Abstract
Targeting of therapeutic agents to molecular markers expressed on the surface of cells requiring clinical intervention holds promise to improve specificity of delivery, enhancing therapeutic effects while decreasing potential damage to healthy tissues. Drug targeting to cellular receptors involved in endocytic transport facilitates intracellular delivery, a requirement for a number of therapeutic goals. However, after several decades of experimental design, there is still considerable controversy on the practical outcome of drug targeting strategies. The plethora of factors contributing to the relative efficacy of targeting makes the success of these approaches hardly predictable. Lack of fully specific targets, along with selection of targets with spatial and temporal expression well aligned to interventional requirements, pose difficulties to this process. Selection of adequate sub-molecular target epitopes determines accessibility for anchoring of drug conjugates and bulkier drug carriers, as well as proper signaling for uptake within the cell. Targeting design must adapt to physiological variables of blood flow, disease status, and tissue architecture by accommodating physicochemical parameters such as carrier composition, functionalization, geometry, and avidity. In many cases, opposite features need to meet a balance, e.g., sustained circulation versus efficient targeting, penetration through tissues versus uptake within cells, internalization within endocytic compartment to avoid efflux pumps versus accessibility to molecular targets within the cytosol, etc. Detailed characterization of these complex physiological factors and design parameters, along with a deep understanding of the mechanisms governing the interaction of targeted drugs and carriers with the biological environment, are necessary steps toward achieving efficient drug targeting systems.
Keywords: Drug delivery, active targeting, ligand, cell surface binding, internalization, subcellular transport
INTRODUCTION
A great variety of complex and intertwined factors governs the efficacy of drugs aimed to provide prophylaxis or, most commonly, therapeutic alleviation of clinical conditions. This includes intrinsic drug features, such as the specificity of action of the drug itself and properties such as its molecular size and chemical composition, determining its overall solubility in body fluids, penetration within tissues, and uptake by cells (reviewed by [1, 2]). In addition to this, the therapeutic outcome largely depends on various processes related to the handling of the drug by the body, which determine drug circulation half-life, as well as drug clearance by certain organs, potential metabolic transformation, degradation, and disposal [1, 2]. New and refined means of drug discovery and development continue to render agents with novel and more powerful therapeutic propertie., In addition, the design of systems capable of more efficiently and safely carrying and delivering drugs to areas of the body requiring intervention offers the opportunity to improve not only the overall therapeutic outcome of the new drugs being discovered, but also that of agents that, despite been clinically approved, display otherwise sub-optimal effects. Targeting of drugs with desirable properties to precise sites of the body affected by disease holds, therefore, great promise with regard to achieving enhanced therapeutic results while reducing side effects. This provides a means to lower the administered dose and, in turn, the cost of clinical treatments. As such, drug targeting is a very active area of research and development.
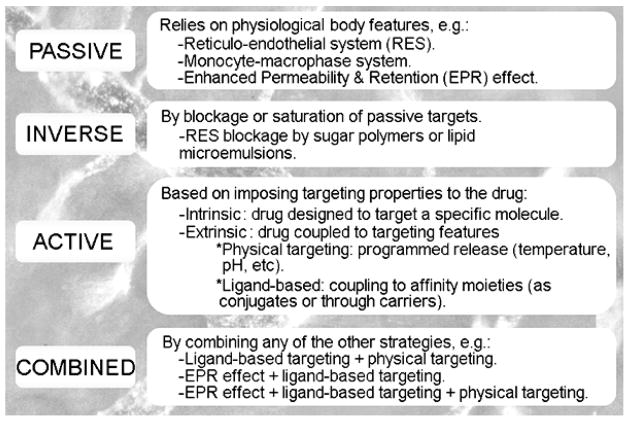
The concept of drug targeting was first proposed early in the twentieth century by Paul Ehrlich, who coined the term “magic bullet”, referring to a theoretical entity composed of a therapeutic agent linked to a component capable of recognizing a disease target, hence, providing precise transport of the drug (reviewed by [3]). This notion became a plausible option after achieving production of monoclonal antibodies in the early 1970’s [4], supporting high affinity and specificity of binding toward particular antigens of interest. Since then, most prominently during the last two decades, the seminal idea of drug targeting has greatly evolved to encompass a great variety of strategies that range from relatively simple concepts, similar to those devised by Ehrlich, to complex approaches involving design of new materials with highly controlled properties (reviewed by [5, 6]). These strategies are often classified within four major categories, including passive, inverse, active, and combined targeting (Figure 1).
Figure 1.
Drug Targeting Strategies.
Passive targeting refers to the accumulation of drugs into particular regions of the body, due to the natural features and physiological role of said tissues. This is the case for drug accumulation in organs of the reticulo-endothelial system (RES), mainly the liver and spleen, which “capture” foreign substances and objects that reach the systemic circulation, as well as the monocyte/macrophage system at the cellular level, given the specialization of these cells in taking into their bodies said foreign materials for disposal (reviewed by [7, 8]). Drugs aimed to cope with pathological conditions in these tissues accumulate at these sites passively. Another common example of passive targeting is illustrated by the enhanced permeability and retention (EPR) effect associated to the tumors’ vasculature (first described by [9]), where the loose junctions between endothelial cells that line the tumor blood vessels allow passive leakage of circulating drugs into the tumor parenchyma. Since no targeting properties are “externally” imposed on the drug itself, passive targeting is often referred to as no targeting.
Inverse targeting is related to the previous strategy in that the goal of this approach is to block sites in the body associated to passive targeting, to allow a drug to better accumulate in other areas. This has been attained by administering certain sugar polymers or lipid microemulsions that saturate the RES prior to administering a therapeutic compound of interest, shifting its biodistribution pattern [10].
The most recognized form of targeting is that where targeting properties are imposed on the drug, which is known as active targeting. This approach comprises strategies aimed to design drugs that exert very specific activities toward precise molecules in the body, whose structure/function needs to be therapeutically modified. Hence, this targeting is an intrinsic signature of the drug itself. Most other active targeting approaches, though, refer to strategies where the targeting property is extrinsically imposed on the drug, e.g., by coupling to other component possessing targeting features (reviewed by [6]). In this case, a drug can be coupled to a component that does not display affinity and binding toward a particular target, but allows for programmed release of the drug under particular environmental signatures of the disease site, such as temperature, pH, etc., which is know as physical targeting (reviewed by [5, 11, 12]. In other cases, active targeting is achieved by coupling the drug to a component (called ligand) that displays affinity and therefore binds to a particular element present at the disease site (reviewed by [13]). Furthermore, ligand-based targeting can be achieved by either coupling the drug to the targeting ligand directly, e.g., biologically or by chemical conjugation, or indirectly through a carrier that encapsulates the drug and displays the targeting ligand (reviewed by [14–17]). Such strategies of ligand-based targeting are the most commonly referred forms of active targeting, and many authors use the term “active targeting” to refer to this type of approach.
Furthermore, numerous approaches combine several elements of the drug targeting categories described above, such as the case of targeted delivery of cancer therapeutics, where relatively insoluble and toxic drugs can be encapsulated into pH-sensitive polymeric materials coupled to ligands that recognize cancer markers (reviewed by [6]). Such composites can favor the solubility and diminish the toxicity of chemotherapeutics, accumulate at tumor sites due to the EPR effect, bind to and enter cancer cells driven by the ligand, where the drug can be released due to the low pH of the cancer-cell environment or within intracellular lysosomal compartments. This illustrates the degree of flexibility and potential for fine-tune design of targeted drug delivery systems.
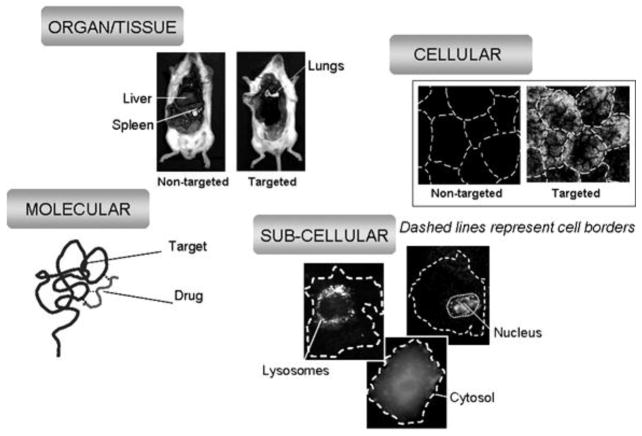
In addition, all of these strategies of drug targeting need to be considered under the light of the different levels of complexity and organization encountered in biological systems; that is, from the macro-scale view of organs and tissues, to the molecular and even atomic level of drug interaction with its precise targets (Figure 2). A site affected by disease is typically confined to a particular organ or tissue in the body (e.g., the brain versus the lung or the liver), likely associated to particular cell type within that organ or tissue (e.g., dopaminergic neurons versus other neurons or glial cells in the brain) and, moreover, located in certain sub-cellular compartment (e.g., the nucleus versus cytosol, the mitochondria versus the Golgi apparatus, etc). While the drug must attain specific and effective action against the molecular determinant involved in a disease, drug targeting strategies must overcome challenges posed by a variety of barriers in the body in order provide safe and efficient transport of the therapeutic agent from the organ, to cell, to sub-cellular level.
Figure 2.
Levels of Drug Targeting
Importantly, no drug targeting strategy serves as a universal platform (reviewed by [18, 19]). Different strategies are designed to overcome different barriers, and often the advantages offered by a particular strategy regarding a certain barrier represent disadvantages from the perspective of overcoming other obstacles. These problems have largely impacted the translational success of drug targeting systems, (reviewed by [6, 19]). Only a few protein-, liposomal-, and polymer-based examples have successfully reached the clinics, due to a major effort investment in design but lack of concomitant systematic studies, the inherent complexity of these systems, partial understanding of their properties in vivo, and still incomplete fundamental knowledge on key regulatory parameters of the physiological environment. For instance, by taking advantage of natural physiological features of the body, passive targeting requires fewer modifications of the drug formulation than in the case of active targeting. This simplifies design and production, therefore reducing cost and facilitating the translational process (reviewed by [6]). However, the potential efficiency of passive targeting is rather restricted, such as the case of the EPR effect for reaching tumors, narrowing down the field of applicability of these systems as compared to the broad Pharma interest. Even in the case of cancer therapeutics, taking advantage of the passive EPR effect requires relatively prolonged circulation of the therapeutic agent or its carrier, and this poses additional design complexity and production obstacles [19]. However, despite design of long circulating formulations, passive transport into the tumor area effect is often counteracted by the high hydrostatic tumor pressure and lymphatic drainage within the tumor parenchyma, resulting in suboptimal drug accumulation at this site [19]. Similar requirements and complications apply to the design of strategies taking advantage of other passive accumulation mechanisms, e.g., for drug delivery to RES organs or inflammatory conditions characterized by increased vascular permeability.
In turn, active drug targeting strategies, e.g., using ligand-targeted carriers, may provide more controllable pharmacokinetics and bioavailability features. However, these strategies are also pronged with numerous obstacles. For example, they require complex design and multiple components, making production difficult, not fully controllable, and rather costly. Conjugation of drugs to targeting moieties and, mainly, their loading in targeted-carriers carriers increases the size of these formulations as compared to free non-coupled therapeutics. As a consequence, these strategies often suffer from lower diffusion and penetration through tissues (e.g., the tumor parenchyma or that of other therapeutic sites), with formulations remaining entrapped in the proximity of blood vessels, unable to reach the target cells (reviewed by [18, 19]). Further, other obstacles arise from the inherent tissue heterogeneity, e.g., regarding composition, structure, and function, heterogeneity in the expression of the target, and numerous intracellular barriers (described in more detail in the following sections) [18, 19].
Altogether, these difficulties hinder practical translation of drug targeting strategies, requiring long and costly developmental processes, with only a minimal fraction of experimental technologies making their way to the marketplace. Understanding the advantages and disadvantages of these different drug targeting strategies and how they relate to the physiological system, is crucial to more logically design these therapeutic approaches while holding realistic expectations regarding their performance. This review will discuss some of the challenges affecting the design and characterization of drug targeting, particularly focusing on ligand-targeted drug delivery systems.
THE TARGET
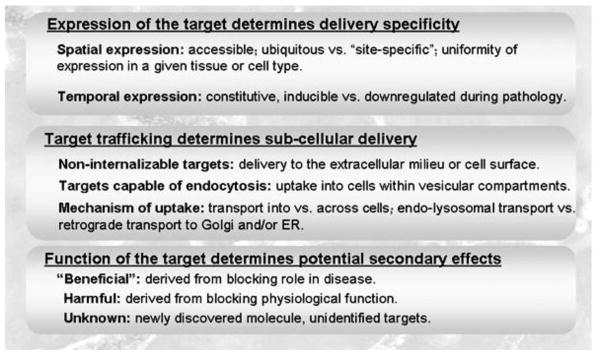
As described above, ligand-targeted drug delivery requires coupling (either directly or through a carrier) of the drug of interest to an affinity moiety that recognizes a feature present in areas of the body affected by disease and (preferably) missing from healthy tissues, favoring drug delivery where needed (reviewed by [20]). Most typically, this is implemented by targeting certain molecular signatures associated to the diseased cells, such as a specific protein. Table 1 below shows only some representative examples of molecular targets used in drug delivery (reviewed by [21–31]). A universal target is not possible, since selection of the target to which a drug will be addressed depends on the particular requirements of the clinical application of interest. When selecting a target for a drug delivery intervention, a number of factors need to be carefully considered (Figure 3).
Table 1.
Some representative targets used in ligand-mediated drug delivery.
| Target Molecule | Affinity Moiety | Target Location | Target Transport | Application | References |
|---|---|---|---|---|---|
| ACE | Ab | Cell surface & caveoli | Unclear | Lung endothelial injury | 45, 46 |
| Aminopeptidase N | NGR peptide | Cell surface & caveoli | Caveolar endocytosis | Vasculature in solid tumors | 112 |
| Aminopeptidase P | Ab & Ab-derived peptides | Caveoli | Caveolar transcytosis | Lung endothelium | 51 |
| αvβ3 & αvβ5 integrins | RGD peptides, aptamers | Cell surface & lipid rafts | Clathrin endocytosis | Vasculature in solid tumors | 113, 114 |
| B-lymphocyte antigen CD20 | Antibody (Rituxan*, Zevalin*, Bexxar*) | Cell surface & lipid rafts | Surface location | B cell lymphomas | 115, 116 |
| EDB-Fn | Ab | Extracellular matrix | N/A | Cancer | 117 |
| EGF receptor (ErbB1) | Ab (Erbitux*) aptamers | Cell surface & lipid rafts | Clathrin endocytosis & macropinocytosis | Metastatic colorectal cancer | 118 |
| Endoglin | Ab, Ab-derived peptide, aptamers | Cell surface - caveoli | Clathrin & caveolar endocytosis | Vasculature in solid tumors | 119 |
| ErbB2 | Ab (Herceptin*) & aptamers | Cell surface | Cell surface & different pathways | Breast & ovarian cancer | 120 |
| Folate receptor | Folate | Cell surface | Unclear | Cancer & inflammation | 67 |
| Glycosylated molecules | Lectins | Cell surface | Different pathways | Multiple applications | 71 |
| gp60 | Albumin & Ab | Caveoli | Caveolar transcytosis | Vascular targeting | 61 |
| ICAM-1 | Peptides, Ab, Ab-derived peptides, aptamers | Cell surface – lipid rafts | CAM endocytosis & transcytosis | Inflammation, autoimmunity, LSDs | 20, 24 |
| IL-2 receptor | Ab & peptides | Cell surface | Clathrin & caveolar independent endocytosis | Cancer & immunity | 88, 212 |
| IGF-1 receptor | Ab & Ab-derived peptides | Cell surface | Clathrin endocytosis & transcytosis | Cancer | 62 |
| Insulin receptor | Ab, Ab-derived peptides, aptamers | Cell surface - caveoli | Different endocytic pathways & transcytosis | Cancer & LSDs | 122 |
| LDL receptor family | Receptor- associated protein RAP | Cell surface | Clathrin endocytosis & transcytosis | Cancer, inflammation, atherosclerosis, LSDs | 123, 124 |
| LHRH receptor | Peptides (Lupron*, Zoladex*) | Cell surface | Clathrin endocytosis | Prostate cancer | 125 |
| LFA-1 | Ab, peptides, aptamers | Cell surface – lipid rafts | Unclear | Inflammation & immune response | 24 |
| Mannose-6-phosphate receptor | Mannose-6-phosphate & insulin-like growth factor II peptide | Cell surface, lysosome, Golgi | Clathrin endocytosis | Cancer & LSDs | 126, 127 |
| MMPs | Ab & peptides | Extracellular matrix | N/A | Cancer & inflammation | 128, 129 |
| MUC1 | Ab aptamers | Cell surface | Clathrin endocytosis | Breast & bladder cancer | 130 |
| PECAM-1 | Ab & Ab-derived peptides | Cell surface | CAM endocytosis | Inflammation | 23 |
| Selectins | Ab, oligosaccharides, aptamers | Cell surface | Clathrin endocytosis | Tumor vasculature & inflammation | 131 |
| Transferrin receptor | Transferrin, Ab, Ab-derived peptides, aptamers | Cell surface | Clathrin endocytosis & transcytosis | Cancer and blood-brain barrier | 27, 73 |
| VCAM-1 | Ab, Ab-derived peptides, aptamers | Cell surface | Clathrin endocytosis | Tumor vasculature, inflammation, atherosclerosis | 132 |
| VEGF receptor | Ab (Avastin*), peptides, aptamers | Cell surface | Clathrin endocytosis | Vasculature in solid tumors | 133, 134 |
Ab = antibody; ACE = angiotensin converting enzyme; Aptamer = only nucleic acid-based affinity molecules are shown; CAM = cell adhesion molecule; EDB-Fn = extradomain B fibronectin; EGF = epithelial growth factor; GI = gastrointestinal; gp60 = albumin receptor (sialo)glycoprotein 60; ICAM-1 = intercellular adhesion molecule 1; IGF-1 = insulin-like growth factor; IL = interleukin; LDL = low density lipoprotein; LFA-1 = leukocyte function antigen 1; LHRH = luteinizing hormone-releasing hormone; LSD = lysosomal storage disorder; MMP = matrix metalloprotease; MUC1 = mucin 1; N/A = not applicable; PECAM-1 = platelet-endothelial cell dhesion molecule 1; VCAM-1 = vascular cell adhesion molecule 1; VEGF = vascular endothelial growth factor.
= Commercial Ab name.
Figure 3.
Selection of the Target
Target Expression
First, it is obvious that the expression of the target will influence the delivery specificity. This concerns both the spatial and temporal patterns of expression. Regarding the site of expression, for instance, if the target is expressed by one sole cell type or if it is ubiquitous through the body will facilitate site-specific or broad delivery, respectively, which may find application for diseases confined to a particular tissue versus multi-organ syndromes.
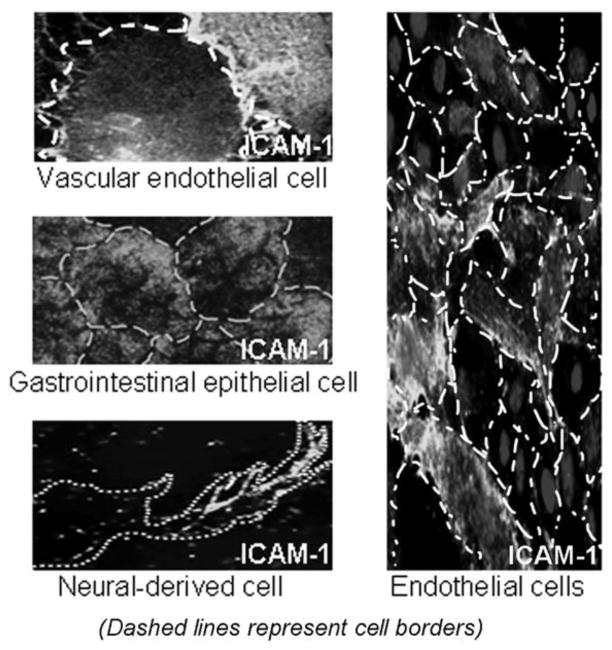
When considering site-specific drug delivery options, it is important to realize that most molecules are not exclusively expressed by a single cell or tissue type, even though their expression may be more prominent in certain sites of the body (reviewed by [18, 19]). As an example (Figure 4, left), intercellular adhesion molecule 1 (ICAM-1) is an Ig-like adhesion molecule preferentially expressed by vascular endothelial cells, and serves as a counter-receptor for leukocyte adhesion and extravasation during inflammation [32, 33]. As such, ICAM-1 targeting is used for drug delivery to the endothelium (reviewed by [34]). However, this molecule is also expressed by many other cells types in the body (reviewed by [35]). This aspect in turn can be exploited, e.g., for broad distribution of enzyme replacement therapies for multi-organ lysosomal storage disorders (reviewed by [36, 37]). The case of ICAM-1 is just an example of a phenomenon broadly observed; indeed most of the targets illustrated in Table 1 (e.g., transferrin receptor, IGF-1, LDL receptor family, ERbB2, etc.) have been associated with several cell types, tissues, and physiological/pathological states. Hence, arguably, increasing the avidity of targeted drug delivery systems may drive the drug to unwanted sites that express lower amounts of a given target versus those displaying moderate avidity, which may more selectively target areas affected by disease, if they present higher expression levels.
Figure 4.
Lack of total specificity (left) and uniformity (right) of target expression.
In addition, it is highly unlikely that a given molecular target is expressed uniformly through a given tissue (see Figure 4, right, showing endothelial cells in culture expressing various levels of ICAM-1). Even in the case of more uniform and synchronized “clonal” cell cultures one can observe great cell-to-cell variability regarding the levels of expression of molecules (reviewed by [18, 19]). This is, once again, the case for most cell surface determinants, including many of the common targets in experimental and clinical interventions, e.g., ErbB2, selectins, Ig-family CAMs, EGF receptor, etc. Hence, it is important to examine these aspects in available cell culture and animal models prior to embarking on the design and development of complex drug targeting strategies.
Considering the time component, a target that is constitutively expressed may facilitate prophylaxis, whereas a target that is inducible during disease is better poised for therapeutic intervention. However, the expression of some molecules can be also down-regulated upon certain stimulation (e.g., the pathology itself or the therapeutic formulation), rendering them inaccessible for drug delivery purposes (reviewed by [38]). Whereas the knowledge of the biological features and regulation of the selected molecular target is key to design targeted drug delivery strategies, this information is not always available and one may encounter unexpected outcomes.
Target Accessibility
The target must also be accessible to the ligand administered exogenously, e.g., by being located on the cell surface versus intracellular compartments, not masked by other determinants which may naturally interact with the target or form a part of a complex where the target is embedded. Although this seems a readily identifiable parameter driving success of targeting, many other aspects that contribute to said success are often overlooked. For instance, the extracellular domain of some transmembrane proteins expressed at the cell surface can be cleaved from the plasma membrane, e.g., during inflammation or in the tumor environment, due to relatively high levels of extracellular proteases at these sites (reviewed by [39]). In addition, it is becoming apparent that most molecules can be expressed in a variety of isoforms, including single amino acid changes, insertions or deletions of full protein domains or sub-domains, that arise from point polymorphic variations, alternative splicing, etc (reviewed by [40]). Different post-translational modifications of a target, e.g., arising from alternative glycosylation patterns, can also add to this isomorphic variability (reviewed by [41]). Hence, although the molecule may be expressed on the tissue of interest, one must consider that a given targeting moiety selected against isoform A will not be able to recognize isoform B of the same target, which may be expressed in a particular organ but not other, under certain developmental status, or under the influence of a pathological stimulus. Unfortunately, for the most part, there are no systematic studies of such parameters relative to expression of molecules that may be interesting from the drug targeting standpoint, hindering our ability to make more logical and less risky target selections.
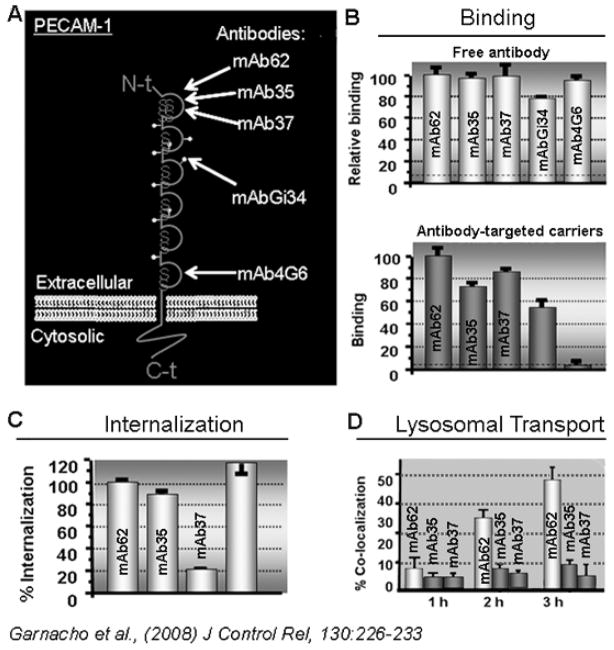
Importantly, and most often overlooked, the very precise epitope of the target molecule that is recognized by a targeting moiety, is key to the success of a given targeting strategy. For instance, although extracellular, certain epitopes may be sufficiently close to the plasma membrane or the interface of partner proteins or other elements as to pose steric hindrances regarding efficient binding of drug carriers. Furthermore, in the case of targeting molecules at the cell surface, leading to endocytic uptake for intracellular drug delivery, targeting epitopes that can be “sensed” by the cell in order to elicit said endocytic response and the subsequent intracellular route is key. An example to illustrate this aspect (Figure 5) is that of carriers targeted to platelet-endothelial adhesion molecule 1 (PECAM-1), another Ig-like adhesion molecule expressed by endothelial cells and involved in inflammation (Figure 5A) [42, 43]. Antibodies recognizing membrane-proximal domains of PECAM-1 can bind to endothelial cells but are unable to target ~200 nm carriers to the cell surface (Figure 5B) [44]. Furthermore, only anti-PECAM carriers that target PECAM-1 epitopes involved in homophilic PECAM-1 interaction or other functions induce carrier endocytosis (Figure 5C) [44]. Also, anti-PECAM recognizing different “internalizable” epitopes traffic to different compartments within the cell (endosomes versus lysosomes; Figure 5D) [44]. Similar observations have been reported in other systems, e.g., in the case of targeting angiotensin converting enzyme, where the efficiency of targeting the endothelium in vivo in animal models varied greatly depending on the molecular epitope targeted [45, 46]. Therefore, the epitope targeted can greatly influence the targeting outcome.
Figure 5.
Effect of targeting drug carriers to different epitopes of a target.
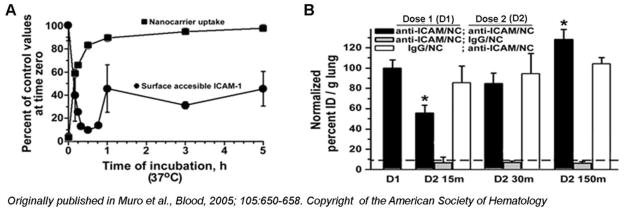
Further, natural ligands of a given receptor target are typically present in the body, which may compete for access and binding of a drug addressed to said target (reviewed by [13]). Also, binding of the targeted drug to its target on the surface of cells may induce internalization by endocytosis (see below Sub-cellular Transport of the Target). Although this is preferable in the case where drugs must be delivered intracellularly, internalization of the targeted drug-receptor complex within the cell might cause disappearance of the target from the cell surface (at least partially or temporarily), which may decrease the ability of targeted drugs remaining in the circulation to continue binding their intended target cells or tissue. This has been shown for anti-ICAM polymer nanocarriers (Figure 6), which bind to ICAM-1 on endothelial cells and are then endocytosed by the cells [47]. Uptake of anti-ICAM nanocarriers causes the engaged ICAM-1 molecules to internalize, hence disappearing from the plasma membrane [48]. Subsequently, ICAM-1 separates from anti-ICAM nanocarriers within endosomal compartments and ICAM-1 recycles back to the plasma membrane ~1 h after internalization (Figure 6A). As a consequence, the targeting ability of a second dose of anti-ICAM-1 nanoparticles injected in mice depends on whether enough time has elapsed from the first administered dose, as to allow ICAM-1 to recycle back to the plasma membrane, where it is fully accessible to the circulating nanocarriers (Figure 6B) [48].
Figure 6.
Endocytosis of the Target Temporarily Hinders its Access by Targeting Systems.
Sub-cellular Transport of the Target
Since the precise sub-cellular localization of the drug is important for most therapeutic applications, this is yet an additional aspect to consider when selecting a target for drug delivery. Table 1 shows the transport of some representative targets used in drug delivery, and Table 2 completes this information by referring to strategies to target particular sub-cellular compartments. Targets that are stably expressed at the cell surface support delivery of drugs for which extracellular activity is important. Drug targets that can be internalized within cells (e.g., by endocytosis, where the cell engulfs with the plasma membrane extracellular materials that bind to a given receptor and internalize them surrounded by a membranous vesicle within the cell body) are more amenable for interventions requiring intracellular location of the drug (reviewed by [49]). However, in certain cases, molecules not naturally associated to endocytic internalization respond as such when targeted with artificial drug delivery systems, whereas other targets may become unable to drive internalization upon binding of said vehicles. As an example, this is the case for targeting of ICAM-1 or aminopeptidase P. Whereas ICAM-1 is not associated to endocytosis of its natural ligands, antibodies, or peptides (and it was expected to support drug targeting to the cell surface), binding of drug carriers displaying multiple copies of affinity molecules against ICAM-1 (e.g., antibodies or peptides) was found to induce an unconventional endocytic pathway in endothelial cells, resulting in internalization of the targeted carriers and their drugs [47, 50]. Contrarily, while lung aminopeptidase P associated to caveoli-mediated transcytosis of antibodies and peptide ligands across the lung endothelium, it failed to mobilize larger objects displaying said ligands (e.g., viruses or particles) [51].
Table 2.
Some strategies of targeting used for sub-cellular drug delivery.
| Sub-cellular Target | Targeting Moiety | References |
|---|---|---|
| Membrane anchors: | ||
| Human low-affinity nerve growth factor | 135 | |
| Acylation | 136 | |
| Palmitoylation | 137 | |
| Cell penetration: | ||
| Tat peptide | 79 | |
| Penetratin (antennapedia) | 138 | |
| Peptoids | 80 | |
| Lysosomes: | ||
| Integrins | 113, 114 | |
| EGF receptor | 118 | |
| Folate receptor | 67 | |
| ICAM-1 | 20, 24 | |
| LHRH receptor | 125 | |
| LFA-1 | 24 | |
| Mannose-6-phosphate receptor | 126, 127 | |
| MUC1 | 130 | |
| Selectins | 131 | |
| VCAM-1 | 132 | |
| VEGF receptor | 133, 134 | |
| Endosomal escape: | ||
| Tat peptide | 79 | |
| Haemagglutinin | 139 | |
| GALA & KALA peptides | 81 | |
| Anthrax toxin | 139 | |
| Diphtheria toxin | 139 | |
| Golgi & ER: | ||
| Cholera toxin | 64 | |
| Shiga toxin | 65 | |
| Mitochondria: | ||
| Cytochrome oxidase subunits | 83 | |
| Retinoic-inteferon-induced mortality proteins | 83 | |
| Nucleus: | ||
| Nuclear localization sequences | 84 | |
| SV40 large T antigen | 84 | |
| Transcytosis: | ||
| Aminopeptidase P | 51 | |
| ICAM-1 | 140 | |
| IGF-1 receptor | 62 | |
| Insulin receptor | 122 | |
| LDL receptor | 123, 124 | |
| Transferrin receptor | 27, 73 |
C34 = carbon 34; EGF = epithelial growth factor; ER = endoplasmic reticulum; gp60 = albumin receptor (sialo)glycoprotein 60; ICAM-1 = intercellular adhesion molecule 1; IGF-1 = insulin-like growth factor; LDL = low density lipoprotein; LFA-1 = leukocyte function antigen 1; LHRH = luteinizing hormone-releasing hormone; MUC1 = mucin 1; SV = simian vacuolating virus; VCAM-1 = vascular cell adhesion molecule 1; VEGF = vascular endothelial growth factor.
= Commercial Ab name
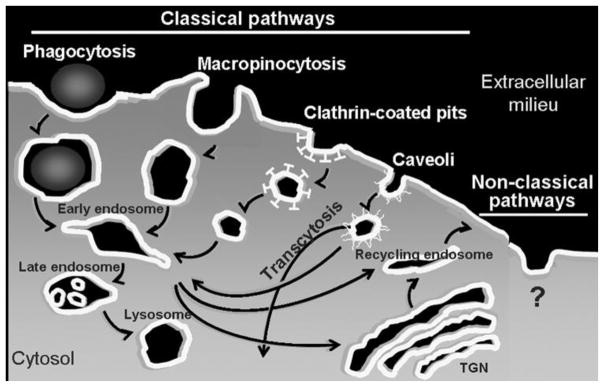
Furthermore, endocytic receptors are associated to specific pathways (Figure 7 and Table 1), whose natural constrains are expected to determine the uptake mechanism of drugs targeted to said receptors (reviewed by [49, 52, 53]). For instance, phagocytosis or macropinocytosis are associated to the uptake of particulate ligands or fluid, respectively, into several micrometer-sized vesicular compartments, highly amenable for cell internalization of drug delivery systems within a variety of sizes and architectures. Yet, these pathways are displayed by certain specialized cells of the immune systems, preventing delivery to many other cell types. In contrast, clathrin- or caveli-mediated endocytosis are present in most cells in the body, yet endocytic vesicles associated to these pathways have a diameter ~100 nm or ~70 nm, respectively, restricting the parameters of design of drug delivery systems. There are a few other pathways that do not operate through these “classical” routes. An example is that of cell adhesion molecule (CAM) endocytosis mediated by ICAM-1 when artificially engaged by multivalent drug carriers [47], as well as that of GPI-anchored proteins, IL-2β receptor, and proteoglycans and bound growth factors, e.g., FGF2 (reviewed by [54]). Remarkably, CAM-mediated endocytosis operates in a variety of cells (endothelial, epithelial, macrophages, fibroblasts, neuronal, etc.) and allows internalization of drug carriers whose size ranges from a few hundred nanometers to several micrometers [55]. Yet, this and other non-classical pathways seem restricted to very particular receptors and our understanding of their function and regulatory mechanisms is far less complete.
Figure 7.
Endocytosis and vesicular transport
The route of entry within the cell often determines the intracellular traffic of the internalized material and its fate (Figure 7) (reviewed by [49, 52, 53]). For instance, endocytic vesicles and their contents can traffic to the cell surface by recycling endosomes (directly or through Golgi compartments) or, in polarized cell monolayers, vesicles can be transported through the cell body and exocytosed to the abluminal space by transcytosis. Such transport, amenable for drug delivery across cellular barriers, is associated with some clathrin- and mainly caveoli-related mechanisms [56–60]. Several examples of such a transport have been reported, most commonly regarding targeting of the caveolar-associated gp60 (an albumin receptor) or aminopeptidase P, and clathrin-associated transferrin receptor or insulin-like growth factor receptor 1, which have been shown to transport therapeutics across the endothelium in the lungs (through caveoli) and the brain (through clathrin) using designs such as fusion proteins, antibody or peptide conjugation, or coupling to drug delivery carriers (Table 2) [27, 51, 61, 62].
Less commonly, clathrin- and caveoli-mediated endocytosis leads to traffic to other sub-cellular destinations, such as the Golgi apparatus or the endoplasmic reticulum [63]. This is the case for certain pathogen toxins and drug delivery strategies that capitalize on them, such as that related to cholera toxin and shiga toxin (Table 2) [64, 65]. Yet, materials internalized by most endocytic mechanisms are sorted to acidic and hydrolytic lysosomal compartments (Table 2) [66]. This is most often the case for drug carriers displaying multiple copies of a targeting ligand, even when that ligand targets a receptor naturally associated to other transport route. An illustrative example is that of the folate (mono)conjugates, which target the folate receptor in tumor cells, following by recycling of the folate ligand-receptor pair to the plasma membrane, whereas multivalent folate carriers traffic to acidic endosomes and lysosomes (reviewed by [67]). Residency within the lysosome is beneficial for very particular applications, e.g., enzyme replacement of genetically deficient enzymes for treatment of lysosomal storage disorders (reviewed by [36, 37]), or in cases where the acidic lysosomal pH induces release of a membrane-permeable drug from a pH-sensitive carrier (reviewed by [5]). However, this transport route leads to degradation of most other materials, representing a major obstacle for efficient drug delivery. This is also the case for therapeutic agents that are non-membrane-permeable and whose action is required in other sub-cellular compartments (e.g., the cytosol or the cell nucleus), which remain entrapped and are eventually degraded within lysosomes (reviewed by [68, 69]).
Effects of Blocking the Target
Finally, a key aspect often forgotten when selecting a target for drug delivery pertains to the physiological function of the selected target and how this may be affected upon binding of the targeted drug or drug carrier (reviewed by [70]). Said binding event might prevent recognition and attachment of natural ligands for which the targets serves as a receptor, modify its conformation, and/or affect its function or regulation. In some instances, the final outcome may lead to beneficial “side effects”, when the target itself contributes to disease, whereas for most strategies the outcome might be detrimental. Strategies based on targeting newly discovered molecules or even unidentified targets (e.g., those underscored by phage display and other technologies panned against full cells and tissues), although unquestionably valuable, pose initial concerns due to the lack of knowledge about the biological function of the molecular determinants they target.
THE TARGETING MOIETY
Achieving binding to the selected target is accomplished by using elements that display affinity toward that target. A variety of molecules can be used for this purpose. These include proteins and peptides, which represent by far the most common affinity elements employed for ligand-based drug targeting. Antibodies are often used given their high affinity and specificity, particularly to establish proof-of-principle but also from a translational perspective, since they can be easily modified for clinical applications into humanized antibodies, antibody fragments, diabodies, etc. Antibodies optimized for particular disease conditions can further serve as platforms to design high affinity peptides derived from the antibody hypervariable region, and these forms can also be utilized to generate recombinant fusion proteins containing the single-chain antibody fragment into their sequence, in the case of protein therapeutics. Other protein-based targeting moieties include natural ligands of cell receptors such as lectins and hormones (reviewed by [71]), or carrier proteins such as LDL [72] or transferrin [73], as well as molecules derived from pathogens or toxins such as cell permeating and fusogenic peptides. Peptides randomly derived from technologies such as phage-display are also commonly used for targeting purposes (reviewed by [74]). In addition, nucleic acids such as aptamers, and small molecules including vitamins, as in the cases of folate [67] or vitamin B12 [75], and certain sugars such as the monosaccharide mannose, have also been explored as drug targeting elements as they can bind to their respective receptors on the cell surface.
The examples of the folate receptor and transferrin receptor are among the most studied ones regarding drug targeting using different targeting moieties and modifications of a given targeting moiety. Folate receptor is one of the carriers for vitamin B9 in the body. Vitamin B9 exist as a small hydrophilic molecule that is most broadly internalized within cells via a reduced folate carrier (reviewed by [76]). However, there is a another type of receptor mostly expressed on cancer cells and inflammatory areas, which is expressed as a GPI-anchored protein associated to lipid-raft domains on the plasmalemma (Table 1). Although the mechanism of endocytosis via this receptor remains lnot fully understood, it is known that the ligand-receptor complex is internalized within cells, routed to early endosomes, and eventually recycled back to the plasmalemma yet leading to several cycles of internalization-recycling, amenable for steady state uptake of drugs. This delivery system has been extensively explored for drug delivery via folate, antibodies, and peptides, either as soluble drug-conjugates or coupled to drug delivery systems, with different transport properties as already mentioned [67]. Interestingly, modifications of the same affinity moiety, folate, has been shown to provide differential drug delivery capabilities. For example, since the endosomal environment is more reducing than acidic, disulphide bonds are reduced very for release of folate-conjugated drug than those exploiting pH-sensitive acyl hydrazone linkage-conjugates [76].
In the case of the transferrin receptor (Table 1), this molecule is naturally involved in uptake and transport of iron into cells through the body, and it has been reported to be overexpressed on the brain vasculature and tumor cells, amenable for treatment of conditions affecting the central nervous systems and cancer (reviewed by [27, 73]). The natural ligand of this receptor in transferrin, a protein carrier for ferric-iron. Iron-bound transferrin binds to the its receptor on the cell surface, inducing endocytosis via clathrin-coated pits, with subsequent traffic to endosomal compartments. Here, lowering of the pH prompts release of iron, which is then transported into the cytosol, while the ligand-receptor complex is recycled back to the plasmalemma, and iron-free transferrin detaches from its receptor at this neutral pH. Alternatively, in polarized cell barriers (e.g., in the brain or epithelial linings), iron-bound transferrin travels across the cell body by trancytosis, being released in the abluminal side, hence, penetrating the cell barrier (reviewed by [76]).
Perhaps less explored, but also interesting from the perspective of drug targeting, are those targeting elements that, although not providing binding to cell surface determinants or receptors involved in endocytic transport, still offer sub-cellular directionality (reviewed by [64]). As an example lipid anchors that can be achieved by acylation, myristoylation, or palmitoylation strategies may improve insertion of a drug in cell membranes, for either cell surface or endocytic transport (Table 2). Cell permeating or fusogenic peptides favor drug delivery to the cell cytosol (reviewed by [71, 78]). This is the case for HIV-derived Tat peptide, antennapedia penetratin peptide. Tat and penetretin peptides are positively charged and bind to the negatively-charged sugar moieties on the cell membrane through electrostatic interactions, leading to either passive uptake via absorptive endocytosis or temporal disruption of the plasmalemma at the binding sites, with opening of pores that allow direct transport into the cytosol [79]. Also, some peptoids can be design to operate similarly. Peptoids are peptidomimetics where the side chains are appended to the nitrogen atom of the peptide backbone instead of the α-carbons, hence, they offer enhanced stability due to the fact that they cannot be degraded by proteases [80]. GALA and KALA peptides, as well as several peptides derived from pathogen toxins, such as haemagglutinin, anthrax toxin, and diphtheria toxin, can disrupt endosomal membranes upon endocytosis within cells, supporting escape into the cytosol [81, 82]. As mentioned above, shiga toxin- and cholera toxin-derived peptides support transport to the endoplasmic reticulum [64, 65]. Additionally, sorting signals are also being explored to provide intracellular transport to various compartments. Sorting signals are peptide sequences naturally present in many intracellular proteins to address them to their respective intracellular locations (e.g., nuclear, endoplasmic reticulum, lysosomes, mitochondrion, etc) [83, 84]. For instance, peptides derived from cytochrome oxidase subunits or retinoic-interferon-induced mortality proteins can target mitochondria [83], and several nuclear localization sequences (mammalian or derived from the large T antigen of Simian vacuolating virus 40 SV40) can be used to target the cell nucleus [84].
As in the case of selection of an appropriate target for drug delivery, selection of an adequate targeting moiety depends on the required features pertaining to a particular clinical application and other design aspects of the targeting strategy, and a balance between the advantages and disadvantages offered by a given choice. For instance, antibodies, receptor-targeting peptides, and some intracellular sorting signals are more specific regarding recognition of molecular signatures of cells, yet these elements often do not provide the ability to cross cellular membranes, posing obstacles to enter the cell cytosol, e.g., directly from the extracellular milieu or from endocytic compartments after endocytosis. Due to this obstacle, cell permeating or fusogenic peptides are used (reviewed by [85, 86]), offering endolysosomal escape and entrance into the cytosol, with subsequent transport to other cellular compartments. Yet, most strategies capable of this action, such as those described in the previous paragraph, bind and destabilize cellular membranes based on broadly displayed signatures (e.g., charge, etc) and therefore suffer from lack of specificity.
THE COUPLING STRATEGY
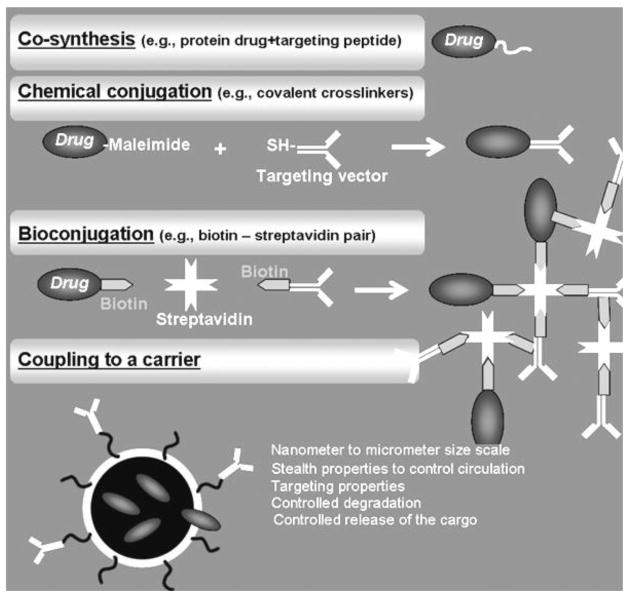
Once the molecular target and amenable targeting moiety have been selected, coupling of the latter to the drug of interest can be achieved by a variety of strategies (Figure 8), including direct coupling approaches such as co-synthesis (applicable to the case of therapeutic proteins that can be synthesized as fusion proteins) or conjugation of both elements after their independent synthesis or production (reviewed by [6, 87, 88]). Conjugation strategies encompass covalent conjugation via chemical cross-linkers, or bioconjugation through non-covalent attachment using biological affinity partners, such as the case of the biotin-streptavidin pair, yet this type of coupling also requires chemical modification of the individual elements to either partner (reviewed by [89, 90]). Depending on the number of “conjugating” modifications imposed on the targeting moiety and/or the drug, conjugates can either display a single molecule of drug attached to a single molecule of the targeting moiety or, most often, a multiple number of drug molecules cross-linked to multiple copies of the targeting moiety.
Figure 8.
Coupling of the Drug to the Targeting Moiety.
Drugs can also be loaded in targeted drug delivery carriers. These are macromolecular assemblies within the sub-micrometer or few micrometers size range, which can incorporate therapeutics of different nature, from small chemicals, peptides and proteins, to oligonucleotides and genes. They can improve the solubility and bioavailability of their cargo, and also control to some extent their circulation, biodistribution in the body, and release rate, altogether enhancing their efficacy [15, 16, 91, 92]. A great diversity of these carriers, particularly in the nanoscale size range (nanocarriers), has been designed during the last two decades, including nanotubes and other carbon nanostructures, branched dendrimers, phospholipid liposomes, amphiphilic polymers formulated as linear structures, self-assembled micelles, or polymer (hollow, porous, solid) particles, etc (reviewed by [6]), where targeting is achieved by coupling of the targeting moieties on the carriers surface.
Conjugates versus Carriers
Conjugates and carriers associate to different properties posing advantages and disadvantages depending on the particular therapeutic application. Conjugates (considering monomolecular composites) are typically smaller in size, which favors diffusion and, to some extent, tissue penetration, yet this also enhances clearance, e.g., by lymphatic drainage (reviewed by [6, 18, 19]). They may exert greater diffusion into cells, but also lesser induction of specific endocytosis due to their lower targeting valency. This latter parameter may also result in lower avidity and targeting in the physiological environment, but exert less immunogenic potential. In turn, carriers are typically larger in size, which may hinder their diffusion within tissues, although this may favor retention due to lower lymphatic drainage (reviewed by [6, 18, 19]). Also, their design is more amenable for modifications aimed to lower their clearance, e.g., by modifying their stealth properties and/or geometry. Carriers do not diffuse into cells but are more prompt to induce endocytic uptake, avoiding efflux pumps and providing some more control over their intracellular transport. Their greater avidity favors in vivo targeting, but they can also induce immunogenicity due to multiplicity of (targeting) elements displayed on their surface. The complexity of their composition and architecture makes carriers less amenable for fast translation into the clinical arena, yet they offer greater drug protection, as well as more controlled pharmacokinetics and release patterns [15, 16, 91, 92]. Hence, both types of formulations have advantages and counterbalancing disadvantages, altogether making it difficult to achieve translational optimization.
Valency and Avidity
The number of targeting moieties (valency) attached to a drug conjugate or carrier is a key parameter determining its avidity and targeting ability. The avidity of the targeting moiety must be sufficient to achieve effective binding to the disease site in a physiological environment, e.g., in the presence of natural ligands competing for binding to the target, withstanding the dragging forces of the shear stress in the circulation, etc. In this regard, formulation as a multivalent composite (multimolecular conjugates or carriers) provides a means to increase the avidity toward the target (reviewed by [93–95]). Generally speaking, because of the multiple copies of the drug held by the conjugate or carrier, a single event of binding to the target provides a higher dose of the drug. Hence, this strategy is appealing for drug delivery purposes. As an example, anti-ICAM polymer nanocarriers holding ~250 anti-ICAM molecules per particle display markedly enhanced (~100-fold) avidity for ICAM-1 over free antibody counterparts [96], and increasing the density of anti-ICAM on the surface of said polymer nanocarriers enhances lung targeting over liver and spleen clearance [97]. Counter-intuitively, in some cases, increasing the dose of carrier administered improves the degree and specificity of targeting at a greater extent than the surface density of the targeting antibody [97]. There are several cases showing that, indeed, the surface density of the targeting moiety coupled on a drug carrier needs to be carefully balanced. For instance, the avidity of multivalent targeted formulations needs to overcome the threshold for effective targeting in a physiological environment, e.g., under dragging forces of the shear stress caused by the blood flow in the circulation or in the presence of natural ligands for the selected receptor. Conversely, if the avidity is too high, then binding to off-target areas may occur (e.g., if the receptor targeted is expressed is low amounts in other organs or tissues) or binding can be impaired due to steric hindrances that depend of the expression level and location of the receptor on the cell surface [13, 97, 98]. Unfortunately, such a balance is difficult to speculate a priori and require systematic studies tailored for each particular target, cell type, carrier, pathological status, and therapeutic intervention
In addition, modification of the valency of a targeting ligand might affect not only avidity but also other parameters. For instance, ICAM-1 targeting with monoclonal antibodies provides stable binding to ICAM-1 expressed on endothelial cells in culture and in vivo [50, 96], which can be used for cell surface delivery. However, (as described above) multivalent conjugates and carriers induce endocytosis due to ICAM-1 cross-linking, which provides intracellular drug delivery [47]. Inversely, multivalent binding might impair the natural endocytic transport of certain molecules that serve as cell receptors for natural monovalent ligands or affect its intracellular traffic, as in the case of aminopeptidase P or the folate receptor, respectively (reviewed by [51, 67]).
Importantly, multivalent conjugates or carriers display considerably larger sizes than monomolecular counterparts, which in some cases poses an obstacle to efficiently access certain molecular targets at the cell surface. As an example, this has been shown for some peptides displayed on viral particles addressed to caveolar regions of the plasmalemma [51], targeting to the ganglioside GM1, and carriers bearing certain antibodies addressed toward the transferrin receptor [99, 100].
Size and Shape
Recent investigations have shown the overall geometry of a drug conjugate or carrier greatly impacts a variety of drug delivery parameters, including circulation half-time, biodistribution, cellular uptake, and intracellular transport.
Clearance of foreign substances in the body occurs by the RES, immune system, and renal filtration [101]. Materials administered in the circulation are mainly cleared by the spleen and liver, and the lymph nodes remove substances that drain from the tissue parenchyma via the lymphatics. Due to their larger size, multivalent targeted carriers (versus smaller monomeric conjugates) are excluded from filtration through the kidneys and penetrate poorly into the parenchyma of tissues, being rapidly removed by the liver and spleen. This hinders their ability to reach a given tissue, e.g., tumor penetration via the EPR effect (reviewed by [18, 19]). Coupling to poly(ethylene glycol) or PEG is a common strategy used to overcome this obstacle, minimizing interactions with plasma opsonins, the complement, professional phagocytes, etc [101]. However, PEG also reduces the ability of a conjugate or carrier to bind to a target on the cell surface. Another strategy is to couple peptides derived from the protein CD47 onto carriers. CD47 binds to SIRPα on leukocytes, which inhibits phagocytosis, therefore prolonging circulation [102]. However, coating of CD47 onto carriers would consequently reduce the surface density of a given targeting moiety. An alternative approach is to modify the carrier geometry, e.g., elongation in one axis enhances alignment in the blood flow, reducing wall collisions and extravasation associated to clearance, and shifting biodistribution from the liver and spleen to the other organs. This has been shown for both non-targeted as well as targeted carriers [55, 103, 104].
The geometry of targeted drug delivery systems also impacts cellular uptake, although to different extents and manners depending on the carrier, its targeting moiety, and the cell type of interest. For instance, macrophages internalize 0.2 μm versus 2 μm IgG-coated spherical particles with similar kinetics, yet through different mechanisms (clathrin endocytosis versus Fc-receptor mediated phagocytosis) [105]. In contrast to macrophages, in most parenchymal cells the kinetics of internalization of carrier particles slows down with increasing carrier size [106]. Also, a size restriction for uptake of carriers via the transferrin receptor has been observed, where ~60 nm transferrin-coupled liposomes were able to target this receptor, but ~120 nm counterparts did not [100]. A similar effect has been reported in the case of targeting to ganglioside GM1 on intestinal cells using cholera toxin B as a ligand, where the free soluble ligand (~6 nm) efficiently bound on the intestinal epithelium, yet conjugation to particles (~30 nm) reduced targeting, and this was totally abolished by increasing particle size (~1 μm) [99]. Yet, in the case of anti-ICAM carriers (Figure 9, left), the size of spherical carriers (between 0.2 μm and 5 μm) does not impact the kinetics or mechanism of uptake by endothelial cells, which occurs via non-classical CAM-mediated endocytosis [55]. Arguably, this may be in part related to the biological function of the molecular targets addressed. For instance, binding of soluble transferrin to its receptor suffices in order to induce signaling cascades leading to formation of clathrin-coated pits [107, 108]. In turn, β2 integrins that represent the natural ICAM-1 ligands are exposed as multiple binding units presented on the surface of several-micrometers-sized leukocytes, and they induced similar endothelial signaling cascades as those related to anti-ICAM carriers [109, 110].
Figure 9.
Effect of size and shape on the cell interaction of ICAM-1 targeted carriers.
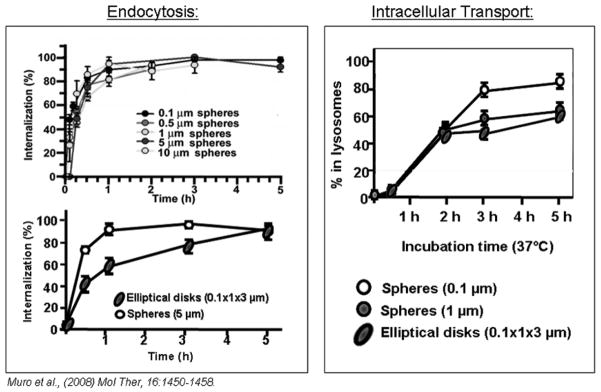
Apart from size, shape of carriers also impacts internalization by cells. For instance, the shape of non-targeted micron-sized particles modulates the rate of phagocytosis by macrophages [111], and this is also the case targeted carriers, e.g., multivalent anti-ICAM conjugates (of relatively amorphous shapes) over 500 nm are not efficiently internalized by endothelial cells versus their spherical polymer counterparts [47, 50, 55], and the kinetics of uptake of spherical anti-ICAM polymer carriers is faster than that of elongated disks of the same composition and targeting antibody density [55].
Sub-cellular trafficking is also influenced in different manners by the geometry of targeted drug delivery systems. For instance, in the case of Fc-mediated phagocytosis of IgG-coated carriers by macrophages, micron carrier particles traffic to lysosomes faster than smaller counterparts 105]. In contrast, in the case of CAM-mediated endocytosis of ICAM-1-targeted carriers, smaller sub-micrometer size particles traffic more efficiently to lysosomes than their micrometer-range counterparts (Figure 9, right) [55]. Interestingly, in this case, although shape versus size of carriers has a greater impact on uptake greater, size (not shape) influences intracellular traffic more profoundly [55]. Hence, as illustrated, it seems these outcomes highly depend on the particular target selected and it is difficult to predict a priori the impact of geometry of a targeted drug delivery system on its internalization kinetics, mechanism, and intracellular fate.
CONCLUSION
Drug targeting holds great promise as a means to enhance the therapeutic outcome of clinical treatments by improving site-specificity and safety of drugs. Yet, after several decades of a considerable experimental effort and apart from a few success stories, the translational output of drug targeting systems does not seem to have met the expectations. Despite undeniable advances, the high number of variables impacting the design of drug delivery systems, lack of homogeneity and level of complexity of the biological environment, along with our somewhat still limited knowledge of their governing factors, have delayed translation of drug targeting systems into the clinical realm. It is important to realize that no targeting strategy can serve as a universal platform, since design variables must adjust to the particular requirements of a given intervention. Even within a specific objective, the success of a drug targeting approach depends upon achieving several opposite features: the advantages offered by a drug delivery strategy regarding a certain barrier represent disadvantages from the perspective of overcoming other obstacles. The balance between these advantages and disadvantages depends in turn on the particular therapeutic intervention, altogether making the success of drug delivery strategies an empirical factor, highly unpredictable. The obstacles impacting clinical translation of drug targeting systems are beginning to be recognized and a deep evaluation of the path walked shall serve to improve the practical potential of this unquestionably promising field.
Acknowledgments
The author thanks Dr. Muzykantov (University of Pennsylvania, Philadelphia, PA) and Dr. Garnacho (University of Seville, Seville, Spain) for their contribution to research on anti-ICAM carriers reviewed in this article, Daniel Serrano (University of Maryland, College Park, MD) for careful reading of the manuscript, and funding from the National Institutes of Health (Grant R01-HL98416) and the American Heart Association (Grant 09BGIA2450014).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dingemanse J, Appel-Dingemanse S. Integrated pharmacokinetics and pharmacodynamics in drug development. Clin Pharmacokinet. 2007;46(9):713–737. doi: 10.2165/00003088-200746090-00001. [DOI] [PubMed] [Google Scholar]
- 2.Takimoto CH. Basic pharmacokinetics and pharmacodynamic principles. Cancer Treat Res. 2001;106:85–101. doi: 10.1007/978-1-4615-1657-6_4. [DOI] [PubMed] [Google Scholar]
- 3.Torchilin VP. Drug targeting. Eur J Pharm Sci. 2000;11(Suppl 2):S81–91. doi: 10.1016/s0928-0987(00)00166-4. [DOI] [PubMed] [Google Scholar]
- 4.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman AS. The origins and evolution of “controlled” drug delivery systems. J Control Release. 2008;132(3):153–163. doi: 10.1016/j.jconrel.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Torchilin VP. Passive and active drug targeting: drug delivery to tumors as an example. Handb Exp Pharmacol. 2010;(197):3–53. doi: 10.1007/978-3-642-00477-3_1. [DOI] [PubMed] [Google Scholar]
- 7.Almeida JP, Chen AL, Foster A, Drezek R. In vivo biodistribution of nanoparticles. Nanomedicine (Lond) 2011;6(5):815–835. doi: 10.2217/nnm.11.79. [DOI] [PubMed] [Google Scholar]
- 8.Chrastina A, Massey KA, Schnitzer JE. Overcoming in vivo barriers to targeted nanodelivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011;3(4):421–437. doi: 10.1002/wnan.143. [DOI] [PubMed] [Google Scholar]
- 9.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Pt 1):6387–6392. [PubMed] [Google Scholar]
- 10.Lee HJ, Ahn B-N, Paik WH, Shim C-K, Lee MG. Inverse targeting of reticuloendothelial system-rich organs after intravenous administration of adriamycin-loaded neutral proliposomes containing poloxamer 407 to rats. Inter J Pharm. 1996;131(1):91–96. [Google Scholar]
- 11.El-Sayed ME, Hoffman AS, Stayton PS. Smart polymeric carriers for enhanced intracellular delivery of therapeutic macromolecules. Expert Opin Biol Ther. 2005;5(1):23–32. doi: 10.1517/14712598.5.1.23. [DOI] [PubMed] [Google Scholar]
- 12.Stayton PS, El-Sayed ME, Murthy N, Bulmus V, Lackey C, Cheung C, Hoffman AS. ‘Smart’ delivery systems for biomolecular therapeutics. Orthod Craniofac Res. 2005;8(3):219–225. doi: 10.1111/j.1601-6343.2005.00336.x. [DOI] [PubMed] [Google Scholar]
- 13.Vyas SP, Sihorkar V. Endogenous carriers and ligands in non-immunogenic site-specific drug delivery. Adv Drug Deliv Rev. 2000;43(2–3):101–164. doi: 10.1016/s0169-409x(00)00067-3. [DOI] [PubMed] [Google Scholar]
- 14.Duncan R. In: Biomedical aspects of drug targetin. Torchilin V, Muzykantov V, editors. Kluwer Academic Publishers; Boston/Dodrecht/London: 2003. pp. 193–209. [Google Scholar]
- 15.Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov. 2003;2(5):347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 16.Langer R. Drug delivery and targeting. Nature. 1998;392(6679 Suppl):5–10. [PubMed] [Google Scholar]
- 17.Torchilin VP. Multifunctional nanocarriers. Adv Drug Deliv Rev. 2006;58(14):1532–1555. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Bae YH. Drug targeting and tumor heterogeneity. J Control Release. 2009;133(1):2–3. doi: 10.1016/j.jconrel.2008.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bae YH, Park K. Targeted drug delivery to tumors: myths, reality and possibility. J Control Release. 2011;153(3):198–205. doi: 10.1016/j.jconrel.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muro S, Muzykantov VR. Targeting of antioxidant and anti-thrombotic drugs to endothelial cell adhesion molecules. Curr Pharm Des. 2005;11(18):2383–2401. doi: 10.2174/1381612054367274. [DOI] [PubMed] [Google Scholar]
- 21.Allen TM. Ligand-targeted therapeutics in anticancer therapy. Nat Rev Cancer. 2002;2(10):750–763. doi: 10.1038/nrc903. [DOI] [PubMed] [Google Scholar]
- 22.Molema G. Design of vascular endothelium-specific drug-targeting strategies for the treatment of cancer. Acta Biochim Pol. 2005;52(2):301–310. [PubMed] [Google Scholar]
- 23.Muzykantov VR. Biomedical aspects of targeted delivery of drugs to pulmonary endothelium. Expert Opin Drug Deliv. 2005;2(5):909–926. doi: 10.1517/17425247.2.5.909. [DOI] [PubMed] [Google Scholar]
- 24.Dunehoo AL, Anderson M, Majumdar S, Kobayashi N, Berkland C, Siahaan TJ. Cell adhesion molecules for targeted drug delivery. J Pharm Sci. 2006;95(9):1856–1872. doi: 10.1002/jps.20676. [DOI] [PubMed] [Google Scholar]
- 25.Lu Y, Yang J, Sega E. Issues related to targeted delivery of proteins and peptides. AAPS J. 2006;8(3):E466–478. doi: 10.1208/aapsj080355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schrama D, Reisfeld RA, Becker JC. Antibody targeted drugs as cancer therapeutics. Nat Rev Drug Discov. 2006;5(2):147–159. doi: 10.1038/nrd1957. [DOI] [PubMed] [Google Scholar]
- 27.Pardridge WM. Brain drug development and brain drug targeting. Pharm Res. 2007;24(9):1729–1732. doi: 10.1007/s11095-007-9387-0. [DOI] [PubMed] [Google Scholar]
- 28.Giordano RJ, Edwards JK, Tuder RM, Arap W, Pasqualini R. Combinatorial ligand-directed lung targeting. Proc Am Thorac Soc. 2009;6(5):411–415. doi: 10.1513/pats.200903-014AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massey KA, Schnitzer JE. Targeting and imaging signature caveolar molecules in lungs. Proc Am Thorac Soc. 2009;6(5):419–430. doi: 10.1513/pats.200903-011AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Low PS, Kularatne SA. Folate-targeted therapeutic and imaging agents for cancer. Curr Opin Chem Biol. 2009;13(3):256–262. doi: 10.1016/j.cbpa.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 31.Yu B, Zhao X, Lee LJ, Lee RJ. Targeted delivery systems for oligonucleotide therapeutics. AAPS J. 2009;11(1):195–203. doi: 10.1208/s12248-009-9096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dustin ML, Rothlein R, Bhan AK, Dinarello CA, Springer TA. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1) J Immunol. 1986;137(1):245–254. [PubMed] [Google Scholar]
- 33.Rothlein R, Dustin ML, Marlin SD, Springer TA. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J Immunol. 1986;137(4):1270–1274. [PubMed] [Google Scholar]
- 34.Muro S, Muzykantov VR. In: Organelle-specific pharmaceutical nanotechnology. Weissig V, D’Souza GGM, editors. John Wiley & Songs, Inc; Hoboken, New Jersey: 2011. pp. 449–474. [Google Scholar]
- 35.Muro S. In: Endothelial Biomedicine. Aird WC, editor. Cambridge University Press; 2007. pp. 1058–1070. [Google Scholar]
- 36.Hsu J, Muro S. In: Human genetic diseases. Plaseska-Karanfilska D, editor. InTech; Rijeka, Croatia: 2011. pp. 241–266. [Google Scholar]
- 37.Muro S. New biotechnological and nanomedicine strategies for treatment of lysosomal storage disorders. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2(2):189–204. doi: 10.1002/wnan.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muzykantov VR. Targeting pulmonary endothelium. Kluwer Academic Publishers; Boston-Dodrecht-London: 2002. [Google Scholar]
- 39.Hayashida K, Bartlett AH, Chen Y, Park PW. Molecular and cellular mechanisms of ectodomain shedding. Anat Rec (Hoboken) 2010;293(6):925–937. doi: 10.1002/ar.20757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andreadis A, Gallego ME, Nadal-Ginard B. Generation of protein isoform diversity by alternative splicing: mechanistic and biological implications. Annu Rev Cell Biol. 1987;3:207–242. doi: 10.1146/annurev.cb.03.110187.001231. [DOI] [PubMed] [Google Scholar]
- 41.Seo J, Lee KJ. Post-translational modifications and their biological functions: proteomic analysis and systematic approaches. J Biochem Mol Biol. 2004;37(1):35–44. doi: 10.5483/bmbrep.2004.37.1.035. [DOI] [PubMed] [Google Scholar]
- 42.Muller WA, Ratti CM, McDonnell SL, Cohn ZA. A human endothelial cell-restricted, externally disposed plasmalemmal protein enriched in intercellular junctions. J Exp Med. 1989;170(2):399–414. doi: 10.1084/jem.170.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yan HC, Pilewski JM, Zhang Q, DeLisser HM, Romer L, Albelda SM. Localization of multiple functional domains on human PECAM-1 (CD31) by monoclonal antibody epitope mapping. Cell Adhes Commun. 1995;3(1):45–66. doi: 10.3109/15419069509081277. [DOI] [PubMed] [Google Scholar]
- 44.Garnacho C, Albelda SM, Muzykantov VR, Muro S. Differential intra-endothelial delivery of polymer nanocarriers targeted to distinct PECAM-1 epitopes. J Control Release. 2008;130(3):226–233. doi: 10.1016/j.jconrel.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balyasnikova IV, Karran EH, Albrecht RF, 2nd, Danilov SM. Epitope-specific antibody-induced cleavage of angiotensin-converting enzyme from the cell surface. Biochem J. 2002;362(Pt 3):585–595. doi: 10.1042/0264-6021:3620585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Danilov S, Atochina E, Hiemisch H, Churak-ova T, Moldobayeva A, Sakharov I, Deichman G, Ryan U, Muzykantov VR. Interaction of mAb to angiotensin-converting enzyme (ACE) with antigen in vitro and in vivo: antibody targeting to the lung induces ACE antigenic modulation. Int Immunol. 1994;6(8):1153–1160. doi: 10.1093/intimm/6.8.1153. [DOI] [PubMed] [Google Scholar]
- 47.Muro S, Wiewrodt R, Thomas A, Koniaris L, Albelda SM, Muzykantov VR, Koval M. A novel endocytic pathway induced by clustering endothelial ICAM-1 or PECAM-1. J Cell Sci. 2003;116(Pt 8):1599–1609. doi: 10.1242/jcs.00367. [DOI] [PubMed] [Google Scholar]
- 48.Muro S, Gajewski C, Koval M, Muzykantov VR. ICAM-1 recycling in endothelial cells: a novel pathway for sustained intracellular delivery and prolonged effects of drugs. Blood. 2005;105(2):650–658. doi: 10.1182/blood-2004-05-1714. [DOI] [PubMed] [Google Scholar]
- 49.Muro S, Koval M, Muzykantov V. Endothelial endocytic pathways: gates for vascular drug delivery. Curr Vasc Pharmacol. 2004;2(3):281–299. doi: 10.2174/1570161043385736. [DOI] [PubMed] [Google Scholar]
- 50.Murciano JC, Muro S, Koniaris L, Christofidou-Solomidou M, Harshaw DW, Albelda SM, Granger DN, Cines DB, Muzykantov VR. ICAM-directed vascular immunotargeting of antithrombotic agents to the endothelial luminal surface. Blood. 2003;101(10):3977–3984. doi: 10.1182/blood-2002-09-2853. [DOI] [PubMed] [Google Scholar]
- 51.Oh P, Borgstrom P, Witkiewicz H, Li Y, Borgstrom BJ, Chrastina A, Iwata K, Zinn KR, Baldwin R, Testa JE, Schnitzer JE. Live dynamic imaging of caveolae pumping targeted antibody rapidly and specifically across endothelium in the lung. Nat Biotechnol. 2007;25(3):327–337. doi: 10.1038/nbt1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bareford LM, Swaan PW. Endocytic mechanisms for targeted drug delivery. Adv Drug Deliv Rev. 2007;59(8):748–758. doi: 10.1016/j.addr.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422(6927):37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 54.Stan RV. Endocytosis pathways in endothelium: how many? Am J Physiol Lung Cell Mol Physiol. 2006;290(5):L806–808. doi: 10.1152/ajplung.00533.2005. [DOI] [PubMed] [Google Scholar]
- 55.Muro S, Garnacho C, Champion JA, Leferovich J, Gajewski C, Schuchman EH, Mitragotri S, Muzykantov VR. Control of endothelial targeting and intracellular delivery of therapeutic enzymes by modulating the size and shape of ICAM-1-targeted carriers. Mol Ther. 2008;16(8):1450–1458. doi: 10.1038/mt.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stan RV. Structure and function of endothelial caveolae. Microsc Res Tech. 2002;57(5):350–364. doi: 10.1002/jemt.10089. [DOI] [PubMed] [Google Scholar]
- 57.Schnitzer JE. Caveolae: from basic trafficking mechanisms to targeting transcytosis for tissue-specific drug and gene delivery in vivo. Adv Drug Deliv Rev. 2001;49(3):265–280. doi: 10.1016/s0169-409x(01)00141-7. [DOI] [PubMed] [Google Scholar]
- 58.Simionescu M, Simionescu N. Endothelial transport of macromolecules: transcytosis and endocytosis. A look from cell biology. Cell Biol Rev. 1991;25(1):5–78. [PubMed] [Google Scholar]
- 59.Predescu D, Palade GE. Plasmalemmal vesicles represent the large pore system of continuous microvascular endothelium. Am J Physiol. 1993;265(2 Pt 2):H725–733. doi: 10.1152/ajpheart.1993.265.2.H725. [DOI] [PubMed] [Google Scholar]
- 60.Minshall RD, Malik AB. Transport across the endothelium: regulation of endothelial permeability. Handb Exp Pharmacol. 2006;176(Pt 1):107–144. doi: 10.1007/3-540-32967-6_4. [DOI] [PubMed] [Google Scholar]
- 61.Tiruppathi C, Song W, Bergenfeldt M, Sass P, Malik AB. Gp60 activation mediates albumin transcytosis in endothelial cells by tyrosine kinase-dependent pathway. J Biol Chem. 1997;272(41):25968–25975. doi: 10.1074/jbc.272.41.25968. [DOI] [PubMed] [Google Scholar]
- 62.Weroha SJ, Haluska P. IGF-1 receptor inhibitors in clinical trials--early lessons. J Mammary Gland Biol Neoplasia. 2008;13(4):471–483. doi: 10.1007/s10911-008-9104-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parton RG, Lindsay M. Exploitation of major histocompatibility complex class I molecules and caveolae by simian virus 40. Immunol Rev. 1999;168:23–31. doi: 10.1111/j.1600-065x.1999.tb01280.x. [DOI] [PubMed] [Google Scholar]
- 64.Rajendran L, Knolker HJ, Simons K. Subcellular targeting strategies for drug design and delivery. Nat Rev Drug Discov. 2010;9(1):29–42. doi: 10.1038/nrd2897. [DOI] [PubMed] [Google Scholar]
- 65.Johannes L, Decaudin D. Protein toxins: intracellular trafficking for targeted therapy. Gene Ther. 2005;12(18):1360–1368. doi: 10.1038/sj.gt.3302557. [DOI] [PubMed] [Google Scholar]
- 66.Fuchs R, Male P, Mellman I. Acidification and ion permeabilities of highly purified rat liver endosomes. J Biol Chem. 1989;264(4):2212–2220. [PubMed] [Google Scholar]
- 67.Xia W, Low PS. Folate-targeted therapies for cancer. J Med Chem. 2010;53(19):6811–6824. doi: 10.1021/jm100509v. [DOI] [PubMed] [Google Scholar]
- 68.Cho YW, Kim JD, Park K. Polycation gene delivery systems: escape from endosomes to cytosol. J Pharm Pharmacol. 2003;55(6):721–734. doi: 10.1211/002235703765951311. [DOI] [PubMed] [Google Scholar]
- 69.Varkouhi AK, Scholte M, Storm G, Haisma HJ. Endosomal escape pathways for delivery of biologicals. J Control Release. 2011;151(3):220–228. doi: 10.1016/j.jconrel.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 70.Muzykantov V. Targeting drugs to pulmonary endothelium. Expert Opinion Drug Delivery. 2005;2:909–926. doi: 10.1517/17425247.2.5.909. [DOI] [PubMed] [Google Scholar]
- 71.Bies C, Lehr CM, Woodley JF. Lectin-mediated drug targeting: history and applications. Adv Drug Deliv Rev. 2004;56(4):425–435. doi: 10.1016/j.addr.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 72.Zheng G, Chen J, Li H, Glickson JD. Rerouting lipoprotein nanoparticles to selected alternate receptors for the targeted delivery of cancer diagnostic and therapeutic agents. Proc Natl Acad Sci U S A. 2005;102(49):17757–17762. doi: 10.1073/pnas.0508677102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pardridge WM. Drug targeting to the brain. Pharm Res. 2007;24(9):1733–1744. doi: 10.1007/s11095-007-9324-2. [DOI] [PubMed] [Google Scholar]
- 74.Molek P, Strukelj B, Bratkovic T. Peptide phage display as a tool for drug discovery: targeting membrane receptors. Molecules. 2011;16(1):857–887. doi: 10.3390/molecules16010857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Serra L, Domenech J, Peppas NA. Engineering design and molecular dynamics of mucoadhesive drug delivery systems as targeting agents. Eur J Pharm Biopharm. 2009;71(3):519–528. doi: 10.1016/j.ejpb.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yoon DJ, Liu CT, Quinlan DS, Nafisi PM, Kamei DT. Intracellular trafficking considerations in the development of natural ligand-drug molecular conjugates for cancer. Ann Biomed Eng. 2011;39(4):1235–1251. doi: 10.1007/s10439-011-0280-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Foged C, Nielsen HM. Cell-penetrating peptides for drug delivery across membrane barriers. Expert Opin Drug Deliv. 2008;5(1):105–117. doi: 10.1517/17425247.5.1.105. [DOI] [PubMed] [Google Scholar]
- 78.Jones AT. Gateways and tools for drug delivery: endocytic pathways and the cellular dynamics of cell penetrating peptides. Int J Pharm. 2008;354(1–2):34–38. doi: 10.1016/j.ijpharm.2007.10.046. [DOI] [PubMed] [Google Scholar]
- 79.Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55(6):1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- 80.Schroder T, Niemeier N, Afonin S, Ulrich AS, Krug HF, Brase S. Peptoidic amino- and guanidinium-carrier systems: targeted drug delivery into the cell cytosol or the nucleus. J Med Chem. 2008;51(3):376–379. doi: 10.1021/jm070603m. [DOI] [PubMed] [Google Scholar]
- 81.Li W, Nicol F, Szoka FC., Jr GALA: a designed synthetic pH-responsive amphipathic peptide with applications in drug and gene delivery. Adv Drug Deliv Rev. 2004;56(7):967–985. doi: 10.1016/j.addr.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 82.Mastrobattista E, Hennink WE, Schiffelers RM. Delivery of nucleic acids. Pharm Res. 2007;24(8):1561–1563. doi: 10.1007/s11095-007-9349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Muratovska A, Lightowlers RN, Taylor RW, Wilce JA, Murphy MP. Targeting large molecules to mitochondria. Adv Drug Deliv Rev. 2001;49(1–2):189–198. doi: 10.1016/s0169-409x(01)00134-x. [DOI] [PubMed] [Google Scholar]
- 84.Li S, Huang L. Nonviral gene therapy: promises and challenges. Gene Ther. 2000;7(1):31–34. doi: 10.1038/sj.gt.3301110. [DOI] [PubMed] [Google Scholar]
- 85.El-Sayed A, Futaki S, Harashima H. Delivery of macromolecules using arginine-rich cell-penetrating peptides: ways to overcome endosomal entrapment. AAPS J. 2009;11(1):13–22. doi: 10.1208/s12248-008-9071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fabre JW, Collins L. Synthetic peptides as non-viral DNA vectors. Curr Gene Ther. 2006;6(4):459–480. doi: 10.2174/156652306777934865. [DOI] [PubMed] [Google Scholar]
- 87.Carter PJ. Introduction to current and future protein therapeutics: a protein engineering perspective. Exp Cell Res. 2011;317(9):1261–1269. doi: 10.1016/j.yexcr.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 88.Schrama D, Reisfeld RA, Becker JC. Antibody targeted drugs as cancer therapeutics. Nat Rev Drug Discov. 2006;5(2):147–159. doi: 10.1038/nrd1957. [DOI] [PubMed] [Google Scholar]
- 89.Debbage P. Targeted drugs and nanomedicine: present and future. Curr Pharm Des. 2009;15(2):153–172. doi: 10.2174/138161209787002870. [DOI] [PubMed] [Google Scholar]
- 90.Vaidya A, Agarwal A, Jain A, Agrawal RK, Jain SK. Bioconjugation of polymers: a novel platform for targeted drug delivery. Curr Pharm Des. 2011;17(11):1108–1125. doi: 10.2174/138161211795656873. [DOI] [PubMed] [Google Scholar]
- 91.Moghimi SM, Hunter AC, Murray JC. Nanomedicine: current status and future prospects. FASEB J. 2005;19(3):311–330. doi: 10.1096/fj.04-2747rev. [DOI] [PubMed] [Google Scholar]
- 92.Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55(3):329–347. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 93.Hudson PJ, Kortt AA. High avidity scFv multimers; diabodies and triabodies. J Immunol Methods. 1999;231(1–2):177–189. doi: 10.1016/s0022-1759(99)00157-x. [DOI] [PubMed] [Google Scholar]
- 94.Rudnick SI, Adams GP. Affinity and avidity in antibody-based tumor targeting. Cancer Biother Radiopharm. 2009;24(2):155–161. doi: 10.1089/cbr.2009.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Raha S, Paunesku T, Woloschak G. Peptide-mediated cancer targeting of nanoconjugates. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011;3(3):269–281. doi: 10.1002/wnan.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Muro S, Dziubla T, Qiu W, Leferovich J, Cui X, Berk E, Muzykantov VR. Endothelial targeting of high-affinity multivalent polymer nanocarriers directed to intercellular adhesion molecule 1. J Pharmacol Exp Ther. 2006;317(3):1161–1169. doi: 10.1124/jpet.105.098970. [DOI] [PubMed] [Google Scholar]
- 97.Calderon AJ, Bhowmick T, Leferovich J, Burman B, Pichette B, Muzykantov V, Eckmann DM, Muro S. Optimizing endothelial targeting by modulating the antibody density and particle concentration of anti-ICAM coated carriers. J Control Release. 2011;150(1):37–44. doi: 10.1016/j.jconrel.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shokeen M, Pressly ED, Hagooly A, Zheleznyak A, Ramos N, Fiamengo AL, Welch MJ, Hawker CJ, Anderson CJ. Evaluation of multivalent, functional polymeric nanoparticles for imaging applications. ACS Nano. 2011;5(2):738–747. doi: 10.1021/nn102278w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Frey A, Giannasca KT, Weltzin R, Giannasca PJ, Reggio H, Lencer WI, Neutra MR. Role of the glycocalyx in regulating access of microparticles to apical plasma membranes of intestinal epithelial cells: implications for microbial attachment and oral vaccine targeting. J Exp Med. 1996;184(3):1045–1059. doi: 10.1084/jem.184.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hatakeyama H, Akita H, Maruyama K, Suhara T, Harashima H. Factors governing the in vivo tissue uptake of transferrin-coupled polyethylene glycol liposomes in vivo. Int J Pharm. 2004;281(1–2):25–33. doi: 10.1016/j.ijpharm.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 101.Moghimi SM, Szebeni J. Stealth liposomes and long circulating nanoparticles: critical issues in pharmacokinetics, opsonization and protein-binding properties. Prog Lipid Res. 2003;42(6):463–478. doi: 10.1016/s0163-7827(03)00033-x. [DOI] [PubMed] [Google Scholar]
- 102.Stachelek SJ, Finley MJ, Alferiev IS, Wang F, Tsai RK, Eckells EC, Tomczyk N, Connolly JM, Discher DE, Eckmann DM, Levy RJ. The effect of CD47 modified polymer surfaces on inflammatory cell attachment and activation. Biomaterials. 2011;32(19):4317–4326. doi: 10.1016/j.biomaterials.2011.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, Discher DE. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol. 2007;2(4):249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shuvaev VV, Ilies MA, Simone E, Zaitsev S, Kim Y, Cai S, Mahmud A, Dziubla T, Muro S, Discher DE, Muzykantov VR. Endothelial targeting of antibody-decorated polymeric filomicelles. ACS Nano. 5(9):6991–6999. doi: 10.1021/nn2015453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Koval M, Preiter K, Adles C, Stahl PD, Steinberg TH. Size of IgG-opsonized particles determines macrophage response during internalization. Exp Cell Res. 1998;242(1):265–273. doi: 10.1006/excr.1998.4110. [DOI] [PubMed] [Google Scholar]
- 106.Brewer JM, Tetley L, Richmond J, Liew FY, Alexander J. Lipid vesicle size determines the Th1 or Th2 response to entrapped antigen. J Immunol. 1998;161(8):4000–4007. [PubMed] [Google Scholar]
- 107.Conrad ME, Umbreit JN. Iron absorption and transport-an update. Am J Hematol. 2000;64(4):287–2. doi: 10.1002/1096-8652(200008)64:4<287::aid-ajh9>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 108.Fishman JB, Rubin JB, Handrahan JV, Connor JR, Fine RE. Receptor-mediated transcytosis of transferrin across the blood-brain barrier. J Neurosci Res. 1987;18(2):299–304. doi: 10.1002/jnr.490180206. [DOI] [PubMed] [Google Scholar]
- 109.Carman CV, Springer TA. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J Cell Biol. 2004;167(2):377–388. doi: 10.1083/jcb.200404129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Millan J, Hewlett L, Glyn M, Toomre D, Clark P, Ridley AJ. Lymphocyte transcellular migration occurs through recruitment of endothelial ICAM-1 to caveola- and F-actin-rich domains. Nat Cell Biol. 2006;8(2):113–123. doi: 10.1038/ncb1356. [DOI] [PubMed] [Google Scholar]
- 111.Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci U S A. 2006;103(13):4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pasqualini R, Koivunen E, Kain R, Lahdenranta J, Sakamoto M, Stryhn A, Ashmun RA, Shapiro LH, Arap W, Ruoslahti E. Aminopeptidase N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesis. Cancer Res. 2000;60(3):722–727. [PMC free article] [PubMed] [Google Scholar]
- 113.Ruoslahti E, Rajotte D. An address system in the vasculature of normal tissues and tumors. Annu Rev Immunol. 2000;18:813–827. doi: 10.1146/annurev.immunol.18.1.813. [DOI] [PubMed] [Google Scholar]
- 114.Kumar CC, Malkowski M, Yin Z, Tanghetti E, Yaremko B, Nechuta T, Varner J, Liu M, Smith EM, Neustadt B, Presta M, Armstrong L. Inhibition of angiogenesis and tumor growth by SCH221153, a dual alpha(v)beta3 and alpha(v)beta5 integrin receptor antagonist. Cancer Res. 2001;61(5):2232–2238. [PubMed] [Google Scholar]
- 115.Borisch B, Semac I, Soltermann A, Palomba C, Hoessli DC. Anti-CD20 treatments and the lymphocyte membrane: pathology for therapy. Verh Dtsch Ges Pathol. 2001;85:161–166. [PubMed] [Google Scholar]
- 116.Cirstoiu-Hapca A, Bossy-Nobs L, Buchegger F, Gurny R, Delie F. Differential tumor cell targeting of anti-HER2 (Herceptin) and anti-CD20 (Mabthera) coupled nanoparticles. Int J Pharm. 2007;331(2):190–196. doi: 10.1016/j.ijpharm.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 117.Menrad A, Menssen HD. ED-B fibronectin as a target for antibody-based cancer treatments. Expert Opin Ther Targets. 2005;9(3):491–500. doi: 10.1517/14728222.9.3.491. [DOI] [PubMed] [Google Scholar]
- 118.Graham J, Muhsin M, Kirkpatrick P. Cetuximab. Nat Rev Drug Discov. 2004;3(7):549–550. doi: 10.1038/nrd1445. [DOI] [PubMed] [Google Scholar]
- 119.Fonsatti E, Nicolay HJ, Altomonte M, Covre A, Maio M. Targeting cancer vasculature via endoglin/CD105: a novel antibody-based diagnostic and therapeutic strategy in solid tumours. Cardiovasc Res. 2010;86(1):12–19. doi: 10.1093/cvr/cvp332. [DOI] [PubMed] [Google Scholar]
- 120.Noonberg SB, Benz CC. Tyrosine kinase inhibitors targeted to the epidermal growth factor receptor subfamily: role as anticancer agents. Drugs. 2000;59(4):753–767. doi: 10.2165/00003495-200059040-00003. [DOI] [PubMed] [Google Scholar]
- 121.Lamaze C, Dujeancourt A, Baba T, Lo CG, Benmerah A, Dautry-Varsat A. Interleukin 2 receptors and detergent-resistant membrane domains define a clathrin-independent endocytic pathway. Mol Cell. 2001;7(3):661–671. doi: 10.1016/s1097-2765(01)00212-x. [DOI] [PubMed] [Google Scholar]
- 122.Boado RJ, Zhang Y, Xia CF, Wang Y, Pardridge WM. Genetic engineering of a lysosomal enzyme fusion protein for targeted delivery across the human blood-brain barrier. Biotechnol Bioeng. 2008;99(2):475–484. doi: 10.1002/bit.21602. [DOI] [PubMed] [Google Scholar]
- 123.Chung NS, Wasan KM. Potential role of the low-density lipoprotein receptor family as mediators of cellular drug uptake. Adv Drug Deliv Rev. 2004;56(9):1315–1334. doi: 10.1016/j.addr.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 124.Prince WS, McCormick LM, Wendt DJ, Fitzpatrick PA, Schwartz KL, Aguilera AI, Koppaka V, Christianson TM, Vellard MC, Pavloff N, Lemontt JF, Qin M, Starr CM, Bu G, Zankel TC. Lipoprotein receptor binding, cellular uptake, and lysosomal delivery of fusions between the receptor-associated protein (RAP) and alpha-L-iduronidase or acid alpha-glucosidase. J Biol Chem. 2004;279(33):35037–35046. doi: 10.1074/jbc.M402630200. [DOI] [PubMed] [Google Scholar]
- 125.Schally AV, Comaru-Schally AM, Plonowski A, Nagy A, Halmos G, Rekasi Z. Peptide analogs in the therapy of prostate cancer. Prostate. 2000;45(2):158–166. doi: 10.1002/1097-0045(20001001)45:2<158::aid-pros10>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 126.Desnick RJ, Schuchman EH. Enzyme replacement and enhancement therapies: lessons from lysosomal disorders. Nat Rev Genet. 2002;3(12):954–966. doi: 10.1038/nrg963. [DOI] [PubMed] [Google Scholar]
- 127.LeBowitz JH, Grubb JH, Maga JA, Schmiel DH, Vogler C, Sly WS. Glycosylation-independent targeting enhances enzyme delivery to lysosomes and decreases storage in mucopolysaccharidosis type VII mice. Proc Natl Acad Sci U S A. 2004;101(9):3083–3088. doi: 10.1073/pnas.0308728100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vartak DG, Gemeinhart RA. Matrix metalloproteases: underutilized targets for drug delivery. J Drug Target. 2007;15(1):1–20. doi: 10.1080/10611860600968967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Koivunen E, Arap W, Valtanen H, Rainisalo A, Medina OP, Heikkila P, Kantor C, Gahmberg CG, Salo T, Konttinen YT, Sorsa T, Ruoslahti E, Pasqualini R. Tumor targeting with a selective gelatinase inhibitor. Nat Biotechnol. 1999;17(8):768–774. doi: 10.1038/11703. [DOI] [PubMed] [Google Scholar]
- 130.Ren J, Agata N, Chen D, Li Y, Yu WH, Huang L, Raina D, Chen W, Kharbanda S, Kufe D. Human MUC1 carcinoma-associated protein confers resistance to genotoxic anticancer agents. Cancer Cell. 2004;5(2):163–175. doi: 10.1016/s1535-6108(04)00020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Everts M, Koning GA, Kok RJ, Asgeirsdottir SA, Vestweber D, Meijer DK, Storm G, Molema G. In vitro cellular handling and in vivo targeting of E-selectin-directed immunoconjugates and immunoliposomes used for drug delivery to inflamed endothelium. Pharm Res. 2003;20(1):64–72. doi: 10.1023/a:1022298725165. [DOI] [PubMed] [Google Scholar]
- 132.Nahrendorf M, Keliher E, Panizzi P, Zhang H, Hembrador S, Figueiredo JL, Aikawa E, Kelly K, Libby P, Weissleder R. 18F-4V for PET-CT imaging of VCAM-1 expression in atherosclerosis. JACC Cardiovasc Imaging. 2009;2(10):1213–1222. doi: 10.1016/j.jcmg.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Brekken RA, Overholser JP, Stastny VA, Waltenberger J, Minna JD, Thorpe PE. Selective inhibition of vascular endothelial growth factor (VEGF) receptor 2 (KDR/Flk-1) activity by a monoclonal anti-VEGF antibody blocks tumor growth in mice. Cancer Res. 2000;60(18):5117–5124. [PubMed] [Google Scholar]
- 134.Binetruy-Tournaire R, Demangel C, Malavaud B, Vassy R, Rouyre S, Kraemer M, Plouet J, Derbin C, Perret G, Mazie JC. Identification of a peptide blocking vascular endothelial growth factor (VEGF)-mediated angiogenesis. EMBO J. 2000;19(7):1525–1533. doi: 10.1093/emboj/19.7.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hildinger M, Dittmar MT, Schult-Dietrich P, Fehse B, Schnierle BS, Thaler S, Stiegler G, Welker R, von Laer D. Membrane-anchored peptide inhibits human immunodeficiency virus entry. J Virol. 2001;75(6):3038–3042. doi: 10.1128/JVI.75.6.3038-3042.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Petersen J, Dandri M, Mier W, Lutgehetmann M, Volz T, von Weizsacker F, Haberkorn U, Fischer L, Pollok JM, Erbes B, Seitz S, Urban S. Prevention of hepatitis B virus infection in vivo by entry inhibitors derived from the large envelope protein. Nat Biotechnol. 2008;26(3):335–341. doi: 10.1038/nbt1389. [DOI] [PubMed] [Google Scholar]
- 137.Covic L, Gresser AL, Talavera J, Swift S, Kuliopulos A. Activation and inhibition of G protein-coupled receptors by cell-penetrating membrane-tethered peptides. Proc Natl Acad Sci U S A. 2002;99(2):643–648. doi: 10.1073/pnas.022460899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Elliott G, O’Hare P. Intercellular trafficking and protein delivery by a herpesvirus structural protein. Cell. 1997;88(2):223–233. doi: 10.1016/s0092-8674(00)81843-7. [DOI] [PubMed] [Google Scholar]
- 139.Mastrobattista E, Koning GA, Storm G. Immunoliposomes for the targeted delivery of antitumor drugs. Adv Drug Deliv Rev. 1999;40(1–2):103–127. doi: 10.1016/s0169-409x(99)00043-5. [DOI] [PubMed] [Google Scholar]
- 140.Ghaffarian R, Bhowmick T, Muro S. Transport of α-galactosidase coupled to ICAM-1-targeted nanocarriers across gastrointestinal epithelial cells. Mol Genet Metab. 2012;105(2):S30. doi: 10.1016/j.jconrel.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]