Abstract
The cyclohexenone core of welwitindolinones was synthesized by a Rh(I)-catalyzed [5+1]-cycloaddition of an allenylcyclopropane with CO. A penta-substituted cyclopropane was prepared successfully by a Rh(II)-catalyzed intramolecular cyclopropanation of alkenes with chlorodiazoacetates.
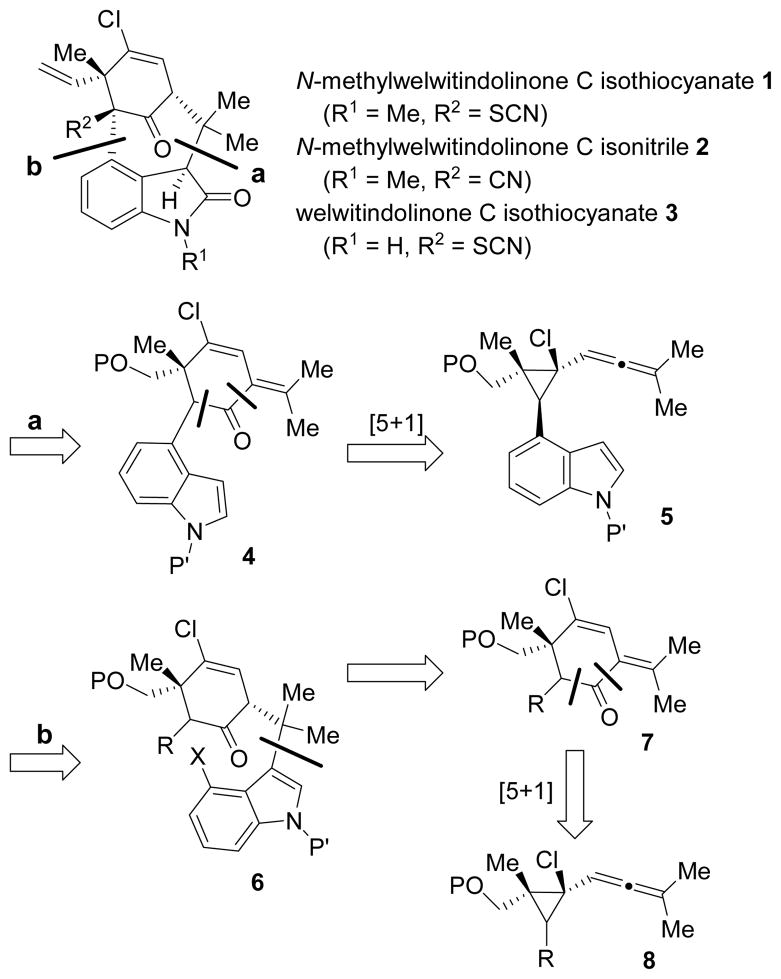
Indole alkaloid welwitindolinones (e.g. 1–3, Scheme 1) and related natural products were isolated from blue-green algae Hapalosiphon welwitschii and westiella intricate.1 N-Methylwelwitindolinone C isothiocyanate 1 reversed the drug-resistance of cancer cells in the presence of various anticancer drugs, including actinomycin D, colchicine, daunomycin, taxol, and vinblastine.2 Because of the challenging structures and their interesting biological activity, numerous synthetic efforts have been devoted to the synthesis of welwitindolinone Cs3 and related natural products, such as welwitindolinone A.4 Recently, research groups of Rawal5 and Garg6 independently accomplished elegant syntheses of welwitindolinone Cs and their oxidized congeners.
Scheme 1.
Proposed Strategies for Welwitindolinones
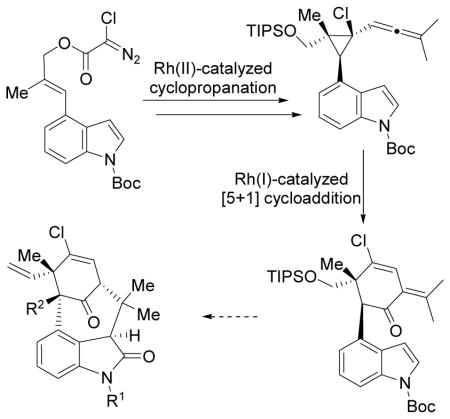
In most previous strategies for welwitindolinone Cs, functional groups on the six-membered ring especially the vinyl chloride group were introduced or proposed to be introduced at late stage, which was often challenging.7 We envisioned that a Rh-catalyzed [5+1] cycloaddition of allenylcyclopropane 5 with CO might afford the fully functionalized cyclohexenone core 4 efficiently. Alternatively, [5+1] cycloaddition of allenylcyclopropane 8 may yield cyclohexenone 7. Closing of the sevenmembered ring may be realized by addition to the isopropylidene group in intermediate 4 through strategy a or α-arylation of the ketone group in intermediate 6 through strategy b.
We recently developed a stereoselective method for the synthesis of highly functionalized cyclohexenones via Rhcatalyzed [5+1] cycloaddition of allenylcyclopropanes derived from 1,3-acyloxy migration of propargyl esters.8,9 We examined the regioselectivity for the cleavage of the cyclopropane ring and found that the C-C bond adjacent to an electron-rich aryl group or away from a quaternary carbon was selectively cleaved.8,10 This provided the basis for the proposed regioselective [5+1] cycloaddition of cyclopropanes 5 or 8.
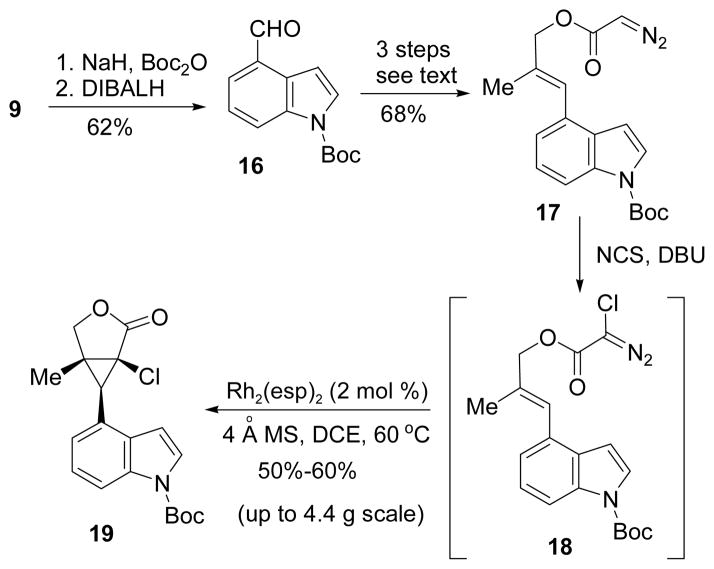
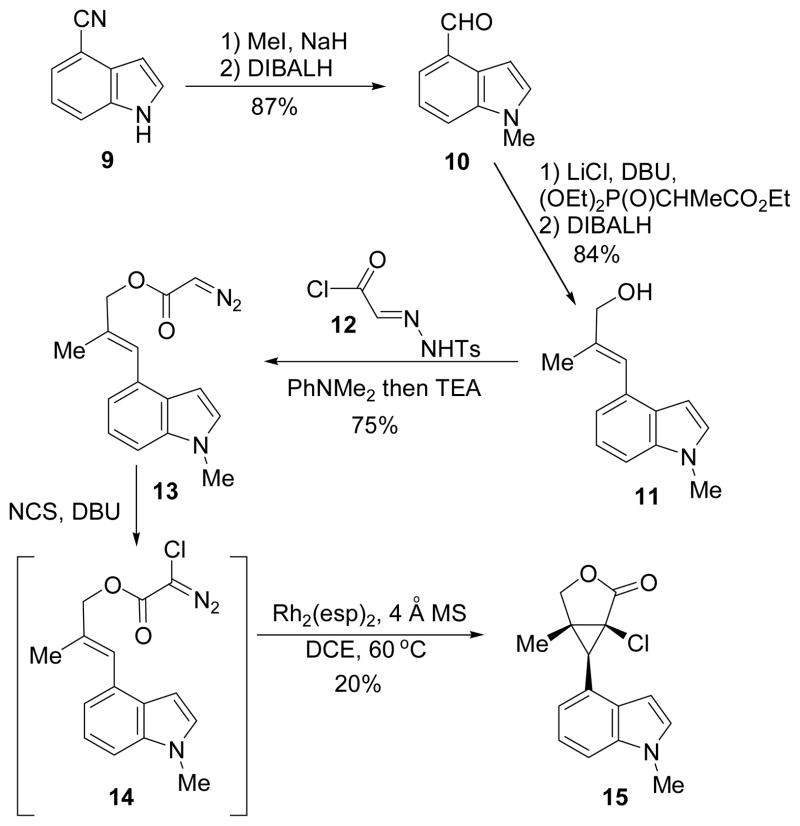
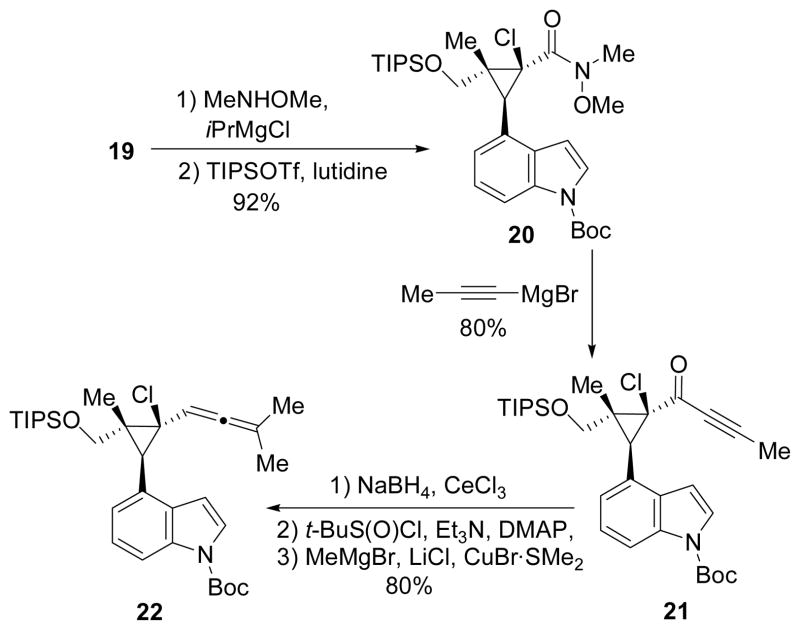
The synthesis began with the preparation of allylic alcohol 11 from commercially available 4-cyanoindole 9 through a sequence of methylation, reduction, olefination, and reduction. Esterification and diazo compound formation were achieved in one step using reagent 12.11 Chlorination of diazo compound 13 yielded an unstable halodiazoacetate that was directly used in the cyclopropanation reaction. Based on a previous report,12 Du Bois’ Rh2(esp)2 was an efficient catalyst for mediating intermolecular cyclopropanation of alkenes and halodiazoacetates.13 We also found that Rh2(esp)2 was superior to other rhodium catalysts such as Rh2(OAc)4 for this intramolecular cyclopropanation. The best isolated yield we obtained for product 15, however, was only about 20%. We suspected that the electron-rich indole ring might interfere with the electrophilic cyclopropanation. Substrates with an electronwithdrawing group on the indole nitrogen were then examined.
Boc-protected indole 16 was prepared in two steps from 4-cyanoindole indole 9 (Scheme 3). Diazoacetate 17 could be synthesized according to protocols described in Scheme 2. Chlorination followed by intramolecular cyclopropanation using Rh2(esp)2 catalyst afforded bicyclic product 19 in 50–60% yield after two steps starting with 1.2–4.4 g of diazo compound 17. This represents the first successful example of intramolecular cyclopropanation of alkenes with chlorodiazoaceates and the reaction could be scaled up to several grams.
Scheme 3.
Intramolecular Cyclopropanation of an Alkene Substituted with a N-Boc protected Indole
Scheme 2.
Intramolecular Cyclopropanation of an Alkene Substituted with a N-Methyl Indole
Opening of the lactone and protection of the resulting primary alcohol afforded Weinreb amide14 20. Addition of propynyl magnesium bromide to this amide then yielded ynone 21, which was reduced to a mixture of two diastereomeric propargyl alcohols. Attempts to prepare allene 22 through a SN2′ reaction led to either decomposition when the leaving group was mesylates/triflate or no reaction when the leaving was acetate.15 Eventually, displacement of a sulfoxide leaving group16 proved to be fruitful and provided the desired allene 22 in 80% yield from ynone 21.
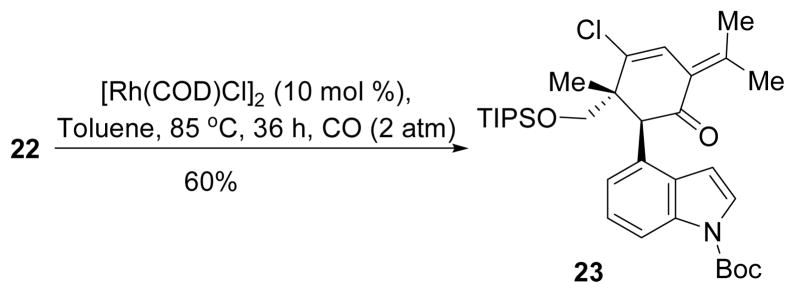
With allenylcyclopropane 22 in hand, we then tried the [5+1] cycloaddition under different conditions. After screening various solvents (toluene, xylene, DCE, CHCl3, dioxane), catalysts ([Rh(CO)2Cl]2, [Rh(COD)2Cl]2, [RhCl(PPh3)3], Ir(CO)2Cl]2), CO pressure (1 atm, 2 atm, and 5 atm), and temperature, we were able to isolate 60% yield of the desired [5+1] cycloaddition product 23 under conditions shown in Scheme 5. The cyclopropane C-C bond adjacent to the indole ring and away from the quaternary carbon was selectively cleaved during the cycloaddition. The relative stereochemistry of the product and the regioselectivity for the cleavage of cyclopropane C-C σ-bond were determined by nOe and HMBC, respectively.17
Scheme 5.
Rh-catalyzed [5+1] cycloaddition of Allenylcyclopropane and CO
In summary, we have developed an efficient strategy to access the cyclohexenone core of welwitindolinone Cs. Highly sterically congested penta-substituted cyclopropanes were prepared successfully by an intramolecular cyclopropanation of trisubstituted alkenes with chlorodiazoacetates. The [5+1] cycloaddition product 23 has a cyclohexenone core with most of the required functionalities for welwitindolinone Cs including a quaternary carbon, a vinylchloride group, an indole ring, and a ketone group with an isopropylidene substituent. Efforts to complete the synthesis of welwitindolinone Cs and their analogues by installing the second quaternary carbon and closing the seven-membered ring are currently underway in our laboratory,18 in addition to the study of [5+1] cycloaddition of other allenylcyclopropanes.
Supplementary Material
Scheme 4.
Preparation of Penta-substituted Allenylcyclopropane
Acknowledgments
We thank the University of Wisconsin and the NIH (R01 GM088285) for financial support and a Young Investigator Award (to W.T.) from Amgen.
Footnotes
Supporting Information Available 1HNMR, 13CNMR, IR, HRMS for starting materials and products. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.a) Stratmann K, Moore RE, Bonjouklian R, Deeter JB, Patterson GML, Shaffer S, Smith CD, Smitka TA. J Am Chem Soc. 1994;116:9935. [Google Scholar]; b) Jimenez JI, Huber U, Moore RE, Patterson GML. J Nat Prod. 1999;62:569. doi: 10.1021/np980485t. [DOI] [PubMed] [Google Scholar]
- 2.a) Smith CD, Zilfou JT, Stratmann K, Patterson GML, Moore RE. Mol Pharmacol. 1995;47:241. [PubMed] [Google Scholar]; b) Zhang XQ, Smith CD. Mol Pharmacol. 1996;49:288. [PubMed] [Google Scholar]
- 3.For efforts towards the synthesis of welwitindolinones, see: Konopelski JP, Deng HB, Schiemann K, Keane JM, Olmstead MM. Synlett. 1998:1105.Wood JL, Holubec AA, Stoltz BM, Weiss MM, Dixon JA, Doan BD, Shamji MF, Chen JM, Heffron TP. J Am Chem Soc. 1999;121:6326.Kaoudi T, Quiclet-Sire B, Seguin S, Zard SZ. Angew Chem Int Ed. 2000;39:731.Jung ME, Slowinski F. Tetrahedron Lett. 2001;42:6835.Deng HB, Konopelski JP. Org Lett. 2001;3:3001. doi: 10.1021/ol016379r.Lopez-Alvarado P, Garcia-Granda S, Alvarez-Rua C, Avendano C. Eur J Org Chem. 2002:1702.Ready JM, Reisman SE, Hirata M, Weiss MM, Tamaki K, Ovaska TV, Wood JL. Angew Chem Int Ed. 2004;43:1270. doi: 10.1002/anie.200353282.Mackay JA, Bishop RL, Rawal VH. Org Lett. 2005;7:3421. doi: 10.1021/ol051043t.Baudoux J, Blake AJ, Simpkins NS. Org Lett. 2005;7:4087. doi: 10.1021/ol051239t.Greshock TJ, Funk RL. Org Lett. 2006;8:2643. doi: 10.1021/ol0608799.Lauchli R, Shea KJ. Org Lett. 2006;8:5287. doi: 10.1021/ol0620747.Xia J, Brown LE, Konopelski JP. J Org Chem. 2007;72:6885. doi: 10.1021/jo071156l.Richter JM, Ishihara Y, Masuda T, Whitefield BW, Llamas T, Pohjakallio A, Baran PS. J Am Chem Soc. 2008;130:17938. doi: 10.1021/ja806981k.Boissel V, Simpkins NS, Bhalay G, Blake AJ, Lewis W. Chem Commun. 2009:1398. doi: 10.1039/b820674k.Boissel V, Simpkins NS, Bhalay G. Tetrahedron Lett. 2009;50:3283.Tian X, Huters AD, Douglas CJ, Garg NK. Org Lett. 2009;11:2349. doi: 10.1021/ol9007684.Trost BM, Mcdougall PJ. Org Lett. 2009;11:3782. doi: 10.1021/ol901499b.Brailsford JA, Lauchli R, Shea KJ. Org Lett. 2009;11:5330. doi: 10.1021/ol902173g.Freeman DB, Holubec AA, Weiss MW, Dixon JA, Kakefuda A, Ohtsuka M, Inoue M, Vaswani RG, Ohki H, Doan BD, Reisman SE, Stoltz BM, Day JJ, Tao RN, Dieterich NA, Wood JL. Tetrahedron. 2010;66:6647. doi: 10.1016/j.tet.2010.04.131.Heidebrecht RW, Gulledge B, Martin SF. Org Lett. 2010;12:2492. doi: 10.1021/ol1006373.Ruiz M, Lopez-Alvarado P, Menendez JC. Org Biomol Chem. 2010;8:4521. doi: 10.1039/c0ob00382d.
- 4.a) Baran PS, Richter JM. J Am Chem Soc. 2005;127:15394. doi: 10.1021/ja056171r. [DOI] [PubMed] [Google Scholar]; b) Reisman SE, Ready JM, Hasuoka A, Smith CJ, Wood JL. J Am Chem Soc. 2006;128:1448. doi: 10.1021/ja057640s. [DOI] [PubMed] [Google Scholar]; c) Baran PS, Maimone TJ, Richter JM. Nature. 2007;446:404. doi: 10.1038/nature05569. [DOI] [PubMed] [Google Scholar]; d) Reisman SE, Ready JM, Weiss MM, Hasuoka A, Hirata M, Tamaki K, Ovaska TV, Smith CJ, Wood JL. J Am Chem Soc. 2008;130:2087. doi: 10.1021/ja076663z. [DOI] [PubMed] [Google Scholar]
- 5.a) Bhat V, Allan KM, Rawal VH. J Am Chem Soc. 2011;133:5798. doi: 10.1021/ja201834u. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Bhat V, Mackay JA, Rawal VH. Org Lett. 2011;13:3214. doi: 10.1021/ol201122f. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Bhat V, Rawal VH. Chem Commun. 2011;47:9705. doi: 10.1039/c1cc13498a. [DOI] [PubMed] [Google Scholar]; d) Bhat V, Mackay JA, Rawal VH. Tetrahedron. 2011;67:10097. doi: 10.1016/j.tet.2011.09.088. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Allan KM, Kobayashi K, Rawal VH. J Am Chem Soc. 2012;134:1392. doi: 10.1021/ja210793x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.a) Huters AD, Quasdorf KW, Styduhar ED, Garg NK. J Am Chem Soc. 2011;133:15797. doi: 10.1021/ja206538k. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Quasdorf KW, Huters AD, Lodewyk MW, Tantillo DJ, Garg NK. J Am Chem Soc. 2012;134:1396. doi: 10.1021/ja210837b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.For reviews on strategies and approaches for welwitindolinones, see: Avendano C, Menendez JC. Curr Org Synth. 2004;1:65.Brown LE, Konopelski JP. Org Prep Proc Intl. 2008;40:411.Wood JL. Nat Chem. 2012;4:341. doi: 10.1038/nchem.1335.Huters AD, Styduhar ED, Garg NK. Angew Chem Int Ed. 2012;51:3758. doi: 10.1002/anie.201107567.
- 8.Shu D, Li X, Zhang M, Robichaux PJ, Tang W. Angew Chem Int Ed. 2011;50:1346. doi: 10.1002/anie.201006881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.For selected examples of [5+1] cycloaddition of vinyl- and allenylcyclopropanes, see: Taber DF, Kanai K, Jiang Q, Bui G. J Am Chem Soc. 2000;122:6807.Kurahashi T, De Meijere A. Synlett. 2005:2619.Iwasawa N, Owada Y, Matsuo T. Chem Lett. 1995:115.Owada Y, Matsuo T, Iwasawa N. Tetrahedron. 1997;53:11069.Murakami M, Itami K, Ubukata M, Tsuji I, Ito Y. J Org Chem. 1998;63:4. doi: 10.1021/jo9718859.Taber DF, Sheth RB, Tian W. J Org Chem. 2009;74:2433. doi: 10.1021/jo802493k.Jiang GJ, Fu XF, Li Q, Yu ZX. Org Lett. 2012;14:692. doi: 10.1021/ol2031526.
- 10.For a comprehensive review on transition metal mediated reactions involving cyclopropanes, see: Rubin M, Rubina M, Gevorgyan V. Chem Rev. 2007;107:3117. doi: 10.1021/cr050988l.
- 11.a) Blankley CJ, Sauter FJ, House HO. Org Synth. 1969;49:22. [Google Scholar]; b) Corey EJ, Myers AG. Tetrahedron Lett. 1984;25:3559. [Google Scholar]
- 12.Bonge HT, Pintea B, Hansen T. Org Biomol Chem. 2008;6:3670. doi: 10.1039/b814374a.For computational study of cyclopropanation with halodiazoacetates, see: Bonge HT, Hansen T. J Org Chem. 2010;75:2309. doi: 10.1021/jo100113b.
- 13.Espino CG, Fiori KW, Kim M, Du Bois J. J Am Chem Soc. 2004;126:15378. doi: 10.1021/ja0446294. [DOI] [PubMed] [Google Scholar]
- 14.Nahm S, Weinreb SM. Tetrahedron Lett. 1981;22:3815. [Google Scholar]
- 15.For selected reviews on the synthesis of allenes, see: Brummond KM, Deforrest JE. Synthesis. 2007:795.Yu S, Ma S. Chem Commun. 2011;47:5384. doi: 10.1039/c0cc05640e.For selected reviews on transition metal mediated reactions involving allenes, see: Hashmi ASK. Angew Chem Int Ed. 2000;39:3590.Ma S. Chem Rev. 2005;105:2829. doi: 10.1021/cr020024j.Inagaki F, Kitagaki S, Mukai C. Synlett. 2011:594.Aubert C, Fensterbank L, Garcia P, Malacria M, Simonneau A. Chem Rev. 2011;111:1954. doi: 10.1021/cr100376w.
- 16.a) Westmijze H, Vermeer P. Synthesis. 1979:390. [Google Scholar]; b) Elsevier CJ, Vermeer P. J Org Chem. 1989;54:3726. [Google Scholar]
- 17.See Supporting Information for details.
- 18.Initial attempts to close the seven-membered ring by Lewis acidmediated direct cyclization of intermediate 23 were not successful.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.