Abstract
Depression is a significant and impairing mood disorder with onset possible as early as age 3 and into adulthood. Given this varying pattern of age of onset, identifying the relationship between brain development and depression across the lifespan has proven elusive. This review identifies some of the factors that may have limited the advancement of our knowledge in this area and discusses how synthesizing established models of depression and normative brain development may help to overcome them. More specifically, it is suggested that current neurobiological models of depression fail to account for the developmental variance associated with early neural network development and the potential influence of experience on this process. The utility of applying an established framework of normative brain development to this topic is described and its potential utility for conceptualizing the influence of depression on brain function across the life span is addressed. Future directions including longitudinal neuroimaging studies of early onset depression and groups at risk for this disorder are proposed.
Keywords: depression, brain, brain development, interactive specialization, preschool depression, pediatric depression
Depression has been increasingly recognized as a significant and impairing mood disorder with widespread public health implications. Current estimates suggest that up to 16% of the general population will experience at least one major depressive episode in their lifetime and that approximately 80–90% will go onto to have additional occurrences (Kessler et al., 2005; Mueller et al., 1999; Solomon et al., 1995). Interestingly, the probability of experiencing future episodes of depression may be age dependent, with an earlier onset (e.g., in childhood) associated with greater risk for- and increased frequency of recurrence (Birmaher et al., 2002; Lewinsohn et al., 1999). Additionally, studies have generally suggested a more complex clinical picture in pediatric depression as well, with increased comorbidity and functional impairment. Given that an earlier onset of depression may signal a more chronic and impairing form of this disorder (Birmaher et al., 2002; Birmaher and Axelson, 2006; Harrington et al., 1996; Perlis et al., 2004), it is remarkable how little is known about its neurodevelopmental course. The growing consensus that childhood may represent a developmental period when the brain is potentially more amenable to prevention and treatment efforts further underscores the need for such information (Fox et al., 2010).
Skepticism about the application of traditional definitions of major depressive disorder (MDD) in early childhood and the pragmatic challenges of using neuroimaging techniques in children has undoubtedly slowed research into depression related effects on brain development. However, we also suggest that the varying timing of depression onset has not allowed for a straightforward interpretation of MDD within a traditional developmental disorder framework. Specifically, many of the more “traditional” developmental disorders such as autism or attention deficit/hyperactivity disorder are considered disorders of childhood and require symptom manifestation prior to a specific age (for example 3 years of age in autism; APA, 2000). Though depression can also be identified in childhood, depression frequently emerges post-puberty and, as such, tends to be viewed as a disorder of adulthood that can and often is diagnosed at earlier ages. This age related distinction (i.e., disorders of childhood or adulthood), while not arbitrary, presents a very real quandary for addressing the developmental neurobiology of a given “adult” disorder. Of primary importance for the current discussion is the common inference that a fixed or static neurobiological model of depression can be directly applied at any age, leading one to overlook the dynamic and highly plastic nature of the brain development process. The assumed direct applicability of adult neurobiological models to pediatric depression is often notable in studies and reviews focused on this condition. For example, studies and reviews addressing brain related findings in pediatric depression commonly frame their discussion using current neurobiological models derived from the adult literature (e.g., restricting or largely focusing their literature review or analyses on brain regions included in these models). While highly informative and thought provoking, this previous work has not discussed, nor fully considered, how current theories of normative brain development processes should be incorporated and considered in neurobiological models of depression. This is not to say that specific periods of brain development (e.g., changes in brain structure or function during adolescence) and general concepts (e.g., neuroplasticity) have not been considered in previous reviews of pediatric depression, as they have (e.g., Davey et al., 2008; Forbes and Dahl, 2012). However, these discussions have generally stopped short of using well-developed theoretical frameworks to inform the process of brain development across the lifespan in this disorder. Rather, they have largely been constrained to identifying patterns of group differences at a given point in development (e.g., childhood) and evaluating the identified differences as consistent or not consistent with different developmental time periods (e.g., adulthood). To be fair, the currently available literature informing brain function and structure in pediatric mood disorders does not allow for much more.
Recent neurobiological reviews of pediatric depression suggest that the field is now at a tipping point for identifying advantageous paths forward in this developing area of study (Hulvershorn et al., 2011; Monk, 2008). As such, the goal of the current review is to suggest one such path forward. Specifically, we suggest that synthesizing established models of depression and normative functional brain development would help provide an important theoretical step forward for identifying how the potential effects of this disorder on brain function emerge across the lifespan. In order to illustrate this approach and how it fits within the broader field of depression research, we discuss the use of a developmental psychopathology perspective (Cicchetti, 1984) as an overarching framework to study depression and the more recent inclusion of general systems neuroscience principles into this perspective (Cicchetti and Tucker, 1994). Following this, we propose that incorporating a well-developed theory of normative brain development (Johnson, 2001) into this discussion may provide unique insights through empirically testable predictions about the relationship between depression and the process of functional brain development. As an illustrative example of this, we selectively review research examining emotion regulation and its associated neurobiology in healthy and depressed children, adolescents, and adults. Given recent in-depth reviews discussing reward processing and other etiologically relevant endophenotypes in depressed adolescents and adults (including two within this special issue), we take a broader approach to this topic by focusing on the process of brain development and how it can inform a lifespan approach to depression. That is, this review does not aim to provide an in-depth discussion of on any one developmental period but rather will apply this “process model” of normative brain development to a domain of specific interest in depression to provide an example of how it might be applied. Therefore, in this review we will restrict our discussion to the regulation of negative affect over the course of normative development and in depression. We conclude the review by suggesting future directions that may help address some of the outstanding gaps in our knowledge about depression and its interaction with normative brain development processes.
It is our hope that the following discussion will help contribute to the creation of a unifying framework for brain research in depression, allowing for the potential identification of developmentally specific as well as age independent or common underlying neurobiological effects of this disorder across the life span. What is presented here is far from a complete account of what is known about the relationship between depression and brain development; rather it is intended to propose a direction and agenda for future research on this topic.
Developmental Psychopathology as an Overarching Framework for the Study of Brain Development in Depression
While rapid advances in technology have offered new and exciting opportunities to examine depression in unprecedented ways, their continued use in the absence of a developmentally informed conceptual framework is unlikely to move our understanding of psychopathological brain processes beyond the “what” and “where” of differences to the more central questions of “when” and “how” did they arise (Cicchetti, 1984). The adaption of such a conceptual framework is uniquely important for the study of developmental phenomena, which by their very nature are perhaps best captured by an examination of process rather than outcome. We believe that such a framework should have several features in order to be useful for this purpose. Succinctly, such a framework must sufficiently capture the complex nature of factors affecting mood disorder onset and course as well as define development as an ongoing process. Further, the given framework must be broad enough to consider the interplay between multiple relevant factors (e.g., psychological, biological, environment), allow for the incorporation of other complimentary theories related to more specific processes of interest not fully captured within it (in our case brain development), and explicitly define development as a process that has no hard and fast end point (i.e., does not end at a specific age or milestone). The previously articulated developmental psychopathology perspective (Cicchetti, 1984; Cicchetti and Toth, 1998; Sroufe and Rutter, 1984) offers a powerful framework that includes each of these elements and comes with a well-established history of being applied to depression (Cicchetti and Toth, 1998). We believe that adopting this general framework provides an important starting point for more detailed discussions of specific aspects contained within it, such as brain development, while still recognizing the importance of taking a multi-level approach to the study of depression.
The Brain as a Complex Self-Organizing System
Central to the developmental psychopathology perspective has been the view of neurobiological development as a self-organizing process, meaning that the “active” individual participates in determining what experiences and environments (e.g., directing their attention to specific stimuli or settings) contribute to the ongoing process of brain development (Cicchetti and Tucker, 1994). Importantly, within this perspective, experience is broadly construed and includes not only external events but also internal ones as well, such as cognitive processes or mood states (Cicchetti and Tucker, 1994). It is important to emphasize that a self-organizing view of brain development extends beyond basic models of plasticity (Huttenlocher, 2002), not only viewing brain development as a process of interacting aspects of nature (e.g., experience-expectant) and nurture (i.e., experience) but also emphasizing the active role of the individual in determining what experiences are encountered.
Brain development as a self-organizing process has generally not been used to generate specific hypotheses regarding neurobiological development in mood disorders. This is likely due in large part to the limited availability of normative research informing the developmental trajectories of the regions and networks of interest. However, given the growing body of neuroanatomical literature suggesting that cortical and subcortical regions implicated in neurobiological models of mood disorders progress along differing trajectories of maturation (Giedd et al., 1999; Giedd et al., 1996a; Giedd et al., 1996b), it is likely that generating theoretically informed hypotheses about the developing specialization of these regions will be critical for addressing whether or not they are key regions of interest across development and whether the effects of depression on them are dependent upon the developmental period during which this disorder manifests. As detailed below, we believe that integrating the self-organizing view of brain development already captured by the developmental psychopathology perspective with recent theoretical work on the emergence of functional specialization during this process will be highly useful for generating specific hypotheses concerning these questions and designing future studies to begin answering them.
Interactive Specialization and the Development of Functional Specialization in the Brain
Interactive Specialization (IS), a recently proposed conceptual framework of normative brain development (Johnson, 2000; Johnson, 2001; Johnson, 2011), suggests that brain regions begin to take on increasingly specific functional roles (i.e., functional specialization) as activity-dependent interactions with other regions shape and eventually restrict their sensitivity to specific sets of stimuli (e.g., faces or events). Thus, similar to the use-dependent properties of neurons described in studies of neural plasticity (Huttenlocher, 2002), IS suggests that brain regions and related networks are progressively “fine tuned” (i.e., constrained) into a mature form following repeated exposure and involvement with a given task (Johnson, 2001). However, it should be noted that IS also suggests that the development of a new skill or onset of an experiential event (e.g., adolescence) may alter previously established interactions between brain regions and lead to large scale reorganization of brain function as a result. Thus, IS emphasizes interregional connectivity between brain regions as important for emerging functional specialization as well as the possibility of later occurring experience dependent reorganization across development.
The IS framework has previously been compared to other general theories of brain development, including maturational and skill learning viewpoints (Johnson, 2001). Briefly, the maturational viewpoint of brain development suggests that new skills or behaviors are associated with the anatomical maturation of a specific brain region. Underlying this relationship is an assumption that neuroanatomical development can be used to identify the specific age when a brain region will become fully “functional.” As such, in the maturational model, the specialized function of a brain region emerges over time in a linear and deterministic fashion and is static once established, ruling out periods of dynamic reorganization of brain function and associated networks across development. Alternatively, skill-learning views of brain development suggest that brain regions used for complex skill acquisition in adults are highly similar to those necessary for the emergence of new skills earlier in development. Thus, while the exact form of the skill to be acquired at a given developmental period may differ the pattern of brain activity necessary to support it may not.
In general, while the theories discussed above are not necessarily mutually exclusive, the IS framework is unique when compared to the maturational and skill learning perspectives given its specific predictions about developing functional specialization within the brain and the underlying assumption that skill development is dependent upon the interregional interactions of cortical areas rather than fully preprogrammed maturational processes or patterns of skill acquisition (see Figure 1 for further detail). Importantly, it also recognizes that brain development is a transactional process, where both genes and behavior play an important role in the developmental of functional specialization (Johnson, 2011). These distinctions are important given a growing body literature suggesting that functional brain development is a prolonged process; where changing patterns of within and between network connectivity (Dosenbach et al., 2010) are likely related to skill development, genetics, and open to environmental influence. (Bluhm et al., 2009; Emerson and Cantlon, 2012; Gaffrey et al., In press; Thomason et al., 2008; Thomason et al., 2009).
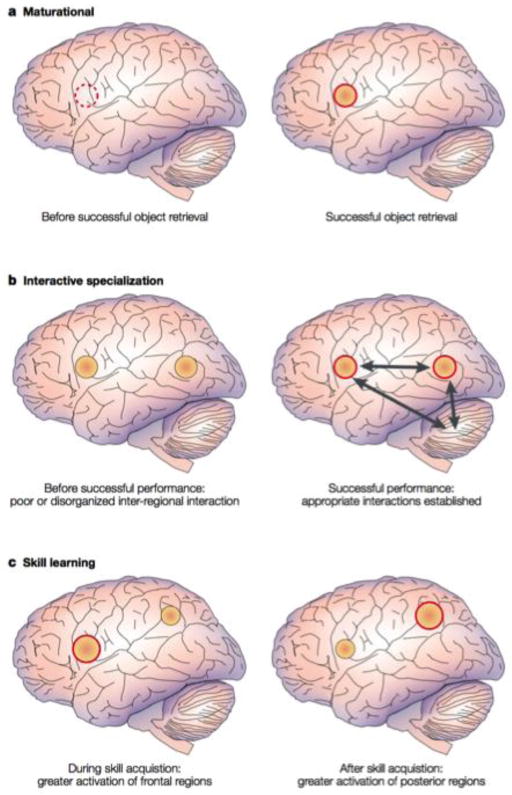
Figure 1. Competing neurobiological models of brain development and later skill emergence.
The figure illustrates (a) a maturational account where skill emergence is associated with cortical regions previously silent prior to maturation, (b) an interactive specialization account where skill emergence is associated with developing interactions between cortical/subcortical regions, and (c) a skill learning account where skill emergence is associated with a transition from greater frontal to posterior activity following the eventual establishment of a given skill. Reprinted by permission from Macmillan Publishers LTD: Nature Reviews Neuroscience, Johnson, M.H. (2001). Functional brain development in humans. 2(7), Pg. 479.
Interactive Specialization as a Conceptual Framework for Studying Brain Development in Depression: Emotion Regulation as an Example
As a domain-general framework for brain development (Johnson, 2011), IS does not provide explicit predictions about the potential effects of individual psychological or biological differences on specific neural networks. Rather, it hypothesizes a developmental process and provides a general set of testable predictions that can be used to explore the development of previously proposed networks associated with a construct (e.g., emotion regulation) and the potential influence of environmental events on them. Normative patterns of functional brain development predicted by IS include that 1) increasing specialization of a brain region for a given stimulus or task(s) will be evidenced by a more selective response pattern within that region (e.g., increase in responding to faces and decrease in responding to objects for a face specialized region); 2) increasing specialization will be associated with increasing localization (i.e., shrinking of cortical tissue/number of regions active in response to a stimulus); 3) regions similarly responsive to a given stimulus at an earlier developmental point may no longer continue to co-activate during tasks once different patterns of functional specialization emerge for each; 4) developing functional specialization for cognitive skills or behavior will be associated with widespread changes across multiple regions; and, 5) individual regions will mutually influence the development of functional specialization in each other and facilitate the emergence of tightly integrated, specialized networks (Johnson, 2011). In line with the IS view that the developing functional specialization of a brain region or network is an emergent process, the influence of experience on this process is also predicted to vary as a function of developmental timing, with early experiences likely resulting in more variable consequences for ongoing brain function and organization when compared to those occurring after networks are likely already firmly established (i.e., in adulthood; Johnson, 2011). In the remainder of this section, we use the IS framework to undertake a selective review of available developmental neuroimaging literature examining emotional response and regulation (considered to be central in the pathophysiology of depression) in healthy and depressed individuals. While we focus specifically on emotion regulation in this chapter, it should be kept in mind that the IS model can also be applied to other key emotion and cognitive processes central to depression.
Interactive Specialization and Emotion Regulation
Brain regions and networks underlying emotion response and regulation (referred to as emotion regulation going forward) have been a topic of increasing interest in both studies of normative brain development as well as pediatric depression. While continually evolving, neurobiological models of emotion regulation have generally implicated corticolimbic circuits involving dorsal “cognitive control” and ventral “emotion generating” regions (Pessoa, 2008). This has been particularly true of models concerning the pathophysiology of mood disorders such as depression, where the relationships between dorsal and ventral regions have been hypothesized as critical for the onset and course of MDD (Drevets et al., 2008; Mayberg, 1997; Phillips et al., 2003). These models have typically suggested patterns of hypo-responsivity in dorsal regions such as the dorsal lateral prefrontal cortex and hyper-responsivity in ventral brain structures such as the amygdala (see Figure 2 for an example). Research into emotion regulation and its associated brain regions and networks has largely focused on healthy and mood disordered adults. However, while still few in number, more recent studies have started to directly examine the relationship between the emergence of emotion regulation and developing brain regions and networks in children and adolescents. These studies have primarily utilized two types of tasks, one presumed to implicitly tap this construct (i.e., capture its more “automatic” aspects) and another designed to explicitly assess specific emotion regulation strategies (e.g., cognitive reappraisal). In line with behavioral research examining emotion regulation (Cole et al., 1994), early findings from this work raise the intriguing possibility that brain regions and networks supporting this skill also undergo a dynamic and prolonged period of development in childhood. Below, we discuss studies selectively chosen based on their use of multiple age groups and tasks designed to examine emotion regulation and assess the degree to which IS provides meaningful predictions concerning brain development and emotion regulation.
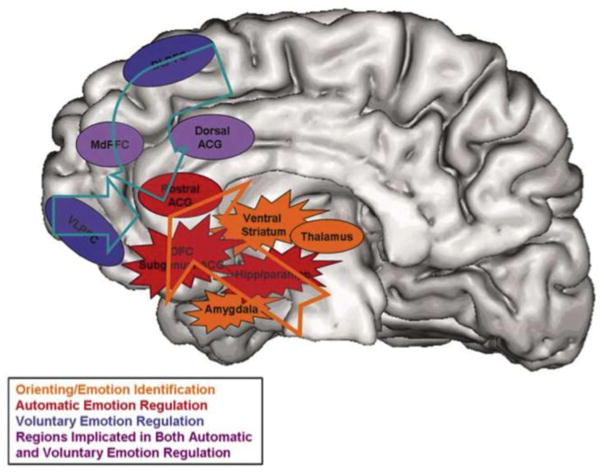
Figure 2. Representative neurobiological model of emotion regulation and its disruption in mood disorders.
Neurobiological model of emotion regulation depicting dorsal and ventral regions commonly implicated in mood disorders, including depression. Arrows in the figure depict a disrupted relationship between control (depicted as ovals) and emotion generating (depicted as stars) regions. Refer to the figure for an explanation of the colors used. Reprinted by permission from Macmillan Publishers LTD: Molecular Psychiatry, Phillips, M.L., Ladouceur, C.D., & Drevets, W.C., (2008). A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. 13(9), Pg. 849.
Emotion Regulation and Brain Development in Typically Developing Groups
Implicit Regulation
Developmental fMRI studies focusing on implicit emotion regulation have tended to use images of human faces displaying specific emotions. Fear faces have been the most frequently used, given their well established relationship with amygdala activity, a region believed to be highly important for the recognition and evaluation of emotionally relevant stimuli. One of the first studies to examine potential developmental differences using this approach was carried out by Thomas and colleagues (2001b) using the amygdala as a region of interest (ROI). When comparing functional activity to fearful faces relative to a fixation cue, left amygdala activity was seen in both children and adults. However, when fearful and neutral faces were compared adults showed greater activation to fearful faces while children demonstrated increased activity to faces with neutral expressions. One suggested explanation for this difference included the potential for neutral faces to be found more ambiguous by children and, given a lack of neutrality, to require increased vigilance to decode or interpret them. However, more recent functional imaging studies using a similar design have reported discrepant results when children and adults are compared. Specifically, using the amygdala as an a priori ROI, Monk and colleagues (2003) have reported that older children and adolescents exhibit increased activity in the right amygdala when compared to adults during the viewing of fearful relative to neutral faces. Similar results were recently found in a larger sample of adolescents and adults from the same research group (Guyer et al., 2008). While the potential reasons for the discrepancy between these studies has been fully articulated elsewhere (Monk, 2008), one important consistency was that developmental differences in amygdala activity were detected only during conditions when the active processing of a face’s emotional content was not required (e.g., passive viewing or judging nose width in Monk et al., 2003; passive viewing only examined in Thomas et al., 2001). More recently, Todd and colleagues (Todd et al., 2011) examined amygdala response to happy and angry facial expressions of emotion in children and adults. ROI analyses focusing on the amygdala revealed a linear relationship between age and activity to faces, suggesting a developing sensitivity to facial expressions of emotion in the amygdala with age. In addition, the authors reported developmental differences in amygdala activity for happy relative to angry faces, with children showing greater activity for happy relative to angry, and adults showing the opposite pattern.
A more recent fMRI study by Perlman and Pelphrey (2011) assessed implicit emotion regulation using mood induction and facial expressions of fear in children and adults. Though not directly compared, a unique pattern of results was found for each age group during the viewing of fearful faces. Specifically, children only demonstrated significant functional activity within the left amygdala during periods of both positive and negative mood while adults demonstrated significant functional activity within the right amygdala during positive mood induction and recovery from negative mood, possibly reflecting developmental differences in motivation or the modulation of fearful face processing by attentional demands. In an additional set of analyses, the authors examined whether ventral medial prefrontal cortex (VMPFC) demonstrated regulatory influence over the amygdala during periods of induced emotion in the child group. Using Grainger causality mapping, effective connectivity between the VMPFC and the left amygdala was found during negative mood induction, indicating that activity within the VMPFC preceded and potentially regulated amygdala reactivity during this block. Increased effective connectivity between the left amygdala and a larger region including VMPFC and anterior cingulate cortex (ACC) was also found in the child group during negative mood recovery. Follow-up analyses revealed that age and ACC-left amygdala connectivity were positively related, suggesting the potential for increasing regulation (i.e., reducing activity) of the amygdala by the ACC with age. Consistent with IS, the results of this study provide initial support for the role of changing functional relationships between brain regions associated with emotion regulation as one progresses through childhood and suggest that additional research exploring the developing pattern of this interaction would be highly informative.
As evident in the studies reviewed above, investigations of implicit emotion regulation using facial expressions of emotion have generally used an ROI approach focusing on the amygdala. Even when the amygdala is not chosen as the only a priori region of interest, findings are generally interpreted using an “amygdalo-centric” viewpoint (i.e., focusing on the amygdala and its relationship with other regions [selected as additional ROIs] potentially connected to it). While the role of the amygdala in processing emotional stimuli is clearly established and drives this approach to study design and data analysis (Pessoa, 2008), one limitation is that other potentially developmentally important regions may be overlooked. Given that a well-established body of literature strongly suggests that processing human faces involves a network of regions (Haxby et al., 2000; Palermo and Rhodes, 2007; Vuilleumier and Pourtois, 2007), the use of this approach alone in studies of implicit emotion regulation may have significant limitations. More specifically, recent neurobiological models (see Figure 3 for an example) have suggested that both a “core” and “extended” network of brain regions are involved in the processing of human faces. Regions within the core face-processing network include the inferior occipital, fusiform, and superior temporal sulci while the extended face-processing network is suggested to be more flexibly involved depending upon the nature of the processing required. For example, regions highly overlapping with those included in neurobiological models of emotion regulation, such as the amygdala, anterior cingulate gyrus, ventromedial/orbital prefrontal cortex, and insula are believed to play a critical role in attending to, evaluating, interpreting, and reacting to facial expressions of emotion (Palermo and Rhodes, 2007). Given that a developing body of neuroimaging evidence suggests that the functional specialization of regions within these networks undergo a prolonged period of development (Cantlon et al., 2011; Cohen Kadosh et al., 2011; Gathers et al., 2004; Joseph et al., 2011), as does the connectivity between them (Cohen Kadosh et al., 2011), the potential importance of taking a broader (i.e., network) analytical approach to studies of implicit emotion regulation using human faces is apparent.
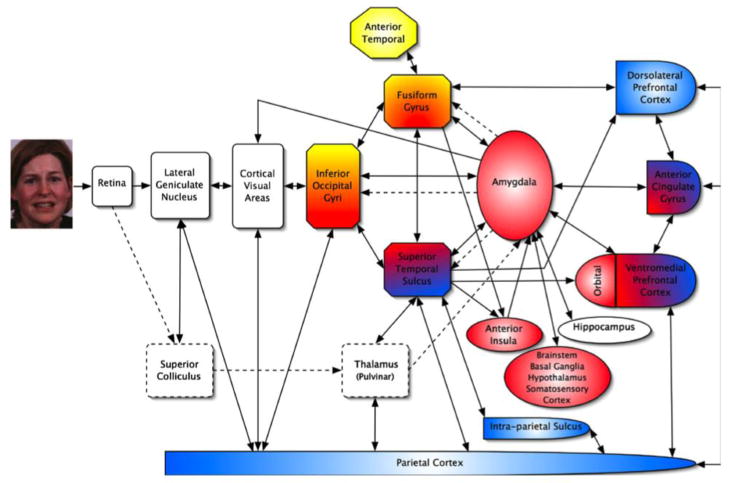
Figure 3. Representative neurobiological model of core- and extended face processing networks.
A simplified neurobiological model of face processing including core- and extended face processing networks. Regions included in the core face processing network include inferior occipital gyri, fusiform gyrus, and superior temporal sulcus. The extended network is composed of regions including the amygdala, ventromedial/orbitofrontal cortices, anterior insula, and anterior cingulate gyrus (among others). Regions shaded in yellow are intended to represent those involved in processing identity and associated semantic information, those in red represent regions involved in emotion analysis, and those in blue represent regions involved in spatial attention. As can be seen in the figure, some regions are suggested to have multiple roles. Reprinted from Neuropsychologia, 45(1), Palermo, R., & Rhodes, G., Are you always on my mind? A review of how face perception and attention interact., Pg. 76, 2007, with permission from Elsevier.
Nevertheless, while patterns of functional activity outside of preselected amygdala ROIs cannot be evaluated in many of these studies, these findings suggest that the functional specialization of the amygdala emerges over the course of childhood and is not functionally isomorphic in children and adults. Further, these data suggest that this structure may be differentially sensitive to attentional demands and specific facial expressions at varying developmental stages. While still in need of further study, the work of Perlman and Pelphrey (2011) provide some initial evidence that the amygdala may demonstrate differing patterns of reactivity and regulation dependent upon specific mood state and development period examined as well. Thus, the available data provides some support for IS predictions regarding the changing nature of functional specialization within this region across development.
Explicit Regulation
Explicit regulation of emotion has also been investigated in a small number of studies with children. In one study by Levesque and colleagues (2004), young girls were shown sad film excerpts depicting the death of a loved one and asked to actively suppress their emotional reactions by taking the stance of a detached observer. Results from the study indicated that a number of prefrontal regions were involved in the active suppression of sadness in these young girls, including lateral, orbital, and ventral lateral prefrontal and rostral anterior cingulate regions. Interestingly, in a previous study, this research group had used the identical procedure in a group of adult women (Levesque et al., 2003). When comparing study results the authors noted that the young girls recruited a greater number of frontal regions than adult women when suppressing their reactions to sad films. This qualitative comparison is consistent with the IS framework (i.e., fewer, more focal areas of activity in the frontal cortex as one matures). However, as with the Perlman and Pelphrey (2011) study noted above, the absence of a direct comparison between the two age groups precludes strong conclusions about the presence of developmentally sensitive patterns of functional activity during emotion regulation.
A more recent study by McRae and colleagues (2012) provides a clearer picture of developmental changes in functional brain activity associated with explicit emotion regulation. Using a previously validated cognitive reappraisal task, the authors examined whether brain regions identified during reappraisal demonstrated a linear or nonlinear pattern of change in functional activity across development (ages 10–23 years). The results revealed a linear relationship between chronological age and activity within the left inferior frontal gyrus (IFG), suggesting increasing IFG activity during reappraisal with age. Interestingly, the authors also identified regions within posterior cingulate, medial prefrontal, and temporal cortices that had greater activity in adolescents relative to younger and older ages. While the relationship between functional activity and reappraisal success was not examined directly, a significant positive relationship was found between reappraisal success (i.e., the difference between reported negative affect during the viewing and reappraisal of negative pictures) and age; suggesting that changes in functional activity associated with age may be related to maturation of cognitive capacity for reappraisal. Interestingly, the authors reported that a large proportion (65%) of their negative stimuli included human faces. As with the implicit studies reviewed above, it is important to note that the stimuli used in emotion regulation tasks may lead to the involvement of larger networks or regions beyond those generally considered in the cortico-limbic models of emotion regulation. Additionally, using the entire sample (i.e., children, adolescents, adults) to identify regions active during reappraisal may have prevented identifying regions active only within a specific age group. Nevertheless, when viewed through IS, the findings reported by McRae and colleagues (2012) are consistent with IS predictions of increasing functional specialization of regions believed to be important for the explicit regulation of emotion with age as well as the potentially dynamic nature of developmental changes within a larger network of regions.
Summary
In sum, the normative studies of implicit and explicit emotion regulation reviewed above provide a growing body of data supporting IS predictions of increasing functional specialization of skill related processing regions with age (IS predictions 1 & 2), changing patterns of relationships between the brain regions supporting this ongoing process (IS prediction 5), and the importance of accounting for regions that demonstrate transient patterns of functional specialization (e.g., decreasing activity with age) in addition to those gradually becoming more sensitive (IS predictions 3 & 4). Given that the majority of the studies reviewed above were restricted to examining areas of developmental difference and their association with chronological age, the interactive relationships between these regions and how they may have changed with development is not clear. Thus, future research specifically targeting this area will be needed to further inform IS predictions regarding the importance of interregional communication and the development of functional specialization. Nevertheless, the currently available data does suggest that the relationship between brain development and emotion regulation is likely to be a dynamic one requiring a developmentally informed neurobiological model. Future studies of emotion regulation and brain development in depression would be best served by accounting for these normative patterns and using conceptual frameworks such as IS to more fully inform study predictions and interpret results.
Emotion Regulation and Brain Development in Depression
Studying brain development and the emergence of emotion regulation in normative samples has proven critically important for increasing our understanding of these basic developmental processes. However, it is important to note that most of these studies have used a cross sectional approach and assume a known end point. That is, the relationship between brain development and emerging emotion regulation has been conceptualized as moving towards a mature level of brain function and organization, defined using values or patterns identified in young adults. While not without some limitations (e.g., knowledge of normal individual variation), this is a reasonable and useful approach to studying normative brain development. However, such an approach to the study of brain development in depression may be less straightforward. As suggested previously, age of onset (as well as age at episode experience), may critically influence how networks underlying a given skill and/or function emerge. For example, since brain regions involved in emotion regulation may display differing patterns of functional activity, stimulus specificity/sensitivity, and connectivity over the course of development, it is likely that depression may have unique effects on brain regions and networks supporting this skill depending upon when in development it occurs. Given that age of depression onset is rarely reported, cross-sectional approaches comparing pediatric and adult studies of depressed individuals “side-by-side” are undoubtedly confounded by this issue and make a developmental interpretation from such comparisons difficult at best and misleading at worst. Unfortunately, studies accounting for these factors (e.g., episode onset and offset, age at onset, etc.) are not readily available, leaving only a qualitative “side-by-side” comparison of emotion regulation related neuroimaging findings in pediatric and adult depression to begin addressing this question. As such, we briefly discuss studies of emotion regulation in children and adults with depression keeping these limitations in mind.
Implicit Regulation
As with normative groups, studies of implicit emotion regulation in depression have generally included facial expressions of emotion. Studies using this approach in depressed adults have frequently reported patterns of elevated amygdala activity when comparisons with age matched controls are undertaken (Fu et al., 2004; Sheline et al., 2001; Whalen et al., 2002). However, in contrast to adult findings, studies using facial expressions of emotion (most often fear) in depressed children and adolescents have reported less consistent findings. For example, Thomas and colleagues (2001a) reported a blunted amygdala response during a facial recognition paradigm using fear faces when comparing depressed girls to their healthy peers. In a study of memory encoding, Roberson-Nay and colleagues (2006) reported elevated left amygdala activation in depressed adolescents during the successful encoding of emotional faces, findings consistent with the adult literature. In a more recent study of children and adolescents at risk for depression based on familial history (Monk et al., 2008), high-risk participants were found to have increased amygdala (and nucleus accumbens) activity during the passive viewing of fear faces when compared to their low-risk peers. Interestingly, no group differences were reported during face conditions requiring an explicit task (e.g., attending to nose width), suggesting that attentional demands may have played an important role in modulating amygdala activity in each group. As with the studies of normative face processing reviewed above, the use of ROI based approaches focusing on the amygdala in many of these studies precludes a full interpretation of them within the IS framework. The use of multiple paradigms and wide age ranges within study groups is also a complicating factor. Nevertheless, mixed findings in depressed children, adolescents, and adults during various face processing tasks do raise the intriguing possibility that depression may affect amygdala functioning in a developmentally unique fashion dependant upon the task used and the age at which an individual is studied.
A more recent fMRI study of girls at high risk for depression due to a maternal history of the disorder used mood induction techniques to examine implicit sad mood regulation (Joormann et al., 2011). In this study, participants were asked to complete a set sequence of events while being scanned, including the recall of a positive autobiographical memory, followed by the viewing of a sad film clip (young girl dying of cancer), then sad mood elaboration, and finishing with the recall of a second positive memory. When compared with their low-risk peers, greater activity within the orbitofrontal cortex, parahippocampus/amygdala complex, and thalamus was found in the high-risk group. Conversely, low-risk girls were found to have greater activity within anterior cingulate and dorsolateral prefrontal cortices as well as in posterior regions including the precuneus, cuneus, fusiform gyrus, and lingual gyrus. Interestingly, findings from this study suggest that reduced cortical involvement spans multiple brain regions (i.e., not just frontal regions) during implicit mood regulation in girls at high-risk for depression. However, given that this task has not been used in adults with depression or with a similar risk status, it is difficult to determine whether any of the noted differences are developmentally specific. Nevertheless, in line with an IS interpretation, the findings do raise the intriguing possibility that being at increased risk for depression is associated with disrupted functioning across a number of regions (i.e., not only frontal areas) which may play a developmentally sensitive role in implicit sad mood regulation during childhood.
Explicit Regulation
In comparison to face processing, research examining explicit emotion regulation in depression has been the subject of few studies. Beauregard and colleagues (2006) conducted the first fMRI study of explicit emotion regulation in depressed adults. In this experiment the authors had depressed and healthy adults view film clips depicting neutral and sad events, followed by instructions to feel as they normally would or to suppress their sad feelings by distancing themselves from the material. When compared during the suppression condition, depressed adults were found to have greater activity within right dorsal anterior cingulate, right anterior temporal pole, right amygdala, and right insula. Johnstone and colleagues (2007) also recently used fMRI to examine explicit emotion regulation in depressed adults. Using negative and positive images, participants were asked to passively attend to the picture or down regulate their emotional response by reappraising the situation depicted within the picture. When compared to their healthy peers, depressed adults exhibited greater activation in right lateral and ventrolateral prefrontal cortices, suggesting the absence of a left-lateralized pattern of activation for these regions as seen in controls. Follow-up analyses revealed a negative relationship between VMPFC and amygdala activity during reappraisal in healthy individuals and a positive relationship between these two regions in the depressed group. Further analyses suggested that the VMPFC mediated the relationship between left ventrolateral prefrontal cortex (region identified during reappraisal) and the amygdala in healthy controls, a relationship that was absent in the depressed group. Sheline and colleagues (2009) also recently examined activity within Default Mode Network (DMN; believed to be important for self referential thought) regions during cognitive reappraisal in healthy and depressed individuals. The depressed group failed to show task related decreases in a number of DMN regions (including portions of the dorsal anterior cingulate and amygdala) during reappraisal, suggesting a failure to appropriately regulate activity within this network.
To our knowledge, a recent study by Perlman and colleagues (Perlman et al., 2012) provides the only available data informing explicit emotion regulation in pediatric depression. Using a cognitive reappraisal paradigm, the authors reported increased activity in the right amygdala as well as visual processing regions (e.g., lingual gyrus) while depressed adolescents were required to maintain their initial emotional reaction to a negative image. In addition, healthy controls were found to have increased amygdala connectivity with emotion regulation (e.g., medial prefrontal cortex) and social cognition (e.g., superior temporal gyrus) regions during this condition. Interestingly, a significant relationship between amygdala connectivity and poorer psychosocial functioning was only present for depressed adolescents. In line with IS and studies of normative explicit emotion regulation (McRae et al., 2012), these findings raise the intriguing possibility that emotion dysregulation may be associated with disrupted functioning across a number of regions (i.e., not only frontal or limbic areas) in depressed adolescents. In addition, when compared to previous studies of explicit emotion regulation in depressed adults (Beauregard et al., 2006; Johnstone et al., 2007), patterns of disrupted functioning across the brain also supports the IS prediction that ongoing development of functional specialization at earlier ages may be associated with more variable patterns of functional disruption in depression.
Summary
Given a somewhat consistent pattern of disrupted amygdala and frontal area functioning during emotion regulation in depressed adults, it is tempting to predict that these same regions will be the only ones affected in depressed children and adolescents. However, in line with IS and the normative data reviewed above, it is likely that these and other regions outside of them will be uniquely disrupted depending upon when in development depression occurs. To some degree this IS prediction is already supported by the mixed findings between pediatric and adult depression during implicit and explicit emotion regulation reviewed above. For example, early visual processing regions identified as differentially involved in depressed or at-risk adolescents during implicit and explicit emotion regulation may be more or less co-activated and/or connected with emotion regulation regions depending when in development depressed and healthy individuals are compared. However, additional longitudinal research is necessary to fully explore these possibilities and importantly to investigate interactive developmental processes as hypothesized by IS.
Functional Brain Network Development and Emotion Regulation in Normative Development and Depression
Recent theoretical models of depression have suggested that depression may be a disorder of distributed neural networks (Drevets et al., 2008; Mayberg, 1997; Stahl, 2003); where synchronized changes within and between circuits contribute to disorder onset, presentation, course and remission (Mayberg and Stackman Jr., 2010). A growing body of research in children and adolescents suggests that the manner in which functional brain networks are connected varies with age (de Bie et al., 2011; Fair et al., 2008a; Fair et al., 2009; Fair et al., 2007; Fransson et al., 2011; Stevens et al., 2009). In many studies, patterns of connectivity within the brain demonstrate developmental ‘curves’ where connections between anatomically close regions weaken and more distal connections strengthen, resulting in distributed (i.e., across the brain) and cohesive networks that stabilize in adulthood (Dosenbach et al., 2010; Fair et al., 2008b; Fair et al., 2009; Supekar et al., 2010). This pattern has also been found to coincide with developing communities of regions (e.g., the Default Mode Network; Fair et al., 2008b) that shift from anatomically based configurations to more functionally defined groupings (Fair et al., 2009).
To date, the Default Mode Network (DMN), defined by brain regions that demonstrate reduced neural activity during most goal directed activities (Raichle et al., 2001), is one of the most studied in depression given its suggested importance in self-referential thought and emotion (Buckner et al., 2008; Wiebking et al., 2011). Functional connectivity (i.e., the statistically significant association of measured fMRI activity between brain regions) studies of the DMN in depressed adults have generally indicated a pattern of increased connectivity between regions that overlaps with a failure of these regions to ‘deactivate’ during shifts away from a rest state to an external focus of attention (Berman et al., 2010; Greicius et al., 2007; Sheline et al., 2009; Zhou et al., 2010). While there is little data available to inform functional connectivity in currently or previously depressed children and adolescents, disrupted connectivity between regions within ventral anterior portions of frontal cortex (i.e., subgenual anterior cingulate) commonly associated with the DMN and dorsal cortical regions potentially important for emotion regulation has been reported (Cullen, et al., 2009; Gaffrey, et al., 2010). The importance of disrupted connectivity between DMN and emotion regulation regions is particularly evident in prior work from our group suggesting that reduced functional coupling between subgenual cingulate and dorsomedial prefrontal cortex is associated with dysregulated behavioral expressions of sadness in school age children with a history of preschool depression (Gaffrey, et al., 2010). More recently, our group has reported increased connectivity between posterior and pregenual cingulate DMN regions in these children as well, noting that this relationship was also associated with reduced emotion regulation ability (Gaffrey et al., In press). Interestingly, previous research suggests the connectivity between these regions undergoes the longest period of maturation within the DMN (Supekar et al., 2010), raising the possibility that the early experience of depression alters the normative developmental trajectory of this relationship. Whether or not disrupted connectivity in currently or previously depressed children represents a predisposing trait for- or scar from the experience of depression is still an open question. However, the above noted findings raise the intriguing possibility that a history of depression during childhood or adolescence is associated with altered patterns of functional connectivity between regions commonly associated with neurobiological models of the emotion regulation and default mode functioning.
Summary
The changing nature of functional connectivity in normative brain development matches the IS prediction that regions with similar patterns of stimulus or task responsivity will gradually integrate into specialized networks (IS predictions 4 & 5). In addition, and in line with IS, a prolonged period of functional network development suggests this process is open to environmental influence (see The Brain as a Complex Self-Organizing System above for greater detail). Currently available data in depressed children and adolescents suggests the early experience of this disorder may involve patterns of both increased and decreased connectivity. As would be predicted by IS, the early experience of depression has been associated with increased connectivity between regions suggested to be important for self-referential thought and emotion (i.e., DMN) and decreased connectivity between regions believed to be important for emotion and its regulation (IS prediction 5). However, it should be noted that future longitudinal research is necessary to replicate these findings and disentangle the causative relationship between disrupted functional connectivity, network development, and depression.
Summary
Within this article we have raised the suggestion that there is a need for a guiding theory relating brain development, emerging abilities, and depression. In support of this suggestion, we discussed that such a theory would fit within a broader framework emphasizing the necessity of a multilevel approach to the study of depression, namely developmental psychopathology, and that it would extend related principles already incorporated within this framework. As such, we proposed that the Interactive Specialization (IS) approach to post natal brain development put forth by Johnson (2000, 2001, 2011) provides a useful framework to achieve this goal. In support of the IS framework we conducted a selective review of research examining the relationship between brain development and emotion regulation, an area of functioning central to depression (Campbell-Sills and Barlow, 2007), in both healthy and depressed individuals. As predicted by IS, studies of healthy individuals suggested that the normative relationship between brain development and emotion regulation was far from a uniformly linear process, with variability both within and across age groups the norm rather than the exception. More specifically, studies of both implicit and explicit emotion regulation suggest that brain regions are differentially involved depending upon the task used and the age of the individual studied. Further, the nature of activity and functional specialization within these regions demonstrates patterns of progressive, recessive, and transient change with age, and that the interactions (i.e., connectivity) between regions undergo similar transitions as well.
A greater emphasis on the potential synergy of understanding normative as well as atypical patterns of brain development and emotion regulation may help move forward our understanding of the neurodevelopmental trajectory of depression. This is of critical importance given the increasing recognition of depression as a neurodevelopmental disorder (Bale et al., 2010). As briefly reviewed above, qualitative comparisons of pediatric and adult depressed samples using a similar or dissimilar approach have dominated discussions of brain development in depression. While the identification of developmental differences represents a logical starting point for including development in neurobiological models of depression, it provides little insight into the interaction between normative patterns of brain development and the occurrence of depression during this process. With these limitations in mind, we conclude with a discussion of future directions below.
Future Directions
At the most basic level, interpreting whether neuroimaging findings from a clinical group are deviant or disorder specific is dependent upon an understanding of what the expected “normative” values or patterns should be. Examinations of this type allow for some level of understanding of what may be characteristically different in one group when compared to another at a specific age. However, this type of comparison does not allow for one to fully capture the ontological nature of these differences. That is, it does not address whether identified differences are representative of a deviant trajectory of development for a given brain region(s) or network(s), a delay of the expected normative pattern of development, or a pattern of normative development followed by deviation. Critically, the distinction of deviant, delayed, or some combination thereof can only be answered in light of data informing the normative brain development process. Thus, future work examining normative patterns of brain development using longitudinal methods is needed to establish a foundation for studies of depression and developmental psychopathology more broadly.
As stated in the introduction, there is a pressing need to place normative developmental principles into the developmental study of depression related neurobiology. If one examines the regions commonly shared in neurobiological models of depression this becomes even more apparent. While continually being refined, these models have consistently implicated both cortical and subcortical regions as critical to the phenomenology of depression. However, little attention has been paid to the differing patterns of maturation for each region and the potential influence this may have on the developing patterns of functional interaction between them. When one considers the framework for these models, research in depressed adults, this is understandable. However, as our understanding of normative brain development is rapidly advancing, their direct application to depression across the lifespan needs to be reconsidered. This is not to suggest that current models should be discarded but, rather, modified in the light of this growing body of evidence for developmental variation.
We believe that the extant literature examining brain function and organization in depression suggests the very real need for longitudinal studies of brain development in this disorder (Hulvershorn et al., 2011; Monk, 2008). This is made even more apparent considering the increasing consensus that depression is indeed a neurodevelopmental disorder (Bale et al., 2010). When and how to begin longitudinal studies examining the neurobiology of depression is the next logical question. One straightforward approach is to identify the earliest known form of depression and begin prospectively following this group forward. A condition that we have commonly referred to as Preschool-Onset Depression (POD) is a clinically significant and valid depressive syndrome in preschool age children, with established findings of symptom specificity (Luby et al., 2002), familial transmission (Luby et al., 2006), biological correlates (Luby et al., 2003), impairment across multiple contexts (Gaffrey et al., 2011a; Luby et al., 2009) and, more recently, alterations in functional brain activity (Gaffrey et al., 2011b; Gaffrey et al., In press; Gaffrey et al., 2010). Thus, longitudinal studies of brain function and organization in this early occurring form of depression are likely to provide unique information about early endophenotypes of depression, provide further insight into the neurobiological continuity between pediatric and adult depression, and may promise to identify developmentally informed critical periods when intervention efforts may be more effective for preventing the future occurrence of MDD. In addition, another logical population to study is children who are at risk for depression by virtue of a familial history of depression, as studies have shown that these children are at an increased risk for the development of depression (Goodman and Gotlib, 1999). This population would provide an interesting comparison group to children with POD, as they would allow us to begin to understand the potentially unique roles that risk for- and the early occurrence of- depression have on the developmental trajectory of brain function and skill emergence as well as subsequent relationships between these effects and later outcomes. Such studies are needed to disentangle the effects of an early episode of depression from endophenotypic changes that might be present prior to a clinical episode within the IS model. Indeed, these comparisons may be key to investigations of the interactive processes as hypothesized by the IS model.
In line with a developmental psychopathology perspective (Cicchetti and Curtis, 2006), future longitudinal studies of brain development in depression should take a multilevel and integrated approach, recognizing that depression is a complex disorder likely involving the influence of many genes as well as epigenetic mechanisms. For example, a developing body of literature has suggested that the influence of stressful life events on depression onset may be dependant upon an individual having a specific genetic risk (e.g., 5HTTLPR risk allele; Caspi et al., 2003; Karg et al., 2011). Research has also demonstrated that depression guides some individuals towards specific experiences, such as interpersonal conflict, that further contributes to presentation and course (Rudolph, 2008; Rudolph et al., 2000). Importantly, the types of experiences likely to influence brain development may be specific to a developmental period of interest as well, such as parenting (Belsky and de Haan, 2011) and stressful experiences early in life (Casey et al., 2011). As such, it is important to keep in mind that both genes and environment have a hand in guiding brain development and that including these factors will be critical for developing a fully integrated neurobiological model of depression.
Conclusions
In conclusion, the increasing use and sophistication of neuroimaging techniques has been instrumental in furthering our understanding of the developing brain. It has revealed a normative pattern of brain development that is both dynamic and highly complex. The potential for this work to contribute to our understanding of depression across the life course is considerable. However, as discussed above, the use of a guiding theoretical framework to inform study design and hypothesis generation is necessary to fully tap this potential. Given its fit within a broader developmental psychopathology perspective and emphasis on the relationship between neural network development and skill emergence, we proposed that the Interactive Specialization approach (Johnson, 2011) appears ideally suited for this purpose. As indicated in the final section of this review, longitudinal studies of brain development in depression are now needed to capitalize on this growing momentum and to provide a deeper understanding of how the mechanisms that give rise to depression manifest across the lifespan. It is our hope that such studies will eventually lead to a more complete neurobiological model of depression and generate developmentally sensitive approaches to treatment and prevention that reduce the burden of this impairing disorder.
Footnotes
The authors have no financial interest(s) or conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- APA. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, D.C: 2000. Text Revision. [Google Scholar]
- Bale TL, et al. Early life programming and neurodevelopmental disorders. Biological Psychiatry. 2010;68:314–9. doi: 10.1016/j.biopsych.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauregard M, et al. Dysfunction in the neural circuitry of emotional self-regulation in major depressive disorder. Neuroreport. 2006;17:843–6. doi: 10.1097/01.wnr.0000220132.32091.9f. [DOI] [PubMed] [Google Scholar]
- Belsky J, de Haan M. Annual Research Review: Parenting and children’s brain development: the end of the beginning. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2011;52:409–28. doi: 10.1111/j.1469-7610.2010.02281.x. [DOI] [PubMed] [Google Scholar]
- Berman MG, et al. Depression, rumination and the default network. Soc Cogn Affect Neurosci. 2010 doi: 10.1093/scan/nsq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, et al. Course and outcome of child and adolescent major depressive disorder. Child and Adolescent Psychiatric Clinics of North America. 2002;11:619–638. doi: 10.1016/s1056-4993(02)00011-1. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Axelson D. Course and outcome of bipolar spectrum disorder in children and adolescents: a review of the existing literature. Development and Psychopathology. 2006;18:1023–35. doi: 10.1017/S0954579406060500. [DOI] [PubMed] [Google Scholar]
- Bluhm RL, et al. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. Journal of Psychiatry and Neuroscience. 2009;34:187–94. [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, et al. The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Campbell-Sills L, Barlow DH. Incorporating Emotion Regulation into Conceptualizations and Treatments of Anxiety and Mood Disorders. In: Gross JJ, editor. Handbook of Emotion Regulation. The Guilford Press; New York: 2007. pp. 542–559. [Google Scholar]
- Cantlon JF, et al. Cortical representations of symbols, objects, and faces are pruned back during early childhood. Cerebral Cortex. 2011;21:191–9. doi: 10.1093/cercor/bhq078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, et al. Transitional and translational studies of risk for anxiety. Depression and Anxiety. 2011;28:18–28. doi: 10.1002/da.20783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cicchetti C, Tucker D. Development and self-regulatory structures of the mind. Development & Psychopathology. 1994;6:533–549. [Google Scholar]
- Cicchetti D. The emergence of developmental psychopathology. Child Development. 1984;55:1–7. [PubMed] [Google Scholar]
- Cicchetti D, Curtis WJ. The developing brain and neuroplasticity: Implications for normality, psychopathology, and resilience. In: Cicchetti D, Cohen DJ, editors. Developmental Psychopathology: Developmental Neuroscience. Wiley; New York: 2006. pp. 1–64. [Google Scholar]
- Cicchetti D, Toth S. The development of depression in children and adolescents. American Psychologist. 1998;53:221–241. doi: 10.1037//0003-066x.53.2.221. [DOI] [PubMed] [Google Scholar]
- Cohen Kadosh K, et al. Developmental changes in effective connectivity in the emerging core face network. Cerebral Cortex. 2011;21:1389–94. doi: 10.1093/cercor/bhq215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole PM, et al. The development of emotion regulation and dysregulation: a clinical perspective. Monographs of the Society for Research in Child Development. 1994;59:73–100. [PubMed] [Google Scholar]
- Davey CG, et al. The emergence of depression in adolescence: development of the prefrontal cortex and the representation of reward. Neuroscience and Biobehavioral Reviews. 2008;32:1–19. doi: 10.1016/j.neubiorev.2007.04.016. [DOI] [PubMed] [Google Scholar]
- de Bie HM, et al. Resting-state networks in awake five- to eight-year old children. Human Brain Mapping. 2011 doi: 10.1002/hbm.21280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, et al. Prediction of individual brain maturity using fMRI. Science. 2010;329:1358–61. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, et al. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson RW, Cantlon JF. Early math achievement and functional connectivity in the fronto-parietal network. Development Cognitive Neuroscience. 2012;2:S139–S151. doi: 10.1016/j.dcn.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, et al. The maturing architecture of the brain’s default network. Proc Natl Acad Sci U S A. 2008a;105:4028–32. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, et al. The maturing architecture of the brain’s default network. Proceedings of the National Academy of Sciences of the United States of America. 2008b;105:4028–32. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, et al. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. 2009;5:e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, et al. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci U S A. 2007;104:13507–12. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE. Research Review: altered reward function in adolescent depression: what, when and how? Journal of Child Psychology and Psychiatry and Allied Disciplines. 2012;53:3–15. doi: 10.1111/j.1469-7610.2011.02477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox SE, et al. How the timing and quality of early experiences influence the development of brain architecture. Child Development. 2010;81:28–40. doi: 10.1111/j.1467-8624.2009.01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P, et al. The functional architecture of the infant brain as revealed by resting-state fMRI. Cerebral Cortex. 2011;21:145–54. doi: 10.1093/cercor/bhq071. [DOI] [PubMed] [Google Scholar]
- Fu CH, et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Archives of General Psychiatry. 2004;61:877–89. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- Gaffrey MS, et al. The 2-week duration criterion and severity and course of early childhood depression: implications for nosology. Journal of Affective Disorders. 2011a;133:537–45. doi: 10.1016/j.jad.2011.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffrey MS, et al. Association between depression severity and amygdala reactivity during sad face viewing in depressed preschoolers: an fMRI study. Journal of Affective Disorders. 2011b;129:364–70. doi: 10.1016/j.jad.2010.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffrey MS, et al. Default Mode Network connectivity in children with a history of preschool onset depression. Journal of Child Psychology and Psychiatry. doi: 10.1111/j.1469-7610.2012.02552.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffrey MS, et al. Subgenual cingulate connectivity in children with a history of preschool-depression. Neuroreport. 2010;21:1182–8. doi: 10.1097/WNR.0b013e32834127eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathers AD, et al. Developmental shifts in cortical loci for face and object recognition. Neuroreport. 2004;15:1549–53. doi: 10.1097/01.wnr.0000133299.84901.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, et al. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cerebral Cortex. 1996a;6:551–60. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Giedd JN, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. Journal of Comparative Neurology. 1996b;366:223–30. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Goodman SH, Gotlib IH. Risk for psychopathology in the children of depressed mothers: A developmental model for understanding mechanisms of transmission. Psychological Review. 1999;106:458–490. doi: 10.1037/0033-295x.106.3.458. [DOI] [PubMed] [Google Scholar]
- Greicius MD, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological Psychiatry. 2007;62:429–37. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, et al. A Developmental Examination of Amygdala Response to Facial Expressions. J Cogn Neurosci. 2008;20:1–18. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington R, et al. Developmental pathways in depression: Multiple meanings, antecedents, and endpoints. Development & Psychopathology. 1996;8:601–616. [Google Scholar]
- Haxby JV, et al. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Hulvershorn LA, et al. Toward dysfunctional connectivity: a review of neuroimaging findings in pediatric major depressive disorder. Brain Imaging Behav. 2011;5:307–28. doi: 10.1007/s11682-011-9134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR. Neural Plasticity: The Effects of Environment on the Development of the Cerebral Cortex. Harvard University Press; Cambridge, MA: 2002. [Google Scholar]
- Johnson MH. Functional brain development in infants: elements of an interactive specialization framework. Child Development. 2000;71:75–81. doi: 10.1111/1467-8624.00120. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Functional brain development in humans. Nat Rev Neurosci. 2001;2:475–83. doi: 10.1038/35081509. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Interactive Specialization: A domain-general framework for human functional brain development? Developmental Cognitive Neuroscience. 2011;1:7–21. doi: 10.1016/j.dcn.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T, et al. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27:8877–84. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, et al. Neural correlates of automatic mood regulation in girls at high risk for depression. Journal of Abnormal Psychology. 2011 doi: 10.1037/a0025294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph JE, et al. Progressive and regressive developmental changes in neural substrates for face processing: testing specific predictions of the Interactive Specialization account. Dev Sci. 2011;14:227–41. doi: 10.1111/j.1467-7687.2010.00963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karg K, et al. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Archives of General Psychiatry. 2011;68:444–54. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Levesque J, et al. Neural circuitry underlying voluntary suppression of sadness. Biological Psychiatry. 2003;53:502–10. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- Lévesque J, et al. Neural basis of emotional self-regulation in childhood. Neuroscience. 2004;129:361–369. doi: 10.1016/j.neuroscience.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, et al. Natural course of adolescent major depressive disorder: I. Continuity into young adulthood. J Am Acad Child Adolesc Psychiatry. 1999;38:56–63. doi: 10.1097/00004583-199901000-00020. [DOI] [PubMed] [Google Scholar]
- Luby J, et al. Preschool major depressive disorder: preliminary validation for developmentally modified DSM-IV criteria. J Am Acad Child Adolesc Psychiatry. 2002;41:928–37. doi: 10.1097/00004583-200208000-00011. [DOI] [PubMed] [Google Scholar]
- Luby JL, et al. The clinical significance of preschool depression: Impairment in functioning and clinical markers of the disorder. Journal of Affective Disorders. 2009;112:111–119. doi: 10.1016/j.jad.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby JL, et al. Risk factors for preschool depression: The mediating role of early stressful life events. Journal of Child Psychology and Psychiatry. 2006;47:1292–1298. doi: 10.1111/j.1469-7610.2006.01672.x. [DOI] [PubMed] [Google Scholar]
- Luby JL, et al. Alterations in stress cortisol reactivity in depressed preschoolers relative to psychiatric and no-disorder comparison groups. Arch Gen Psychiatry. 2003;60:1248–55. doi: 10.1001/archpsyc.60.12.1248. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. Journal of Neuropsychiatry and Clinical Neurosciences. 1997;9:471–81. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Stackman RW., Jr . Targeted modulation of neural circuits: A new treatment strategy of neuropsychiatric disease. In: Vertes RP, Stackman RW Jr, editors. Electrophysiological Recording Techniques. Humana Press; New York: 2010. pp. 257–279. [Google Scholar]
- McRae K, et al. The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults. Soc Cogn Affect Neurosci. 2012;7:11–22. doi: 10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS. The development of emotion-related neural circuitry in health and psychopathology. Development and Psychopathology. 2008;20:1231–50. doi: 10.1017/S095457940800059X. [DOI] [PubMed] [Google Scholar]
- Monk CS, et al. ‘Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression’: Correction. American Journal of Psychiatry. 2008;165:266–266. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- Monk CS, et al. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20:420–8. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Mueller TI, et al. Recurrence after recovery from major depressive disorder during 15 years of observational follow-up. American Journal of Psychiatry. 1999;156:1000–6. doi: 10.1176/ajp.156.7.1000. [DOI] [PubMed] [Google Scholar]
- Palermo R, Rhodes G. Are you always on my mind? A review of how face perception and attention interact. Neuropsychologia. 2007;45:75–92. doi: 10.1016/j.neuropsychologia.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Perlis RH, et al. Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the systematic treatment enhancement program for bipolar disorder (STEP-BD) Biological Psychiatry. 2004;55:875–81. doi: 10.1016/j.biopsych.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Perlman G, et al. Amygdala response and functional connectivity during emotion regulation: A study of 14 depressed adolescents. Journal of Affective Disorders. 2012;139:75–84. doi: 10.1016/j.jad.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman SB, Pelphrey KA. Developing connections for affective regulation: age-related changes in emotional brain connectivity. Journal of Experimental Child Psychology. 2011;108:607–20. doi: 10.1016/j.jecp.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. On the relationship between emotion and cognition. Nat Rev Neurosci. 2008;9:148–58. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- Phillips ML, et al. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biological Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Raichle ME, et al. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson-Nay R, et al. Increased amygdala activity during successful memory encoding in adolescent major depressive disorder: An FMRI study. Biol Psychiatry. 2006;60:966–73. doi: 10.1016/j.biopsych.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Rudolph KD. Developmental influences on interpersonal stress generation in depressed youth. Journal of Abnormal Psychology. 2008;117:673–9. doi: 10.1037/0021-843X.117.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD, et al. Toward an interpersonal life-stress model of depression: the developmental context of stress generation. Development and Psychopathology. 2000;12:215–34. doi: 10.1017/s0954579400002066. [DOI] [PubMed] [Google Scholar]
- Sheline YI, et al. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–8. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Sheline YI, et al. The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A. 2009;106:1942–7. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon DA, et al. Course of illness and maintenance treatments for patients with bipolar disorder. Journal of Clinical Psychiatry. 1995;56:5–13. [PubMed] [Google Scholar]
- Sroufe LA, Rutter M. The Domain of Developmental Psychopathology. Child Development. 1984;55:17–29. [PubMed] [Google Scholar]
- Stahl SM. Symptoms and circuits, part 1: major depressive disorder. Journal of Clinical Psychiatry. 2003;64:1282–3. doi: 10.4088/jcp.v64n1101. [DOI] [PubMed] [Google Scholar]
- Stevens MC, et al. Changes in the interaction of resting-state neural networks from adolescence to adulthood. Human Brain Mapping. 2009;30:2356–66. doi: 10.1002/hbm.20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, et al. Development of functional and structural connectivity within the default mode network in young children. Neuroimage. 2010;52:290–301. doi: 10.1016/j.neuroimage.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KM, et al. Amygdala response to fearful faces in anxious and depressed children. Archives of General Psychiatry. 2001a;58:1057–63. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- Thomas KM, et al. Amygdala response to facial expressions in children and adults. Biol Psychiatry. 2001b;49:309–16. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- Thomason ME, et al. Default-mode function and task-induced deactivation have overlapping brain substrates in children. Neuroimage. 2008;41:1493–503. doi: 10.1016/j.neuroimage.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, et al. BDNF genotype modulates resting functional connectivity in children. Front Hum Neurosci. 2009;3:55. doi: 10.3389/neuro.09.055.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd RM, et al. The changing face of emotion: age-related patterns of amygdala activation to salient faces. Soc Cogn Affect Neurosci. 2011;6:12–23. doi: 10.1093/scan/nsq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia. 2007;45:174–94. doi: 10.1016/j.neuropsychologia.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, et al. Functional neuroimaging studies of the amygdala in depression. Seminars in Clinical Neuropsychiatry. 2002;7:234–42. doi: 10.1053/scnp.2002.35219. [DOI] [PubMed] [Google Scholar]
- Wiebking C, et al. Are emotions associated with activity during rest or interoception? An exploratory fMRI study in healthy subjects. Neuroscience Letters. 2011 doi: 10.1016/j.neulet.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Zhou Y, et al. Increased neural resources recruitment in the intrinsic organization in major depression. Journal of Affective Disorders. 2010;121:220–30. doi: 10.1016/j.jad.2009.05.029. [DOI] [PubMed] [Google Scholar]