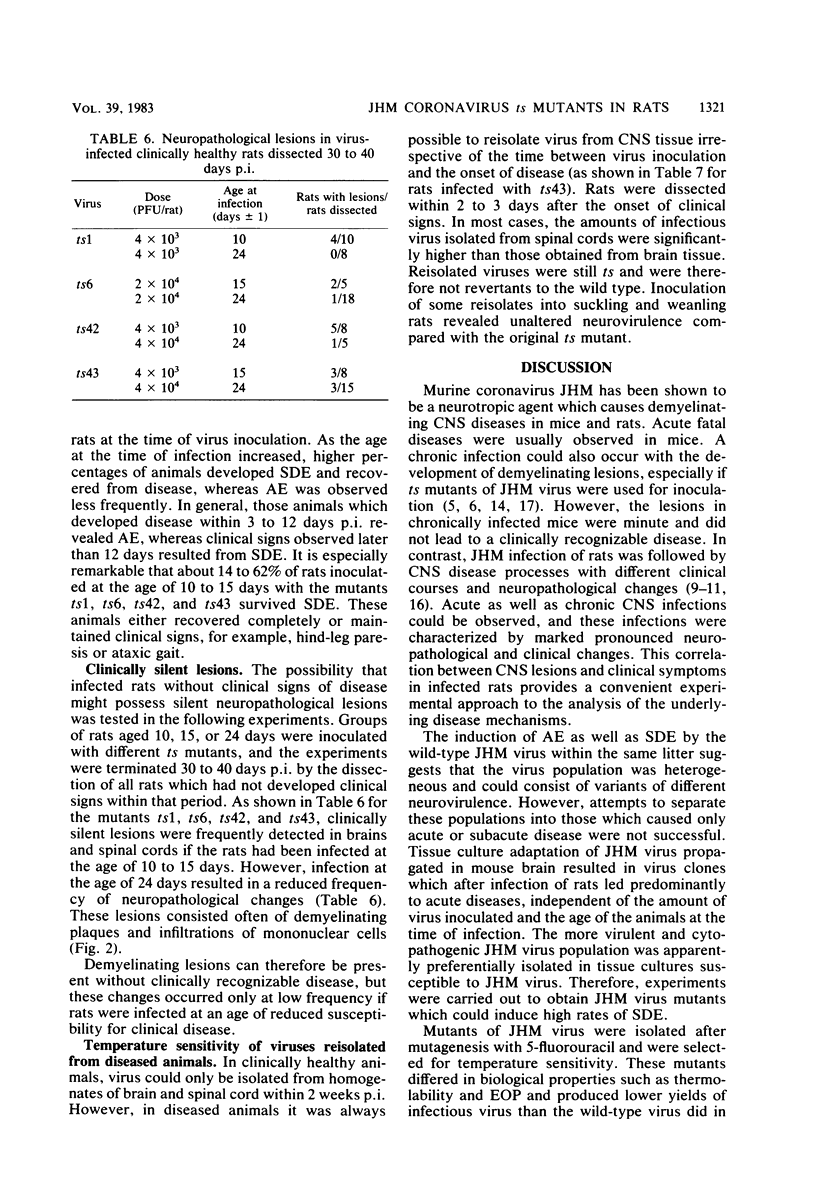
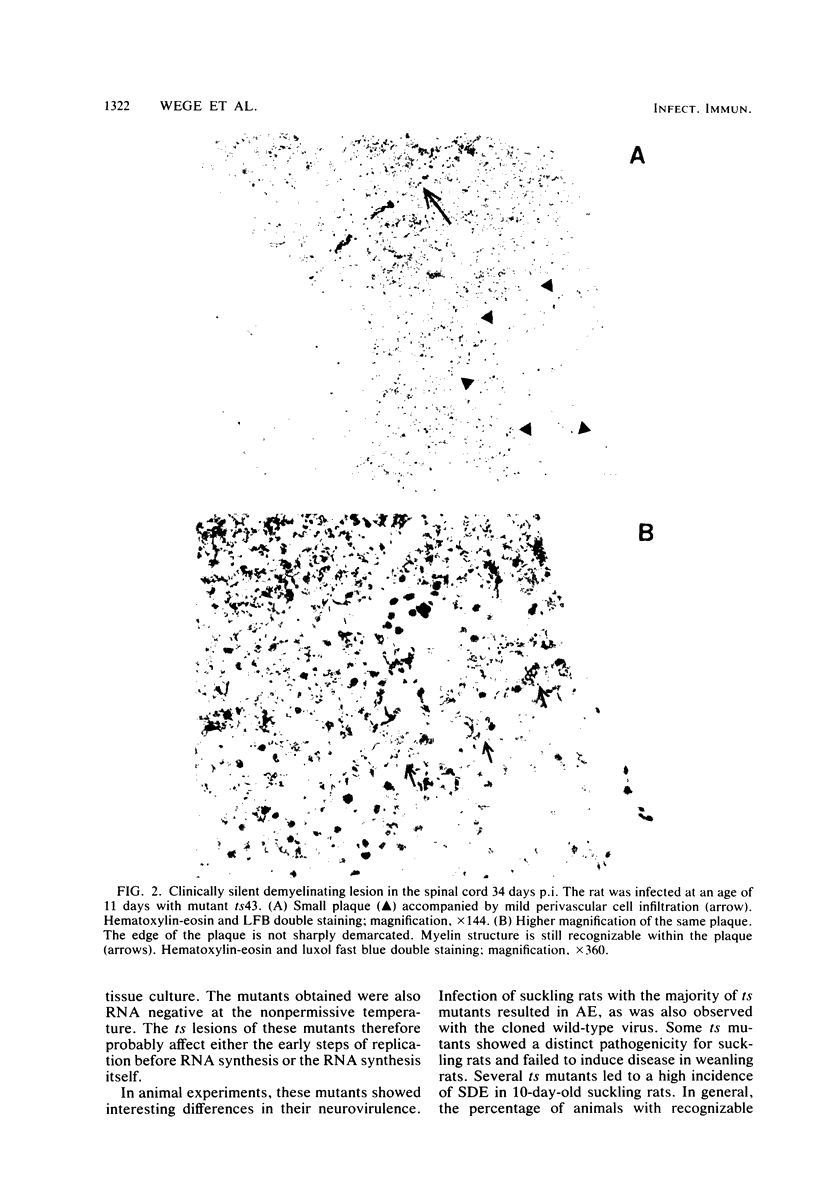
Abstract
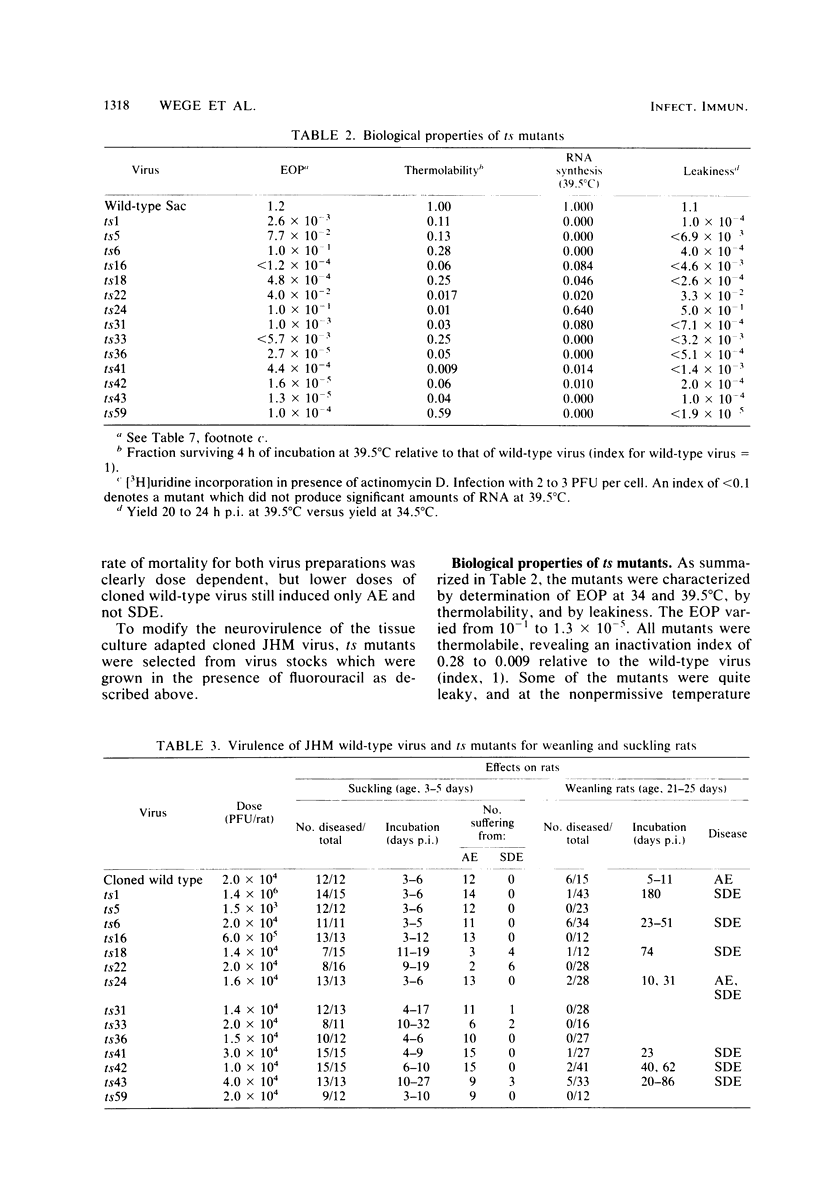
The murine coronavirus strain JHM is highly neurotropic in rats and has a marked tendency to cause demyelinating central nervous system diseases after intracerebral inoculation. The clinical diseases observed range from an acute encephalomyelitis occurring within 2 weeks postinfection to a subacute demyelinating encephalomyelitis developing several weeks or months postinfection. Uncloned wild-type virus induced both acute and subacute diseases, whereas cloned JHM virus grown in tissue culture caused only acute disease without the pronounced lesions of primary demyelination. In contrast, temperature-sensitive mutants selected from that clone were capable of inducing subacute demyelinating encephalomyelitis after prolonged incubation times. Viruses recovered from diseased animals were still temperature sensitive. Inoculation of temperature-sensitive mutants into suckling rats (age, 10 to 15 days) produced high rates of subacute demyelinating diseases running a more chronic course; these diseases often were not fatal. Those rats which did not show clinical signs frequently revealed inflammatory demyelinating lesions. These findings indicate that the rate and type of clinical disease are dependent on the neurovirulence of the virus mutant used for inoculation and the age of the animals at the time of infection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett P. N., Atkins G. J. Virulence of temperature-sensitive mutants of Sindbis virus in neonatal mice. Infect Immun. 1979 Dec;26(3):848–852. doi: 10.1128/iai.26.3.848-852.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois-Dalcq M. E., Doller E. W., Haspel M. V., Holmes K. V. Cell tropism and expression of mouse hepatitis viruses (MHV) in mouse spinal cord cultures. Virology. 1982 Jun;119(2):317–331. doi: 10.1016/0042-6822(82)90092-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin D. E., Mullinix J., Narayan O., Johnson R. T. Age dependence of viral expression: comparative pathogenesis of two rodent-adapted strains of measles virus in mice. Infect Immun. 1974 Apr;9(4):690–695. doi: 10.1128/iai.9.4.690-695.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haspel M. V., Duff R., Rapp F. Isolation and preliminary characterization of temperature-sensitive mutants of measles virus. J Virol. 1975 Oct;16(4):1000–1009. doi: 10.1128/jvi.16.4.1000-1009.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haspel M. V., Lampert P. W., Oldstone M. B. Temperature-sensitive mutants of mouse hepatitis virus produce a high incidence of demyelination. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4033–4036. doi: 10.1073/pnas.75.8.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobler R. L., Dubois-Dalcq M., Haspel M. V., Claysmith A. P., Lampert P. W., Oldstone M. B. Selective localization of wild type and mutant mouse hepatitis virus (JHM strain) antigens in CNS tissue by fluorescence, light and electron microscopy. J Neuroimmunol. 1981 Mar;1(1):81–92. doi: 10.1016/0165-5728(81)90010-2. [DOI] [PubMed] [Google Scholar]
- Lampert P. W. Autoimmune and virus-induced demyelinating diseases. A review. Am J Pathol. 1978 Apr;91(1):176–208. [PMC free article] [PubMed] [Google Scholar]
- Mogensen S. C. Role of macrophages in natural resistance to virus infections. Microbiol Rev. 1979 Mar;43(1):1–26. doi: 10.1128/mr.43.1.1-26.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima K., Wege H., Meyermann R., ter Meulen V. Demyelinating encephalomyelitis induced by a long-term corona virus infection in rats. A preliminary report. Acta Neuropathol. 1979 Mar 15;45(3):205–213. doi: 10.1007/BF00702672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima K., Wege H., ter Meulen V. Early and late CNS-effects of corona virus infection in rats. Adv Exp Med Biol. 1978;100:395–409. doi: 10.1007/978-1-4684-2514-7_28. [DOI] [PubMed] [Google Scholar]
- Neighbour P. A., Rager-Zisman B., Bloom B. R. Susceptibility of mice to acute and persistent measles infection. Infect Immun. 1978 Sep;21(3):764–770. doi: 10.1128/iai.21.3.764-770.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickel K., Müller M. A., ter Meulen V. Analysis of age-dependent resistance to murine coronavirus JHM infection in mice. Infect Immun. 1981 Dec;34(3):648–654. doi: 10.1128/iai.34.3.648-654.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb J. A., Bond C. W., Leibowitz J. L. Pathogenic murine coronaviruses. III. Biological and biochemical characterization of temperature-sensitive mutants of JHMV. Virology. 1979 Apr 30;94(2):385–399. doi: 10.1016/0042-6822(79)90469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb J. A., Martin R. G. Genetic analysis of simian virus 40. I. Description of microtitration and replica-plating techniques for virus. Virology. 1970 Aug;41(4):751–760. doi: 10.1016/0042-6822(70)90439-3. [DOI] [PubMed] [Google Scholar]
- Sorensen O., Perry D., Dales S. In vivo and in vitro models of demyelinating diseases. III. JHM virus infection of rats. Arch Neurol. 1980 Aug;37(8):478–484. doi: 10.1001/archneur.1980.00500570026003. [DOI] [PubMed] [Google Scholar]
- Stohlman S. A., Weiner L. P. Chronic central nervous system demyelination in mice after JHM virus infection. Neurology. 1981 Jan;31(1):38–44. doi: 10.1212/wnl.31.1.38. [DOI] [PubMed] [Google Scholar]
- Wege H., Müller A., ter Meulen V. Genomic RNA of the murine coronavirus JHM. J Gen Virol. 1978 Nov;41(2):217–227. doi: 10.1099/0022-1317-41-2-217. [DOI] [PubMed] [Google Scholar]
- Weiner L. P., Stohlman S. A. Viral models of demyelination. Neurology. 1978 Sep;28(9 Pt 2):111–114. doi: 10.1212/wnl.28.9_part_2.111. [DOI] [PubMed] [Google Scholar]
- Wiśniewski H. M. Immunopathology of demyelination in autoimmune diseases and virus infections. Br Med Bull. 1977 Jan;33(1):54–59. doi: 10.1093/oxfordjournals.bmb.a071397. [DOI] [PubMed] [Google Scholar]
- ter Meulen V., Hall W. W. Slow virus infections of the nervous system: virological, immunological and pathogenetic considerations. J Gen Virol. 1978 Oct;41(1):1–25. doi: 10.1099/0022-1317-41-1-1. [DOI] [PubMed] [Google Scholar]