Abstract
Background
Vascular calcification is highly prevalent in patients with type II diabetes mellitus (T2DM). Little is known about whether T2DM is causative.
Methods
Low density lipoprotein receptor mutant (LDLr−/−) mice were fed with customized diabetogenic and/or procalcific diets to induce atherosclerosis, cartilaginous metaplasia and calcification, along with obesity, hyperglycemia, hyperinsulinemia, and hypercholesterolemia at various levels, and euthanized for study after 18–24 weeks on diet.
Results
We found that T2DM accelerated cartilaginous and calcific lesion development by ~3- and 13-folds as determined by incidence of vascular cartilaginous metaplasia and calcification in LDLr−/− mice. Lowering dietary fat from ~60% to ~40% kcal reduced body weight and serum glucose and insulin levels, leading to a 2-fold decrease in aortic calcium content. Correlation analysis of calcium content with a calculated insulin resistance index, HOMA-IR, showed a positive correlation of insulin resistance with vascular calcification. Finally, we used genetic fate mapping strategy to trace cells of SM origin in these animals. Vascular SMCs were found to be a major cell source contributing to osteochondrogenic differentiation and calcification. Receptor for advanced glycation end-products (RAGE) was up-regulated, co-localizing with osteochondrogenic SMCs.
Conclusions
Through quantitative measure of aortic calcium content, we provided experimental findings that LDLr−/− mice, like T2DM patients, are predisposed to vascular calcification. Our study is also the first to establish a distinct role of hyperglycemia and hypercholesterolemia in osteochondrogenic differentiation of SMCs and determined these cells as a major source contributing to cartilaginous and calcifying lesions of T2DM blood vessels, possibly mediated by RAGE.
Keywords: atherosclerosis, receptor for glycation end-products, smooth muscle cells, type 2 diabetes mellitus, vascular calcification
I. Introduction
Diabetes mellitus affects an estimated 25.8 million people and is the 7th leading cause of death in the United States. Type 2 diabetes mellitus (T2DM) is the most common form of this disease, accounting for 90–95% of cases.1 Blood vessels of T2DM patients showed increased connective tissue and calcium-phosphate salt deposition in atherosclerotic intimae and medias, namely vascular calcification,2–6 featured with cartilaginous metaplasia.2, 3 In a study of 1,059 patients with T2DM, vascular calcification, especially in arterial media, was found to be a strong independent predictor of total cardiovascular and coronary heart disease mortality, as well as a significant predictor of future coronary heart disease events, stroke, and lower-limb amputation.7 Compared to diabetics without vascular calcification, diabetics with vascular calcification had a 1.5-fold increase in mortality, a 1.6-fold increase in coronary artery disease, a 2.4-fold increase in proteinuria, a 1.7-fold increase in retinopathy, and a 5.5-fold increase in amputation.4
Although long been considered a degenerative process involving passive accumulation of calcium-phosphate salts associated with tissue necrosis, a growing number of studies have highlighted vascular calcification as an active, cell-mediated process resembling embryonic bone formation and remodeling.3, 8, 9 Supporting evidence in calcifying T2DM blood vessels includes the appearance of molecules that initiate and regulate osteochondrogenic differentiation, such as BMP2, Msx2, Runx2, osterix, and Sox9.8–12 In later stages, cells expressing bone and/or cartilage-restricted proteins, such as alkaline phosphatase, bone sialoprotein, bone Gla protein, type II collagen, and osteopontin were often co-localized with calcium-phosphate minerals within the T2DM vessel wall.3, 9–11 Interestingly, in a lineage tracing study using SM22α-Cre recombinase and Rosa26-LacZ reporter alleles, vascular smooth muscle cells (SMCs) were found to reprogram their lineage into osteochondrogenic precursor- and chondrocyte-like cells in calcifying arterial medias of MGP−/− mice, identifying a crucial role of these cells in mediating vascular calcification.13
Despite the vast number of complications and public health burden associated with vascular calcification in diabetic patients, little is known about the specific role and mechanisms of T2DM in osteochondrogenic differentiation and calcification of blood vessels. In addition to amorphous calcium-phosphate salt deposition, calcification of human T2DM blood vessels is often associated with widespread cartilaginous matrices, chondrocyte-like cells, and even endochondral bone elements.2, 3 While much of the effort in developing T2DM mouse models has focused on selective breeding of certain insulin resistant strains and gene targeting of insulin receptor and its cellular targets,14–16 no animal model thus far has been able to reproduce the pathology of human T2DM blood vessels.2, 3 Low density lipoprotein receptor mutant (LDLr−/−) mice, when fed with a “Western” diet, was shown to develop not only atherosclerosis but also calcium-phosphate salt deposition in coronary arteries and valves, confined primarily to the adventitia and perivascular connective tissue.12 Although there were no cartilaginous or bony elements found in the calcified arteries, these mice had increased serum glucose and insulin levels.12 In the present paper, we attempted to develop vascular calcification mouse models with various degrees of insulin resistance, hyperglycemia, and hypercholesterolemia. These mice were used to study whether these factors play a role in the initiation and/or progression of cartilaginous metaplasia and calcification seen in T2DM blood vessels.
2. Materials and methods
2.1. Mouse models and genetic fate mapping
LDLr−/− mice were purchased from the Jackson Laboratory (002207). The mice were bred onto the SM22α-Cre recombinase (SM22α-Cre, gift from Dr. Herz, UT) and Rosa26 Cre reporter (R26R-LacZ, gift from Dr. Soriano, FHCRC) transgenic background to produce SM22α-Cre+/0:R26R-LacZ+/0:LDLr−/− mice. As characterized in our previous study,13 SM22α-Cre and R26R-LacZ transgenic alleles allow Cre recombination to occur in SM22α-positive cells early in embryogenesis and onward, resulting in a permanent mark of these cells with active β-galactosidase. Therefore, SMCs that have lost their marker proteins in calcifying lesions are identifiable by X-gal staining, which converts any intracellular β-galactosidase activity into a blue precipitate.
At 10–12 weeks old (≥20g), baseline body weight and fasted blood glucose levels of SM22α-Cre+/0:R26R-LacZ+/0:LDLr−/− mice and LDLr−/− mice were recorded. SM22α-Cre+/0:R26R-LacZ+/0:LDLr−/− mice were randomly assigned into three groups, fed with a customized diabetogenic/procalcific diet (Table 1, HFCD1; Bio-Serv), a procalcific “Western” diet that does not induce T2DM (D12108, Research Diets, Inc.),17 or normal chow (NC). LDLr−/− mice were randomly assigned into four groups, fed with two customized diabetogenic/procalcific diets (Table 1, HFCD2-3; Bio-Serv), a known diabetogenic diet containing no additional cholesterol (F1850, Bio-Serv)18, or NC. Progression of diabetes was monitored by body weight every two weeks and fasted blood glucose every four weeks under diets. At 18–24 weeks, SM22α-Cre+/0:R26R+/0:LDLr−/− and LDLr−/− mice were sacrificed with 50–180 mg/kg pentobarbital intraperitoneally followed by exsanguination via cardiac puncture for blood collection. Aortic arches of all SM22α-Cre+/0:R26R+/0:LDLr−/− mice and 2–4 LDLr−/− mice per group were collected for histological analysis, starting from the brachiocephalic branch to 1 mm passed the left subclavian branch. All other LDLr−/− mice (10–13 per group) were used to quantify calcium content of aortic arches. A total of 48 SM22α-Cre+/0:R26R+/0:LDLr−/− mice and 60 LDLr−/− mice were examined. All animals were maintained in a specific pathogen-free environment and genotypes were determined as described.13, 19 All protocols are in compliance with the NIH Guideline for the Care and Use of Laboratory Animals and have been approved by the Institutional Animal Care and Use Committee, University of Washington.
Table 1.
Composition of Specific Procalcific and/or Diabetogenic Diets
| Diets
|
||||||
|---|---|---|---|---|---|---|
| NC | D12108 | HFCD1 | HFCD2 | HFCD3 | F1850 | |
| Protein (% kcal) | 23.6 | 20 | 15.1 | 15.2 | 17.4 | 14.7 |
| Fat (% kcal) | 11.9 | 39.9 | 57.5 | 57.9 | 40.1 | 58.7 |
| Carbohydrate (%kcal) | 64.5 | 40 | 27.4 | 26.9 | 42.5 | 26.7 |
| Cholesterol (%) | 149 ppm | 1.25 | 1.25 | 0.20 | 0.20 | 0.03 |
| Fat source | Soybean oil | Cocoa butter Soybean oil |
Lard | Lard | Lard | Lard |
| Fatty Acid Profile | ||||||
| Saturated FA (%) | 17.8 | 55.6 | 39.2 | 39.2 | 39.2 | 39.7 |
| Monounsaturated FA (%) | 22.1 | 33 | 45.1 | 45.1 | 45.1 | 49.8 |
| Polyunsaturated FA (%) | 60.1 | 11.5 | 11.2 | 11.2 | 11.2 | 10.4 |
| Features | ||||||
| Obesity | No | No | Yes | Yes | Yes | Yes |
| Hyperglycemia | No | No | Yes | Yes | Yes | Yes |
| Vascular cartilaginous metaplasia | No | Yes | Yes | Yes | Yes | Rare |
| Vascular calcification | No | Yes | Yes | Yes | Yes | Rare |
2.2. X-gal staining
Tissue dissected from SM22α-Cre+/0:R26R-LacZ+/0:LDLr−/− mice were stained using a β-galactosidase stain kit (Special Media) as described previously.13
2.3. Histochemical and immunohistochemical staining
X-gal-stained SM22α-Cre+/0:R26R-LacZ+/0:LDLr−/− tissues were post-fixed with Methyl Carnoy’s fixative prior to processing and embedding in paraffin. Aortic arches collected from LDLr−/− mice were fixed directly with Methyl Carnoy’s fixative and embedded in paraffin. Five-micrometer sections were collected from the brachiocephalic artery end for ~400 μm for histochemical and immunohistochemical analyses. Movat Pentachrome stain was used to visualize atherosclerosis and cartilaginous metaplasia. Calcium-phosphate minerals were detected by von Kossa staining. Antibodies recognizing SM22α (ab10135, Abcam), SMMHC (ab683, Abcam), SM α-actin (A2547, Sigma), Runx2/Cbfa1 (MAB2006, R&D systems), Sox-9 (sc-20095, Santa Cruz), RAGE (ab3611, Abcam), aggrecan (AB1031, Millipore), type II collagen (AB761, Millipore), and type X collagen (LSL-LB-0092, Cosmo Bio Co., LTD.) were used to detect SMCs, osteochondrogenic cells, chondrocytes, receptor for advanced glycation-end products (RAGE), and cartilaginous matrices. Selected sections were counterstained with methyl green or nuclear fast red (H-3403, Vector) as indicated in Figure legends.
2.4. Blood and Sera Analysis
Mice were fasted for 4 hours prior to blood collection into serum separator tubes. Sera were analyzed for cholesterol and triglyceride levels by Laboratory Medicine at the University of Washington via spectrophotometry. Serum insulin levels were measured by ELISA (90080, Crystal Chem, Inc). Index of insulin resistance was calculated using the homeostasis model assessment of insulin resistance (HOMA-IR), given by the formula, fasting blood glucose (mmol/L) × insulin concentration (mIU/L) / 22.5. HOMA-IR is a mathematical model developed by Matthews et al20 and shown to be a reliable, rapid, and cost-effective alternative to the standard glucose clamp method for measuring insulin resistance.21
2.5. Quantification of aortic arch calcium content
Around 6 mm of the aortic arch from each LDLr−/− mouse was collected and lyophilized to a constant weight. Calcium was extracted from the lyophilized tissue with 0.6 N HCl and determined colorimetrically using the o-Cresolphthalein complexone kit (Teco Diagnostics, C503-480).13 Values were normalized to dry weight of the arches.
2.6. Statistics
Data, shown as means ± SD, were analyzed with Student’s t-test or ANOVA to determine the significance of differences. Data were considered to be statistically significant at a p value < 0.05.
3. Results
3.1. Development of T2DM mouse models for vascular calcification study
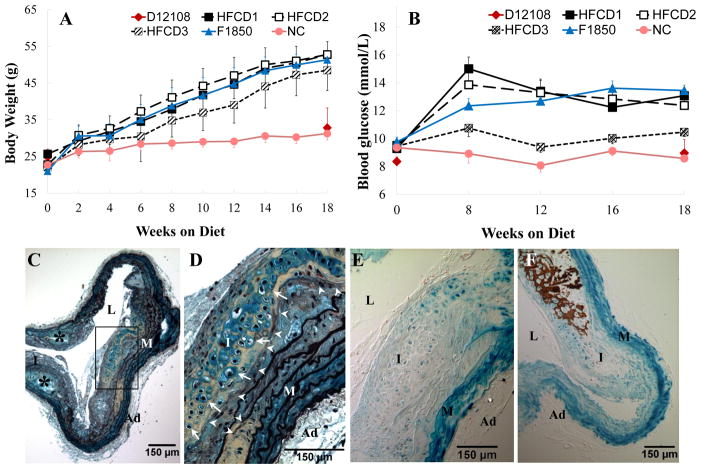
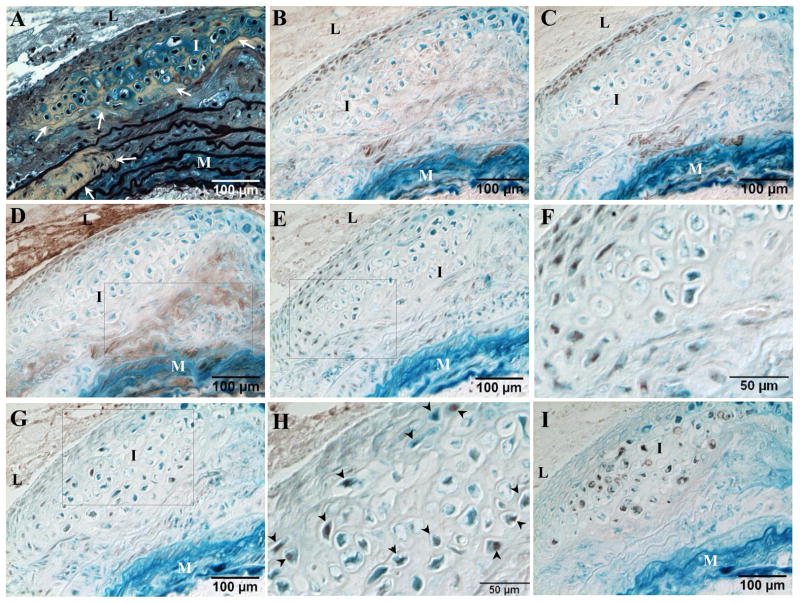
To develop mouse models that may be used to study the role of T2DM and hypercholesterolemia in vascular calcification, we customized three diets (Table 1, HFCD1-3) by changing dietary fat and cholesterol levels of a known diabetogenic diet (F1850)18 to induce cartilaginous metaplasia and calcification in blood vessels of LDLr−/− mice, with varying levels of hyperglycemia and hypercholesterolemia. A known non-diabetogenic diet (D12108) that induces vascular cartilaginous metaplasia and calcification of LDLr−/− mice17 and normal chow (NC) were used as dietary controls. As shown in Table 1, mice fed with diets consisting of ~40–60% kcal fat with similar levels of saturated and monosaturated fatty acids (HFCD1-3 and F1850) developed T2DM as determined by the increased body weight (Figure 1A) and serum glucose levels (Figure 1B). As expected, lowering dietary fat content reduced body weight and blood glucose levels (HFCD3 vs HFCD1-2 and F1850). Cartilaginous metaplasia, visualized by Movat pentachrome staining, is characterized as a collagen- (yellow) and proteoglycan- (blue) rich matrix containing large, round cells that have relatively large area of clear cytoplasm surrounded by a lacunar rims (Figures 1C and 1D, white arrows), replicated features of human T2DM cardiovasculature.2, 3 Cartilaginous metaplasia primarily occurred in arteries of mice fed with cholesterol-rich diets, including both diabetogenic (HFCD1-3) and non-diabetogenic (D12108), starting in the deep intima and inner medial layers of blood vessels in the absence of calcium-phosphate salt deposition (Figure 1E, lack of dark brown vs brown in Figure 1F), with chondrocyte-like cells adjacent to areas of elastic lamina breakage (arrowheads) outside of lipid-laden areas (asterisks).
Figure 1. Characterization of LDLr−/− mice fed with various customized diets.
LDLr−/− mice were challenged with diabetogenic, procalcific diets (HFCD1-3), non-diabetogenic, procalcific diet (D12108), or diabetogenic diet (F1850) for 18 weeks. Normal chow (NC) was used as dietary control. T2DM development was monitored by body weight (A) and fasted blood glucose levels (B). C and D. Movat pentachrome staining of an aortic arch from an LDLr−/− mouse fed with HFCD1 for 18 weeks showing cartilaginous metaplasia (yellow collagen staining), chondrocyte-like cells (white arrows), and elastic lamina breakages (white arrow heads). E and F. Von Kossa staining to visualize calcification. Cell nuclei were counterstained with nuclear fast red. Note the absence of calcification in an adjacent section of D (E) and the occurrence of calcification in an advanced lesion (F, brown). D and E, boxed area of C. M=media, L=lumen, I=intima, Ad=adventitia.
3.2. T2DM accelerates the development of cartilaginous metaplasia and calcification in LDLr−/− mouse vessels
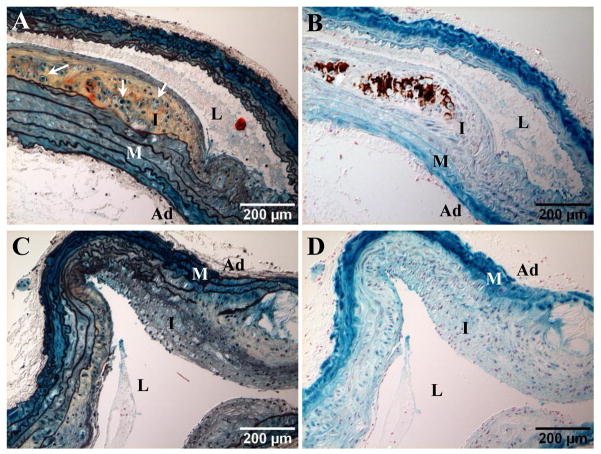
To understand whether T2DM plays a role in the development of cartilaginous metaplasia and calcification of blood vessels, we analyzed aortic arches of LDLr−/− mice fed with diabetogenic (HFCD1) and non-diabetogenic (D12108) diets consisting of identical dietary cholesterol (1.25%). As shown in Table 2, mice fed with HFCD1 and D12108 diets developed similar levels of hypercholesterolemia (43.4±2.1 vs 37.3±1.8 mmol/L; p=0.1049). While D12108 did not induce diabetes, indicated by similar body weights, fasted blood glucose levels, and calculated HOMA-IR (index of insulin resistance) as that of NC controls, mice fed with HFCD1 developed T2DM. These mice were ~1.6 times heavier than D12108 and NC groups, with elevated blood glucose (~1.5 times higher) and insulin resistance (>7 times higher in HOMA-IR). Importantly, vascular cartilaginous metaplasia and calcification were more apparent in mice that had developed diabetes when compared with their non-diabetic counterparts that were challenged with a diet consisting of same amount of cholesterol and which developed similar levels of hypercholesterolemia (Table 2). Of 22 HFCD1 mice examined, all of them developed cartilaginous metaplasia (100%), 14 of which had calcium-phosphate deposition (64%) in aortic arches by 18 weeks diet fed. Only 33% of non-diabetic D12108 mice developed vascular cartilaginous metaplasia (6 out of 18), with 5% of the aortic arches calcified (1 out of 18) by 18 weeks, suggesting that T2DM triggered an accelerated progression of vascular calcification. In fact, diabetic HFCD1 mice had larger cartilaginous lesions (Figure 1C) and were at more advanced stages of lesion development, assessed by the occurrence of cells with similar morphology to hypertrophic chondrocytes seen in endochondral ossification (Figures 1D and Figure 2A, arrows) and the concurrence of calcium-phosphate deposition (Figure 2B, brown).
Table 2.
Effect of diabetogenic and procalcific diet on serum parameters and incidence of cartilaginous metaplasia and calcification in aortic arches of T2DM and non-T2DM LDLr−/− mice
| Diets
|
|||
|---|---|---|---|
| NC | D12108 | HFCD1 | |
| Serum total cholesterol (mmol/L) | 6.2±0.4 | 37.3±1.8§ | 43.4±2.1§ |
| Body weight (g) | 31.2±1.8 | 32.8±5.4 | 52.7±1.3§ |
| Fasted blood glucose (mmol/L) | 8.47±0.4 | 8.96±0.9 | 13.08±0.3§ |
| HOMA-IR | 11.9±1.3 | 10.7±1.3 | 77.6±8§ |
| Cartilaginous metaplasia in aortic arches | 0/8 (0%) | 6/18 (33%) | 22/22 (100%) |
| Aortic arch calcification | 0/8 (0%) | 1/18 (5%) | 14/22 (64%) |
Data are presented as Mean±SEM and analyzed against NC controls.
P < 0.05
Figure 2. Cartilaginous metaplasia and calcification in blood vessels of LDLr−/− mice.
Aortic arches of SM22α-Cre+/0:R26R-LacZ+/0:LDLr−/− mice fed with HFCD1 (A and B) and D12108 (C and D) diets for 18 weeks. A and C, Movat pentachrome staining; arrows denote cells of chondrocyte morphology in cartilaginous areas. B and D. Von Kossa staining to visualize calcification (dark brown). M=media, L=lumen, I=intima, Ad=adventitia.
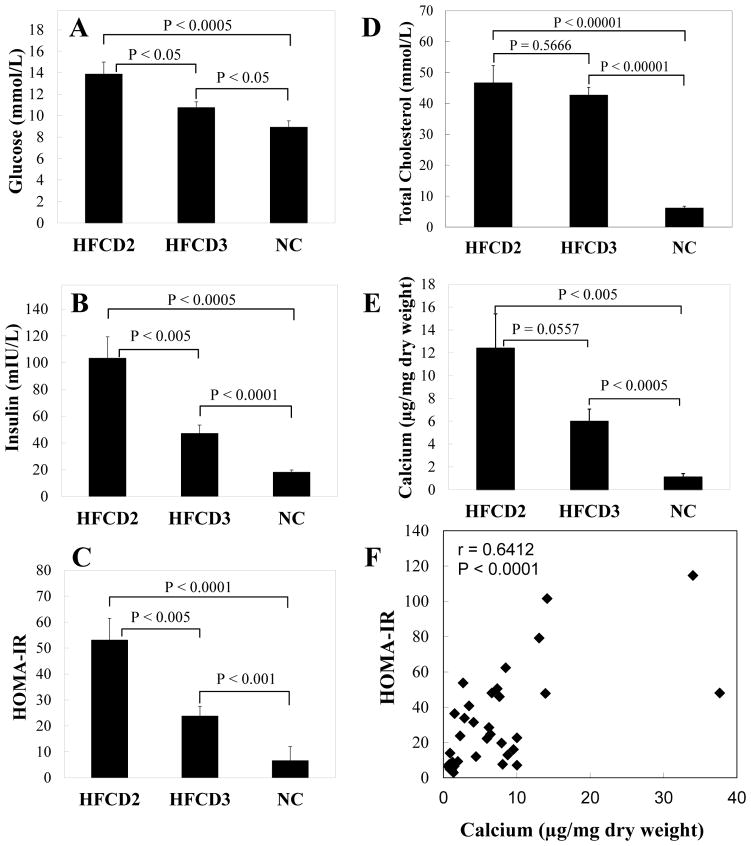
We further determined whether vascular calcification is associated with levels of insulin resistance and hyperglycemia. As shown in Figures 1A and 1B, LDLr−/− mice fed with HFCD2 (57.9% kcal fat and 0.2% cholesterol) and HFCD3 (40.1% kcal fat and 0.2% cholesterol) diets were obese and hyperglycemic. HFCD2 mice were heavier (13.4%, p<0.01) and had higher blood glucose (29%, p<0.05) and insulin levels (2.2 folds, p<0.005) compared to HFCD3 mice (Figures 3A and 3B). There was also a 2.2 fold-increase in HOMA-IR index of mice on HFCD2 versus HFCD3 (Figure 3C, p<0.005), suggesting that these animals were more insulin resistant. Importantly, even with similar serum cholesterol levels (Figure 3D), mice with higher body weight and insulin resistance had almost doubled the calcium content in their aortic arches (Figure 3E). Finally, Pearson correlation analysis of values collected from these mice revealed that insulin resistance is positively correlated with calcium content of aortic arches (r=0.6412, p<0.0001), identifying a compelling relationship between insulin resistance and the extent of vascular calcification.
Figure 3. Correlation of vascular calcification with blood glucose, insulin, and insulin resistance.
LDLr−/− mice were fed with diabetogenic, procalcific diets (HFCD2-3) for 24 weeks, with normal chow (NC) as diet control. Fasted blood glucose (A), insulin (B), and total cholesterol (E) levels were measured as described in “Methods”. Insulin sensitivity was assessed by the homeostatic model assessment of insulin resistance (HOMA-IR), calculated by the formula, fasting blood glucose (mmol/L) × insulin concentration (mIU/L)/22.5 (C). Calcium was extracted from aortic arches of animals and quantified as described in “Methods” (E). F. Correlation of HOMA-IR and calcium content of aortic arches. Data shown are mean±SEM, n=10–13.
3.3. Hypercholesterolemia is likely required for the initiation of vascular calcification in T2DM LDLr−/− mice
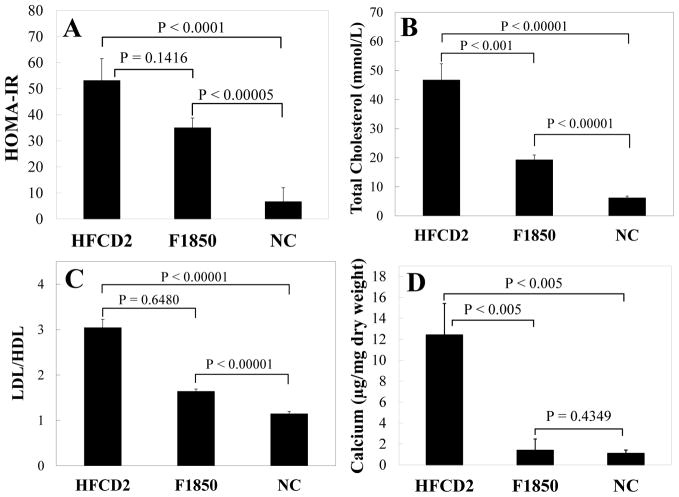
Our study also points to a likely requirement of hypercholesterolemia in the development of vascular calcification. LDLr−/− mice fed with diets consisting of ~58% kcal fat with (HFCD2) and without (F1850) 0.2% cholesterol developed similar levels of obesity and hyperglycemia by 18 weeks on diet (Figures 1A and 1B, black open squares vs blue triangles), with the HFCD2 group having a slightly higher calculated HOMA-IR index at 8 weeks (Figure 4A). Addition of dietary cholesterol led to a substantial increase in serum cholesterol (>35 mmol/L) and LDL/HDL cholesterol ratio (Figures 4B and 4C), corresponding to a large increase in calcium content of blood vessels (Figure 4D). Importantly, mice fed with F1850 diet, consisting of no additional dietary cholesterol (0.03% as presented in fat source), showed only a slightly higher LDL/HDL cholesterol ratio compared to NC controls. There was no significant difference in calcium content between F1850 animals and NC control (p=0.4349). These findings suggest that hypercholesterolemia, especially elevated LDL cholesterol, may be an important requirement for initiation of vascular calcification under T2DM settings.
Figure 4. The role of hypercholesterolemia in vascular calcification of T2DM LDLr−/− mice.
LDLr−/− mice were fed with respective diets for 24 weeks. Fasted blood glucose and insulin levels were measured and HOMA-IR (A) was calculated as described in Figure 3. Total cholesterol (B), LDL/HDL cholesterol ratio (C), and calcium content of aortic arches (D) were determined as described in “Methods”. Data shown are mean±SEM, n=10–13.
3.4. Vascular SMCs are the major contributors to osteochondrogenic precursor- and chondrocyte-like cells in T2DM LDLr−/− vessels
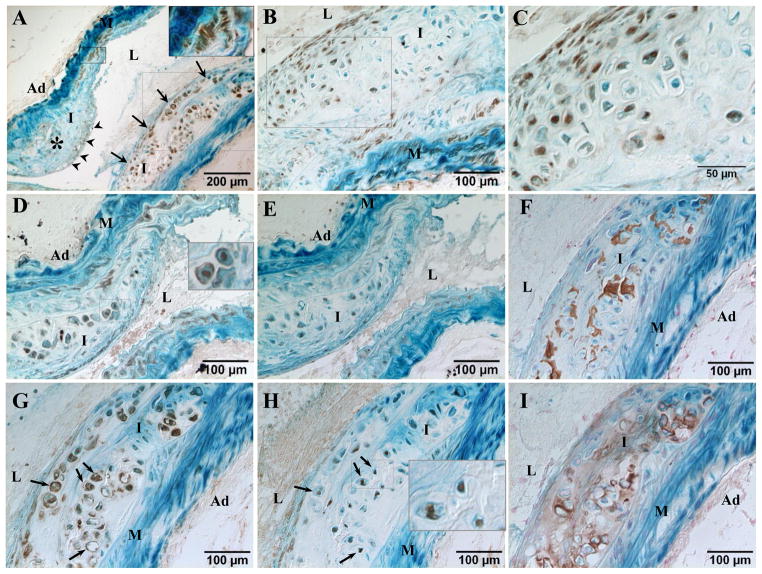
Our recent genetic fate mapping studies of non-atherosclerotic medial calcification have identified SMCs as the major cell sources giving rise to vascular cartilaginous metaplasia and calcification.13 To determine whether SMCs are critical for T2DM-accelerated vascular calcification, we employed the same genetic fate mapping strategy to permanently label cells of SM origin. Thus, SM22α-Cre+/0:R26R-LacZ+/0:LDLr−/− mice were fed with a diabetogenic/procalcific diet (HFCD1) to induce T2DM and osteochondrogenic differentiation. After 18 weeks on diet, their aortic arches were collected and stained with X-gal to identify cells that have once expressed SM22α. As shown in Figure 5, the majority of cells located within the cartilaginous intima and media (5A, arrows) were stained blue by X-gal, identifying their SM origin (Figures B-I). These cells no longer express SMC lineage proteins such as SM22α, SM α-actin, and SMMHC (5B-5D, lack of brown). Instead, they acquired osteochodrogenic and chondrocytic properties, as determined by the presence of brown nuclear staining for osteochondrogenic transcription factor Runx2/Cbfa1 (5E and 5F) and chondrocytic transcription factor Sox9 (5G). Cartilaginous matrix protein collagen type II (5H, brown rim) were also observed in the surrounding areas of the blue X-gal stained cells. Of particular interest, some SMC marker-positive cells co-expressed Runx2/Cbfa1, suggesting a transitional state in lineage reprogramming of these cells into osteochondrogenic cells, consistent with our previous findings in arterial medial calcification of MGP−/− mice13 and in non-diabetic atherosclerotic mice,19 as well as human atherosclerotic arteries that showed hybrid “myochondrocytes” by Bobryshev.9
Figure 5. SMCs were the major contributors to osteochondrogenic precursor- and chondrocyte-like cells in T2DM LDLr−/− vessels.
SM22α-Cre+/0:R26R-LacZ+/0:LDLr−/− mice were fed with HFCD1 for 18 weeks. Aortic arches were stained with X-gal to label cells of SM origin (blue). Cells of chondrocyte morphology and cartilaginous metaplasia were visualized via Movat pentachrome staining (A, white arrows). B-I. Immunohistochemical staining of adjacent sections. B-D. SMC lineage proteins. SM22α (B), SM α-actin (C), and SMMHC (D). Note that chondrocyte-like cells within the cartilaginous intima and media are negative for SMC lineage proteins. E. Osteochondrogenic transcription factor, Runx2/Cbfa1 (brown nuclear). G. Chondrogenic transcription factor, Sox9 (brown nuclear). F and H. Higher magnification of boxed region showing localization of Runx2/Cbfa1 (F) and Sox9 (H, arrowheads) within X-gal positive cells. I. Type II collagen. M=media, L=lumen, I=intima, Ad=adventitia.
3.5. RAGE may be a critical factor involved in SMC lineage reprogramming into osteochondrogenic precursor- and chondrocyte-like cells in T2DM LDLr−/− vessels
Forced-expression of RAGE in vascular SMCs were found to induce osteogenic differentiation of cells and increase matrix calcification, especially when the cells were cultured in the presence of serum derived from patients with diabetic nephropathy.22 To determine whether RAGE is involved in osteochondrogenic differentiation of SMCs in cartilaginous and calcifying lesions of T2DM blood vessels, we stained aortic arch sections for RAGE immunohistochemically. As shown in Figure 6A, RAGE was found mostly in areas of cartilaginous metaplasia (arrows) and, to a lesser extent, in medial SMCs (insert) and cells located on the lumen side of the lipid core that were not stained by X-gal (arrowheads). Notably, the majority of RAGE-positive cells within cartilaginous areas were stained blue by X-gal, identifying its expression in SMCs undergoing lineage reprogramming. In early osteochondrogenic differentiation of these cells, RAGE was found primarily in the cell nulei (6B and 6C), co-localizing with osteochondrogenic and chondrocytic transcription factors, Runx2/Cbfa1 (5E) and Sox9 (5G), with a few of these cells also positive for RAGE on the membrane (6D, insert). In calcifying lesions (6F), RAGE expression was strongest on cell membranes (6G, arrows), co-localizing with Sox9 (6H, arrows), especially in chondrocyte-like cells identifiable by their large, round shape and lacunar rim containing type X collagen (6I, brown), indicating a possible role of RAGE in osteochondrogenic differentiation and chondrocyte maturation.
Figure 6. Expression of RAGE in calcifying blood vessels of T2DM LDLr−/− mice.
SM22α-Cre+/0:R26R-LacZ+/0:LDLr−/− mice were fed with HFCD1 for 18 weeks. Aortic arches were stained with X-gal to label cells of SM origin (blue). Adjacent sections were stained immunohistochemically for RAGE (A-D, and G), Runx2/Cbfa2 (E), Sox9 (H), and Collagen X (I). C. Higher magnification of boxed region in B. Vascular calcification was visualized via von Kossa staining (F). M=media, L=lumen, I=intima, Ad=adventitia.
4. Discussion
The lack of appropriate animal models to emulate the pathology of human T2DM cardiovascular complication has hindered our understanding of the disease, especially the molecules and pathways critical for disease initiation and progression, which may serve as a basis to improve clinical management decisions and/or develop effective strategies to treat this complication. In the present study, we challenged LDLr−/− mice, a knockout strain susceptible to developing non-insulin dependent diabetes, atherosclerosis, uremia, and vascular ectopic calcification,12, 18, 23 with various customized high-fat, high-cholesterol diets. We found that diets consisting of ~40–60% kcal fat (with lard as fat source) and 0.2–1.25% cholesterol induced not only T2DM, as evidenced by obesity, hyperglycemia and hyperinsulinemia, but also cartilaginous metaplasia and calcification in atherosclerotic vessels, similar to human T2DM blood vessels.2, 3 Importantly, T2DM accelerated the development of vascular cartilaginous and calcific lesions. Compared to non-T2DM LDLr−/− mice with similar levels of hypercholesterolemia, T2DM mice showed a significantly higher incidence of cartilaginous and calcific lesions in their blood vessels (HFCD1 vs D12108). Lowering dietary fat from ~60% to ~40% kcal reduced body weight and serum glucose and insulin levels, leading to a reduction of vascular calcification (HFCD3 vs HFCD2). Indeed, analysis of calcium content in diseased blood vessels of LDLr−/− mice with a calculated insulin resistance index (HOMA-IR) showed that insulin resistance was positively correlated with the extent of vascular calcification. Our results are the first to clarify a role of T2DM in vascular cartilaginous and calcific lesion progression, which in part explains the high incidence of this complication in T2DM patients.
While little is known about the role of lipid metabolism in cardiovascular calcification, its abnormalities is a well-known cause of atherosclerosis and has been extensively studied24. In a retrospective study of 104 patients with coronary and aortic valve calcification, ectopic calcification was measured periodically by electron beam tomography. During a 3-year follow-up, around 82% of these patients had a progressive build-up of ectopic calcification, indicated by annually increasing calcification score at a rate of 27.3% for coronary artery calcification and 24.5% for aortic valve calcification. Interestingly, serum levels of LDL cholesterol were found to be an independent factor, associated with higher annual calcification scores in both coronary and aortic valve calcification.25 The association of hypercholesterolemia, especially LDL cholesterol levels, with progression of cardiovascular calcification has since then been reported by several other groups.6, 26, 27 Indeed, culture of vascular SMCs in the presence of 7-ketocholesterol, a major oxycholesterol observed in atherosclerotic lesions, promoted phosphate-induced matrix calcification.28 This also occurred in cultures of calcifying vascular cells, a clonal vascular wall cells with high potential for ectopic calcification, when the cells were exposed to oxidized LDL.29 In the present study, we used a genetic fate mapping strategy to track cells derived from SMCs and found that vascular SMCs were the major cell source contributing to vascular cartilaginous and calcific lesion development, consistent with previous findings in non-atherosclerotic vascular medial calcification13 and atherosclerotic vascular intimal calcification.9 Under T2DM conditions, SMCs are likely to have adapted their phenotype and differentiate into osteochondrogenic precursors and chondrocytes. Importantly, our studies suggest an important role of cholesterol in the development of vascular calcification. Hypercholesterolemia (>30 mmol/L in our experimental conditions) is likely required for initiation of vascular osteochondrogenic differentiation and hence calcification. LDLr−/− mice fed with diabetogenic F1850 diet, consisting of only 0.03% dietary cholesterol, developed T2DM and a mild increase in LDL cholesterol levels, but showed no significant increase in vascular calcium content, with levels similar to mice fed with normal chow.
The precise mechanisms of T2DM as an accelerator of vascular cartilaginous metaplasia and calcification are currently unknown. Hyperglycemia appears in the initial stages of the disease and has long been hypothesized to explain at least partial effects of diabetes on cardiovascular complications. In support of this, interaction of advanced glycation end-products (AGEs), a non-enzymatic glycosylation of proteins and lipids under hyperglycemia, with RAGE, a multi-ligand receptor that interacts with AGEs, members of S100/calgranulin family, and amphoterin,30 was found to be involved in signal transduction cascades that regulate T2DM-accelerated atherosclerosis.31 Mice deficient in RAGE have a reduced susceptibility to atherosclerosis, as evidenced by significantly smaller lesion sizes, reduced expression of inflammatory mediators and leukocyte recruitment, decreased nuclear factor-κB activity and oxidative stress, and improved endothelial function.32, 33 Diabetic patients showed increased serum levels of AGEs, such as glycated albumin and hemoglobin, correlating with the occurrence of peripheral vascular calcification.34 Soluble RAGE, a truncated decoy of RAGE that lacks transmembrane and signaling domains, was decreased in serum of patients with calcific aortic valve stenosis.35 In the present study, increased RAGE expression and membrane translocation were found in cartilaginous and calcific areas of atherosclerotic blood vessels, co-localizing with osteochondrogenic and chondrocytic transcription factors, Runx2 and Sox 9, as well as chondrocyte marker proteins, types II and X collagen.
Finally, the sustained expression of RAGE may be critical for T2DM-associated predisposition of vascular osteochondrogenic differentiation and calcification. The membrane-proximal cytoplasmic region of RAGE was found to directly bind to Erk by a D-domain-like docking site36 and thus activates Erk signaling,37 a critical pathway known to up-regulate the expression of Runx2 in a variety of cell types, including SMCs,13 and is required for Runx2 phosphorylation, an active form of Runx2 that drives osteoblastic differentiation.38 Thus, in vitro transfection of vascular SMCs with adenoviruses expressing RAGE increased osteogenic differentiation of the cells and matrix calcification.22 Taken together with our findings of increased RAGE expression and co-localization with Runx2, RAGE signaling is likely important for T2DM-accelerated vascular osteochondrogenic differentiation and calcification, possibly through Runx2 activation.
Summary.
Vascular calcification is highly prevalent in patients with T2DM. Using LDLr−/− mice, we found that T2DM accelerated the development of vascular calcification and hypercholesterolemia was likely required for the development of calcification lesions. We also genetically traced cells of SM origin. SMCs were a major cell source attributable to osteochondroprogenitor- and chondrocyte-like cells in calcifying lesions, possibly mediated through RAGE signaling.
Acknowledgments
We gratefully acknowledge Dr. Renee C. LeBoeuf, Department of Medicine, University of Washington, for her expertise in diabetic mouse models as well as the invaluable discussion of the project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Diabetes Association. National diabetes fact sheet 2011. 2011. [Google Scholar]
- 2.Qiao JH, Mertens RB, Fishbein MC, Geller SA. Cartilaginous metaplasia in calcified diabetic peripheral vascular disease: morphologic evidence of enchondral ossification. Hum Pathol. 2003 Apr;34(4):402–7. doi: 10.1053/hupa.2003.72. [DOI] [PubMed] [Google Scholar]
- 3.Shanahan CM, Cary NR, Salisbury JR, Proudfoot D, Weissberg PL, Edmonds ME. Medial localization of mineralization-regulating proteins in association with Monckeberg’s sclerosis: evidence for smooth muscle cell-mediated vascular calcification. Circulation. 1999;100(21):2168–76. doi: 10.1161/01.cir.100.21.2168. [DOI] [PubMed] [Google Scholar]
- 4.Everhart JE, Pettitt DJ, Knowler WC, Rose FA, Bennett PH. Medial arterial calcification and its association with mortality and complications of diabetes. Diabetologia. 1988 Jan;31(1):16–23. doi: 10.1007/BF00279127. [DOI] [PubMed] [Google Scholar]
- 5.Niskanen L, Siitonen O, Suhonen M, Uusitupa MI. Medial artery calcification predicts cardiovascular mortality in patients with NIDDM. Diabetes Care. 1994 Nov;17(11):1252–6. doi: 10.2337/diacare.17.11.1252. [DOI] [PubMed] [Google Scholar]
- 6.Katz R, Wong ND, Kronmal R, Takasu J, Shavelle DM, Probstfield JL, Bertoni AG, Budoff MJ, O’Brien KD. Features of the metabolic syndrome and diabetes mellitus as predictors of aortic valve calcification in the Multi-Ethnic Study of Atherosclerosis. Circulation. 2006 May 2;113(17):2113–9. doi: 10.1161/CIRCULATIONAHA.105.598086. [DOI] [PubMed] [Google Scholar]
- 7.Lehto S, Niskanen L, Suhonen M, Ronnemaa T, Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16(8):978–83. doi: 10.1161/01.atv.16.8.978. [DOI] [PubMed] [Google Scholar]
- 8.Tyson KL, Reynolds JL, McNair R, Zhang Q, Weissberg PL, Shanahan CM. Osteo/chondrocytic transcription factors and their target genes exhibit distinct patterns of expression in human arterial calcification. Arterioscler Thromb Vasc Biol. 2003 Mar 1;23(3):489–94. doi: 10.1161/01.ATV.0000059406.92165.31. [DOI] [PubMed] [Google Scholar]
- 9.Bobryshev YV. Transdifferentiation of smooth muscle cells into chondrocytes in atherosclerotic arteries in situ: implications for diffuse intimal calcification. J Pathol. 2005 Apr;205(5):641–50. doi: 10.1002/path.1743. [DOI] [PubMed] [Google Scholar]
- 10.Aikawa E, Nahrendorf M, Sosnovik D, Lok VM, Jaffer FA, Aikawa M, Weissleder R. Multimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve disease. Circulation. 2007 Jan 23;115(3):377–86. doi: 10.1161/CIRCULATIONAHA.106.654913. [DOI] [PubMed] [Google Scholar]
- 11.Bostrom K, Watson KE, Horn S, Wortham C, Herman IM, Demer LL. Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest. 1993 Apr;91(4):1800–9. doi: 10.1172/JCI116391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Towler DA, Bidder M, Latifi T, Coleman T, Semenkovich CF. Diet-induced diabetes activates an osteogenic gene regulatory program in the aortas of low density lipoprotein receptor-deficient mice. J Biol Chem. 1998 Nov 13;273(46):30427–34. doi: 10.1074/jbc.273.46.30427. [DOI] [PubMed] [Google Scholar]
- 13.Speer MY, Yang HY, Brabb T, Leaf E, Look A, Lin W-L, Frutkin AD, Dichek DA, Giachelli CM. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ Res. 2009 Feb 5;104(6):733–41. doi: 10.1161/CIRCRESAHA.108.183053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobayashi K, Forte TM, Taniguchi S, Ishida BY, Oka K, Chan L. The db/db mouse, a model for diabetic dyslipidemia: molecular characterization and effects of Western diet feeding. Metabolism. 2000 Jan;49(1):22–31. doi: 10.1016/s0026-0495(00)90588-2. [DOI] [PubMed] [Google Scholar]
- 15.Accili D, Drago J, Lee EJ, Johnson MD, Cool MH, Salvatore P, Asico LD, Jose PA, Taylor SI, Westphal H. Early neonatal death in mice homozygous for a null allele of the insulin receptor gene. Nat Genet. 1996 Jan;12(1):106–9. doi: 10.1038/ng0196-106. [DOI] [PubMed] [Google Scholar]
- 16.Withers DJ, Gutierrez JS, Towery H, Burks DJ, Ren JM, Previs S, Zhang Y, Bernal D, Pons S, Shulman GI, Bonner-Weir S, White MF. Disruption of IRS-2 causes type 2 diabetes in mice. Nature. 1998 Feb 26;391(6670):900–4. doi: 10.1038/36116. [DOI] [PubMed] [Google Scholar]
- 17.Ahmad PJ, Trcka D, Xue S, Franco C, Speer MY, Giachelli CM, Bendeck MP. Discoidin domain receptor-1 deficiency attenuates atherosclerotic calcification and smooth muscle cell-mediated mineralization. Am J Pathol. 2009 Dec;175(6):2686–96. doi: 10.2353/ajpath.2009.080734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schreyer SA, Lystig TC, Vick CM, LeBoeuf RC. Mice deficient in apolipoprotein E but not LDL receptors are resistant to accelerated atherosclerosis associated with obesity. Atherosclerosis. 2003 Nov;171(1):49–55. doi: 10.1016/j.atherosclerosis.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Naik V, Leaf EM, Hu JH, Yang HY, Nguyen NB, Giachelli CM, Speer MY. Sources of cells that contribute to atherosclerotic intimal calcification: an in vivo genetic fate mapping study. Cardiovasc Res. 2012 Jun 1;94(3):545–54. doi: 10.1093/cvr/cvs126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985 Jul;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 21.Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, Monauni T, Muggeo M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000 Jan;23(1):57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- 22.Suga T, Iso T, Shimizu T, Tanaka T, Yamagishi S, Takeuchi M, Imaizumi T, Kurabayashi M. Activation of receptor for advanced glycation end products induces osteogenic differentiation of vascular smooth muscle cells. J Atheroscler Thromb. 2011 Aug 24;18(8):670–83. doi: 10.5551/jat.7120. [DOI] [PubMed] [Google Scholar]
- 23.Davies MR, Lund RJ, Hruska KA. BMP-7 is an efficacious treatment of vascular calcification in a murine model of atherosclerosis and chronic renal failure. J Am Soc Nephrol. 2003 Jun;14(6):1559–67. doi: 10.1097/01.asn.0000068404.57780.dd. [DOI] [PubMed] [Google Scholar]
- 24.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999 Jan 14;340(2):115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 25.Pohle K, Maffert R, Ropers D, Moshage W, Stilianakis N, Daniel WG, Achenbach S. Progression of aortic valve calcification: association with coronary atherosclerosis and cardiovascular risk factors. Circulation. 2001 Oct 16;104(16):1927–32. doi: 10.1161/hc4101.097527. [DOI] [PubMed] [Google Scholar]
- 26.Farivar RS, Cohn LH. Hypercholesterolemia is a risk factor for bioprosthetic valve calcification and explantation. J Thorac Cardiovasc Surg. 2003 Oct;126(4):969–75. doi: 10.1016/s0022-5223(03)00708-6. [DOI] [PubMed] [Google Scholar]
- 27.Alrasadi K, Alwaili K, Awan Z, Valenti D, Couture P, Genest J. Aortic calcifications in familial hypercholesterolemia: potential role of the low-density lipoprotein receptor gene. Am Heart J. 2009 Jan;157(1):170–6. doi: 10.1016/j.ahj.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 28.Saito E, Wachi H, Sato F, Seyama Y. 7-ketocholesterol, a major oxysterol, promotes pi-induced vascular calcification in cultured smooth muscle cells. J Atheroscler Thromb. 2008 Jun;15(3):130–7. doi: 10.5551/jat.e556. [DOI] [PubMed] [Google Scholar]
- 29.Parhami F, Morrow AD, Balucan J, Leitinger N, Watson AD, Tintut Y, Berliner JA, Demer LL. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler Thromb Vasc Biol. 1997;17(4):680–7. doi: 10.1161/01.atv.17.4.680. [DOI] [PubMed] [Google Scholar]
- 30.Yan SF, Ramasamy R, Schmidt AM. Mechanisms of disease: advanced glycation end-products and their receptor in inflammation and diabetes complications. Nat Clin Pract Endocrinol Metab. 2008 May;4(5):285–93. doi: 10.1038/ncpendmet0786. [DOI] [PubMed] [Google Scholar]
- 31.Cipollone F, Iezzi A, Fazia M, Zucchelli M, Pini B, Cuccurullo C, De Cesare D, De Blasis G, Muraro R, Bei R, Chiarelli F, Schmidt AM, Cuccurullo F, Mezzetti A. The receptor RAGE as a progression factor amplifying arachidonate-dependent inflammatory and proteolytic response in human atherosclerotic plaques: role of glycemic control. Circulation. 2003 Sep 2;108(9):1070–7. doi: 10.1161/01.CIR.0000086014.80477.0D. [DOI] [PubMed] [Google Scholar]
- 32.Harja E, Bu DX, Hudson BI, Chang JS, Shen X, Hallam K, Kalea AZ, Lu Y, Rosario RH, Oruganti S, Nikolla Z, Belov D, Lalla E, Ramasamy R, Yan SF, Schmidt AM. Vascular and inflammatory stresses mediate atherosclerosis via RAGE and its ligands in apoE−/− mice. J Clin Invest. 2008 Jan;118(1):183–94. doi: 10.1172/JCI32703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soro-Paavonen A, Watson AM, Li J, Paavonen K, Koitka A, Calkin AC, Barit D, Coughlan MT, Drew BG, Lancaster GI, Thomas M, Forbes JM, Nawroth PP, Bierhaus A, Cooper ME, Jandeleit-Dahm KA. Receptor for advanced glycation end products (RAGE) deficiency attenuates the development of atherosclerosis in diabetes. Diabetes. 2008 Sep;57(9):2461–9. doi: 10.2337/db07-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamada S, Inaba M, Shidara K, Okada S, Emoto M, Ishimura E, Nishizawa Y. Association of glycated albumin, but not glycated hemoglobin, with peripheral vascular calcification in hemodialysis patients with type 2 diabetes. Life Sci. 2008 Sep 26;83(13–14):516–9. doi: 10.1016/j.lfs.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Basta G, Corciu AI, Vianello A, Del Turco S, Foffa I, Navarra T, Chiappino D, Berti S, Mazzone A. Circulating soluble receptor for advanced glycation end-product levels are decreased in patients with calcific aortic valve stenosis. Atherosclerosis. 2010 Jun;210(2):614–8. doi: 10.1016/j.atherosclerosis.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 36.Ishihara K, Tsutsumi K, Kawane S, Nakajima M, Kasaoka T. The receptor for advanced glycation end-products (RAGE) directly binds to ERK by a D-domain-like docking site. FEBS Lett. 2003 Aug 28;550(1–3):107–13. doi: 10.1016/s0014-5793(03)00846-9. [DOI] [PubMed] [Google Scholar]
- 37.Cortizo AM, Lettieri MG, Barrio DA, Mercer N, Etcheverry SB, McCarthy AD. Advanced glycation end-products (AGEs) induce concerted changes in the osteoblastic expression of their receptor RAGE and in the activation of extracellular signal-regulated kinases (ERK) Mol Cell Biochem. 2003 Aug;250(1–2):1–10. doi: 10.1023/a:1024934008982. [DOI] [PubMed] [Google Scholar]
- 38.Ge C, Xiao G, Jiang D, Yang Q, Hatch NE, Roca H, Franceschi RT. Identification and functional characterization of ERK/MAPK phosphorylation sites in the Runx2 transcription factor. J Biol Chem. 2009 Nov 20;284(47):32533–43. doi: 10.1074/jbc.M109.040980. [DOI] [PMC free article] [PubMed] [Google Scholar]