Abstract
Background
Animals exposed to alcohol during the developmental period develop many physiological and behavioral problems due to neuronal loss in various brain areas including the hypothalamus. Because alcohol exposure is known to induce oxidative stress in developing neurons, we tested whether hypothalamic cells from the fetal brain exposed to ethanol may alter the cell-cell communication between neurons and microglia, thereby leading to increased oxidative stress and the activation of apoptotic processes in the neuronal population in the hypothalamus.
Methods
Using enriched neuronal and microglial cells from fetal rat hypothalami, we measured cellular levels of various oxidants (O2-; reactive oxygen species, ROS; nitrite), antioxidants (glutathione; GSH), antioxidative enzymes (glutathione peroxidase, GSH-Px; catalase; superoxide dismutase, SOD) and apoptotic death in neurons in the presence and absence of ethanol or ethanol-treated microglial culture medium. Additionally, we tested the effectiveness of antioxidative agents in preventing ethanol or ethanol-treated microglial conditioned medium actions on oxidative stress and apoptosis in neuronal cell cultures.
Results
Neuronal cell cultures showed increased oxidative stress, as demonstrated by higher cellular levels of oxidants but lower levels of antioxidant and antioxidative enzymes, as well as, increased apoptotic death following treatment with ethanol. These effects of ethanol on oxidative stress and cell death were enhanced by the presence of microglia. Antioxidative agents protected developing hypothalamic neurons from oxidative stress and cellular apoptosis which is caused by ethanol or ethanol-treated microglial culture medium.
Conclusions
These data suggest that exposure of developing hypothalamic neurons to ethanol increases cellular apoptosis via the effects on oxidative stress of neurons directly and via increasing production of microglia-derived factor(s).
Ethanol consumption affects normal fetal brain growth and reduces the number of neurons in the various brain regions, including thehippocampus, cerebral cortex, cerebellum, olfactory bulb, and hypothalamus (Chen et al., 2004; De et al; 1994; Goodlett et al., 1991; Miller, 1995; Miller and Potempa, 1990; West et al., 1984). Within the hypothalamus, prenatal ethanol has been shown to produce functional abnormalities of several neuronal populations including β-endorphin (Sarkar et al., 2007), CRH (Lee et al., 2000), α-MSH, NPY, galanin (Barson et al., 2010), orexin 1 (Stettner et al., 2011), arginine vasopressin (Bird et al., 2006), vasoactive intestinal peptide (Rojas et al., 1999) and luteinizing hormone releasing hormone producing neurons (Scott et al., 1995). In addition prenatal ethanol has been shown to produce alteration in the body’s clock regulatory mechanism within the suprachiasmatic nucleus of the hypothalamus (Chen et al., 2006). Many of the functional defects of the hypothalamus in prenatal ethanol-exposed animals are related to the loss of the neuronal cell population (Baker and Shoemaker, 1995; De et al., 1994; Sarkar et al., 2007)
Oxidative stress has been proposed as a mechanism of ethanol teratogenicity (Cohen-Kerem and Koren, 2003). The formation of ROS, including oxygen free radicals, occurs intracellularly in various tissues of rodent species following ethanol administration (Reinke et al., 1987). In the rat, ethanol also can perturb intracellular antioxidant pathways, including glutathione (GSH), GSH peroxidase, superoxide dismutase and catalase (Schlorff et al., 1999). Free radicals can react chemically with key cell macromolecules, namely, phospholipids, proteins and DNA, which may lead to cell dysfunction and eventual cell demise. In the brain, ethanol can produce oxidative stress by direct and indirect mechanisms, which can enhance ROS production (Montoliu et al., 1995). Ethanol also can increase the sensitivity of the brain to oxidative stress by selective mitochondrial injury (Ramachandran et al., 2001), thus compromising antioxidant pathways such as GSH (Rathinam et al., 2006).
Because heavy alcohol exposure causes oxidative stress in developing neurons (for a review see Cohen-Kerem and Koren, 2003), we hypothesized that rats exposed to ethanol in utero may have oxidative damage to the hypothalamus, altering the cell-cell communication between neurons and other cells including microglia and leading to the activation of apoptotic processes in the neuronal population in the hypothalamus. Oxidative stress, in which production of ROS overwhelms antioxidant defenses, is a feature of many neurological diseases and neurodegeneration (Halliwell, 2006; Lin and Beal, 2006). ROS generated extracellularly and intracellularly directly oxidize and damage macromolecules such as DNA, proteins, and lipids, culminating in neurodegeneration of the CNS. Neurons are most susceptible to direct oxidative injury by ROS, which can also indirectly contribute to tissue damage by activating a number of cellular pathways that lead to the expression of stress-sensitive genes and proteins that cause oxidative injury.
Recently, we have shown that ethanol treatment increased the release of various cytokines such as TNF-α, IL-1β, IL-6 from microglial cells and that TNF-α caused apoptotic cell death of developing hypothalamic neurons in vitro (Boyadjieva and Sarkar, 2010). A number of studies have indicated that the microglia are a source of ROS in the brain (Chao et al., 1992; Colton and Gilbert, 1987). The production of ROS by microglia is a part of the immune defense in the brain. A limited number of studies focused on the role of ROS generated from microglia in apoptosis of neurons (Min et al., 2003; Wang et al., 2008), and also the role of ROS from ethanol-treated microglia in apoptosis of developing hypothalamic neurons is not studied. In this study, we determined whether ethanol-treated microglial cell culture generate ROS and increase apoptotic cell death of fetal hypothalamic neurons in culture.
MATERIALS AND METHODS
Animal Use
Pregnant Sprague-Dawley female rats were obtained from Charles River Laboratories (Wilmington, MA) and were used as the source of fetal rat brains for hypothalamic cell cultures. Animal surgery and care were performed in accordance with institutional guidelines and complied with the National Institutes of Health policy.
Enriched hypothalamic neuronal cell cultures
Primary cultures of fetal hypothalamic neuronal cell cultures were prepared from the mediobasal part of the hypothalamus (containing neuroendocrine neurons, including beta-endorphin, dopamine, thyrotropin-releasing hormone and growth hormone-releasing hormone; Brown, 1998). In brief, pregnant rats at 18 to 20 days of gestation were sacrificed, fetuses were removed by aseptic surgical procedure, brains from the fetuses were immediately removed, hypothalami were separated and placed in ice-cold Hanks’ balanced salt solution containing antibiotic solution (100 U/ml penicillin, 100 μg/ml streptomycin, and 250 ng/ml amphotericin B), 0.1% bovine serum albumin, and 200 μM ascorbic acid (all from Sigma-Aldrich, St. Louis, MO). The block of mediobasal hypothalamic tissue consisted of approximately 1 mm rostral to the optic chiasma and just caudal to the mammillary bodies, laterally to the hypothalamic sulci, and dorsally to 2 mm deep. The hypothalamic cells were washed and then incubated at 37°C for 5 min using the same medium. After dispersion, the cells were plated at a density of 3.0 × 106 cells per 25-mm2 flask and at a density of 1.0 × 106 cells per well in a 24-well plate. Both the flask and plate were coated with polyornithine at a concentration 100 μg/ml and then incubated for 3 h. The cells were maintained in Dulbecco’s modified Eagle’s medium with 10% fetal calf serum at 37°C and 7.5% CO2 in a humidified water-jacketed incubator for 2 days. On day 2, the medium was replaced with HDME containing 10% fetal calf serum, 33.6 μg/ml uridine and 13.2 μg/ml 5-fluorodeoxiuridine (Sigma-Aldrich, St. Louis, MO) to stop the overgrowth of glial cells. On day 4, the medium was replaced with serum-free, chemically defined medium (HDME consisting of 30 nM selenium, 20 nM progesterone, 1 μM iron-free human transferrin, 100 μM putrescine, and 5 μg/ml insulin). Cells were maintained for the next two days with this medium. By this time, the cultures were approximately 85 – 90% neurons, as determined by MAP2 positivity (Boyadjieva et al., 2009; Boyadjieva and Sarkar, 2010; Sarkar et al., 2008).
In study1, hypothalamic neurons were treated with or without ethanol (25, 50 and 100 mM) for a period of 24 h or 48 h for conducting ethanol dose- and time-response effects on cellular levels of nucleosome and various oxidants and antioxidants. In study 2, microglial influences on ethanol actions on cell function were determined by incubating hypothalamic neuronal cell cultures for 24h with conditioned media collected from microglial cell cultures that had been treated with various doses (25, 50 and 100 mM) of ethanol for 24 h. In study 3, hypothalamic neuronal cell cultures were treated for 24 h with 50 mM ethanol with or without various doses (0, 0.1, 1.0 and 10 μM) of EUK-134 (chloro[[2,2′-[1,2-ethanediylbis[(nitrilo-κN)methylidyne]]bis[6-methoxyphenolato-κO]]]-manganese; Cayman Chemical, Ann Arbor, MI) or Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-Carboxylic Acid; Cayman Chemical, Ann Arbor, MI) and then used for the measurements of cellular levels of nucleosome and various oxidants and antioxidants. In study 4, hypothalamic neuronal cell cultures were treated for a period of 24 h with conditioned medium of microglial cell culture (treated for 24 h with 50 mM ethanol) with or without various doses of EUK-134 (U; 0, 0.1, 1.0 or 10.0 μM) or Trolox (T; 0, 0.1, 1.0 or 10.0 μM). In a pilot experiment, we measured the effect of 10 μM Trolox or 10 μM EUK on neuronal apoptosis and found no significant effects on cellular levels of nucleosome. Hence, we did not include the U or T alone groups in the measurements of oxidant and antioxidant levels in neuronal cell extracts. We have used 2 to 3 culture/treatment and repeated each experiment 3 times. Cultures with any cell number abnormality or assay errors were not included in the experiments
Microglial cell cultures
Microglia cells were prepared from E19 rat cerebral cortex using a method modified from that described previously (McCarty and de Vellis, 1980). Cortices were dissociated by passing through a 70-μm Nitex mesh and plated at 2 × 105 cells/cm2. Cultures were fed every four days with DMEM/MEM/Hams 12 (DMF 12) in a 4:5:1 ratio with 10% fetal calf serum (FCS). On day 12, the culture was shaken on a rotary shaker at 800 rpm for 1 h (Frei et al., 1987). The suspended cells were plated on uncoated T25 flasks and incubated for 1 h at 37°C. Then the medium containing suspended cells was discarded and adherent cells were fed with DMEMF12 for 3 days to develop the microglial culture prior to experimentation. To confirm the microglia positive cells, the culture was labeled with microglial marker (antibody Annexin 1) or the astrocyte marker glial fibrillary acidic protein (GFAP). The cultures with positive cells for Annexin 1 were considered microglial cells. Microglial cells were maintained in DMEMF12 in 24 well plates (1×105) and treated for 24 h with 25 mM (0.15% v/v), 50 mM (0.3% v/v), 100 mM (0.6% v/v) or 25 mM ethanol, or vehicle. Then, the medium from cultured microglia cells was collected and centrifuged and the supernatants were used to treat enriched neuronal cells in cultures.
Glutathione (GSH) assay
The cellular levels of total glutathione (GSSG + GSH) in cells were determined by Glutation ASSAY (Calbiochem, USA). Briefly, cells were washed and taken for determination of glutathione levels by following procedures provided by the manufacturer. The sample is first deproteinized with the 5% 5-sulfosalicylic acid solution. Glutathione content of the sample is then assayed using a kinetic assay in which catalytic amounts of glutathione cause a continuous reduction of 5,5′-dithiobis-(2-nitrobenzoic) acid (DTNB) to TNB. The oxidized glutathione formed is recycled by glutathione reductase and NADPH. The product, TNB, is assayed colorimetrically at 412 nm. Each sample was run in duplicates in the assay The GSH levels were presented in umol/l/μg of total protein
Glutathione peroxidase (GSH-Px) assay
The cellular level of GSH-Px activity was measured by a kit (Cat # CGP1; Sigma, St Lois, MO). The kit is based on the oxidation of glutathione (GSH) to oxidized glutathione (GSSG) catalyzed by GPx, which is then coupled to the recycling of GSSG back to GSH utilizing glutathione reductase (GR) and NADPH. Cell extracts were prepared by glutathione peroxidase assay buffer (Cat # G8664) with 50 mM Tris HCl, adjusted with HCl to Ph 7.0 and containing 0.5 mM EDTA and used in the assay using the protocol described in the assay kit. Each sample was run in duplicates in the assay. The GSH-Px activity was measured in mmol/min/ml=Units/ml.
Catalase assay
The catalase activity was determined by a calorimetric assay for the catalase activity in neuronal cell cultures using a kit (Cat# CAT 100; Sigma). Cells were washed with PBS twice, lysed by lyses buffer and used in the assay. The catalase activity was determined by following the instructions of the manufacturer. Each sample was run in duplicates in the assay. The activity was determined in units/ml.
Measurement of superoxide dismutase (SOD) activity
Enriched neuronal cell cultures treated with various agents were detached by gentle trypsinization (0.5% (w/v) trypsin, 0.2% (w/v) EDTA. Cells were counted and washed two times in ice cold 1X PBS, centrifuged for 10 minutes at 250 × g at 2 – 8° C. Cells were then lysed using a cell lysis buffer (500 μl of cell lysis buffer per 1 – 5 ×10 6 cells). Cell lysates were transferred to a 1.5 mL tube and centrifuged for 5 minutes at 12,000 –14,000 × g at 2 – 8° C. The supernatant was placed into a clean 1.5 mL tube and stored on ice and then assayed for SOD immediately or store at −80° C. The supernatant samples were assayed by a kit (Cat# CSOD 100-2; Cell Technology, Mountain View, CA) using the instruction of the manufacture. SOD kit utilizes a water-soluble tetrazolium salt that produces a water-soluble formazan dye upon reduction with a superoxide anion. The rate order reduction of O2− is linearly related to the xanthine oxidase activity and is inhibited by SOD. Absorbance was measured at 450 nm. Absorbance was measured at 450 nm using microplate reader. Cell Technology’s SOD kit utilizes a water-soluble tetrazolium salt, WST-1 (2-(4-Iodophenyl)- 3-(4-nitrophenyl)-5-(2,4-disulfophenyl)- 2H-tetrazolium, monosodium salt) that produces a highly water-soluble formazan dye upon reduction with a superoxide anion. The rate of the reduction with O2.- is linearly related to the xanthine oxidase (XO) activity, and is inhibited by SOD. SOD Standard Curve (from 200 SOD units/ml to 0) was used). Calculation of the SOD activity (inhibition rate %) was done using the following equation. . Each sample was run in duplicates in the assay.
Determination of intracellular O2- production
Intracellular production of O2- was determined by the reduction of nitroblue tetrazolium (Calbiochem, Billerica, MA) as described previously (McDonald et al., 1997) with some modifications. Enriched hypothalamic neurons in culture (50 000 cells/well in 24 wells) were treated with or without ethanol (25, 50 or 100 mM) for a period of 24 or 48 h. At the final point of the experiment, the nitroblue-tetrazolium (1μg/ml) was added and the cells were incubated for 60 min. After treatment, microscopic examination verified the generation of insoluble formazan as dark purple granules. The medium was removed, and the formazan was dissolved in dimethyl-sulfoxide. The lysates were transferred to a 96-well plate, and the absorbance at 570 nm was measured with a spectrophotometer. Each sample was run in duplicates in the assay.
Nitrite assay
As an indicator of nitric oxide production, the amount of nitrite accumulated in the cultures was determined with a calorimetric assay using Griess reagent (1% sulfanilamide, 2.5% H3PO4, 0.1% N-(1-naphthyl) ethylenediamine dihydrochloride). Briefly, 50 μl of Griess reagent and 50 μl of the culture supernatant were incubated in the dark at room temperature for 10 min. After incubation, color intensity was measured at 540 nM using the Spectra Max Plus microplate spectrophotometer. The sample nitrite concentrations were determined from a sodium nitrite standard curve. Each sample was run in duplicates in the assay.
Intracellular reactive oxygen species assay
The production of intracellular reactive oxygen species (ROS) was measured by 2′,7′-dichlorofluorescin (DCF) oxidation method and using the 2′,7′-dichlorohydrofluorescein diacetate (H2DCFH-DA) assay kit and methods were provided by Molecular Probes (Eugene, OR). Molecular Probes offers derivatives of reduced fluorescein and calcein as cell-permeant indicators for ROS. Chemically reduced and acetylated forms of 2′,7′-dichlorofluorescein (DCF) and calcein are nonfluorescent until the acetate groups are removed by intracellular esterases and oxidation occurs within the cell. The carboxy derivative of fluorescein, carboxy-H2DCFDA (C400), carries additional negative charges that improve its retention compared to noncarboxylated forms. H2DCF-DA is a cell-permeable probe converted into DCF-DA by intracellular esterases, and its oxidation results in fluorescent DCF. The final concentration of H2DCF-DA ranges between 2–10 μM. Enriched hypothalamic cells (50 000 cells/well/24 wells plate) were incubated with ethanol (25, 50 or 100 mM) for a period of 24 or 48 h or ethanol-treated microglial culture medium and the intracellular reactive oxygen species was determined. The rat enriched hypothalamic neurons were washed twice with Tyrode’s buffer (TB: 145 mM NaCl, 5 mM KCl, 10 mM glucose, 1.5 mM CaCl2, 1 mM MgCl2, and 10 mM HEPES; adjust pH to 7.4 with NaOH). Cells were then loaded with H2DCF-DA (2 μM of H2DCF-DA) for 45 min in dark in cell culture incubator. After 45 min, the neurons were washed 4 times with TB to remove excess fluorescent indicator before lyses and follow the procedure of the manufacturer. Each sample was run in duplicates in the assay.
Measurement of apoptosis
The cell apoptosis was determined by measuring cellular levels of nucleosome using ELISA (Calbiochem, USA) and the values were presented as apoptotic units/ml. This kit is for relative quantification of histone-complexed DNA fragments (mono- and oligonucleosomes) out of the cytoplasm of cells after the induction of apoptosis. Since the assay does not require prelabeling of cells, it can detect internucleosomal degradation of genomic DNA during apoptosis even in cells that do not proliferate in vitro (for example enriched neurons). The ELISA is specific for nucleosomes containing single- or double-stranded DNA. It is a one-step sandwich ELISA colorimetric assay. After incubating cells with or without ethanol, the cells were incubated with lysis buffer (1.0 ml) for 10 min and centrifuged. The supernatant (cell lysate) was used for determination of the amount of apoptotic nucleosomes present in the sample by binding the nucleosomes in the supernatant with two monoclonal antibodies, antihistone (biotin-labeled) and anti-DNA (peroxidase-conjugated) that were bound to the microplate by the streptavidin. The nucleosome units were calculated per ml of cell lysate. Each sample was run in duplicates in the assay.
Statistical analysis
The data shown in the figures and text are mean ± S.E.M values 5 to 8 cultures from 3 independent experiments. Data comparisons among multiple groups were made using one-way analysis of variance (ANOVA). Post hoc tests involved the Student-Newman-Keuls test. Two-way ANOVA tests were used to compare ethanol dose-response curves between 24 h and 48h. Post hoc tests involved the Bonferroni test. A value of P < 0.05 was considered significant.
RESULTS
Effects of ethanol on cellular apoptosis and cellular levels of antioxidants and oxidant in neuronal cell cultures
In order to determine the changes of antioxidant and oxidant levels that occur during ethanol-induced apoptosis, we treated neuronal cell cultures with various doses of ethanol (0, 25, 50 and 100 mM) for a period of 24 and 48 h, and then measured the cell contents of nucleosome (cellular markers of apoptosis), O2-, ROS and nitrite (oxidative molecules), GSH (antioxidative molecule) and GSH-Px, SOD and catalase (enzymes produce antioxidative molecules).
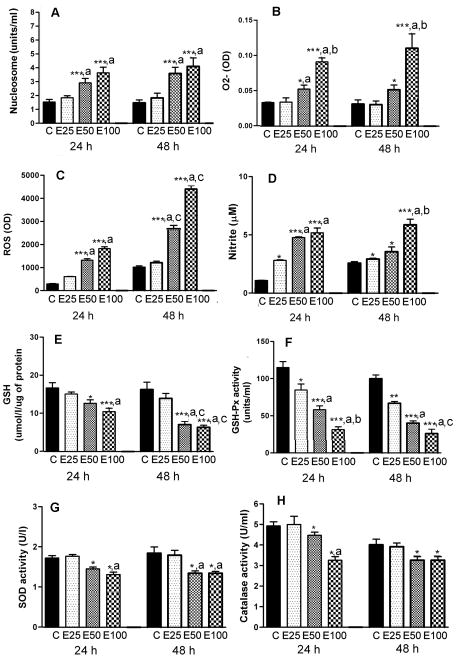
Measurements of cellular apoptosis by determining nucleosome levels revealed that ethanol dose-dependently increased neuronal apoptosis with a 2-fold increase in apoptosis at 50 mM dose and about a 2.6-fold increase at 100 mM dose of ethanol (Fig. 1A). The doses of ethanol tested were equi-effective at 24 h and 48 h.
Figure 1.
Effects of ethanol on cellular apoptosis and cellular levels of antioxidants and oxidants in hypothalamic neuronal cell cultures. Enriched fetal hypothalamic neuronal cell cultures (50 000 cells/well) were exposed to no ethanol (C) or various doses of ethanol (E1–25 mM, E2–50 mM or E3–100 mM) for a period of 24 h or 48 h. The cell extracts were used to measure nucleosome (A), O2- (B), ROS (C), nitrite (D), GSH (E), GSH-Px (F), SOD (G) and catalase activity (H). N=5–8. Significant interaction between dose and time were observed at the level of P<0.001 for ROS (F= 18.61, df=3), P<0.005 for GSH (F= 7.61, df=3) and P<0.002 for GSH-Px (F= 9.162; df=3). *P<0.05, **P<0.01, ***P<0.001, as compared to C. a, P<0.05, as compared to E25. b, P<0.05, as compared to E50, c, P<0.05, as compared to a similar ethanol dose at 24h.
Treatments with ethanol at concentrations of 50 or 100 mM for a period of 24 or 48 h increased in a dose-dependent manner the production of ROS and O2- and the nitrite levels in cultured neurons (Fig. 2B–D). Ethanol concentrations of 50–100 mM increased the O2- level between 1.6–2.6-fold, the ROS level between 3.7–4.5-fold of control. Ethanol concentrations of 25 to 100 mM also increased nitrite levels about 2–4.7-fold of control at 24 h. The stimulatory effects of 50 and 100 mM concentrations of ethanol on the ROS, but not O2- and nitrite levels, were increased at 48 h than that at 24 h.
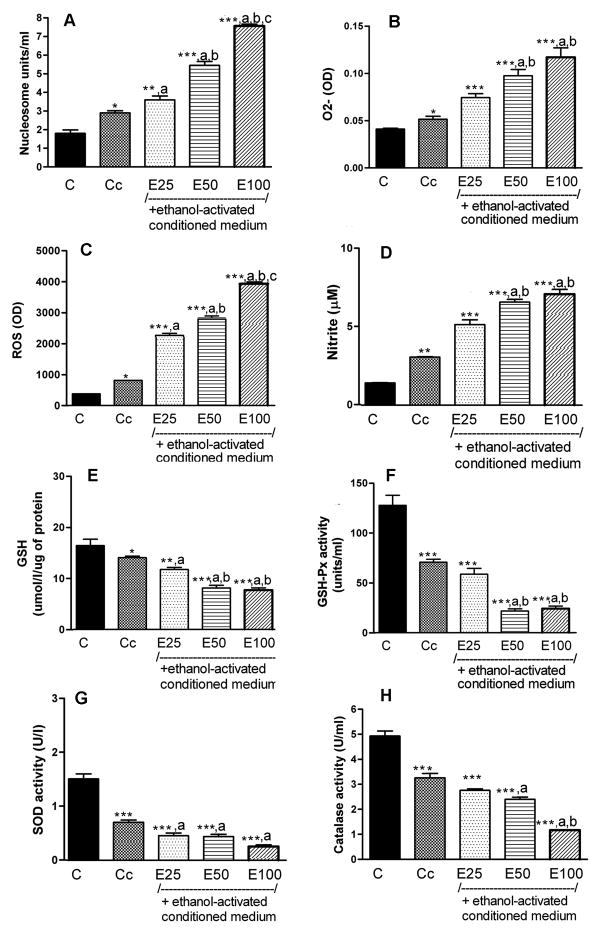
Figure 2.
Effects of ethanol-treated conditioned medium from cultured microglial cells on the levels of nucleosome, intracellular production of reactive oxygen species (ROS), O2-, nitrite, GSH and the activity of GSH-Px, SOD and catalase in hypothalamic neuronal cell cultures. Enriched fetal hypothalamic neuronal cell cultures (50 000 cells/well) were exposed to no ethanol (C), the conditioned medium from non-ethanol-treated microglial cells (Cc) and the conditioned medium collected from microglial cells treated with various doses of ethanol (E1–25 mM, E2–50 mM or E3–100 mM) for a period of 24 h. The cell extracts were used to measure nucleosome (A), O2- (B), ROS (C), nitrite (D), GSH (E), GSH-Px (F), SOD (G) and catalase activity (H). N=5–8; *P<0.05, **P<0.01, ***P<0.001, as compared to C. a, P<0.05, as compared to Cc. b, P<0.05, as compared to E25. c, P<0.05, as compared to E50.
For the determination of antioxidant effects in neuronal cell cultures after different doses of ethanol treatments, we measured cellular levels of glutathione and glutathione producing enzyme activity (GSH-Px). As shown in Fig. 1E and F, ethanol at a dose-range of 25–100 mM was able to reduce the GSH level to between 0.8–1.6-fold and GSH-Px level to 1.5 to 2.8-fold of control at 24 h. The inhibitory effects of ethanol on GSH and GSH-Px levels were pronounced at 48h than those at 24 h.
We also determined ethanol effects on catalase and SOD activities that are known to be involved in production of antioxidant molecules. As shown in Fig. 1G and H, both 50 and 100 mM doses of ethanol decreased these enzymes’ levels between 1.2–1.4-fold of the control levels at 24 and 48 h.
Microglia’s ability to alter ethanol’s effects on cellular apoptosis and cellular levels of antioxidants and oxidants in neuronal cell cultures
Since microglial cells are known to participate in ethanol-induced apoptosis of hypothalamic neurons, we determined the effects of control and ethanol-treated (50 mM for a period of 24 h) microglia cell culture conditioned medium on cellular apoptosis and on antioxidant and oxidant parameters in enriched neurons.
Data shown in Fig. 2A indicated that the conditioned medium from controlled microglial cells increased (1.6-fold) nucleosome levels in neuronal cell cultures. The potency of the microglial conditioned medium to induce neuronal apoptosis markedly increased following activation of microglial cells by ethanol. The levels of nucleosome were highest when neuronal cell cultures were challenged with the conditioned medium from the microglial cell culture treated with 100 mM ethanol (5-fold of control) followed by the medium from cultures treated with 50 mM ethanol (3.5-fold of control), 25 mM ethanol (2.1-fold of control) or 0 mM ethanol (1.4-fold of control)
Figure 2B–D shows that conditioned medium samples collected from microglial cells treated with various doses of ethanol dose-dependently increased the levels of O2-(ethanol, 0–100 mM; 1.25–3.0-fold of control), ROS (ethanol, 0–100 mM; 2.1–8.0-fold of control) and nitrite (ethanol, 0–100 mM; 2.0–4.6-fold of control).
Ethanol-treated microglia cell conditioned medium also decreased the antioxidants’ stress in neuronal cell cultures (Fig. 2E–H). Conditioned medium samples collected from microglial cells treated with various doses of ethanol dose-dependently decreased the level of GSH (ethanol, 0–100 mM; 1.2-0-2.2-fold of control), GSH-Px (ethanol, 0–100 mM; 1.2-0-2.2-fold of control), SOD (ethanol, 0–100 mM; 2.0 5.0-fold of control) and catalase (ethanol, 0–100 mM; 1.4–3.2-fold of control).
Ethanol actions on apoptotic death and the production of oxidative molecules appeared to be enhanced markedly in the presence of ethanol-treated microglial culture medium (compare data of Figure 1 and 2).
Antioxidant’s ability to antagonize the effects of ethanol on oxidative stress and apoptosis of neurons caused by ethanol or ethanol-treated microglial culture medium
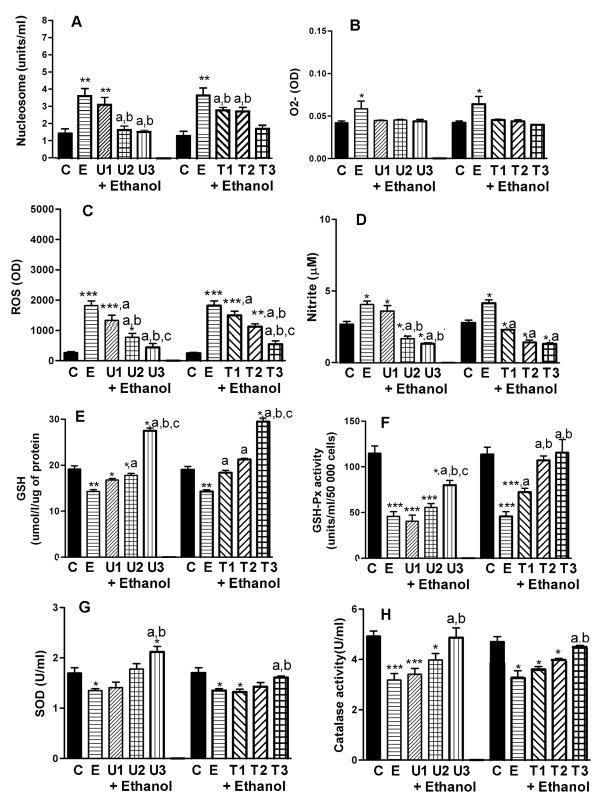
Two well-established antioxidants (Trolox and EUK-134) were used to determine whether suppression of oxidative stress blocks ethanol’s ability to alter the cellular process of neuronal apoptosis in the absence or in the presence of ethanol-treated microglial culture medium influence or both. We have chosen to use a 50 mM dose of ethanol in this study, since this dose was considered a moderate and effective dose of ethanol in altering oxidative stress and neurotoxicity. We also used a 24 h treatment period, since this time period was effective in altering oxidative stress of neurons and their apoptosis.
As shown on Fig. 3A, Trolox or EUK-134 dose-dependently decreased ethanol-induced apoptosis in cultured neurons. Trolox and EUK-134 inhibited the production of O2- (Fig. 3B), ROS (Fig. 3C) and nitrite levels (Fig. 3D). In addition, Trolox and EUK-134 increased the levels of GSH (Fig. 3E), GSH-Px (Fig. 3F), SOD (Fig. 3G) and catalase activity (Fig. 3H). Taken together, these data illustrated that both antioxidants reversed ethanol’s effects on apoptosis and oxidative stress in hypothalamic neurons.
Figure 3.
Effects of antioxidants EUK-134 or Trolox on ethanol-induced changes in the level of nucleosome, intracellular production of reactive oxygen species (ROS), O2-, nitrite, GSH and the activity of GSH-Px, SOD and catalase in hypothalamic neuronal cell cultures. Enriched fetal hypothalamic neuronal cell cultures (50 000 cells/well) were exposed to no ethanol (C) or ethanol (50 mM) with various doses of EUK-134 (U1, 0.1μM EUK-134: U2, 1.0 μM EUK-134; or U3, 10.0 μM EUK-134) or Trolox (T1, 0.1 μM Trolox; T2, 1.0 μM Trolox or T3, 10.0 μM Trolox) or without antioxidants (U or T-0; E) for a period of 24 h. The cell extracts were used to measure nucleosome (A), O2-(B), ROS (C), nitrite (D), GSH (E), GSH-Px (F), SOD (G) and catalase activity (H). N=5–8; *P<0.05, **P<0.01, ***P<0.001, as compared to C. a, P<0.05, as compared to E. b, P<0.05, as compared to U1 or T1. c, P<0.05, as compared to U2 or T2.
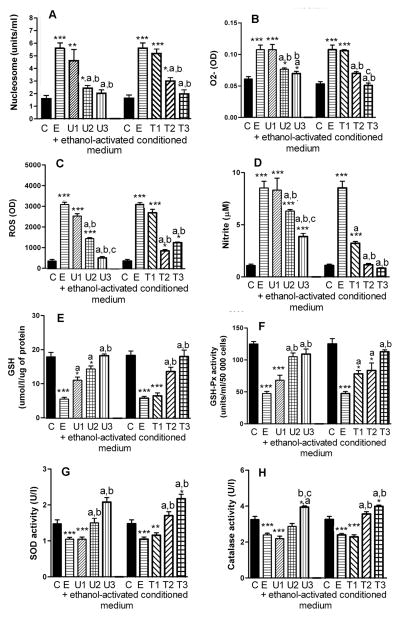
We demonstrated in Fig. 2 that, upon activation by ethanol, microglia cells are capable of promoting apoptosis by altering the oxidative stress of neurons. In this study, we determined whether Trolox and EUK-134 suppress the effects of ethanol-treated microglia culture medium on neuronal cell functions. Treatment with EUK-134 or Trolox dose-dependently reduced the microglial cell culture medium’s ability to enhance the level of nucleosome (Fig. 4A), O2- (Fig. 4B), ROS (Fig. 4C) and nitrite (Fig. 4D). These antioxidants increased the levels of GSH (Fig. 4E) and GSH-Px (Fig. 4F), SOD (Figure 4G) and catalase (Fig. 4H). It is noteworthy, that the 10μM dose of Trolox or EUK-134 completely prevented ethanol-activated microglial oxidative and apoptotic actions on hypothalamic neurons.
Figure 4.
Effects of antioxidants EUK-134 (U) or Trolox (T) on ethanol-treated microglial culture medium-induced changes in the nucleosome, intracellular production of reactive oxygen species (ROS), O2-, nitrite, GSH and the activity of GSH-Px, SOD and catalase in hypothalamic neuronal cell cultures. Enriched fetal hypothalamic neuronal cell cultures (50 000 cells/well) were exposed to no ethanol (C) or an ethanol (50 mM)-treated conditioned medium with various doses of EUK-134 (U1, 0.1μM EUK-134: U2, 1.0 μM EUK-134; or U3, 10.0 μM EUK-134) or Trolox (T1, 0.1 μM Trolox; T2, 1.0 μM Trolox or T3, 10.0 μM Trolox) or without antioxidants (U or T-0; E) for a period of 24 h. The cell extracts were used to measure nucleosome (A), O2- (B), ROS (C), nitrite (D), GSH (E), GSH-Px (F), SOD (G) and catalase activity (H). N=5–8; *P<0.05, **P<0.01, ***P<0.001, as compared to C. a, P<0.05, as compared to E. b, P<0.05, as compared to U1 or T1. c, P<0.05, as compared to U2 or T2.
DISCUSSION
In this study we demonstrated that ethanol generated on oxidative stress in cultured fetal hypothalamic neuronal cell cultures and increased the apoptotic cell death. These effects of ethanol were promoted by the presence of microglia. Furthermore, we showed here that ethanol induces oxidative stress in neurons by increasing the cellular production of O2-, ROS and nitrite while decreasing the level of GSH and the cellular activity of GSH-Px, catalase and SOD activities. In addition, the antioxidants EUK-134 and Trolox protected developing hypothalamic neurons from oxidative stress and cellular apoptosis caused by ethanol or ethanol-treated microglia medium. Therefore, the increased production of ROS and nitrite and decreased levels of cellular glutathione in the cultured hypothalamic neurons observed during the treatments with ethanol and ethanol-treated microglial culture medium likely affect the apoptotic cell death. These data suggest that exposure of developing hypothalamic neurons to ethanol increases cellular apoptosis via effects on the oxidative stress of neurons directly and via increasing production of microglia-derived factor(s).
In the present study we demonstrated that ethanol and ethanol-treated microglial culture medium decreased the levels of antioxidant glutathione (GSH) and the activity of GSH-Px enzyme in neurons. GSH plays the role of endogenous antioxidant (Dringen et al., 1999). The role of GSH in ethanol-related toxicity is well documented in the liver, and there is evidence that GSH depletion can enhance an apoptotic response to CYP2E1-mediated oxidative stress in this organ (Wu and Cederbaum, 2001). In fetal cortical neurons, CYP2E was only slightly expressed, and the source of the ethanol-related ROS increase was undetermined. These studies have illustrated that normalizing or enhancing the cultured cerebral cortical neuron GSH, either by administration of N-acetylcysteine or by coculturing with astrocytes, can prevent ethanol-mediated oxidative stress and subsequent apoptotic death (Ramachandran et al., 2003; Watts et al., 2005). Maffi et al. (2008) suggested that GSH content is an important predictor of neuronal sensitivity to ethanol-mediated oxidative stress and subsequent cell death. The view that GSH content is important for the prevention of ethanol neurotoxicity in the hypothalamus is supported by the present data, which showed a decreased GSH level and activity of GSH-Px during ethanol-induced neuronal apoptosis, and an increased GSH levels and the activity of GSH-Px during protection from ethanol’s neurotoxicity by EUK-134 or Trolox treatments.
This study also focused on ethanol’s effects on the production of ROS, O2- and nitrite in cultured fetal hypothalamic neurons. We used the dichlorofluorescein (DCF) measurement assay to determine ROS production in the hypothalamic cells. It is well established that the increased DCF fluorescence is generally taken as a measure of enhanced steady-state levels of H2O2. In addition to detecting production of ROS in living cells, this technique is optimal for defining rapid onset of cellular events. Previously it has been shown that acute and chronic ethanol treatments increased production of ROS in a variety of cells and species, including humans (Garcia-Ruiz et al., 1995). ROS have been implicated in induction of oxidative stress and as a causal factor in the enhanced apoptosis in fetal brains exposed to ethanol in utero (Ramachandran et al., 2001). Recent studies also suggested that nitrite from iNOS expression strongly synergizes with hypoxia to induce apoptosis in neuronal cell cultures because nitrite inhibits cytochrome oxidase in competition with oxygen, resulting in glutamate release and cytotoxicity (Brown and Neher, 2010). We have previously shown that ethanol increased nitrite production and iNOS in primary hypothalamic cultures (Chen et al., 2003). In this study, we have also shown that ethanol increased nitrite levels in hypothalamic neurons. There is a balance between the flux of superoxide anion and the flux of nitrite into cells for the oxidative stress. It has been demonstrated that the level of oxidative stress is lowest when nitrite flux is high and the flux of SO-anion is low (Offer et al., 2000). Therefore, it could be hypothesized that ethanol may cause more flux of superoxide (SO)-anion and lower flux of nitrite, which activates the oxidative stress in hypothalamic neurons.
The causal relationship of oxidative stress in ethanol-induced hypothalamic neuronal apoptosis also come from our findings that two antioxidants, EUK-134 and Trolx, prevent ethanol-activated apoptotic cell death and the production of ROS, O2- and NO in neurons. The salen manganese complexes (EUKs), synthetic SOD and catalase mimetics, are known to catalytically eliminate both superoxide and hydrogen peroxide (Baker et al., 1998; Baudry et al., 1993). These compounds have been shown to be neuroprotective in several animal models, including those for inflammatory autoimmune disease (Malfroy et al., 1997), amyotrophic lateral sclerosis (Jung et al., 2001), ischemia (Gonzalez et al., 1995), epilepsy (Rong et al., 1999). One of the well-recognized substances from the group of EUKs is EUK-134. It is a combined superoxide dismutase/catalase mimetic, and opposes the effects of H2O2, thereby decreasing phosphorylation levels of signaling enzymes such as Akt, Src tyrosine kinase, and ERK. Treatment of rats with EUK-134 significantly reduces DNA-binding activity of two transcription factors, activator protein-1 (AP-1) and NF-κB, and attenuates kainate-induced neuropathology (Baker et al., 1998). Our present data demonstrate that EUK-134 reversed the effects of ethanol and ethanol-treated microglial cultured medium on nucleosome, ROS, NO and O2-, as well as on GSH and GSH-Rx in neurons. We also showed here that Trolox, which is a well-known analog of vitamin E with antioxidant activity, decreased apoptosis as well as ROS, NO and O2- production and increased the GSH levels and GSH-Px activity in ethanol-treated hypothalamic neurons. Neither EUK-134 or Trolox, at the concentrations used, were stimulatory or inhibitory for neuronal survival without ethanol. This is the first demonstration of the effects of EUK-134 and Trolox in prevention of ethanol- and ethanol-treated microglial culture medium-induced oxidative stress and apoptosis in hypothalamic neurons. The present data with two well established antioxidants suggest that ethanol may cause a disruption of balance between the endogenous antioxidant defense system and the reactive oxygen species which appears to be one of the major factors involved in the ethanol-activated apoptosis in developing hypothalamic neurons.
In this study we have shown that the conditioned medium collected from ethanol-activated microglial cell conditioned medium elevated more amount of oxidative substances and cellular apoptosis than ethanol alone. These data presented provide evidence that microglial cells-derived substance(s) enhances the effects of ethanol on apoptosis, via disruption of the oxidant versus antioxidant balance in developing hypothalamic neurons. Activation of glial cells is a critical event in many neuroinflammatory processes (Block et al., 2007; Streit et al., 2004) and has been linked to neurodegeneration through the production of neurotoxic factors, such as proinflammatory cytokines and free radicals (Combs et al., 1999; McDonald et al., 1997; Zou and Crews, 2010). We previously reported that ethanol treated cultured microglia produce TNF-α, IL-1β, MIP-1, MIP-2 (Boyadjieva and Sarkar, 2010). Microglial cells also have been shown to produce ROS and NO (Block et al., 2007). Whether or not microglia induces oxidative stress and apoptosis in neurons via microglia-derived proinflammatory cytokines and or free radicals remains to be elucidated.
Taken together, these data provide strong evidence that ethanol-treated microglial culture medium enhanced the production of ROS and apoptosis in developing hypothalamic neurons. Our demonstration of the role of activated microglia as well as the protective effect of antioxidants in neuronal apoptosis may represent a future approach for understanding the fetal alcohol spectrum diseases. Toward that aim, it will be necessary to extend the present in vitro study to the hypothalamic system in vivo, and to identify the microglial factors that induce oxidative stress and apoptotic death in neurons.
Acknowledgments
This work was supported by National Institute of Health Grants R37 AA08757.
References
- Baker K, Marcus CB, Huffman K, Kruk H, Malfroy B, Doctrow SR, Gonzalez PK, Zhuang J, Benson PF, Menconi MJ, Fink MP, Baudry M, Etienne S, Bruce A, Palucki M, Jacobsen E. Synthetic combined superoxide dismutase/catalase mimetics are protective as a delayed treatment in a rat stroke model: A key role for reactive oxygen species in ischemic brain injury. J Pharmacol Exp Ther. 1998;284:215–221. [PubMed] [Google Scholar]
- Baker RA, Shoemaker WJ. Effect of prenatal ethanol and stress on levels of beta-endorphin in different brain regions of the rat. Alcohol Clin Exp Res. 1995;19:727–734. doi: 10.1111/j.1530-0277.1995.tb01574.x. [DOI] [PubMed] [Google Scholar]
- Barson JR, Morganstern I, Leibowitz SF. Galanin and consummatory behavior: special relationship with dietary fat, alcohol and circulating lipids. EXS. 2010;102:87–111. doi: 10.1007/978-3-0346-0228-0_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry M, Etienne S, Bruce A, Palucki M, Jacobsen E, Malfroy B. Biochem Biophys Res Commun. 1993;192:964–968. doi: 10.1006/bbrc.1993.1509. [DOI] [PubMed] [Google Scholar]
- Bird DN, Sato AK, Knee DS, Uyehara CF, Person DA, Claybaugh JR. Effects of prenatal ethanol exposure and sex on the arginine vasopressin response to hemorrhage in the rat. Am J Physiol Regul Integr Comp Physiol. 2006;291:R77–82. doi: 10.1152/ajpregu.00740.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Boyadjieva NI, Ortigüela M, Arjona A, Cheng X, Sarkar DK. Beta-endorphin neuronal cell transplant reduces corticotropin releasing hormone hyperresponse to lipopolysaccharide and eliminates natural killer cell functional deficiencies in fetal alcohol exposed rats. Alcohol Clin Exp Res. 2009;33:931–937. doi: 10.1111/j.1530-0277.2009.00911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyadjieva NI, Sarkar DK. Role of microglia in ethanol’s apoptotic action on hypothalamic neuronal cells in primary cultures. Alcohol Clin Exp Res. 2010;34:1835–1842. doi: 10.1111/j.1530-0277.2010.01271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GC, Neher JJ. Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol Neurobiol. 2010;41:242–247. doi: 10.1007/s12035-010-8105-9. [DOI] [PubMed] [Google Scholar]
- Brown RE. An Introduction to Neuroendocrinology. Cambridge University Press; New York, NY: 1998. pp. 41–43. [Google Scholar]
- Chao CC, Hu S, Molitor TW, Shaskan EG, Peterson PK. Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J Immunol. 1992;149:2736–2741. [PubMed] [Google Scholar]
- Chen CP, Kuhn P, Advis JP, Sarkar DK. Prenatal ethanol exposure alters the expression of period genes governing the circadian function of beta-endorphin neurons in the hypothalamus. J Neurochem. 2006;97:1026–1033. doi: 10.1111/j.1471-4159.2006.03839.x. [DOI] [PubMed] [Google Scholar]
- Chen WJ, Maier SE, Parnell SE, West JR. Alcohol and the developing brain: neuroanatomical studies. Alcohol Res Health. 2003;27:174–180. [PMC free article] [PubMed] [Google Scholar]
- Cohen-Kerem R, Koren G. Antioxidants and fetal protection against ethanol teratogenicity. I. Review of the experimental data and implications to humans. Neurotoxicol Teratol. 2003;25:1–9. doi: 10.1016/s0892-0362(02)00324-0. [DOI] [PubMed] [Google Scholar]
- Colton CA, Gilbert DL. Production of superoxide anions by a CNS macrophage, the microglia. FEBS Lett. 1987;223:284–288. doi: 10.1016/0014-5793(87)80305-8. [DOI] [PubMed] [Google Scholar]
- Combs CK, Johnson DE, Cannady SB, Lehman TM, Landreth GE. Identification of microglial signal transduction pathways mediating a neurotoxic response to amyloidogenic fragments of beta-amyloid and prion proteins. J Neurosci. 1999;19:928–939. doi: 10.1523/JNEUROSCI.19-03-00928.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De A, Boyadjieva NI, Pastorcic M, Reddy BV, Sarkar DK. Cyclic AMP and ethanol interact to control apoptosis and differentiation in hypothalamic beta-endorphin neurons. J Biol Chem. 1994;269:26697–26705. [PubMed] [Google Scholar]
- Dringen R, Kussmaul L, Gutterer JM, Hirrlinger J, Hamprecht B. The glutathione system of peroxide detoxification is less efficient in neurons than in astroglial cells. J Neurochem. 1999;72:2523–2530. doi: 10.1046/j.1471-4159.1999.0722523.x. [DOI] [PubMed] [Google Scholar]
- García-Ruiz C, Morales A, Colell A, Ballesta A, Rodés J, Kaplowitz N, Fernández-Checa JC. Feeding S-adenosyl-L-methionine attenuates both ethanol-induced depletion of mitochondrial glutathione and mitochondrial dysfunction in periportal and perivenous rat hepatocytes. Hepatology. 1995;21:207–214. doi: 10.1002/hep.1840210133. [DOI] [PubMed] [Google Scholar]
- Gonzalez PK, Zhuang J, Doctrow SR, Malfroy B, Benson PF, Menconi MJ, Fink MP, Baudry M, Etienne S, Bruce A, Palucki M, Jacobsen E. J Pharmacol Exp Ther. 1995;275:798–806. [PubMed] [Google Scholar]
- Goodlett CR, Thomas JD, West JR. Long-term deficits in cerebellar growth and rotarod performance of rats following “binge-like” alcohol exposure during the neonatal brain growth spurt. Neurotoxicol Teratol. 1991;13:69–74. doi: 10.1016/0892-0362(91)90029-v. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- Jung C, Rong Y, Doctrow S, Baudry M, Malfroy B, Xu Z. Neurosci Lett. 2001;304:157–160. doi: 10.1016/s0304-3940(01)01784-0. [DOI] [PubMed] [Google Scholar]
- Lee S, Schmidt D, Tilders F, Rivier C. Increased activity of the hypothalamic-pituitary-adrenal axis of rats exposed to alcohol in utero: role of altered pituitary and hypothalamic function. Mol Cell Neurosci. 2000;16:515–528. doi: 10.1006/mcne.2000.0890. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Maffi SK, Rathinam ML, Cherian PP, Pate W, Hamby-Mason R, Schenker S. Henderson GI. Glutathione content as a potential mediator of the vulnerability of cultured fetal cortical neurons to ethanol-induced apoptosis. J Neurosci Res. 2008;86:1064–1076. doi: 10.1002/jnr.21562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfroy B, Doctrow SR, Orr PL, Tocco G, Fedoseyeva EV, Benichou G. Cell Immunol. 1997;177:62–68. doi: 10.1006/cimm.1997.1091. [DOI] [PubMed] [Google Scholar]
- McDonald DR, Brunden KR, Landreth GE. Amyloid fibrils activate tyrosine kinase-dependent signaling and superoxide production in microglia. J Neurosci. 1997;17:2284–2294. doi: 10.1523/JNEUROSCI.17-07-02284.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald DR, Brunden KR, Landreth GE. Amyloid fibrils activate tyrosine kinase-dependent signaling and superoxide production in microglia. J Neurosci. 1997;17:2284–2294. doi: 10.1523/JNEUROSCI.17-07-02284.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, Potempa G. Numbers of neurons and glia in mature rat somatosensory cortex: effects of prenatal exposure to ethanol. J Comp Neurol. 1990;293:92–102. doi: 10.1002/cne.902930108. [DOI] [PubMed] [Google Scholar]
- Min KJ, Jou I, Joe E. Plasminogen-induced IL-1beta and TNF-alpha production in microglia is regulated by reactive oxygen species. Biochem Biophys Res Commun. 2003;312:969–974. doi: 10.1016/j.bbrc.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Montoliu C, Sancho-Tello M, Azorin I, Burgal M, Vallés S, Renau-Piqueras J, Guerri C. Ethanol increases cytochrome P4502E1 and induces oxidative stress in astrocytes. J Neurochem. 1995;65:2561–2570. doi: 10.1046/j.1471-4159.1995.65062561.x. [DOI] [PubMed] [Google Scholar]
- Offer T, Russo A, Samuni A. The pro-oxidative activity of SOD and nitroxide SOD mimics. FASEB J. 2000;14:1215–1223. doi: 10.1096/fasebj.14.9.1215. [DOI] [PubMed] [Google Scholar]
- Ramachandran V, Perez A, Chen J, Senthil D, Schenker S, Henderson GI. In utero ethanol exposure causes mitochondrial dysfunction, which can result in apoptotic cell death in fetal brain: a potential role for 4-hydroxynonenal. Alcohol Clin Exp Res. 2001;25:862–871. [PubMed] [Google Scholar]
- Ramachandran V, Watts LT, Maffi SK, Chen J, Schenker S, Henderson G. Ethanol-induced oxidative stress precedes mitochondrially mediated apoptotic death of cultured fetal cortical neurons. J Neurosci Res. 2003;74:577–588. doi: 10.1002/jnr.10767. [DOI] [PubMed] [Google Scholar]
- Rathinam ML, Watts LT, Stark AA, Mahimainathan L, Stewart J, Schenker S, Henderson GI. Astrocyte control of fetal cortical neuron glutathione homeostasis: up-regulation by ethanol. J Neurochem. 2006;96:1289–1300. doi: 10.1111/j.1471-4159.2006.03674.x. [DOI] [PubMed] [Google Scholar]
- Reinke LA, Lai EK, DuBose CM, McCay PB. Reactive free radical generation in vivo in heart and liver of ethanol-fed rats: correlation with radical formation in vitro. Proc Natl Acad Sci U S A. 1987;84:9223–92227. doi: 10.1073/pnas.84.24.9223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong Y, Doctrow SR, Tocco G, Baudry M. EUK-134, a synthetic superoxide dismutase and catalase mimetic, prevents oxidative stress and attenuates kainate-induced neuropathology. Proc Natl Acad Sci U S A. 1999;96:9897–9902. doi: 10.1073/pnas.96.17.9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas JC, Vigueras RM, Reyes G, Rojas P, Cintra L, Aguilar-Roblero R. Morphological changes produced by acute prenatal exposure to ethanol on the immunoreactive vasoactive intestinal polypeptide cells of the suprachiasmatic nucleus of the rat. Proc West Pharmacol Soc. 1999;42:75–76. [PubMed] [Google Scholar]
- Sarkar DK, Kuhn P, Marano J, Chen C, Boyadjieva N. Alcohol exposure during the developmental period induces beta-endorphin neuronal death and causes alteration in the opioid control of stress axis function. Endocrinology. 2007;148:2828–2834. doi: 10.1210/en.2006-1606. [DOI] [PubMed] [Google Scholar]
- Schlorff EC, Husain K, Somani SM. Dose- and time-dependent effects of ethanol on plasma antioxidant system in rat. Alcohol. 1999;17:97–105. doi: 10.1016/s0741-8329(98)00039-1. [DOI] [PubMed] [Google Scholar]
- Scott HC, Zoeller RT, Rudeen PK. Acute prenatal ethanol exposure and luteinizing hormone-releasing hormone messenger RNA expression in the fetal mouse brain. Alcohol Clin Exp Res. 1995;19:153–159. doi: 10.1111/j.1530-0277.1995.tb01484.x. [DOI] [PubMed] [Google Scholar]
- Stettner GM, Kubin L, Volgin DV. Antagonism of orexin 1 receptors eliminates motor hyperactivity and improves homing response acquisition in juvenile rats exposed to alcohol during early postnatal period. Behav Brain Res. 2011;221:324–328. doi: 10.1016/j.bbr.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Mrak RE, Griffin WS. Microglia and neuroinflammation: a pathological perspective. J Neuroinflammation. 2004;1:14. doi: 10.1186/1742-2094-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HK, Park UJ, Kim SY, Lee JH, Kim SU, Gwag BJ, Lee YB. Free radical production in CA1 neurons induces MIP-1alpha expression, microglia recruitment, and delayed neuronal death after transient forebrain ischemia. J Neurosci. 2008;28:1721–1727. doi: 10.1523/JNEUROSCI.4973-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts LT, Rathinam ML, Schenker S, Henderson GI. Astrocytes protect neurons from ethanol-induced oxidative stress and apoptotic death. J Neurosci Res. 2005;80:655–666. doi: 10.1002/jnr.20502. [DOI] [PubMed] [Google Scholar]
- West JR, Dewey SL, Pierce DR, Black AC., Jr Prenatal and early postnatal exposure to ethanol permanently alters the rat hippocampus. Ciba Found Symp. 1984;105:8–25. doi: 10.1002/9780470720868.ch2. [DOI] [PubMed] [Google Scholar]
- Wu D, Cederbaum AI. Removal of glutathione produces apoptosis and necrosis in HepG2 cells overexpressing CYP2E1. Alcohol Clin Exp Res. 2001;25:619–628. [PubMed] [Google Scholar]
- Zou J, Crews F. Induction of innate immune gene expression cascades in brain slice cultures by ethanol: key role of NF-κB and proinflammatory cytokines. Alcohol Clin Exp Res. 2010;34:777–789. doi: 10.1111/j.1530-0277.2010.01150.x. [DOI] [PubMed] [Google Scholar]