:579–87. doi: 10.1161/HYPERTENSIONAHA.111.177410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox E, Taylor H, Andrew M, Han H, Mohamed E, Garrison R, et al. Body mass index and blood pressure influences on left ventricular mass and geometry in African Americans: The Atherosclerotic Risk In Communities (ARIC) Study. Hypertension. 2004;44:55–60. doi: 10.1161/01.HYP.0000132373.26489.58. [DOI] [PubMed] [Google Scholar]
- 15.The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 Suppl 4th Report):555–76. [PubMed] [Google Scholar]
- 16.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–8. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 17.de Simone G, Devereux RB, Daniels SR, Koren MJ, Meyer RA, Laragh JH. Effect of growth on variability of left ventricular mass: assessment of allometric signals in adults and children and their capacity to predict cardiovascular risk. J Am Coll Cardiol. 1995;25:1056–62. doi: 10.1016/0735-1097(94)00540-7. [DOI] [PubMed] [Google Scholar]
- 18.Khoury PR, Mitsnefes M, Daniels SR, Kimball TR. Age-specific reference intervals for indexed left ventricular mass in children. J Am Soc Echocardiogr. 2009;22:709–14. doi: 10.1016/j.echo.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 20.Benjamini YH Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. 1995;57:289–300. [Google Scholar]
- 21.Sorof JM, Turner J, Martin DS, Garcia K, Garami Z, Alexandrov AV, et al. Cardiovascular risk factors and sequelae in hypertensive children identified by referral versus school-based screening. Hypertension. 2004;43:214–8. doi: 10.1161/01.HYP.0000114696.96318.4e. [DOI] [PubMed] [Google Scholar]
- 22.Daniels SD, Meyer RA, Loggie JM. Determinants of cardiac involvement in children and adolescents with essential hypertension. Circulation. 1990;82:1243–8. doi: 10.1161/01.cir.82.4.1243. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Li S, Ulusoy E, Chen W, Srinivasan SR, Berenson GS. Childhood adiposity as a predictor of cardiac mass in adulthood: the Bogalusa Heart Study. Circulation. 2004;110:3488–92. doi: 10.1161/01.CIR.0000149713.48317.27. [DOI] [PubMed] [Google Scholar]
- 24.de Jonge LL, van Osch-Gevers L, Willemsen SP, Steegers EA, Hofman A, Helbing WA, et al. Growth, obesity, and cardiac structures in early childhood: the Generation R Study. Hypertension. 2011;57:934–40. doi: 10.1161/HYPERTENSIONAHA.110.163303. [DOI] [PubMed] [Google Scholar]
- 25.Festa A, D’Agostino R, Jr., Howard G, Mykkanen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS) Circulation. 2000;102:42–7. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 26.Sesso HD, Buring JE, Rifai N, Blake GJ, Gaziano JM, Ridker PM. C-reactive protein and the risk of developing hypertension. Jama. 2003;290:2945–51. doi: 10.1001/jama.290.22.2945. [DOI] [PubMed] [Google Scholar]
- 27.Litwin M, Michalkiewicz J, Niemirska A, Gackowska L, Kubiszewska I, Wierzbicka A, et al. Inflammatory activation in children with primary hypertension. Pediatr Nephrol. 25:1711–8. doi: 10.1007/s00467-010-1548-4. [DOI] [PubMed] [Google Scholar]
- 28.Urbina E, Alpert B, Flynn J, Hayman L, Harshfield GA, Jacobson M, et al. Ambulatory blood pressure monitoring in children and adolescents: recommendations for standard assessment: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee of the council on cardiovascular disease in the young and the council for high blood pressure research. Hypertension. 2008;52:433–51. doi: 10.1161/HYPERTENSIONAHA.108.190329. [DOI] [PubMed] [Google Scholar]
- 29.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of Obesity and Trends in Body Mass Index Among US Children and Adolescents, 1999-2010. Jama. 2012;307 doi: 10.1001/jama.2012.40. published online January 17, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tu W, Eckert GJ, DiMeglio LA, Yu Z, Jung J, Pratt JH. Intensified effect of adiposity on blood pressure in overweight and obese children. Hypertension. 2011;58:818–24. doi: 10.1161/HYPERTENSIONAHA.111.175695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011;365:1876–85. doi: 10.1056/NEJMoa1010112. [DOI] [PubMed] [Google Scholar]