Abstract
It is known that ouabain, a selective inhibitor of Na/K-ATPase, can cause not only activation of signal cascades, which regulate the cell viability, but also can cause free radical accumulation, which can evoke the oxidative stress. We have shown that nanomolar concentrations of ouabain result in the temporary increase in the level of intracellular free radicals but the millimolar concentration of ouabain induces a stable intracellular accumulation of free radicals in rat thymocytes. The increasing level of free radicals resulting from both low and high concentrations of ouabain can be attenuated by the antioxidant, carnosine. Moreover the long-term incubation with ouabain leads to the cell death by necrosis and apoptosis. Ouabain-mediated apoptosis and necrosis were also abolished by carnosine.
Keywords: Na/K-ATPase, free radicals, ouabain, carnosine, thymocytes
INTRODUCTION
Sodium-potassium ATPase (Na/K-ATPase) is the enzyme which maintains the sodium and potassium asymmetry on the outer cell membrane of all animal tissues1. This enzyme participates in processes of intracellular signaling in myocytes2, neurons3, lymphocytes4 and also in some of neuroblastome-like cell lines5. Ouabain, one of the cardiotonic steroid (CTS), induces accumulation of reactive oxygen/nitrogen (ROS/NOS) species in cells6 and activation of signal cascades which regulate the cell viability7. Ouabain action eventually can lead to cell death or to adaptation to oxidative stress depending on the incubation condition8.
Some cells synthesize isoforms of Na/K-ATPase which differ in their affinity to ouabain9. Modulation of ouabain concentration can distinguish the isoforms which are directly associated with regulatory function of cells. Thymocytes express several isoforms of Na/K-ATPase with high (α2 and α3) and low (α1) affinity to CTS10, which allows study of the regulatory properties of Na/K-ATPase in these cells by using different concentrations of ouabain.
Inhibition of Na/K-ATPase by ouabain causes rapid intracellular free radical accumulation, which is related with intracellular Ca mobilization in the case of myocytes11 and neurons3,12. The free radical signals cause phosphorylation of mitogen-activated protein kinases (MAPK)13,14, which mediate specific cell responses. Participation of Na/K-ATPase in cell signal reactions is still poorly studied for thymocytes; thus we undertook this research.
MATERIALS AND METHODS
Reagents
Ouabain, Ethylene glycol-bis (2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA), NaCl, KCl, MgCl2, CaCl2, HEPES, D-glucose, BSA, Triton X-100, formaldehyde, N-nitroarginine, Annexin V-FITC apoptosis detection kit were purchased from Sigma (USA); DCF-DA was supplied by Molecular Probes (USA). L-Carnosine was purchased from “Hamari Chem., Ltd” (99% purity, HPLC).
Preparation of thymocytes
The animal experiments were approved by the institutional Committee for Ethics in Animal Experimentation (Institute for Health and the Environment, University at Albany, Rensselaer, NY). Wistar Kyoto rat pups (10–25 days of age) were used in the experiments. The thymus was isolated, cut in pieces with a razor blade, and placed in Tyrode’s solution (148 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM D-glucose, 10 mM HEPES, pH 7.4). Thymocytes were gently dissociated by rubbing the thymus pieces between two frosted glass slides, as previously described15. The debris was allowed to settle for about 3 min, and then the single cell suspension was filtered by gravity through a Teflon filter (pore size 53 μm), washed twice with cold physiological buffered saline and diluted to 1*106 cells/ml with Tyrode’s solution at 37°C. Different concentration of ouabain were used: 1nM or 100 nM (inhibition only high-sensitive isoform) and 1 mM (inhibition of high-sensitive and low-sensitive isoforms) in order to separate response of different isoforms of Na,K-ATPase16.
Flow cytometry
A BD FACSCalibur (Becton Dickinson, USA) flow cytometer was used in these studies. Cells were loaded by DCF-DA dye (5-(and 6)-carboxy-2’,7’-dichlorodihydrofluorescein diacetate, 100 μM), a marker of ROS/NOS radicals15 and cells were excited with an argon laser (488 nm). Then 10,000 cells were analyzed per sample. On the basis of light scatter, a relatively homogenous population of cells was gated for the study.
Detection of intracellular free radicals
Free radical production was measured after incubation of thymocytes with ouabain and other compounds for different periods of time. The nature of the fluorescent signal is not discussed here since in the literature it is widely accepted that both oxygen and nitrogen radicals can be trapped by DCF-DA15. Cells were loaded for 30 min in the dark, then were resuspended in fresh Tyrode’s solution. N-nitroarginine (NNA, 30 μM) was applied to block NO-synthase. Carnosine (1 mM) was used to prevent free radical accumulation. Cell viability was determined by using of the DNA binding dye propidium iodide (PI, 10 μM). PI was added to cells 5 min before detection.
Detection of apoptotic and necrotic cells by Annexin V-FITC kit
Annexin V-FITC apoptosis detection kit uses Annexin V-FITC conjugates for flow cytometry detection of cell surface changes that occur in initiation of the apoptotic process. Suspensions of thymocytes were treated with two different concentrations of ouabain, −100 nM or 1 mM, corresponding to inhibition of only the low ouabain-sensitive pool of Na/K-ATPase molecules or total inhibition of all the Na/K-ATPase molecules, respectively. Thymocytes were maintained at 37°C for 5 h. After incubation with ouabain, the cells were washed twice in PBS (pH 7.4) and resuspended in 100 ml of freshly prepared binding buffer containing Annexin V-FITC and PI were used according to the manufacturer’s protocol. The cell suspension was incubated for 15 min in the dark at room temperature. After incubation, cells were resuspended in 0.5 ml of binding solution and analyzed in the flow cytometer.
Data analysis
In all experiments, each sample was measured in triplicate and each data point was the result of at least three experimental replications. Repetitive experiments were performed on rat pups obtained from different females. The statistical analysis was made using the software “Biostatistica” (Excel program from the operating system of Windows XP). For each variable analyzed, the mean value and its standard deviation (SD) were calculated.
RESULTS
Incubation of a freshly prepared suspension of thymocytes with ouabain results in intracellular accumulation of free radicals, and the kinetics of this process depends on concentration of ouabain (Fig. 1). In the presence 1nM or 100 nM ouabain the radical levels increased to a maximum and then decreased to baseline after 30 min of incubation. In the presence 1 mM ouabain the radical level was stable over the full incubation time and the maximum was higher in comparison with that from nanomolar concentration of ouabain.
Figure 1.
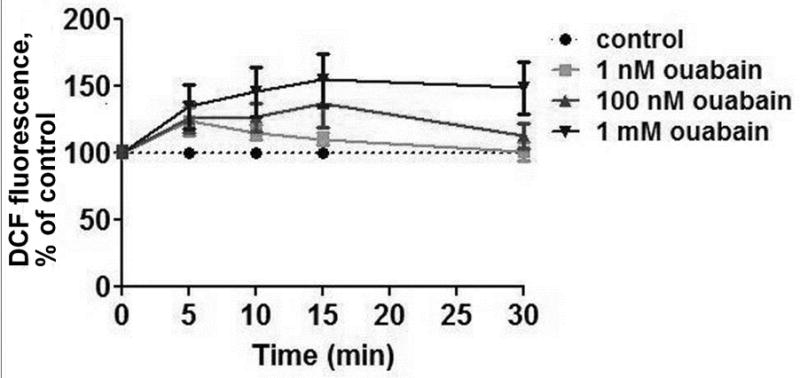
Effect of ouabain on DCF fluorescence levels in rat thymocytes. DCF-preloaded cells were incubated with different concentrations of ouabain (1 nM, 100 nM or 1 mM as noted) for 30 min at 37°C before measurement.
It was shown recently that accumulation of free radicals is accompanied with a rise in intracellular calcium and subsequent activation of NO-synthase in neurons and lymphocytes17,18. This is also the case for thymocytes (Fig. 2), since removal of calcium ions from the incubating medium prevents the accumulation of radicals. This was demonstrated in the case of both low (100 nM) and high (1 mM) concentrations of ouabain.
Figure 2.
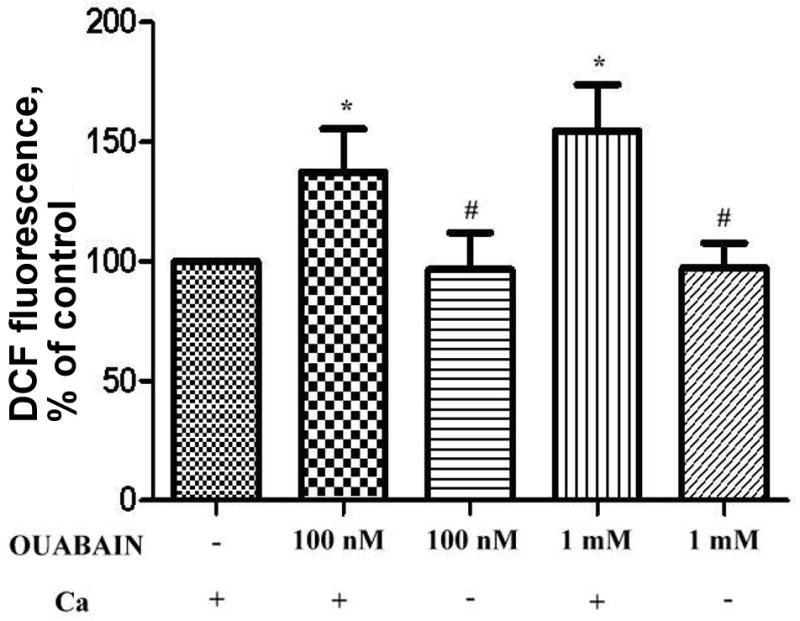
Effect of Ca-free media on DCF fluorescence level in ouabain-treated thymocytes. DCF-loaded cells were pre-incubated in calcium-free Tyrode’s solution containing 1mM EGTA for 30 min at 37°C and then treated with ouabain (15 min at 37°C). Sign * corresponds to significance vs control, # – to significance vs samples containing Ca, n = 3, p < 0,05.
In ouabain-treated thymocytes, NO-synthase significantly participated in the free radicals accumulation as has been previously shown for lymphocytes. In the presence of 30 μM N-nitroarginine (NNA), a NO-synthase inhibitor (10-times excess over the concentration giving total inhibition of the enzyme), the ouabain-mediated free radical signal disappeared while the steady-state radical level was not alternated by NNA (Fig. 3).
Figure 3.
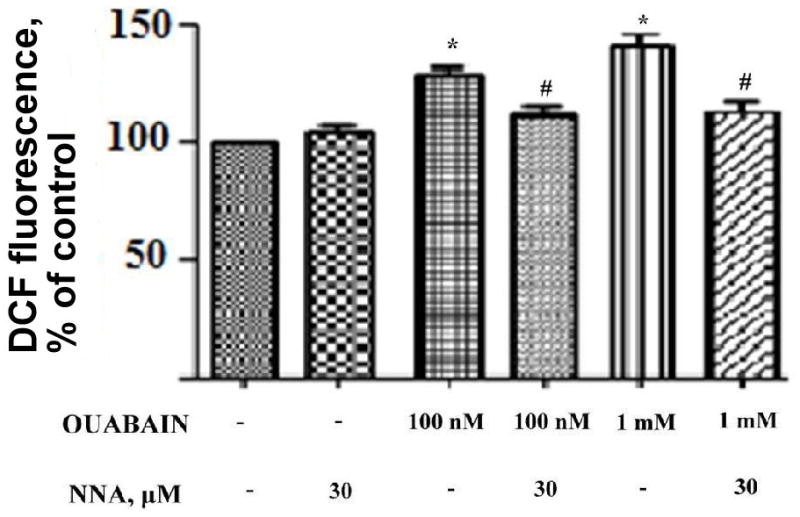
Effect of an inhibitor of NO-synthase on accumulation of free radicals in ouabain-treated thymocytes. DCF-loaded cells were pre-treated with N-nitroarginine (NNA, 30 μM) for 30 min at 37°C followed by incubation with ouabain (for 15 min at 37°C). Sign * corresponds to significance vs control, # – to significance vs samples containing no NNA, n = 3, p < 0,05.
The standard test for the presence of free radicals in cells is an ability of membrane permeable antioxidants to neutralize them. N-acetylcysteine19 and carnosine20 are widely used for this purpose. Both of these compounds are not found in the thymus; however carnosine periodically circulates in blood. After meat intake as food, carnosine levels in blood may reach 0.5–1 mM for a period of time. In specific animal tissues, such as skeletal muscles, heart and brain, carnosine works as a natural regulator of free radical levels, thus protecting excitable tissues from oxidative stress21.
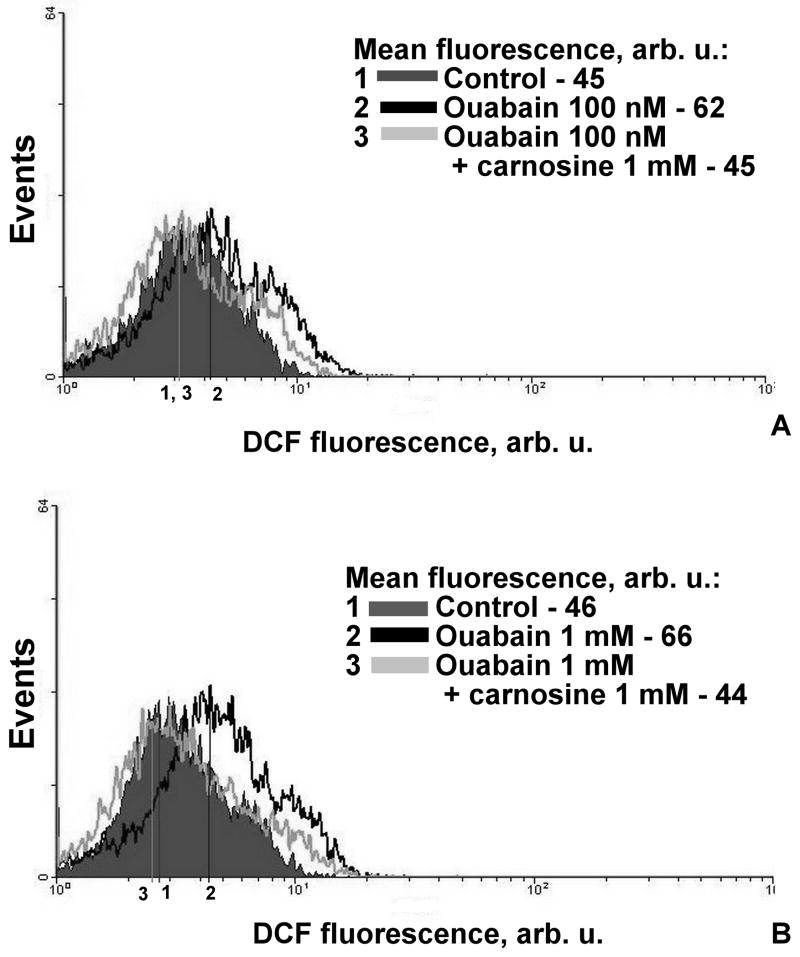
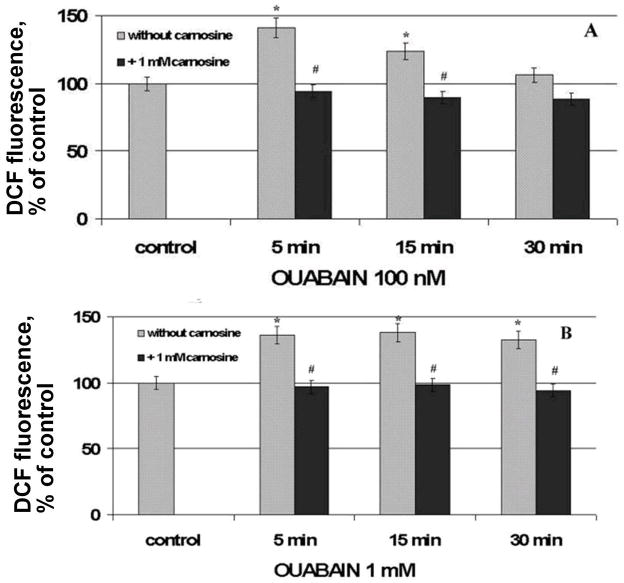
In our experiments we have incubated the cells with 1 mM of carnosine and then treated with different concentrations of ouabain. Ouabain shifts the cells to the area with high DCF fluorescence (low and high concentration) and carnosine arrests this shift (Fig.4). Carnosine prevents accumulation of intracellular radicals in the case of both ouabain concentrations. Fig. 5 presents the data as numerical values.
Figure 4.
Profile of distribution the thymocytes along the DCF fluorescence axis. DCF fluorescence value corresponds to free radicals levels in cells. Thymocytes were incubated with carnosine (1 mM for 40min) and then were treated with low (100 nM, A) or high (1 mM, B) concentrations of ouabain for 15 min at 37°C.
Figure 5.
Effect of carnosine on ouabain-mediated free radical accumulation. DCF-loaded cells were pretreated with 1 mM carnosine for 40 min and then were incubated with different concentrations of ouabain (100 nM and 1 mM) for 5, 15 and 30 min at 37°C. Sign * corresponds to significance vs control, # – to significance vs samples containing no carnosine, n = 3, p < 0,05.
Excessive accumulation of free radicals in cells is a signal leading to cell death14, and thymocytes are very sensitive to this signal. Therefore, we asked the question of whether thymocyte cell death occurs under these conditions. We used dexamethasone, well known inductor of thymocyte apoptotic death, as a positive control22. Incubation of thymocytes at both concentrations of ouabain causes cell death after 5 hours (Fig.6). We observed both apoptotic (Fig.6a) and necrotic cells (Fig.6b) but the proportion of necrotic cells was higher than apoptotic cells in the case of both ouabain concentrations. However the high ouabain concentration evoked a stronger effect than the low ouabain concentration. Dexamethasone increased the number of apoptotic, but not necrotic, cells. This corresponds to the known mechanism action of dexamethasone, inducing apoptosis via specific receptors with no effect on steady-state radical levels in thymocytes23.
Figure 6.
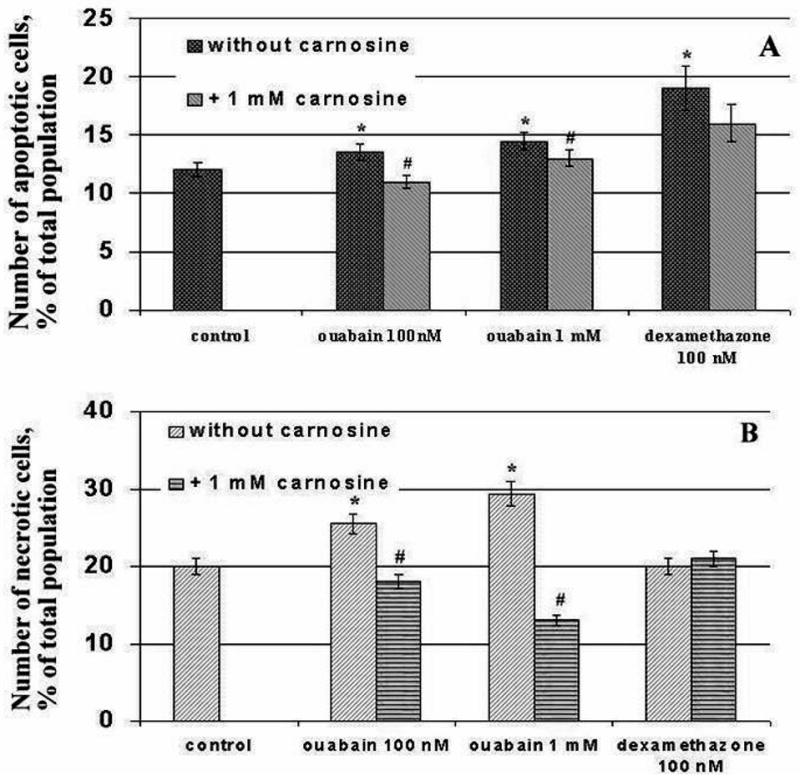
Effect of ouabain on the numbers of apoptotic (A) and necrotic (B) cells under different conditions. Cells were treated with 1 mM carnosine and then incubated with ouabain (100nM, 1mM) and 100 nM dexametasone during 5 h. Sign * corresponds to significance vs control, # – to significance vs samples containing no carnosine, n = 3, p < 0,05.
Carmosine decreased the number of apoptotic cells induced by ouabain while it did not affect the number of apoptotic cells induced by dexametasone. This is probably refllextive of the mechanism of dexamethasone action, which does not involve radicals in signal transduction. Carnosine also prevented the necrotic death induced by ouabain and the number of necrotic cell was lower than in control samples.
DISCUSSION
In the present study we have demonstrated for the first time for thymocytes that specific inhibition of high-sensitive and low-sensitive isoforms of Na/K-ATPase by ouabain induces extracellular calcium accumulation in the cells and activation of NO-synthase followed by accumulation of intracellular free radicals. The increasing level of free radicals contributes to both apoptosis and necrosis in thymocytes. Under these conditions, application of the natural, cell membrane penetrating antioxidant, carnosine, protects the thymocytes from both oxidative stress and either type of cell death.
This raises the question of whether each cell or only certain cells express the distinct isoforms of Na/K-ATPase. Based on the flow cytometry data the thymocytes suspension presents the homogeneous cell population and generates free radicals in response to low and high ouabain similarly (Fig.4). Therefore we can conclude that both high-sensitive and low-sensitive isofoms of Na/K-ATPase exist in each thymocyte.
The difference in response of thymocytes to low and high concentrations of ouabain is probably explained by the fact that different concentrations of ouabain modulate different isoforms of Na/K-ATPase. The nanomolar ouabain concentrations affect only (or mainly) the more ouabain-sensitive (but not the more ouabain-resistant) isoforms while millimolar ouabain concentrations inhibit both types of Na/K-ATPase isoforms. The level of free radicals is temporally increased after the selective action of ouabain on the high-sensitive isoforms of Na,K-ATPase. After 30 min of incubation with 1 nM and 100 nM ouabain it returns to base line. Inhibition of the more ouabain-sensitive isoforms of Na,K-ATPase activates cell signaling by causing a transient accumulation of free radicals that plays a role as a second messenger and its concentration rapidly falls after performing this function. On the other hand, the millimolar concentration of ouabain leads toa stable increase in free radical levels, that does not disappearing with time, and this results in the development of oxidative stress in cells.
We have shown that the NO-synthase inhibitor, NNA, prevented free radical accumulation, but NNA does not affect the basal level (Fig. 3). This means that the reaction of NO synthesis could, directly or indirectly, be the source of ouabain-induced free radical accumulation in thymocytes while the basal level of free radicals has another origin. At the same time, carnosine also did not affect the basal level of free radicals in thymocytes although carnosine has been shown to have direct anti-radical action in some models24. This may indicate that this pool of radicals is not accessible for the cellular signaling and performs other functions. Definitely such assumptions need additional research.
The level of necrotic cells in probes with 1 mM of ouabain was significantly higher than in probes with 100 nM of ouabain. The level of apoptotic cells in probes with 100 nM and 1 mM ouabain were approximately equal but not as large as the level of necrotic cells (Fig 6). A possible explanation for this phenomenon is that inhibition of ouabain-sensitive isoforms of Na/K-ATPase by nanomolar concentrations of ouabain initiates the process of intracellular signaling, the initial stage of which is an increase of “signal” free radicals, whereas the last stage is apoptotic cell death. On the other hand, the action of high ouabain concentration results in inhibition of ouabain-sensitive and ouabain-resistant isoforms of Na/K-ATPase simultaneously and this results in apoptosis due the more ouabain-sensitive isoforms up-regulation of the cell signaling cascade, while necrosis is due to the ouabain-resistant isoform activation of oxidative stress. These assumptions could be tested by studying the influence of ouabain on the expression of pro- and antiapoptotic genes that should be the result of the special analysis.
The effects of low concentrations of ouabain reviewed in this study may have a physiological significance, because ouabain is a hormone in the human organism released by the adrenal glands and hypothalamus25, and it circulates in blood by binding with globulin-like proteins26,27. The influence of ouabain on apoptosis in thymocytes is also interesting because thymocytes are the lymphoid type cells that maturate in the thymus. They undergo antigenic selection to autoantigenes and “spoilt” thymocytes die via apoptosis during the process of such selection. It is possible that the presence in blood of even low concentration of ouabain can influence this process, facilitating transition of thymocytes to apoptotic death.
In summary, this research shows that there are two pools of Na/K-ATPase with different sensitivity to CTS, and that these two pools participate in different reactions of cell signaling. These ouabain-sensitive isoforms are responsible for the reactions regulating apoptosis in thymocytes.
Acknowledgments
L.V.S. was supported by a stipend granted through the Fulbright Program. The work is supported by RFBR (Grant # 09-04-00507 and # 12-04-00593) and by the Institute for Health and the Environment at the University at Albany.
Footnotes
CONFLICT OF INTEREST
The authors have declared that there is no conflict of interest.
References
- 1.Skou J, Esmann M. The Na,K-ATPase. Bioenerg Biomembr. 1992;24(3):249–261. doi: 10.1007/BF00768846. [DOI] [PubMed] [Google Scholar]
- 2.Kometiani P, Li J, Gnudi L, Kahn BB, Askari A, Xie Z. Multiple signal transduction pathways link Na/K-ATPase to growth-related genes in cardiac myocytes. J Biol Chem. 1998;273:15249–15256. doi: 10.1074/jbc.273.24.15249. [DOI] [PubMed] [Google Scholar]
- 3.Bulygina ER, Lyapina LY, Boldyrev AA. Activation of glutamate receptors inhibits Na/K-ATPase of cerebellum granule cells. Biochemistry (Mosc) 2002;67(9):1001–1005. doi: 10.1023/a:1020569802119. [DOI] [PubMed] [Google Scholar]
- 4.Karitskaya I, Aksenov N, Vassilieva I, Zenin V, Marakhova I. Long-term regulation of Na,K-ATPase pump during T-cell proliferation. Pflugers Arch. 2010;460(4):777–789. doi: 10.1007/s00424-010-0843-z. [DOI] [PubMed] [Google Scholar]
- 5.Karpova L, Eva A, Kirch U, Boldyrev A, Scheiner-Bobis G. Sodium pump alpha1 and alpha3 subunit isoforms mediate distinct responses to ouabain and are both essential for survival of human neuroblastoma. FEBS J. 2010;277(8):1853–1860. doi: 10.1111/j.1742-4658.2010.07602.x. [DOI] [PubMed] [Google Scholar]
- 6.Boldyrev A, Bulygina E, Carpenter D, Schoner W. Glutamate receptors communicate with Na+/K+-ATPase in rat cerebellum granule cells: demonstration of differences in the action of several metabotropic and ionotropic glutamate agonists on intracellular reactive oxygen species and the sodium pump. J Mol Neurosci. 2003;21(3):213–222. doi: 10.1385/jmn:21:3:213. [DOI] [PubMed] [Google Scholar]
- 7.Schoner W, Scheiner-Bobis G. Endogenous and exogenous cardiac glycosides: their roles in hypertension, salt metabolism, and cell growth. Am J Physiol Cell Physiol. 2007;293(2):509–536. doi: 10.1152/ajpcell.00098.2007. [DOI] [PubMed] [Google Scholar]
- 8.Boldyrev AA, Arzumanyan ES, Kulebyakin KY, Berezov TT. Novel mechanisms of regulation of brain plasticity. Neurochemical J. 2011;28(4):340–344. [Google Scholar]
- 9.Karpova LV, Bulygina ER, Boldyrev AA. Different neuronal Na/K-ATPase isoforms are involved in diverse signaling pathways. J Cell Biochem Funct. 2010;278:135–141. doi: 10.1002/cbf.1632. [DOI] [PubMed] [Google Scholar]
- 10.Rodrigues-Mascarenhas S, Da Silva de Oliveira A, Dias Amoedo N, Affonso-Mitidieri OR, Rumjanek FD, Rumjanek VM. Modulation of the immune system by ouabain. Ann NY Acad Sci. 2009;1153:153–163. doi: 10.1111/j.1749-6632.2008.03969.x. [DOI] [PubMed] [Google Scholar]
- 11.Xie Z, Kometiani P, Liu J, Li J, Shapiro JI, Askari A. Intracellular reactive oxygen species mediate the linkage of Na/K-ATPase to hypertrophy and its marker genes in cardiac myocytes. J Biol Chem. 1999;274:19323–19328. doi: 10.1074/jbc.274.27.19323. [DOI] [PubMed] [Google Scholar]
- 12.Boldyrev AA, Bulygina ER. Na/K-ATPase and oxidative stress. Ann N Y Acad Sci. 1997;834:666–668. doi: 10.1111/j.1749-6632.1997.tb52345.x. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Haas M, Liang M, Cai T, Tian J, Li S, Xie Z. Ouabain assembles signaling cascades through the caveolar Na/K-ATPase. J Biochem. 2004;279(17):17250–17259. doi: 10.1074/jbc.M313239200. [DOI] [PubMed] [Google Scholar]
- 14.Riemann A, Schneider B, Ihling A, Nowak M, Sauvant C, Thews O, Gekle M. Acidic environment leads to ROS-induced MAPK signaling in cancer cells. PLoS One. 2011;6(7):e22445. doi: 10.1371/journal.pone.0022445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oyama Y, Yamazaki Y, Okada Y, Takahama K, Satoh M, Hayashi H. Toxicity of methylmercury conjugated with L-cysteine on rat thymocytes and human leukemia K562 cells in comparison with that of methylmercury chloride. Environ Toxicol Pharmacol. 2000;9(1–2):49–55. doi: 10.1016/s1382-6689(00)00062-4. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Tian J, Haas M, Shapiro JI, Askari A, Xie Z. Ouabain interaction with cardiac Na+/K+-ATPase initiates signal cascades independent of changes in intracellular Na and Ca concentrations. J Biol Chem. 2000;275:27838–27844. doi: 10.1074/jbc.M002950200. [DOI] [PubMed] [Google Scholar]
- 17.Makhro A, Bulygina E, Boldyrev A. Effects of homocysteine and homocysteic acid on cerebellum granule cells. Neurochemical J. 2006;1:127–132. [Google Scholar]
- 18.Vladychenskaya E, Tyulina O, Urano S, Boldyrev A. Rat lymphocytes express NMDA receptors that take part in regulation of cytokine production. J Cell Biochem Funct. 2011;29(7):527–533. doi: 10.1002/cbf.1771. [DOI] [PubMed] [Google Scholar]
- 19.Cheng J, Wang F, Yu DF, Wu PF, Chen JG. The cytotoxic mechanism of malondialdehyde and protective effect of carnosine via protein cross-linking/mitochondrial dysfunction/reactive oxygen species/MAPK pathway in neurons. Eur J Pharmacol. 2011;650(1):184–194. doi: 10.1016/j.ejphar.2010.09.033. [DOI] [PubMed] [Google Scholar]
- 20.Boldyrev A. Carnosine – the novel function of well known molecule. Biochemistry (Mosc) 2012;77:405–420. doi: 10.1134/S0006297912040013. [DOI] [PubMed] [Google Scholar]
- 21.Kulebyakin K, Karpova L, Lakonsteva E, Krasavin M, Boldyrev A. Carnosine protects neurons against oxydative stress and modulates the time profile of MAPK cascade signaling. Amino Acids. 2011 Nov 19; doi: 10.1007/s00726-011-1135-4. [DOI] [PubMed] [Google Scholar]
- 22.Wang S, Martin SM, Harris PS, Knudson CM. Caspase inhibition blocks cell death and enhances mitophagy but fails to promote T-cell lymphoma. PLoS One. 2011;6(5):e19786. doi: 10.1371/journal.pone.0019786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt S, Rainer J, Ploner E, Presul E, Riml S, Kofler R. Glucocorticoid-induced apoptosis and glucocorticoid resistance: molecular mechanisms and clinical relevance. Cell Death and Differentiation. 2004;11:S45–S55. doi: 10.1038/sj.cdd.4401456. [DOI] [PubMed] [Google Scholar]
- 24.Boldyrev AA, Kurella EG, Rubtsov AM, Tiulina OV, Shara M, Shentiurts M. Direct measurement of the interaction of carnosine and its analogs with free radicals. Biochemistry (Mosc) 1992;57:1360–1365. [PubMed] [Google Scholar]
- 25.Schoner W. Endogenous cardiac glycosides, a new class of steroid hormones. Eur J Biochem. 2002;269:2440–2448. doi: 10.1046/j.1432-1033.2002.02911.x. [DOI] [PubMed] [Google Scholar]
- 26.Mathews WR, Du Charme DW, Hamlyn JM, Harris DW, Mandel F, Clark MA, Ludens JA. Mass spectral characterization of an endogenous digitalis-like factor from human plasma. Hypertension. 1991;17:930–935. doi: 10.1161/01.hyp.17.6.930. [DOI] [PubMed] [Google Scholar]
- 27.Antolovic R, Kost H, Mohadjerani M, Linder D, Linder M, Schoner W. A specific binding protein for cardiac glycosides exists in bovine serum. J Biol Chem. 1998;273:16259–16264. doi: 10.1074/jbc.273.26.16259. [DOI] [PubMed] [Google Scholar]