Abstract
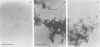
Human isolates of Actinomyces viscosus and Actinomyces naeslundii have been divided into six clusters in a numerical taxonomy study. Surface fibrils of strains representing these clusters were isolated and purified. Chemical analyses revealed that the major component of all fibrils was protein and that although differences in percentages of specific amino acid residues were found, the relative proportions of basic, acidic, polar uncharged, and nonpolar amino acids were rather similar among clusters. All of the fibrils except those from strain B236 (cluster 2) either failed to migrate or penetrated only slightly into gels during sodium dodecyl sulfate-polyacrylamide gel electrophoresis, even after boiling, reduction, or alkylation. Immunological studies by electron microscopic examination of fibril-antibody immunocomplexes, whole bacterial cell agglutination, inhibition of hemagglutination, and immunofluorescence by using antifibril antisera and antibodies demonstrated that strains of typical A. naeslundii (cluster 5) have a specific fibril-associated antigen(s) distinct from those of strains of other clusters. Cross-reactions for atypical A. naeslundii (cluster 3) were few. The fibrils from A. viscosus clusters 1, 2, 4, and 6 demonstrated several cross-reactions. By absorbing antifibril antibodies with cross-reactive strains it was possible to obtain cluster-specific antibodies, as determined by whole cell agglutination, only for cluster 5. Absorbed antifibril antisera for both A. naeslundii clusters 3 and 5 were specific by indirect immunofluorescence, whereas anti-cluster 1 fibril antisera cross-reacted only with other A. viscosus cluster representatives. Purification of Actinomyces fibrils by methods used for appendages of other species yields preparations containing common antigens among taxonomic groups. However, absorbing antifibril antisera, gamma globulin, or both has promise for producing cluster-specific reagents useful in identification.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Buchanan T. M. Antigenic heterogeneity of gonococcal pili. J Exp Med. 1975 Jun 1;141(6):1470–1475. doi: 10.1084/jem.141.6.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan T. M., Swanson J., Holmes K. K., Kraus S. J., Gotschlich E. C. Quantitative determination of antibody to gonococcal pili. Changes in antibody levels with gonococcal infection. J Clin Invest. 1973 Nov;52(11):2896–2909. doi: 10.1172/JCI107486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J. O., Barsumian E. L., Curl S. H., Vatter A. E., Sandberg A. L., Siraganian R. P. Detection and localization of a lectin on Actinomyces viscosus T14V by monoclonal antibodies. J Immunol. 1981 Oct;127(4):1318–1322. [PubMed] [Google Scholar]
- Cisar J. O., Kolenbrander P. E., McIntire F. C. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979 Jun;24(3):742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J. O., Vatter A. E., McIntire F. C. Identification of the virulence-associated antigen on the surface fibrils of Actinomyces viscosus T14. Infect Immun. 1978 Jan;19(1):312–319. doi: 10.1128/iai.19.1.312-319.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J. O., Vatter A. E. Surface fibrils (fimbriae) of Actinomyces viscosus T14V. Infect Immun. 1979 May;24(2):523–531. doi: 10.1128/iai.24.2.523-531.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Webb E. L., Wheeler T. T., Fischlschweiger W., Birdsell D. C., Mansheim B. J. Role of surface fimbriae (fibrils) in the adsorption of Actinomyces species to saliva-treated hydroxyapatite surfaces. Infect Immun. 1981 Sep;33(3):908–917. doi: 10.1128/iai.33.3.908-917.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello A. H., Cisar J. O., Kolenbrander P. E., Gabriel O. Neuraminidase-dependent hamagglutination of human erythrocytes by human strains of Actinomyces viscosus and Actinomyces naeslundii. Infect Immun. 1979 Nov;26(2):563–572. doi: 10.1128/iai.26.2.563-572.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P. Establishment and distribution of Actinomyces viscosus and Actinomyces naeslundii in the human oral cavity. Infect Immun. 1976 Nov;14(5):1119–1124. doi: 10.1128/iai.14.5.1119-1124.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Fillery E. D., Chan K. H., Grove D. A. Sialidase-enhanced lectin-like mechanism for Actinomyces viscosus and Actinomyces naeslundii hemagglutination. Infect Immun. 1980 Feb;27(2):335–343. doi: 10.1128/iai.27.2.335-343.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Walker D. L., Chan K. H. Association of long surface appendages with adherence-related functions of the gram-positive species Actinomyces naeslundii. J Bacteriol. 1978 Jun;134(3):1171–1175. doi: 10.1128/jb.134.3.1171-1175.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillery E. D., Bowden G. H., Hardie J. M. A comparison of strains of bacteria designated Actinomyces viscosus and Actinomyces naeslundii. Caries Res. 1978;12(6):299–312. doi: 10.1159/000260349. [DOI] [PubMed] [Google Scholar]
- Gerencser M. A., Slack J. M. Identification of human strains of Actinomyces viscosus. Appl Microbiol. 1969 Jul;18(1):80–87. doi: 10.1128/am.18.1.80-87.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerencser M. A., Slack J. M. Serological identification of Actinomyces using fluorescent antibody techniques. J Dent Res. 1976 Jan;55:A184–A191. doi: 10.1177/002203457605500110011. [DOI] [PubMed] [Google Scholar]
- Kolenbrander P. E., Williams B. L. Lactose-reversible coaggregation between oral actinomycetes and Streptococcus sanguis. Infect Immun. 1981 Jul;33(1):95–102. doi: 10.1128/iai.33.1.95-102.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Nurmiaho E. L., Ranta H., Edén C. S. New Method for isolation of immunologically pure pili from Escherichia coli. Infect Immun. 1980 Feb;27(2):569–575. doi: 10.1128/iai.27.2.569-575.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumazawa N., Yanagawa R. Chemical properties of the pili of Corynebacterium renale. Infect Immun. 1972 Jan;5(1):27–30. doi: 10.1128/iai.5.1.27-30.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai C. H., Listgarten M. A. Immune labeling of certain strains of Actinomyces naeslundii and Actinomyces viscosus by fluorescence and electron microscopy. Infect Immun. 1979 Sep;25(3):1016–1028. doi: 10.1128/iai.25.3.1016-1028.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda N., Ellen R. P., Grove D. A. Purification and characterization of surface fibrils from taxonomically typical Actinomyces viscosus WVU627. J Bacteriol. 1981 Sep;147(3):1095–1104. doi: 10.1128/jb.147.3.1095-1104.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire F. C., Vatter A. E., Baros J., Arnold J. Mechanism of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34. Infect Immun. 1978 Sep;21(3):978–988. doi: 10.1128/iai.21.3.978-988.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhall W. J., Wilde C. E., 3rd, Sawyer W. D., Haak R. A. High-molecular-weight antigenic protein complex in the outer membrane of Neisseria gonorrhoeae. Infect Immun. 1980 Feb;27(2):475–482. doi: 10.1128/iai.27.2.475-482.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revis G. J., Vatter A. E., Crowle A. J., Cisar J. O. Antibodies against the Ag2 fimbriae of Actinomyces viscosus T14V inhibit lactose-sensitive bacterial adherence. Infect Immun. 1982 Jun;36(3):1217–1222. doi: 10.1128/iai.36.3.1217-1222.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler T. T., Clark W. B. Fibril-mediated adherence of Actinomyces viscosus to saliva-treated hydroxyapatite. Infect Immun. 1980 May;28(2):577–584. doi: 10.1128/iai.28.2.577-584.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]