Abstract
Proinflammatory cytokines induce Guanylate Binding Protein 1 (GBP-1) protein expression in intestinal epithelial tissues. GBP-1 has been described as influencing a number of cellular processes important for epithelial homeostasis, including cell proliferation. However, many questions remain as to the role of GBP-1 in intestinal mucosal homeostasis. We therefore sought to investigate the function of proinflammatory cytokine induced GBP-1 during intestinal epithelial cell proliferation. Through the use of complementary GBP-1 overexpression and siRNA-mediated knockdown studies, we now show that GBP-1 acts to inhibit pro-mitogenic β-catenin/T cell factor (TCF) signaling. Interestingly, proinflammatory cytokine induced GBP-1 was found to be a potent suppressor of β-catenin protein levels and β-catenin serine 552 phosphorylation. Neither GSK3-β nor proteasomal inhibition alleviated GBP-1-mediated suppression of cell proliferation or β- catenin/TCF signaling, indicating a non-canonical mechanism of β-catenin inhibition. Together, these data show that cytokine-induced GBP-1 retards cell proliferation by forming a negative feedback loop that suppresses β-catenin/TCF signaling.
Introduction
Guanylate binding protein 1(GBP-1) is a member of a family of interferon-γ (IFN-γ) inducible large GTPases 1. GBP-1 has been investigated as an innate anti-bacterial and anti-viral response factor with a host of proposed cellular functions, including the regulation of cell proliferation, migration, apoptosis, and epithelial barrier maintenance2-7. However, the molecular mechanisms through which GBP-1 executes many of these processes has yet to be determined.
Structurally, GBPs (GBP-1-7 in humans) are comprised of large globular domains containing a Ras-like GTP binding region and an elongated β-helical domain8,9,10. GBP helical domains have been shown to form GTP hydrolysis dependent homo or hetero-tetramers, whose formation is enhanced in GDP or GMP bound states 11,12. The GTPase activity of GBP-1 is required for its anti-viral functions and effects on cell migration, while the alpha helical domains are sufficient for suppression of endothelial cell proliferation 3,5,6.
It has recently been shown that GBP-1 is up-regulated in intestinal epithelial cells (IECs) at sites of active inflammation in individuals with inflammatory bowel disease (IBD)7,13. In IBD, and animal models of intestinal inflammation, extended exposure of IECs to proinflammatory cytokines perturb epithelial homeostasis and exacerbate disease progression. In order to maintain IEC homeostasis, intracellular Wingless-Int (Wnt) signaling cascades act to regulate glycogen synthase kinase 3β (GSK3β)-dependent proteosomal degradation of β-catenin14-16. Indeed, Wnt-β-catenin signaling is a key regulator of IEC proliferation and survival17. When β- catenin degradation is impaired by Wnt signaling, β-catenin translocates to the nucleus and participates in the transcription of pro-mitogenic target genes such as cyclin D1 15. Our laboratory has shown that prolonged exposure to proinflammatory cytokines decreases mitogenic Wnt-β-catenin signaling, however the role of GBP-1 in these processes has not been evaluated 18.
In this study we show that inflammation induced expression of GBP-1 restricts cellular proliferation in intestinal epithelial cells. A reductionistic cell culture model was used to determine the link between proinflammatory cytokine stimulated GBP-1 and epithelial cell proliferation. Through the use of complementary GBP-1 overexpression and siRNA mediated knockdown studies, we show that GBP-1 acts to inhibit β-catenin/ T cell factor (TCF) signaling. Induction of GBP-1 was found to inhibit β-catenin/TCF transcriptional activation through suppression of β-catenin protein levels. Interestingly, neither GSK3-β nor proteasomal inhibition alleviated GBP-1-mediated suppression of β-catenin/TCF signaling. Together, these data show that GBP-1 retards epithelial cell proliferation and TCF signaling through non-canonical inhibition of β- catenin protein levels.
Results
Exposure of IECs to TNF-α/IFN-γ reduces cellular proliferation co-incident with GBP-1 protein induction
Increased tumor necrosis factor alpha (TNF-α and interferon gamma (IFN-γ) have been reported in intestinal mucosa in inflammatory bowel disease (IBD) and experimental colitis 18-20. Furthermore, proinflammatory cytokines have been proposed to influence epithelial homeostasis during intestinal inflammation. GBP-1 protein expression is stimulated by proinflammatory cytokine exposure, and has been proposed to regulate vital homeostatic functions such as apoptosis and cell growth 5,7,21. Therefore, we focused this study on the potential for GBP-1 to regulate IEC proliferation.
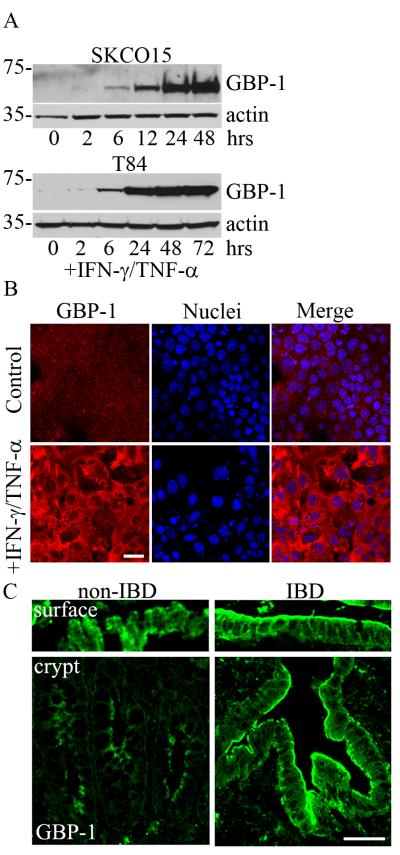
To determine the link between GBP-1 and cell proliferation in the intestinal epithelium, we exposed the model intestinal epithelial cell lines SKCO15 and T84 to exogenous TNF-α/IFN-γ (TNF-α 50ng/ml, IFN-γ 100U/ml). Time course studies were performed and the induction GBP-1 protein expression was assessed by immunoblot analysis (Figure 1A). Indeed, cytokine exposure dramatically increased GBP-1 protein levels from a low basal level in both cell lines (Figure 1A). Although GBP-1 induction is common to both cell lines tested, SKCO15 colonic epithelial cultures are more amenable to experimental manipulations such as transfection and transduction. Therefore, we continued the remainder of studies using these cultures and will be referred to as IECs. The subcellular localization of cytokine stimulated GBP-1 was then assessed by confocal microscopy and immunofluorescence staining of IEC monolayers (Figure 1B). Analogous to the immunoblot results obtained in Figure 1A, an increased intensity of GBP-1 staining was observed in the cytoplasm and plasma membrane of cytokine treated IECs. We next corroborated our in vitro findings by analyzing GBP-1 protein levels and localization in the colonic mucosa of ulcerative colitis patients. Frozen tissue sections were immunostained for GBP-1 and analyzed by confocal microscopy. Similar to cytokine treated monolayers, GBP-1 staining in IECs was more robust in inflamed tissue relative to non-IBD controls (Figure 1C). In normal colonic mucosa, GBP-1 is expressed in surface epithelial cells, which contact the lumen of the intestine (Figure 1C). Interestingly, prominent expression of GBP-1 was observed within the proliferative crypt compartment in addition to surface epithelium of mucosa from IBD patients (Figure 1C).
Figure 1.
In vitro proinflammatory cytokine treatment of IECs increases GBP-1 expression. A: SKCO15 and T84 colonic epithelial cells were treated with TNF-α/IFN-γ for the times indicated and GBP-1 expression was examined via immunoblotting. B: SKCO15 cells were treated with TNF-β/IFN-γ for 24 hours and then immunostained for GBP-1 (red). Bar = 40μm. C: Frozen tissue sections were obtained from patients with ulcerative colitis or from non-IBD colonic tissues. Non-IBD tissue shows diffuse surface and crypt epithelium GBP-1 signal. Actively inflamed regions show strong staining of GBP-1 (green) in surface and crypt epithelium. Bar = 100μm.
GBP-1 expression regulates IEC proliferation
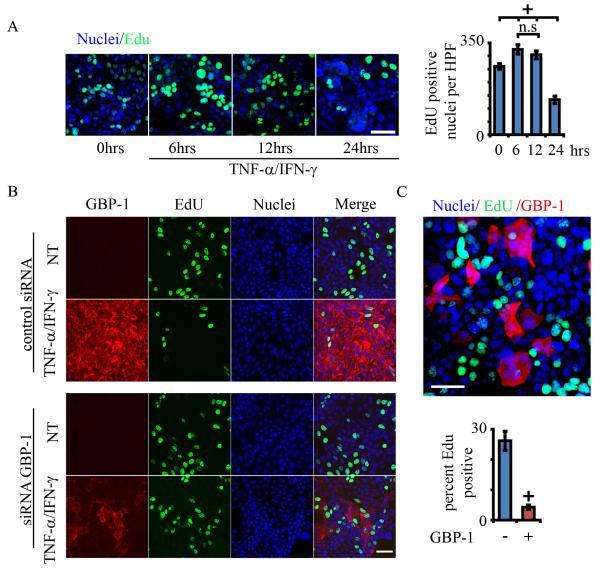
Our previous findings have shown that inflammatory cytokines regulate IEC proliferation 18. In order to assess cell replication after GBP-1 induction, SKCO15 cells were treated with TNF-α/IFN-γ and were assayed for 5- ethynyl-2-deoxyuridine (EdU) incorporation at various times following cytokine treatment. EdU is a thymidine analogue that is readily incorporated into replicating DNA and is subsequently detected by confocal microscopy. EdU incorporation was increased significantly at 6 hours post cytokine treatment (p<0.05), after which it decreased as much as 50% compared to controls by 24 hours (Figure 2A). Control mock experiments using identical methods, but in the absence of TNF-α/IFN-γ, show little change in EdU incorporation rates (Supplemental Figure 1). Together, these data demonstrate an inverse relationship between GBP-1 expression and cellular replication in response to pro-inflammatory cytokine treatment.
Figure 2.
GBP-1 expression attenuates IEC proliferation. A: SK-CO15 cells were treated with TNF-β/IFN-γ for the times indicated and cell proliferation was determined by EdU incorporation (1hr). n=3. + indicates p < 0.05, all samples versus untreated (0 hours) (n.s. not significant, ANOVA). Error bars = SE. B: SKCO15 cells, either treated with TNF-β/IFN-γ (48hrs) or non-treated controls (NT), were transfected with a GBP-1 targeted siRNA (GBP-1 siRNA) or non-silencing siRNA (control siRNA). Immunofluorescence analysis shows silencing of GBP-1 in SKCO15 cells after cytokine treatment. Proliferation was determined by EdU incorporation (green, scale bar = 50μm). C: SKCO15 cells were transiently transfected with a GBP-1 expression plasmid. Immunofluorescence analysis of IEC monolayers for EdU incorporation (green) and GBP-1 (red). ° indicates p < 0.005 versus total nuclei Error bars = SE, n=3.
We next asked if increased expression of GBP-1 plays a role in the cytokine induced cell growth suppression observed above. SKCO15 cells were exposed to TNF-α/IFN-γ for 48hrs and assayed for EdU incorporation. Consistent with our findings in Figure 1, TNF-α/IFN-γ treatment caused a significant decrease in EdU incorporation as compared to controls (Figure 2B, Supplemental Figure 2). In order to assess the role of GBP-1, protein levels were down-regulated using siRNA 7. Transient downregulation of GBP-1 was determined by immunofluorescence labeling and confocal microscopy (~50% transfection efficiency, GBP-1 knockdown, 72% +/− 12, n=4, see SF2)). While TNF-α/IFN-γ treated monolayers showed decreased EdU incorporation under control conditions (non-silencing siRNA), the effect was ameliorated by siRNA mediated GBP-1 depletion, where cytokine exposure only marginally reduced cell growth (lower panel and Supplemental Figure 2). Conversely, GBP-1 over-expression via transient transfection resulted in decreased EdU incorporation, supporting a direct role for GBP-1 in the regulation of cell proliferation (Figure 2C). However, during the course of these experiments it was noted that the apparent transfection efficiency rarely surpassed 50%. Therefore, we directly assessed EdU incorporation in individual GBP-1 overexpressing cells. SKCO15 cells were transiently transfected with GBP-1 and EdU incorporation was assessed 48hrs post transfection. GBP-1 overexpressing cells were identified by immunostaining with a GBP-1 antibody (Figure 2C, lower panel). Indeed, EdU incorporation in transfected cells was greatly reduced compared to the overall EdU incorporation. These findings demonstrate that the TNF-α/IFN-γ inducible protein, GBP-1, inhibits IEC proliferation.
GBP-1 reduces β-catenin protein levels through a proteasome independent mechanism
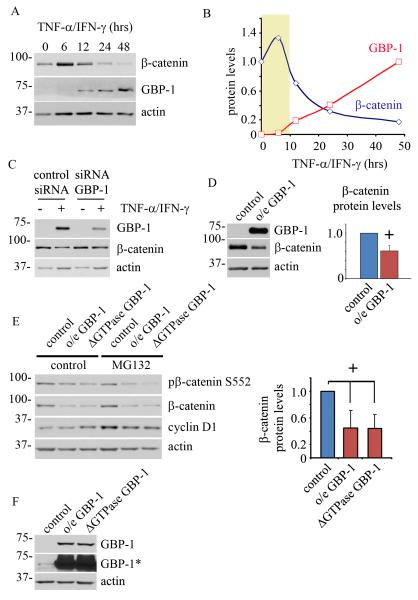
We recently reported that prolonged TNF-α/IFN-γ treatment attenuated epithelial proliferation by suppressing β-catenin signaling 18. Indeed, β-catenin signaling plays a central role in IEC proliferation15. Having demonstrated that high GBP-1 protein expression is sufficient to reduce proliferation in IECs, we next investigated the potential link between GBP-1 and β-catenin. As shown in Figure 3A, β-catenin protein levels vary considerably after TNF-α/IFN-γ exposure, with decreased β-catenin levels coincident with GBP-1 induction. Densitometric analysis of westernblot data revealed a peak in β-catenin levels at 6hrs post cytokine treatment, which gradually decreased over the subsequent 24-48hrs (Figure 3B). Interestingly, GBP-1 protein expression is minimal before 6hrs (highlighted area), but accumulated at a constant rate during the experiment. We next asked if GBP-1 protein levels directly contributed to the decrease in β-catenin protein expression. SKCO15 cells were treated with non-silencing control siRNA or siRNA directed against GBP-1. Epithelial monolayers were subsequently exposed to TNF-α/IFN-γ for 48hrs. As shown in Figure 3C, siRNA mediated suppression of GBP-1 resulted in the inhibition of cytokine-induced decreases in β-catenin protein levels. Conversely, exogenous overexpression of GBP-1 was found to suppress β-catenin levels (Figure 3D). In order to determine the specificity of GBP-1 function on mitogenic signaling, we investigated Erk1/2 signaling, an alternative pathway known to regulate cell proliferation. However, siRNA mediated suppression of GBP-1 failed to alter the Erk proliferative signaling pathway (supplemental Figure SF4). Together, these data show that high levels of GBP-1 suppress β-catenin protein levels.
Figure 3.
GBP-1 suppresses β-catenin protein expression. A: SKCO15 cells were treated with TNF-β/IFN-γ for the times indicated. GBP-1 and β-catenin protein levels were assessed by western blot (representative of three independent experiments). B: Desitometric analysis of β-catenin protein levels during cytokine treatment. C: GBP-1 knockdown by siRNA in SKCO15 cells rescues TNF-β/IFN-γ induced loss of β-catenin protein levels compared with non-silencing siRNA controls. D: Overexpression of GBP-1 is sufficient to suppress β-catenin protein levels compared with controls (empty vector) (0.61+/−0.2, n=4. + p<0.05). E: Lentivirus transfected SKCO15 cells stably expressing GBP-1, GTPase deficient GBP-1 (ΔGTPase), or empty control vector were treated with MG132, an inhibitor of proteasome function, for 8 hours. Right panel, relative β-catenin protein levels were determined by densitometry and compared to controls (0.45+/−0.2 o/e GBP-1 vs controls, 0.44+/−0.2 GTPase deficient GBP-1 (ΔGTPase) vs controls n=6, p<0.05, ANOVA, other comparisons are non-significant). F: SKCO15 cells stably overexpressing wild-type GBP-1 (o/e GBP-1), GTPase deficient GBP-1 (ΔGTPase) or empty vector (control). *, long exposure highlights endogenous GBP-1 expression levels in control cells.
We next sought to investigate the mechanism through which β-catenin expression is attenuated by GBP-1. First, we examined β-catenin mRNA levels by real time PCR and found similar β-catenin message levels between control and GBP-1 overexpressing cells (supplemental Figure SF5). Therefore, it is unlikely that GBP-1 regulates β-catenin at the mRNA level. β-catenin protein levels are regulated by intracellular pathways that target it for proteasomal degradation22. We therefore analyzed β-catenin protein levels by immunoblotting in IECs that stably express GBP-1, with or without chemical inhibition of the proteasome (Figures 3E and F). Either GBP-1 or a GTPase defective GBP-1 mutant (ΔGTPase GBP-1, D184N) was expressed in epithelial cultures in excess of baseline endogenous GBP-1 protein levels (Figure 3F, *). GBP-1 D184N was expressed to evaluate the role of the GTPase function of GBP-1 on β-catenin protein levels. Consistent with our previous findings, both wild type and mutant D184N GBP-1 proteins suppress β-catenin levels (Figure 3E, 0.44+/−0.2 vs.control). However, the proteasomal inhibitor MG132 failed to rescue the effects of GBP-1 overexpression, as β-catenin levels remain suppressed in MG132 treated cultures. As a control for MG132 function, we assessed cyclin D1 protein levels under these conditions. Cyclin D1 levels are tightly regulated through proteasomal degradation and are therefore elevated in MG132 treated samples (Figure 3E). Additionally, we monitored β- catenin phosphorylation at a c-terminal serine residue (serine 552) known to promote β-catenin transcriptional activity 23. Indeed, the levels of β-catenin S552 phosphorylation were decreased in GBP-1 overexpressing cells relative to controls and similar effects were seen after proteasomal inhibition (Figure 3E). However, densitometric comparisons of the ratio of total to S552 phosphorylated β-catenin were not significantly different from controls samples (data not shown). In summary, we find that GBP-1 regulates β-catenin protein levels but not β-catenin mRNA expression.
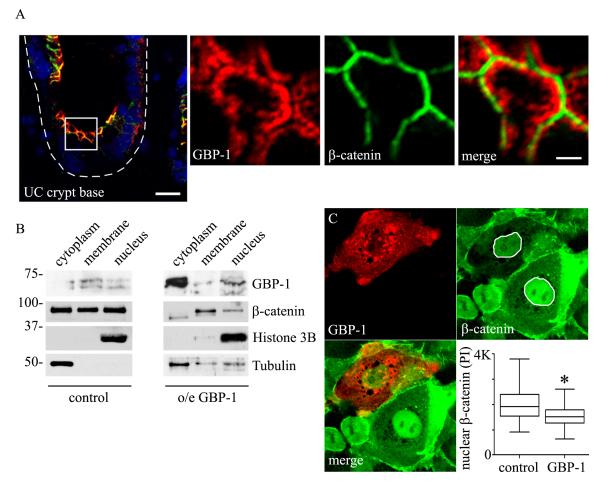
Canonical Wnt pathway activation leads to accumulation of cytoplasmic β-catenin and subsequent β- catenin translocation to the nucleus, where it functions as a transcriptional co-factor15. We therefore investigated the subcellular distribution of β-catenin in GBP-1 expressing cells of patients with ulcerative colitis by immunofluorescence co-staining. As seen in Figure 4A, GBP-1 is expressed in crypt epithelial cells and exhibits a perijunctional subcellular localization with limited co-localization with beta catenin (Figure 4A). We next assessed the subcellular localization of β-catenin in SKCO15 cell stably overexpressing GBP-1. As shown in Figure 4B, under normal conditions subcellular fractionation revealed relatively equal distribution of β-catenin between the cytoplasm, nucleus and membrane fractions. This is in contrast to cells overexpressing GBP-1, which contain the majority of cellular β-catenin within the membrane fraction (Figure 4B). β-catenin is known to regulate mitogenic signaling by accumulating in the nucleus. Therefore, we further evaluated the amount of nuclear β-catenin in cells after transient overexpression of GBP-1 by immunofluorescence labeling and pixel intensity analysis (Figure 4C). Indeed, a significant decrease in nuclear β-catenin protein levels where found in cells overexpressing GBP-1. Together, these data show that GBP-1 expression regulates both β-catenin protein levels and its subcellular localization.
Figure 4.
GBP-1 expression alters the subcellular localization of β-catenin. A: Immunofluorescence co-staining of β-catenin and GBP-1 in human ulcerative colitis samples (UC). Scale bar = 15μm. White box indicates magnified image area. Scale bare = 1.5μm. B: Subcellular fractionation and western blot analysis of control and GBP-1 stably overexpressing cells. C: SKCO15 cells transiently transfected with GBP-1 and analyzed for nuclear β-catenin levels by pixel intensity analysis (PI, *, p<0.01, control n=114, GBP-1, n=56).
GBP-1 suppresses proinflammatory cytokine induced β-catenin/TCF transactivation
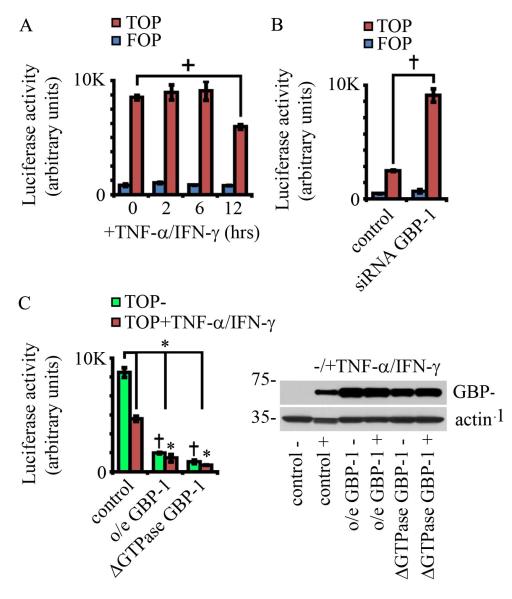
β-catenin participates in pro-mitogenic signaling by enhancing the activity of TCF/lef transcription factors. Given that GBP-1 suppresses both cell proliferation and β-catenin protein levels, we investigated TCF/lef transcriptional activity in epithelial cells after cytokine treatment. SKCO15 cells were transiently transfected with a β-catenin/TCF responsive luciferase reporter plasmid and then treated with TNF-α/IFN-γ (Figure 5A). As seen in Figure 5A, TCF luciferase output was attenuated by 12 hours of cytokine treatment, a time point coincident with rising GBP-1 levels (Figure 1A). We therefore performed the β-catenin/TCF luciferase assay using SK-CO15 cells transiently co-transfected with GBP-1 targeted siRNA. Indeed, siRNA mediated down-regulation of GBP-1 resulted in increased β-catenin/TCF luciferase reporter activity relative to non-silencing control siRNA, in TNF-α/IFN-γ treated IECs (24hrs post TNF-α/IFN-γ treatment, Figure 5B). Additionally, GBP-1 knockdown increased levels of the TCF dependent protein cyclin D1 (supplemental figure SF4B). In order to determine the effects of GBP-1 overexpression, cells stably expressing GBP-1 were transiently transfected with the β-catenin/TCF luciferase reporter and treated with TNF-α/IFN-γ. Similar to our findings in Figure 5A, cytokine treated cells showed significant attenuation of β-catenin/TCF luciferase activity relative to non-treated controls. Consistent with our previous findings, luciferase activity was dramatically decreased in cells expressing either full-length GBP-1 (o/eGBP-1) or GTPase deficient GBP-1 (ΔGTPase GBP-1, D184N Figure 5C). GBP-1 protein levels were then assessed by immunoblot analyses of the lysates obtained from these cells (Figure 5C, right panel).
Figure 5.
GBP-1 acts to suppresses β-catenin/TCF signaling. A: SKCO15 cells were transiently transfected with a β-catenin/TCF responsive luciferase reporter construct (TOP) or a reporter in which the TCF responsive sites have been mutated (FOP). Cells were further treated with TNF-β/IFN-γ for the times indicated. + indicates p < 0.05 (ANOVA, all others not significant). n=3. Error bars =SE. B: SKCO15 cells were transiently co-transfected with a β-catenin/TCF responsive luciferase reporter construct and GBP-1 targeted siRNA (GBP-1 siRNA) or non-silencing siRNA (control siRNA). Luciferase activity was assessed 24hrs after transfection and TNF-β/IFN-γ treatment. ° indicates p < 0.005, n=3. C: SKCO15 cells were transduced with retrovirus and selected so as to stably express wild type GBP-1 (o/e GBP-1) or GTPase deficient GBP-1 (ΔGTPase), or were transduced with the same virus lacking GBP-1 (control). These cells were then transiently transfected with a β- catenin/TCF responsive luciferase reporter construct and treated with TNF-β/IFN-γ for 24 hours (values =TOP-FOP). + indicates p < 0.01 between control and each cytokine treated sample, ° indicates p < 0.005 GBP-1 and ΔGTPase GBP-1 overexpression versus untreated control cells, * indicates p<0.01 verses cytokine treated control cells. All other comparisons are non-significant (ANOVA, n=3). Immunoblot analyses show that GBP-1 levels in cells stably expressing GBP-1 proteins are similar to levels attained during cytokine treatment (right panel).
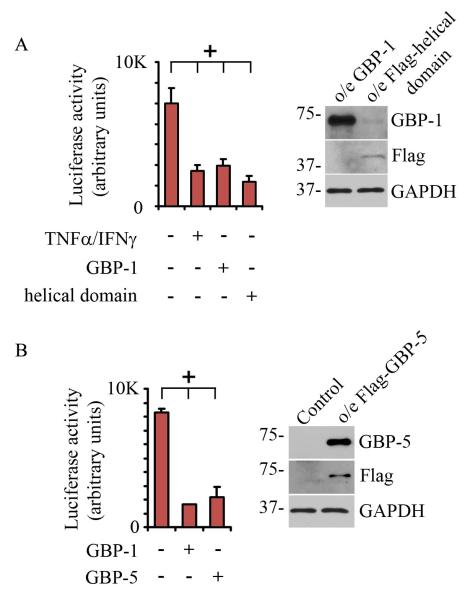
Previous studies have shown that the GBP-1 helical domain suppresses endothelial cell proliferation 5. We therefore investigated the role of the GBP-1 helical domain in the regulation of β-catenin/TCF signaling in IECs. SKCO15 cells were transiently transfected with either full length GBP-1 or a GBP-1 truncation mutant that expresses the C-terminal alpha-helical domains. As seen in Figure 6A, both full-length GBP-1 and the C-terminal alpha-helical domains suppressed β-catenin/TCF activity. Interestingly, β-catenin/TCF suppression due to GBP-1 or GBP-1 helical domain overexpression was similar to the reduction observed due to treatment of IECs with TNF-α/IFN-γ. The effects of TNF-α/IFN-γ and GBP-1 constructs were assessed at 48hrs post cytokine treatment or transfection in order to ensure GBP-1 expression. Cell proliferation relative to non-treated samples remained suppressed at this time point, as shown in supplemental Figure SF7. GBP-1 and GBP-1 helical domain (Flag) protein expression was confirmed by westernblot, as seen in Figure 6A.
Figure 6.
GBP-1 acts to suppresses β-catenin/TCF signaling. A: SKCO15 cells were transiently transfected with a β-catenin/TCF responsive luciferase reporter construct (TOP) or a reporter in which the TCF responsive sites have been mutated (FOP, values = TOP-FOP). Cells were further treated with TNF-β/IFN-γ for 48hrs or transiently transfected with full length GBP-1 or the GBP-1 helical domain where indicated. Protein expression was confirmed by western blot (right panel), * indicates p< 0.05 by ANOVA. B. SKCO15 cells were transiently co-transfected with a β-catenin/TCF responsive luciferase reporter construct and GBP-1 or GBP-5 (values = TOP-FOP). Protein expression was confirmed by western blot (right panel), * indicates p< 0.05 by ANOVA.
As stated above, there are a number of GBPs expressed in human tissues, several of which have been found to be upregulated in response to proinflammatory stimulus, including GBP-5 8. To assess the possibility that β-catenin/TCF suppression is a common feature among GBPs, we investigated the effects of GBP-5 overexpression on β-catenin/TCF output. GBP-5 was transiently overexpressed in SKCO15 cells along with a β-catenin/TCF reporter construct. For comparison, GBP-1 overexpressing cells were also analyzed (Figure 6B). Together, these data confirm that the GBP-1 helical domain is sufficient for suppression of β-catenin/TCF signaling and that this suppression may also be mediated by GBP-5.
Discussion
IEC homeostasis requires coordinated control of cellular apoptosis and proliferation, and during inflammatory episodes it is of grave importance to maintain a balance between these two processes. For example, apoptosis is often increased within inflamed regions of the colon, necessitating increased proliferation to replace apoptotic cells24. Inflammatory bowel disease is associated with acute mucosal lesions, which must be resolved through epithelial wound repair if mucosal barrier integrity is to be reestablished. Indeed, IEC wound repair involves both epithelial cell migration and replication, and failure to seal the mucosal barrier leads to further exposure to inflammatory stimulus. These stimuli include proinflammatory cytokines which further modulate the reparative response. Increases in the proinflammatory cytokines TNF-alpha and IFN-gamma during IBD have been previously reported25. Importantly, long standing inflammation and cell proliferation contribute ultimately to neoplastic conditions seen in chronic inflammatory states 26. GBP-1 has been described as influencing a number of these cellular processes, including cell proliferation, apoptosis and cell migration7,21,27. Therefore, it is vital to understand the mechanisms by which GBP-1 regulates intestinal epithelial mucosa homeostasis.
Previous studies have shown that GBP-1 protein expression is increased in endothelial cells during inflammatory diseases and its expression correlates inversely with endothelial cell proliferation 5,6,28. Increased GBP-1 expression likewise attenuates proliferation of mammary epithelial cells in a mouse mammary tumor model 21. We have extended these findings and have now confirmed that GBP-1 also restricts cell growth in IECs, as well as elucidated the signaling mechanisms through which this occurs. Indeed, the above data demonstrate that GBP-1 acts as a downstream regulator of cytokine function, and is both necessary and sufficient to mediate suppression of cell proliferation and pro-mitogenic β-catenin/TCF signaling. The C-terminal alpha-helical domain of GBP-1 was found to be adequate for this function and GBP-5 reduced β- catenin/TCF signaling, indicating that regulation of β-catenin/TCF signaling may be a common property among GBP family proteins. Interestingly, high magnification confocal immunofluorescence has determined that inflammation-induced GBP-1 localizes within a perijunctional compartment, proximal to β-catenin-containing adhesions. Complementary in vitro cell fractionation studies show that high GBP-1 expression correlates with low cytoplasmic and nuclear β-catenin levels, consistent with low TCF activity. This leads us to speculate that during inflammation, perijunctional GBP-1 prevents cytoplasmic and therefore nuclear accumulation of β- catenin.
Proinflammatory cytokines signal to IECs through the engagement of cell-surface receptors. Receptor activation then stimulates intracellular signals that activate down-stream nuclear transcription. Recent work by our laboratory and others has demonstrated that cytokine stimulation results in the activation of parallel pathways, one involving β-catenin/TCF activation, the other results in Nuclear Factor kappa B and Interferon Response Factor (NF-κB/IRF)-mediated GBP-1 transcription 18,29,30. We now contend that GBP-1 protein induction acts to impinge upon cytokine induced β-catenin/TCF activation. This occurs through the GBP-1 mediated disruption of β-catenin protein levels, effectively forming a negative feedback loop.
Cytokine induced GBP-1 production takes several hours to reach detectable levels and begins to suppress β-catenin at between 6 and 10 hours (Figure 2A). This creates a window of time after cytokine exposure in which cells exhibit increased TCF/lef output and proliferation (Figure 1C and 4A). Our previous studies have shown that short-term exposure of intestinal epithelial cells to IFN-α resulted in activation of β- catenin/TCF signaling and cellular proliferation 18. Indeed, in our study, long term exposure (between 48 and 72 hrs) correlated with induction of apoptosis and was coincident secretion of the Wingless-int (Wnt) inhibitor Dkk1. Dkk1 was demonstrated to inhibit β-catenin signaling through de-repression of Glycogen Synthase Kinase 3 beta (GSK3-β). GSK3- β is then free to inhibit β-catenin. However, GBP-1 appears to represses β- catenin through a different mechanism, as GSK3-β and proteasomal inhibition was unable to rescue the loss of β-catenin/TCF activity (Figure 3E, F and supplemental figure SF6). In addition to suppression of β-catenin protein levels, GBP-1 expression resulted in decreased serine 552 phosphorylated β-catenin. This finding is consistent with low TCF/lef transcriptional activity, as serine 552 phosphorylated β-catenin has a higher activity within the nucleus23. However, relative ratios of β-catenin 552-to-total β-catenin were unchanged (data not shown), leading us to conclude that GBP-1 primarily acts to suppress TCF/lef output through the suppression of β-catenin protein levels and not through the regulation of β-catenin activity. Interestingly, we also observed a cytokine-induced downregulation of mitogenic Erk signaling (supplemental figure SF4A). While we conclude that Erk suppression is independent of GBP-1, this finding indicates that multiple proliferative pathways are reduced during inflammation in our cells. Although it should be considered that our studies utilized model human intestinal epithelial cells derived form tumors, our complementary overexpression and siRNA knockdown studies confirm a role for GBP-1 in the above processes. The exact molecular mechanism involved in GBP-1-mediated β-catenin suppression will be the subject of future studies.
The above findings suggest that proinflammatory cytokine exposure produces multiple signals that act in concert to suppress β-catenin/TCF signaling during long term inflammatory events. It also presents the possibility that continued cytokine exposure produces temporally divergent responses to prolonged stimulus. However, it is clear that further examination of these signaling pathways will be required to determine how the cell chooses between the vital cellular processes of proliferation, survival or cell apoptosis, to maintain homeostasis.
Methods and Materials
Cell culture
SKCO15 and T84 model colonic epithelial cell lines were used due to their enterocyte-stereotypic differentiation, polarization, and response to proinflammatory cytokines 18. Briefly, intestinal epithelial T84 cells were grown in 1:1 DMEM and modified Ham’s F-12 medium, and SKCO15 cells were grown in DMEM with 10% fetal calf serum and antibiotics. Cells were maintained in a humidified incubator with 5% CO2. For immunofluorescence studies, cells were seeded onto collagen-coated glass coverslips. SKCO15 cell lines stably expressing these GBP-1 and GBP-1 mutant proteins (ΔGTPase GBP-1, D184N) did not grow efficiently in cell culture (Figures 3E and F, 4B, 5C and SF5) and were therefore independently established for each experiment.
Western blot and immunoprecipitations
To prepare cells for Western blot analysis, cells were scraped into RIPA lysis buffer (150mM NaCl, 1% NP-40, 0.5% deoxycholic acid, 0.1% SDS, 50mM Tris pH 8.0) containing protease and phosphatase inhibitors (Sigma) and 1mM PSMF, sonicated, and cleared by centrifugation. Protein concentration was determined using a BCA protein assay, and samples were boiled in SDS sample buffer with 50mM dithiothreitol. Equal amounts of protein were separated by SDS-PAGE, and transferred onto PVDF membranes. Membranes were blocked for 1h with 5% wt/vol dry milk or BSA in Tris-buffered saline containing 0.1% Tween-20, and incubated with primary antibodies in blocking buffer overnight at 4°C. Antibodies were detected using HRP-linked secondary antibodies (Jackson ImmunoResearch) and chemiluminescent substrate (Denville Scientific). Image quantitation was performed by densitometry using NIH image J.
Antibodies and Reagents
Antibodies used were as follows: Erk and phospho-Erk antibodies are from Cell Signaling (kit #9911), rabbit polyclonal cyclin D1 (sc-718) from Santa Cruz (Santa Cruz, CA), GBP1 (1B1, SAB4200056), β-catenin (C2206), and anti-actin (A20667) from Sigma-Aldrich (St Louis, MO), GSK3-β (610202) from BD Biosciences (San Diego, CA), and β-catenin pS552 antibody (9566S) form Cell Signaling (Beverly, MA). Rat anti-human GBP-5 monoclonal antibody was a kind gift from M. Stürzel (3D8). Secondary antibodies used were as follows: Alexa-conjugated secondary antibodies were obtained from Molecular Probes (Invitrogen, Carlsbad, CA). HRP-conjugated secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Nuclei were stained with ToPro-3 (Invitrogen). Recombinant human IFN-γ was provided by Genentech and used at a concentration of 100 U/ml. Recombinant human TNF-α was purchased from BioVision and used at a concentration of 50 ng/ml. Growth media was exchanged in all samples when cytokines were added. The GSK3-β inhibitor AR-A014418 or N-(4-Methoxybenzyl)-N’-(5-nitro-1,3-thiazol-2-yl)urea (A3230) and the proteasomal inhibitor MG132 (C2211) are from (Sigma-Aldrich). For transfection studies, Lipofectamine 2000 (Invitrogen) was used for transient overexpression of pCDNA3-h.s.GBP-1, empty vector control pCDNA3.0, or siRNA transfection according to the manufacturer’s instructions. Similar methods were used to transfect pMCV1.4 FLAG-GBP-1 helical /globular domains and FLAG-GBP-5 (form M. Stürzel)8. Transient knockdown was performed using siRNA against GBP-1 (SASI_Hs01_00045495, Sigma-Aldrich) and compared to non-silencing siRNA as a control (QIAGEN ,Valencia, AllStars Negative Control). Human tissue samples were obtained from patients with ulcerative colitis or from non-IBD colonic tissues and evaluated by two expert gastrointestinal pathologists (C.P or A.N.).
Lentivirus and Stable Cell Line Production
Constructs containing full length GPB-1 and GBP-1 D184N were gifts from M. Stürzel and used as templates for PCR to make pLEX-MCS constructs for lentivirus production. GBP-1 and GBP-1 D184N was cloned into pLEX-MCS (Thermo Fisher, Huntsville, AL) by PCR and the product was digested, purified and ligated into SpeI/NotI digested pLEX-MCS using standard cloning techniques. Lentivirus was produced using the Trans-Lentiviral pGIPZ packaging system (Thermo Fisher) according to the manufacturer’s protocol. Lentivirus particle titer was determined by limiting dilution. SKCO15 cells were then grown as above with 15 mm HEPES and 1% nonessential amino acids (Cellgro, Manassas, VA). Stable control pLEX-MCS, GPB-1, and GPB-1 D184N SKCO-15 cell lines were produced by infecting 4×105 cells with lentivirus at an MOI of 3 for 12 hours in Opti-MemI media (Invitrogen). At 48 hours post infection transduced cells were selected with 5μg of puromycin.
Immunofluorescence labeling and pixel intensity analysis
Immunofluorescence localization of proteins of interest was performed as described previously31. Briefly, cells were grown as confluent or sub confluent cultures and fixed for immunofluorescence. SKCO15 were fixed in absolute ethanol for 20 min at −20°C. For the visualization of GBP-1 and nuclear β-catenin, cell monolayers were fixed in 2% paraformaldehyde, pH 7.4 in PBS and permiabilized with 0.5% Triton X-100 for 10 min a 4°C. Non-specific binding was inhibited by an incubation in 1% BSA (1hr) prior to incubation with the indicated primary antibody (1hr) at room temperature (RT). Incubation with Alexa-secondary antibodies followed for 45min, at RT. Fluorescence images were acquired using LSM 510 and META image analysis software v. 4.2 (Zeiss Microimaging Inc., Thornwood, NY, USA). Pixel intensity analysis was performed as previously described32. Briefly, immunofluorescence images were collected as above and nuclear pixel intensities were collected using NIH Image J and analyzed using GraphPad Prism 5. Increases in GBP-1 expression shown in Figure 1C was determined form Pixel Intensity analysis of ulcerative colitis samples. The data was collected 3-4 images per patient. Non-IBD (2), n=19 crypts, IBD (3), n=16 crypts.
Subcellular fractionation
Epithelial cells were grown to confluence and harvested in 10mM HEPES pH 7.4, 100mM KCl, 3mM NaCl, 1mM Na2ATP, 3.5MgCl2, Protease Inhibitor Cocktail (Sigma), Phosphatase Inhibitor Cocktail I and II (Sigma), and 1mM PMSF. Cell lysis was achieved by nitrogen cavitation, at 200psi for 15min, 4°C. Nuclear fractions were collected by 1000g spin for 10min, 4°C. Membrane and cytosolic samples were separated by ultacetrifugation, 125,000g, 50 min at 4°C.
β-catenin TCF/lef Reporter Expression and in vitro cell proliferation assay
TCF reporter construct activity was measured using Dual Luciferase Reporter system (Promega, Madison WI) according to the manufacturer’s instructions. 5-ethynyl-2-deoxyuridine (EdU) incorporation was performed for 1hr and assessed with a Click-iT EdU Cell Proliferation Kit (Invitrogen), according to the supplier’s instructions. BrdU incorporation assays in SF6 were performed using Cell Proliferation ELISA, BrdU (colorimetric) assay (Roche, Indianapolis, IN) according to manufacturer’s instruction. Briefly, Cells were incubated in BrdU containing growth media for 1hr, washed with PBS, and lysed in RIPA lysis buffer. 5ug of total protein was assessed per sample.
RNA Isolation and real time PCR
RNA was isolated by phenol-chloroform extraction, treated with deoxyribonuclease I, and purified with the RNeasy Mini Kit (Qiagen, Valencia, CA). Real-time PCR was performed to determine mRNA levels. PCR amplification was performed using the GeneAmp 5700 sequence detection system (Applied Biosystems, Foster City, CA). PCR was run using the following protocol: initial activation at 94°C for 3 min, 40 cycles of 94°C for 15 s, 52°C for 30 s, and 72°C for 30 s. Primers for real-time RT-PCR were purchased from Realtime-primers.com: β-catenin, F-TGAGGACAAGCCACAAGATTAC, R-TCCACCAGAGTGAAAAGAACG and GAPDH.F-CAACAGCGACACCCACTCCT, R-CACCCTGTTGCTGTAGCCAAA.
Statistics
All experiments shown are representative of three independent experiments unless otherwise indicated. GraphPad Prism 5 and Microsoft Excel software was used for statistical analyses. Data were analyzed with 2- tailed Student t test unless otherwise indicated, including; ANOVA with Bonfirroni’s post test, or Fisher’s Exact Test. Statistical significance was assumed at p < .05. All results are displayed as mean + standard error of the mean (SE) unless otherwise indicated.
Supplementary Material
SF1: Cell proliferation rates in SKCO15 cells after growth media exchange at the time points indicated. Cells not subjected to media change are indicated as time point 0. Proliferation rates are represented as the fraction of EdU positive cells/Total cells counted (n=3, 5-600 cells per experiment, n.s by ANOVA).
SF2: GBP-1 knockdown by siRNA ameliorates TNF-β/IFN-γ induced suppression of cell growth. After 48hrs cytokine treatment, control samples show decreased proportion of EdU positive cells. IECs treated with siRNA against GBP-1 in addition to TNF-β/IFN-γ show no significant change in EdU incorporation compared with controls (n.s.). Statistical significance was determined by Fisher’s exact test (°, p=0.005, n=2,800 and 2,100).
SF3: Desitometric analysis of westernblot data showing GBP-1 knockdown by siRNA, with or without TNF-β/IFN-γ induction (n=4).
SF4: A. GBP-1 regulation of cell proliferation is independent of Erk signaling. SKCO15 cells treated with TNF-α/IFN-γ in addition to control siRNA or siRNA directed against GBP-1. B. As above, SKCO15 cells treated with TNF-α/IFN-γ in addition to control siRNA or siRNA directed against GBP-1. Cyclin D1 levels were assessed by immunoblot.
SF5: GBP-1 does not alter β-catenin transcription. β-catenin transcript levels were determined by real time PCR. Values shown are normalized to GAPDH under control and GBP-1 overexpression conditions.
SF6: GSK3-β inhibition fails to rescue GBP-1 induced suppression of β-catenin/TCF transcription. SKCO15 cells stably expressing GBP-1 or GTPase defective GBP-1 were transfected with TCF/lef reporter constructs and treated with a GSK3-β inhibitor (AR-A014418, 8hrs 10μM).
SF7: TNF-β/IFN-γ treatment (48hrs) results in decreased cell proliferation relative to non-treated control SCKO15 cells. Cell proliferation was determined by ELISA-based BrdU incorporation assay after normalization to total protein (n=6, ++ p<0.1).
Acknowledgements
We would like to thank Caroline Addis, Oskar Laur at the Emory Cloning Core, and Dr. Susan Voss for expert technical assistance. Supported by grants from the National Institutes of Health (DK64399 to C.A.P.; DK55679, and DK59888 to A.N.; and DK64399 [NIH DDRC tissue culture and morphology grant]), and the Crohn’s and Colitis Foundation of America Fellowship Award to C.T.C. and Senior Researcher Award to N.A.L. M.S. was sponsored by a grant from the German Research Association (STU 238/6-1).
Footnotes
Disclosure: We have no conflicts of interest to disclose.
References
- 1.Cheng YS, Colonno RJ, Yin FH. Interferon induction of fibroblast proteins with guanylatebinding activity. J Biol Chem. 1983;258:7746–50. [PubMed] [Google Scholar]
- 2.Kim BH, et al. A family of IFN-gamma-inducible 65-kD GTPases protects against bacterial infection. Science. 2011;332:717–21. doi: 10.1126/science.1201711. [DOI] [PubMed] [Google Scholar]
- 3.Itsui Y, et al. Antiviral effects of the interferon-induced protein guanylate binding protein 1 and its interaction with the hepatitis C virus NS5B protein. Hepatology. 2009;50:1727–37. doi: 10.1002/hep.23195. [DOI] [PubMed] [Google Scholar]
- 4.Anderson SL, Carton JM, Lou J, Xing L, Rubin BY. Interferon-induced guanylate binding protein-1 (GBP-1) mediates an antiviral effect against vesicular stomatitis virus and encephalomyocarditis virus. Virology. 1999;256:8–14. doi: 10.1006/viro.1999.9614. [DOI] [PubMed] [Google Scholar]
- 5.Guenzi E, et al. The helical domain of GBP-1 mediates the inhibition of endothelial cell proliferation by inflammatory cytokines. EMBO J. 2001;20:5568–77. doi: 10.1093/emboj/20.20.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guenzi E, et al. The guanylate binding protein-1 GTPase controls the invasive and angiogenic capability of endothelial cells through inhibition of MMP-1 expression. EMBO J. 2003;22:3772–82. doi: 10.1093/emboj/cdg382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schnoor M, Betanzos A, Weber DA, Parkos CA. Guanylate-binding protein-1 is expressed at tight junctions of intestinal epithelial cells in response to interferon-gamma and regulates barrier function through effects on apoptosis. Mucosal Immunol. 2009;2:33–42. doi: 10.1038/mi.2008.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tripal P, et al. Unique features of different members of the human guanylate-binding protein family. J Interferon Cytokine Res. 2007;27:44–52. doi: 10.1089/jir.2007.0086. [DOI] [PubMed] [Google Scholar]
- 9.Olszewski MA, Gray J, Vestal DJ. In silico genomic analysis of the human and murine guanylate-binding protein (GBP) gene clusters. J Interferon Cytokine Res. 2006;26:328–52. doi: 10.1089/jir.2006.26.328. [DOI] [PubMed] [Google Scholar]
- 10.Prakash B, Praefcke GJ, Renault L, Wittinghofer A, Herrmann C. Structure of human guanylate-binding protein 1 representing a unique class of GTP-binding proteins. Nature. 2000;403:567–71. doi: 10.1038/35000617. [DOI] [PubMed] [Google Scholar]
- 11.Vopel T, et al. Mechanism of GTPase-activity-induced self-assembly of human guanylate binding protein 1. J Mol Biol. 2010;400:63–70. doi: 10.1016/j.jmb.2010.04.053. [DOI] [PubMed] [Google Scholar]
- 12.Britzen-Laurent N, et al. Intracellular trafficking of guanylate-binding proteins is regulated by heterodimerization in a hierarchical manner. PLoS One. 2010;5:e14246. doi: 10.1371/journal.pone.0014246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naschberger E, et al. Increased expression of guanylate binding protein-1 in lesional skin of patients with cutaneous lupus erythematosus. Exp Dermatol. 2011;20:102–6. doi: 10.1111/j.1600-0625.2010.01160.x. [DOI] [PubMed] [Google Scholar]
- 14.Gregorieff A, et al. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology. 2005;129:626–38. doi: 10.1016/j.gastro.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Kikuchi A, Kishida S, Yamamoto H. Regulation of Wnt signaling by protein-protein interaction and post-translational modifications. Exp Mol Med. 2006;38:1–10. doi: 10.1038/emm.2006.1. [DOI] [PubMed] [Google Scholar]
- 16.Staal FJ, Noort Mv M, Strous GJ, Clevers HC. Wnt signals are transmitted through N-terminally dephosphorylated beta-catenin. EMBO Rep. 2002;3:63–8. doi: 10.1093/embo-reports/kvf002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sancho E, Batlle E, Clevers H. Live and let die in the intestinal epithelium. Curr Opin Cell Biol. 2003;15:763–70. doi: 10.1016/j.ceb.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Nava P, et al. Interferon-gamma regulates intestinal epithelial homeostasis through converging beta-catenin signaling pathways. Immunity. 2010;32:392–402. doi: 10.1016/j.immuni.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siegmund B, et al. Neutralization of interleukin-18 reduces severity in murine colitis and intestinal IFN-gamma and TNF-alpha production. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1264–73. doi: 10.1152/ajpregu.2001.281.4.R1264. [DOI] [PubMed] [Google Scholar]
- 20.MacDonald TT, Hutchings P, Choy MY, Murch S, Cooke A. Tumour necrosis factor-alpha and interferon-gamma production measured at the single cell level in normal and inflamed human intestine. Clin Exp Immunol. 1990;81:301–5. doi: 10.1111/j.1365-2249.1990.tb03334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lipnik K, et al. Interferon gamma-induced human guanylate binding protein 1 inhibits mammary tumor growth in mice. Mol Med. 2010;16:177–87. doi: 10.2119/molmed.2009.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang D, et al. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem. 2007;282:11221–9. doi: 10.1074/jbc.M611871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwamoto M, Koji T, Makiyama K, Kobayashi N, Nakane PK. Apoptosis of crypt epithelial cells in ulcerative colitis. J Pathol. 1996;180:152–9. doi: 10.1002/(SICI)1096-9896(199610)180:2<152::AID-PATH649>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura M, Saito H, Kasanuki J, Tamura Y, Yoshida S. Cytokine production in patients with inflammatory bowel disease. Gut. 1992;33:933–7. doi: 10.1136/gut.33.7.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feagins LA, Souza RF, Spechler SJ. Carcinogenesis in IBD: potential targets for the prevention of colorectal cancer. Nat Rev Gastroenterol Hepatol. 2009;6:297–305. doi: 10.1038/nrgastro.2009.44. [DOI] [PubMed] [Google Scholar]
- 27.Weinlander K, et al. Guanylate binding protein-1 inhibits spreading and migration of endothelial cells through induction of integrin alpha4 expression. FASEB J. 2008;22:4168–78. doi: 10.1096/fj.08-107524. [DOI] [PubMed] [Google Scholar]
- 28.Lubeseder-Martellato C, et al. Guanylate-binding protein-1 expression is selectively induced by inflammatory cytokines and is an activation marker of endothelial cells during inflammatory diseases. Am J Pathol. 2002;161:1749–59. doi: 10.1016/S0002-9440(10)64452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grimm T, et al. EBV latent membrane protein-1 protects B cells from apoptosis by inhibition of BAX. Blood. 2005;105:3263–9. doi: 10.1182/blood-2004-07-2752. [DOI] [PubMed] [Google Scholar]
- 30.Briken V, et al. Interferon regulatory factor 1 is required for mouse Gbp gene activation by gamma interferon. Mol Cell Biol. 1995;15:975–82. doi: 10.1128/mcb.15.2.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Severson EA, Lee WY, Capaldo CT, Nusrat A, Parkos CA. Junctional adhesion molecule A interacts with Afadin and PDZ-GEF2 to activate Rap1A, regulate beta1 integrin levels, and enhance cell migration. Mol Biol Cell. 2009;20:1916–25. doi: 10.1091/mbc.E08-10-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laukoetter MG, et al. JAM-A regulates permeability and inflammation in the intestine in vivo. J Exp Med. 2007;204:3067–76. doi: 10.1084/jem.20071416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SF1: Cell proliferation rates in SKCO15 cells after growth media exchange at the time points indicated. Cells not subjected to media change are indicated as time point 0. Proliferation rates are represented as the fraction of EdU positive cells/Total cells counted (n=3, 5-600 cells per experiment, n.s by ANOVA).
SF2: GBP-1 knockdown by siRNA ameliorates TNF-β/IFN-γ induced suppression of cell growth. After 48hrs cytokine treatment, control samples show decreased proportion of EdU positive cells. IECs treated with siRNA against GBP-1 in addition to TNF-β/IFN-γ show no significant change in EdU incorporation compared with controls (n.s.). Statistical significance was determined by Fisher’s exact test (°, p=0.005, n=2,800 and 2,100).
SF3: Desitometric analysis of westernblot data showing GBP-1 knockdown by siRNA, with or without TNF-β/IFN-γ induction (n=4).
SF4: A. GBP-1 regulation of cell proliferation is independent of Erk signaling. SKCO15 cells treated with TNF-α/IFN-γ in addition to control siRNA or siRNA directed against GBP-1. B. As above, SKCO15 cells treated with TNF-α/IFN-γ in addition to control siRNA or siRNA directed against GBP-1. Cyclin D1 levels were assessed by immunoblot.
SF5: GBP-1 does not alter β-catenin transcription. β-catenin transcript levels were determined by real time PCR. Values shown are normalized to GAPDH under control and GBP-1 overexpression conditions.
SF6: GSK3-β inhibition fails to rescue GBP-1 induced suppression of β-catenin/TCF transcription. SKCO15 cells stably expressing GBP-1 or GTPase defective GBP-1 were transfected with TCF/lef reporter constructs and treated with a GSK3-β inhibitor (AR-A014418, 8hrs 10μM).
SF7: TNF-β/IFN-γ treatment (48hrs) results in decreased cell proliferation relative to non-treated control SCKO15 cells. Cell proliferation was determined by ELISA-based BrdU incorporation assay after normalization to total protein (n=6, ++ p<0.1).