Abstract
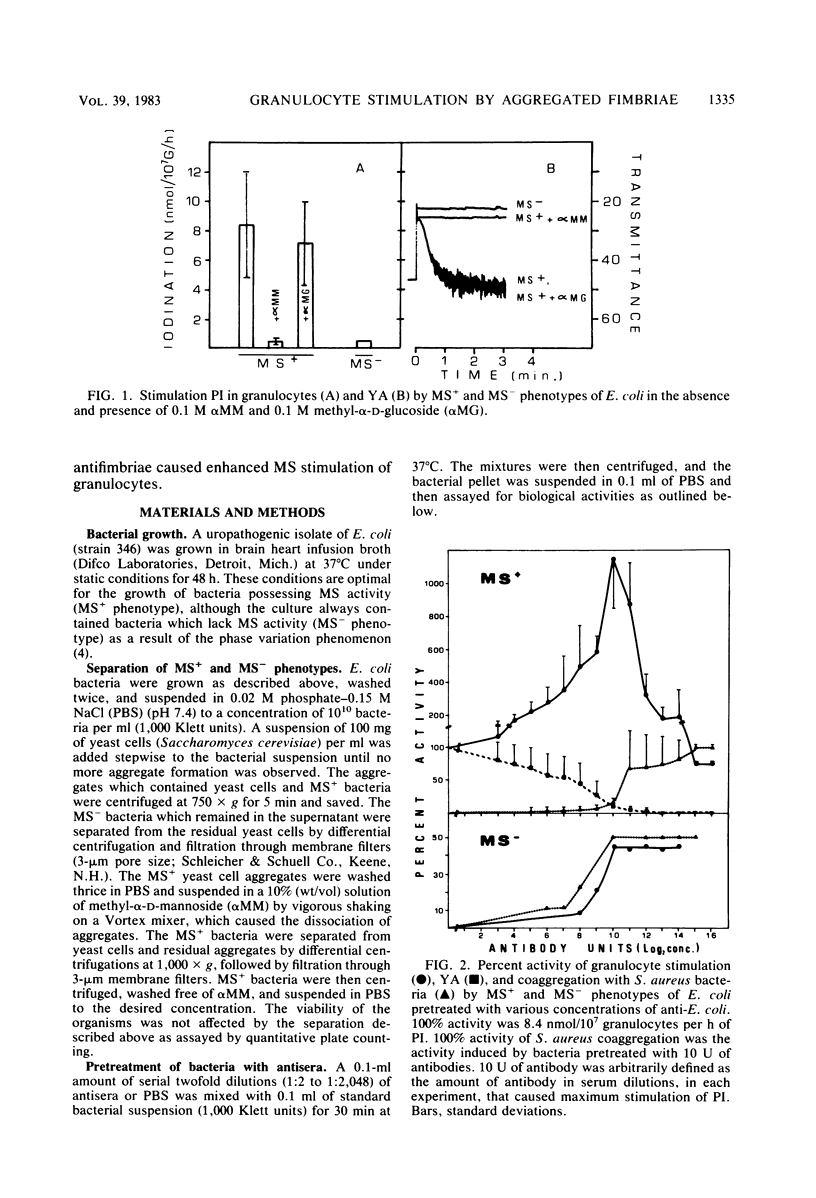
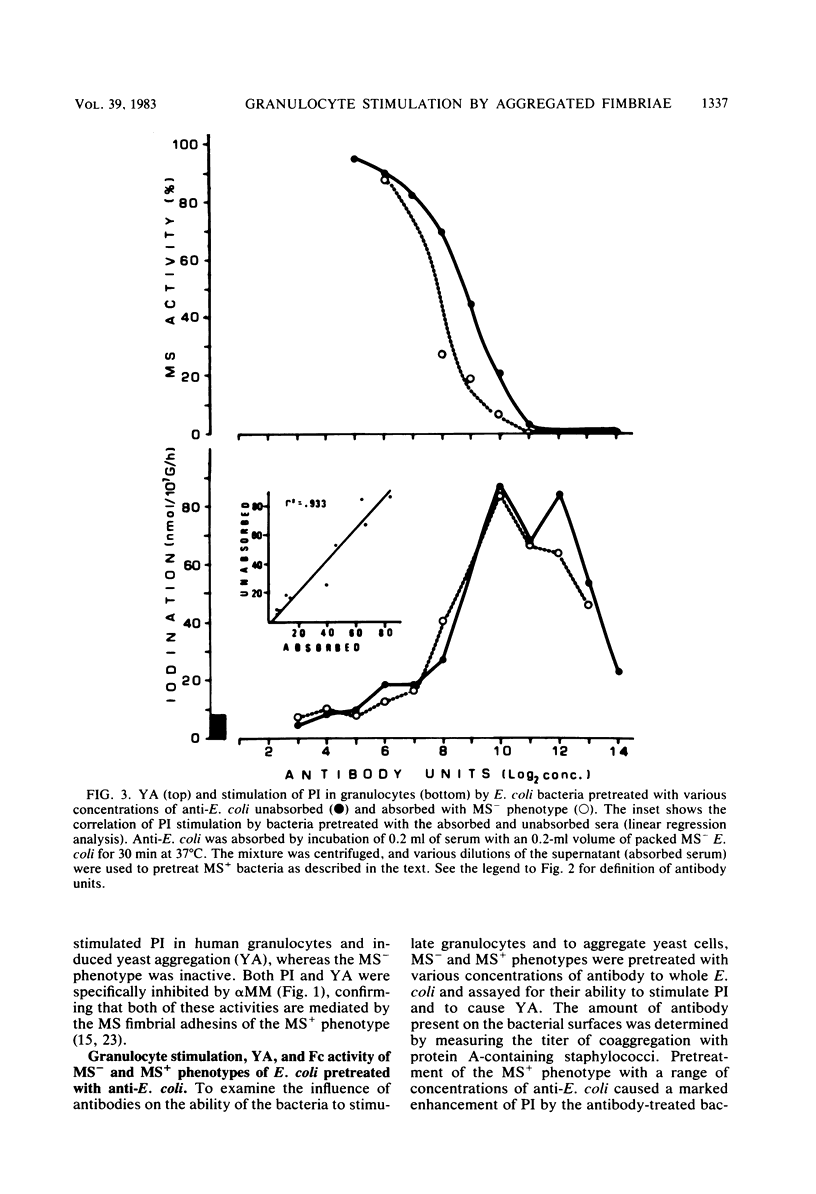
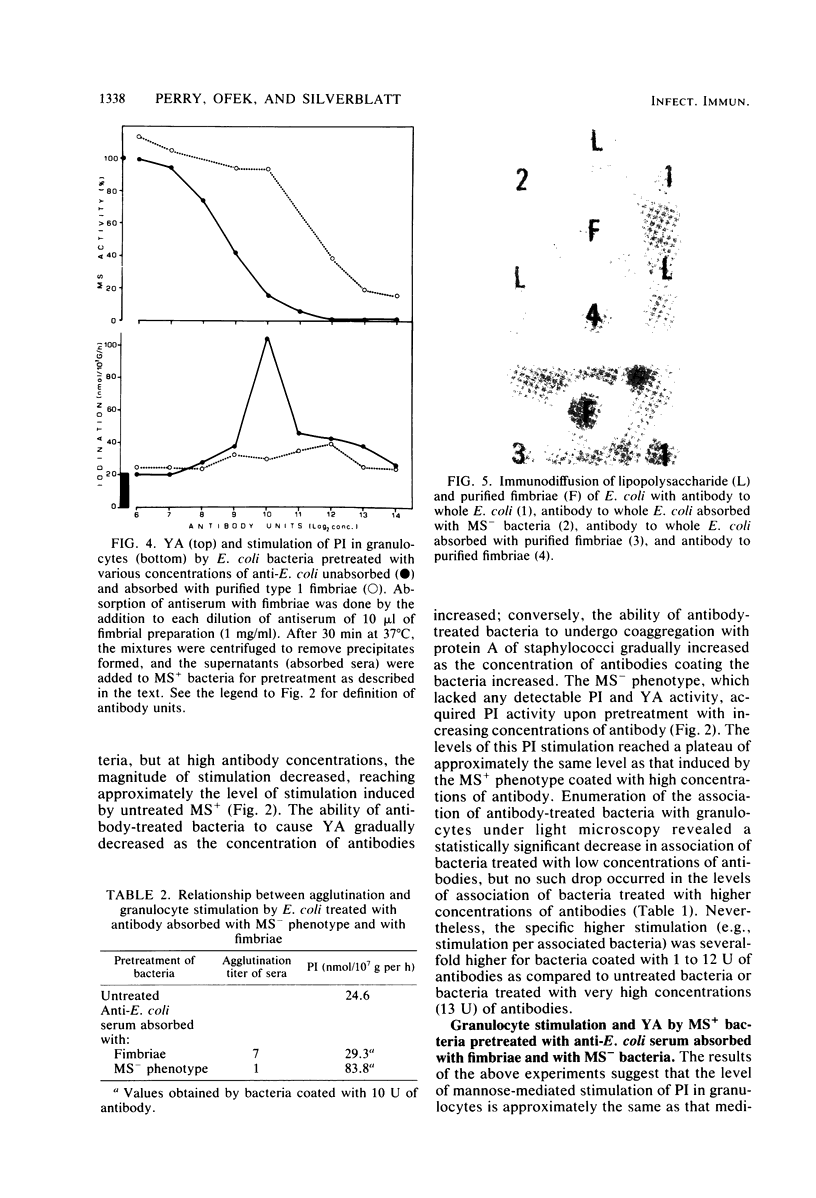
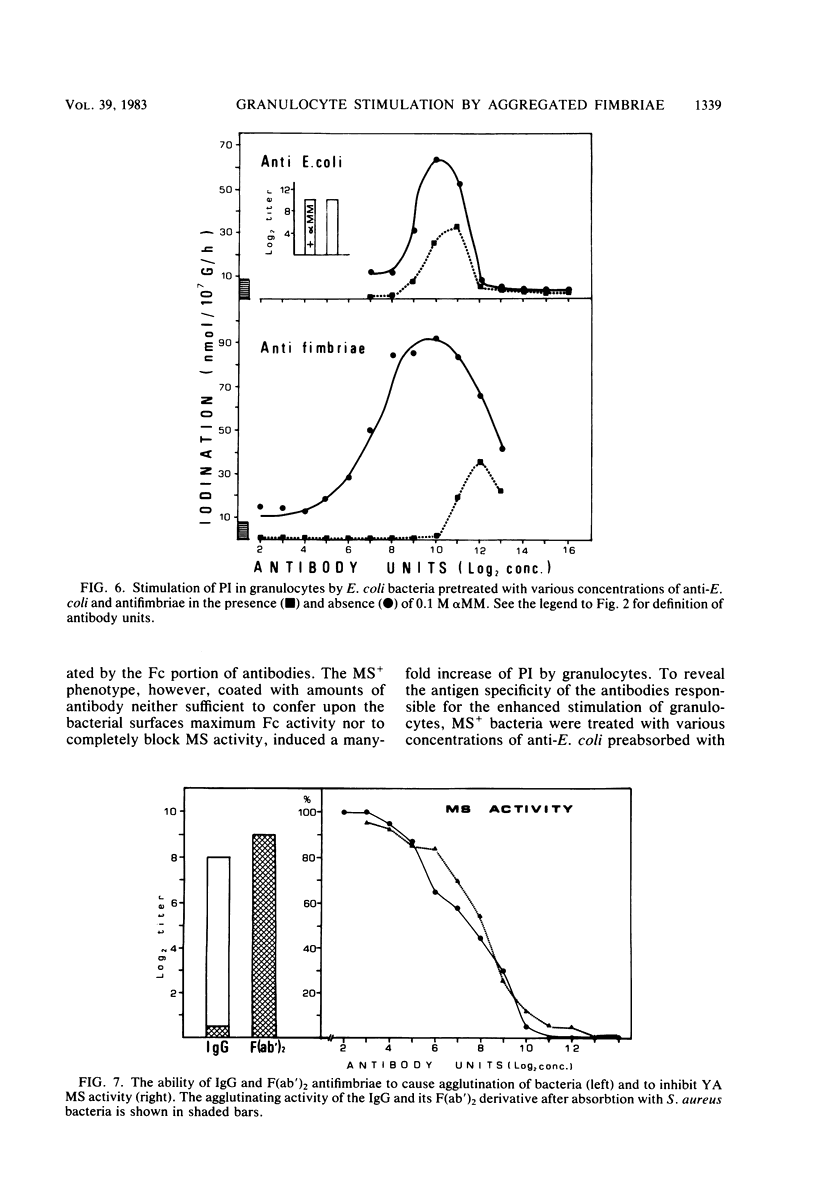
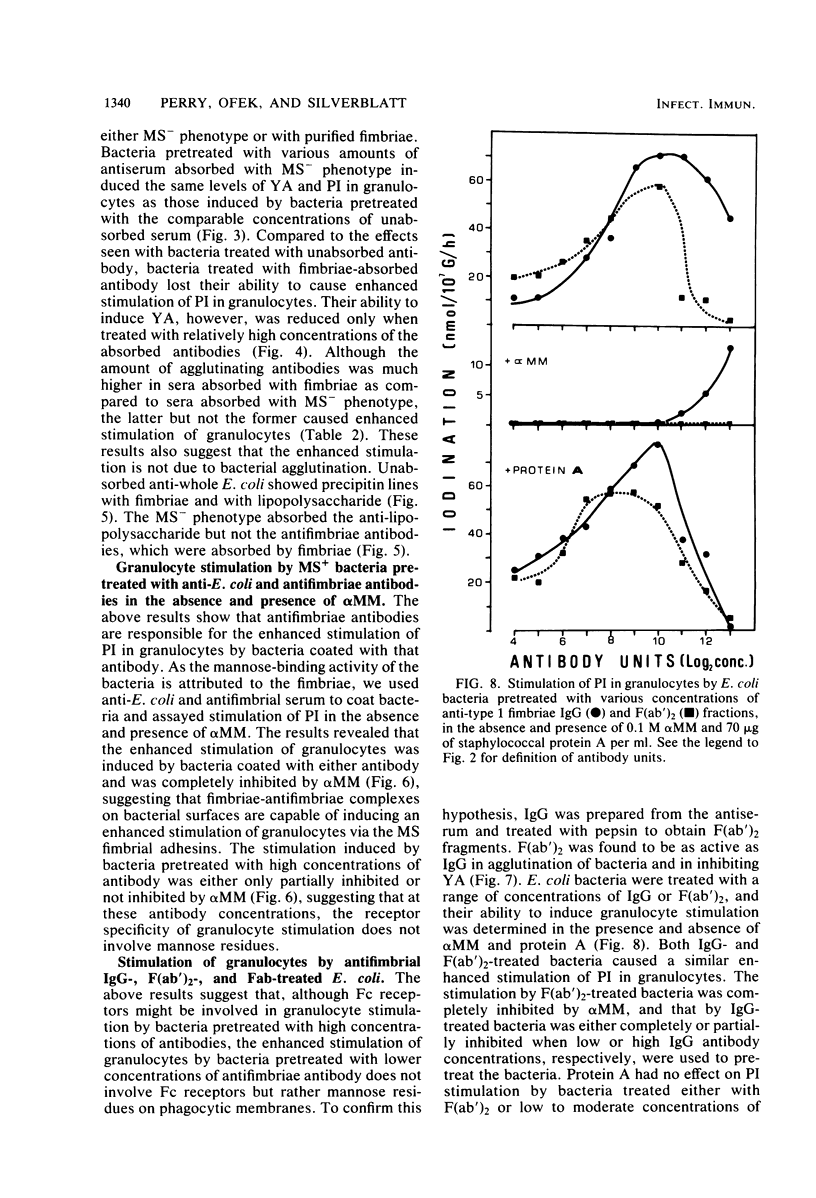
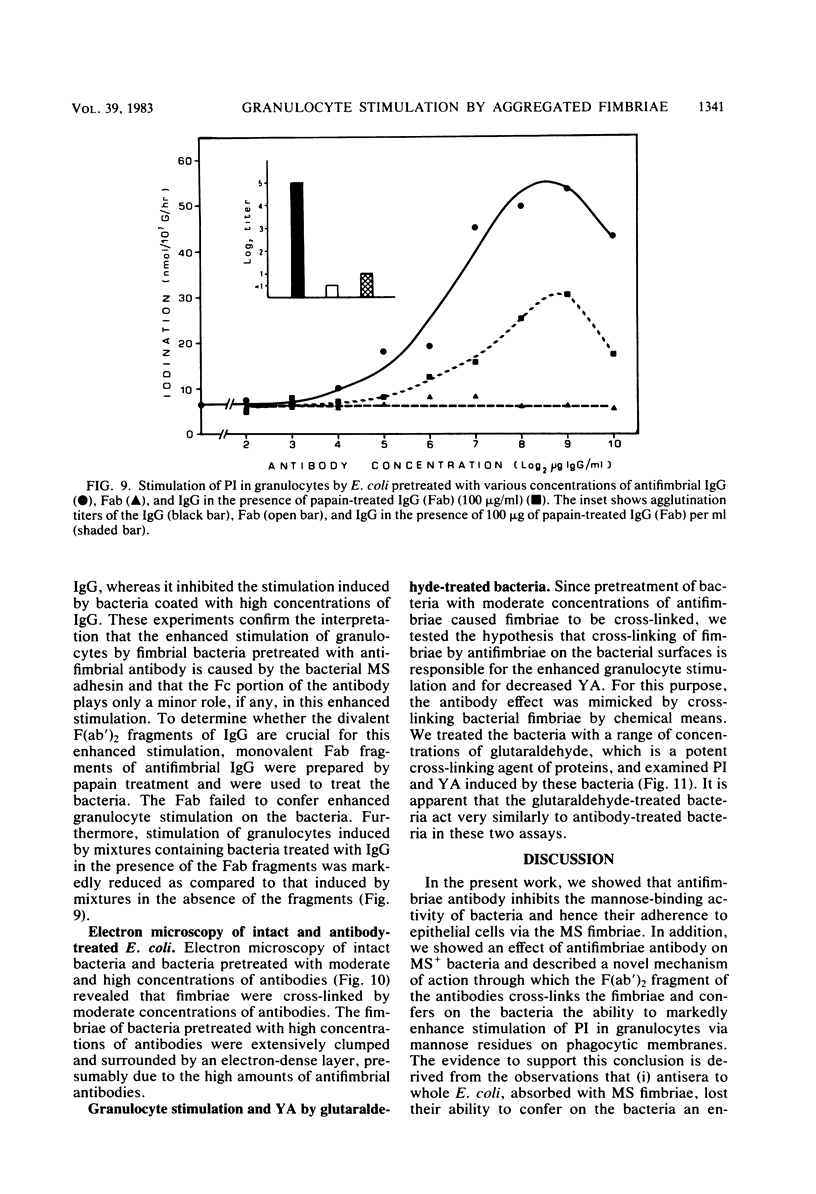
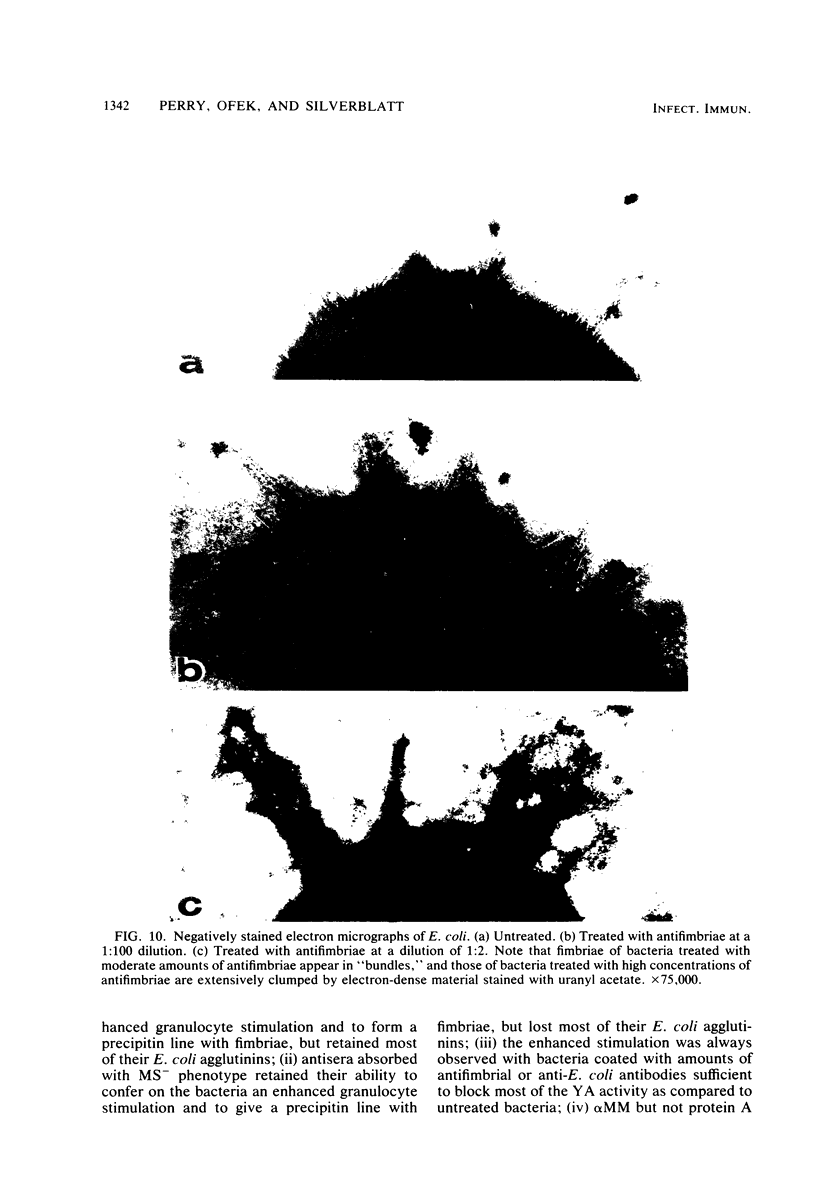
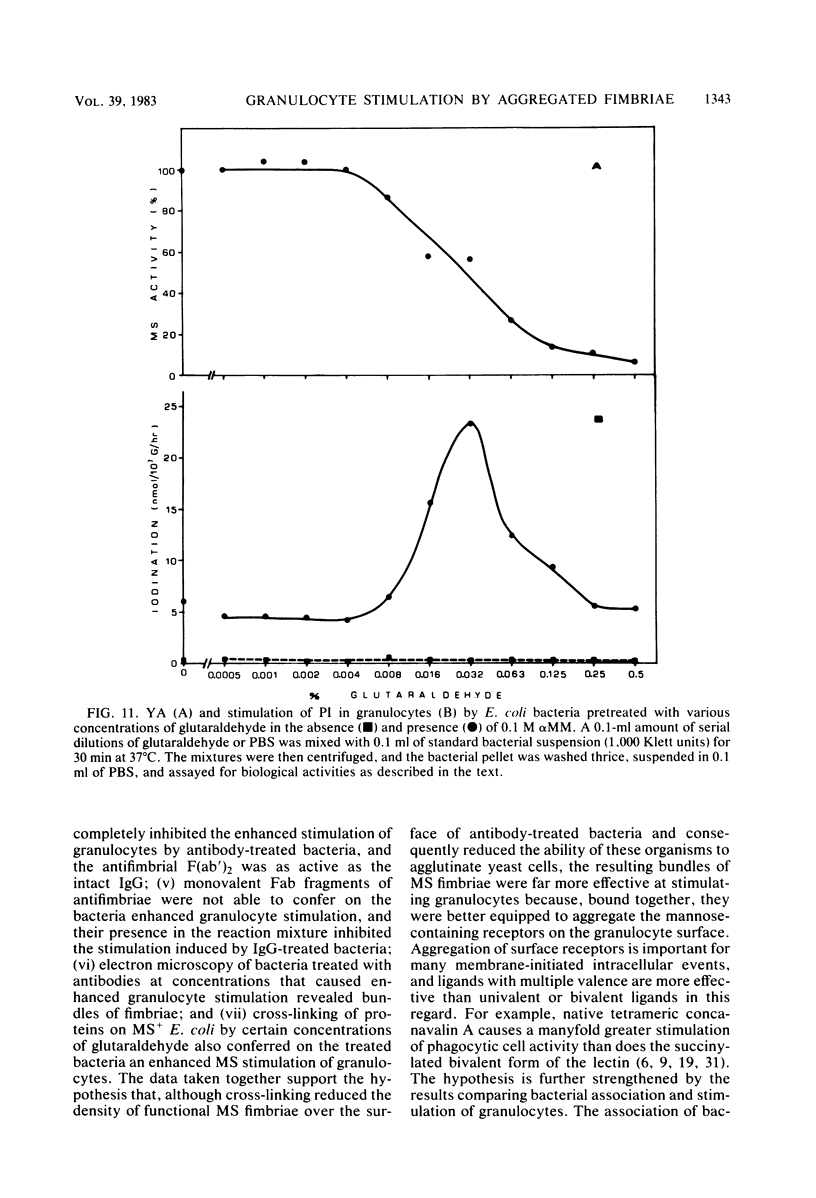
In the present study, we assayed protein iodination in human granulocytes after interaction of the cells with mannose-specific (MS) type 1 fimbriated (MS+) and nonfimbriated (MS-) phenotypes of Escherichia coli pretreated with various amounts of anti-E. coli and antifimbrial antibodies. The MS+ phenotype stimulated protein iodination in granulocytes and possessed potent MS activity as measured by yeast aggregometry. In contrast, the MS- phenotype lacked all these activities. MS+ pretreated with moderate concentrations of antibodies, however, showed up to a 15-fold increase in granulocyte stimulation as compared to granulocyte stimulation induced by the non-antibody-treated MS+ phenotype or by the antibody-treated MS- phenotype. This marked antibody-mediated increase in stimulation of granulocytes was (i) dependent on the antibody concentrations, (ii) markedly reduced by methyl-alpha-D-mannoside, (iii) caused by immunoglobulin G as well as by F(ab')2, (iv) caused only by antifimbrial antibodies, (v) associated with cross-linked fimbriae seen as "bundles" under an electron microscope, and (vi) mimicked by treating MS+ bacteria with a certain range of glutaraldehyde. The data taken together support the hypothesis that, although cross-linking of fimbriae reduced the density of functional MS fimbriae over the surface of antibody-treated bacteria and consequently reduced the ability of these organisms to agglutinate yeast cells, the resulting bundles of MS fimbriae were far more effective at stimulating granulocytes because, bound together, they were better equipped to aggregate the mannose-containing receptors on the granulocyte surface.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Shavit Z., Goldman R., Ofek I., Sharon N., Mirelman D. Mannose-binding activity of Escherichia coli: a determinant of attachment and ingestion of the bacteria by macrophages. Infect Immun. 1980 Aug;29(2):417–424. doi: 10.1128/iai.29.2.417-424.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shavit Z., Ofek I., Goldman R., Mirelman D., Sharon N. Mannose residues on phagocytes as receptors for the attachment of Escherichia coli and Salmonella typhi. Biochem Biophys Res Commun. 1977 Sep 9;78(1):455–460. doi: 10.1016/0006-291x(77)91276-1. [DOI] [PubMed] [Google Scholar]
- Eisenstein B. I. Phase variation of type 1 fimbriae in Escherichia coli is under transcriptional control. Science. 1981 Oct 16;214(4518):337–339. doi: 10.1126/science.6116279. [DOI] [PubMed] [Google Scholar]
- Eshdat Y., Silverblatt F. J., Sharon N. Dissociation and reassembly of Escherichia coli type 1 pili. J Bacteriol. 1981 Oct;148(1):308–314. doi: 10.1128/jb.148.1.308-314.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R., Sharon N., Lotan R. A differential response elicited in macrophages on interaction with lectins. Exp Cell Res. 1976 May;99(2):408–422. doi: 10.1016/0014-4827(76)90598-x. [DOI] [PubMed] [Google Scholar]
- Keisari Y., Pick E. Macrophage-mediated cytolysis off erythrocytes in the guinea pig. I. Activation by stimulators of the oxidative burst. Cell Immunol. 1981 Jul 15;62(1):172–185. doi: 10.1016/0008-8749(81)90311-7. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Klebanoff S. J., Clark R. A. Iodination by human polymorphonuclear leukocytes: a re-evaluation. J Lab Clin Med. 1977 Mar;89(3):675–686. [PubMed] [Google Scholar]
- Korhonen T. K., Leffler H., Svanborg Edén C. Binding specificity of piliated strains of Escherichia coli and Salmonella typhimurium to epithelial cells, saccharomyces cerevisiae cells, and erythrocytes. Infect Immun. 1981 May;32(2):796–804. doi: 10.1128/iai.32.2.796-804.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind I. Protein A production in different strains of Staphylococcus aureus under varied growth conditions. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Dec;82(6):821–828. doi: 10.1111/j.1699-0463.1974.tb02379.x. [DOI] [PubMed] [Google Scholar]
- Mangan D. F., Snyder I. S. Mannose-sensitive stimulation of human leukocyte chemiluminescence by Escherichia coli. Infect Immun. 1979 Dec;26(3):1014–1019. doi: 10.1128/iai.26.3.1014-1019.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Bisno A. L. Resistance of Neisseria gonorrhoeae to phagocytosis: relationship to colonial morphology and surface pili. J Infect Dis. 1974 Mar;129(3):310–316. doi: 10.1093/infdis/129.3.310. [DOI] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H. Mannose binding and epithelial cell adherence of Escherichia coli. Infect Immun. 1978 Oct;22(1):247–254. doi: 10.1128/iai.22.1.247-254.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Mosek A., Sharon N. Mannose-specific adherence of Escherichia coli freshly excreted in the urine of patients with urinary tract infections, and of isolates subcultured from the infected urine. Infect Immun. 1981 Dec;34(3):708–711. doi: 10.1128/iai.34.3.708-711.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick E., Keisari Y. Superoxide anion and hydrogen peroxide production by chemically elicited peritoneal macrophages--induction by multiple nonphagocytic stimuli. Cell Immunol. 1981 Apr;59(2):301–318. doi: 10.1016/0008-8749(81)90411-1. [DOI] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Type I Escherichia coli pili: characterization of binding to monkey kidney cells. J Exp Med. 1977 Nov 1;146(5):1182–1194. doi: 10.1084/jem.146.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon N., Eshdat Y., Silverblatt F. J., Ofek I. Bacterial adherence to cell surface sugars. Ciba Found Symp. 1981;80:119–141. doi: 10.1002/9780470720639.ch9. [DOI] [PubMed] [Google Scholar]
- Silverblatt F. J., Cohen L. S. Antipili antibody affords protection against experimental ascending pyelonephritis. J Clin Invest. 1979 Jul;64(1):333–336. doi: 10.1172/JCI109458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverblatt F. J., Dreyer J. S., Schauer S. Effect of pili on susceptibility of Escherichia coli to phagocytosis. Infect Immun. 1979 Apr;24(1):218–223. doi: 10.1128/iai.24.1.218-223.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. W., Wagner S., Swenson R. M. Local immune response to Escherichia coli pili in experimental pyelonephritis. Infect Immun. 1981 Jan;31(1):17–20. doi: 10.1128/iai.31.1.17-20.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasaka T., Kambara T. Different effects of concanavalin A and its succinylated derivative on superoxide release in peritoneal macrophages. Biochim Biophys Acta. 1979 Jun 12;585(2):229–239. doi: 10.1016/0304-4165(79)90023-0. [DOI] [PubMed] [Google Scholar]