Abstract
Cardiovascular disease is the leading cause of death and disability worldwide, which can be largely attributed to atherosclerosis, a chronic inflammation of the arteries characterized by lesions containing immune and smooth muscle cells, lipids and extracellular matrix. In recent years, the lipid endocannabinoid system has emerged as a new therapeutic target in variety of disorders associated with inflammation and tissue injury, including those of the cardiovascular system. The discovery that Δ-9-tetrahydrocannabinol (Δ9-THC), the main active constituent of marijuana, inhibited atherosclerotic plaque progression via a cannabinoid 2 (CB2) receptor-dependent anti-inflammatory mechanism, and that certain natural and synthetic cannabinoid ligands could modulate the myocardial or cerebral ischaemia–reperfusion-induced tissue damage, have stimulated impetus for a growing number of studies investigating the implication of CB2 receptors in atherosclerosis, restenosis, stroke, myocardial infarction and heart failure. The aim of this review is to update on recent findings and controversies on the role of CB2 receptors in cardiovascular disease. Particular emphasis will be placed on novel insights in the potential cellular targets of CB2 stimulation in cardiovascular system (e.g. endothelial and vascular smooth muscle cells, cardiomyocytes, infiltrating and/or resident monocytes/macrophages and leukocytes, etc.), their interplay and intracellular signalling mechanisms identified, as well as on experimental and clinical studies.
Keywords: atherosclerosis, restenosis, neointima, myocardial infarction and reperfusion injury, stroke, macrophages, smooth muscle cells, endothelial cells
Introduction
The cannabinoid 2 (CB2) receptor was first cloned from the human leukaemia cell line HL-60 in 1993 (Munro et al., 1993). It is highly expressed in spleen and immune cells and at low levels in various peripheral tissues under normal physiological conditions (Galiegue et al., 1995). In the cardiovascular system, CB2 receptor expression has been established in the rat myocardium (Lepicier et al., 2007) and at low levels in human cardiomyocytes (Weis et al., 2010; Mukhopadhyay et al., 2010a), coronary endothelial cells and smooth muscle cells (Rajesh et al., 2007a; 2008). Under pathophysiological conditions such as inflammatory stimulation or tissue injury, increased CB2 receptor expression levels have been reported in the cardiovascular system, which probably reflects a protective response to limit cell or tissue injury (Pacher and Mechoulam, 2011). For example, up-regulation of CB2 receptor expression has been described in primary human endothelial and smooth muscle cells stimulated by pro-inflammatory triggers and/or mitogens (Rajesh et al., 2007a; 2008; Ramirez et al., 2012), in human and mouse atherosclerotic plaques (Steffens et al., 2005), neointimal lesions following balloon injury (Molica et al., 2012) and in the myocardium of chronic heart failure patients (Weis et al., 2010). Conversely, CB2 immunostaining was significantly reduced in carotid artery plaques of stroke versus asymptomatic patients (Montecucco et al., 2011). Therefore, one can speculate that CB2 signalling is part of a protective response against human plaque vulnerability, which is impaired in patients with acute vascular events. Further clinical studies are warranted to validate this hypothesis. Similarly, down-regulation of CB2 was described in kidney biopsies from patients with advanced kidney nephropathy, suggesting impaired protective CB2 regulation that counteracts the deleterious effects of signalling through CB1 receptors (Barutta et al., 2011), consistently with protective effects of CB2 receptor agonists in models of experimental nephropathy (Mukhopadhyay et al., 2010b; Barutta et al., 2011).
As to the experimental evidence for an implication of CB2 in cardiovascular disease, there is a mounting number of studies suggesting a protective role for CB2 receptors in mouse models of atherosclerosis (Steffens et al., 2005; Netherland et al., 2010; Zhao et al., 2010a,b; Hoyer et al., 2011), restenosis (Molica et al., 2012) and myocardial (Defer et al., 2009; Montecucco et al., 2009) and cerebral ischaemia/reperfusion injury (Zhang et al., 2007; 2008; Pacher and Hasko, 2008; Murikinati et al., 2010; Zarruk et al., 2012).
The selectivity of the endocannabinoids and synthetic ligands used in various cardiovascular and other related disorders towards CB1/2 receptors discussed in the following parts is summarized in Table 1 (reviewed in Pertwee, 2005; Pacher and Mechoulam, 2011).
Table 1.
Ranges of Ki values for certain cannabinoid CB1 and/or CB2 receptor agonists or antagonists/inverse agonists for the in vitro displacement of [3H]CP55,940, [3H]HU243 or [3H]BAY38-7271 from CB1- and CB2-specific binding sites (reviewed in Pertwee 2005)
| Agonis/ligand | CB1Ki value (nM) | CB2Ki value (nM) | Reference |
|---|---|---|---|
| Mixed (without marked CB1/2 selectivity) | |||
| Anandamide | 61–543 | 279–1940 | Pertwee (2005) |
| 2-AG | 58–472 | 145–1400 | Pertwee (2005) |
| CP55,940 | 0.5–5 | 0.7–2.8 | Pertwee (2005) |
| HU-210 | 0.06–0.1 | 0.2–0.5 | Pertwee (2005) |
| R-(+)-WIN 55,212-2 | 1.9–123 | 0.3–16 | Pertwee (2005) |
| Δ9-THC | 5–53 | 3–75 | Pertwee (2005) |
| CB1 selective agonists | |||
| ACEA | 1.4–5.3 | 195 to >2000 | Pertwee (2005) |
| R-(+)-methanandamide | 17.9–28.3 | 815–868 | Pertwee (2005) |
| CB2 selective agonists | |||
| JWH015 | 383 | 13.8 | Pertwee (2005) |
| JWH133 | 677 | 3.4 | Pertwee (2005) |
| O-1966 | 4071–6039 | 20.9–25.1 | Wiley et al. (2002) |
| O-3853 | 1361–1657 | 3.5–8.5 | Zhang et al. (2007) |
| HU-308 | >10 000 | 22.7 | Pertwee (2005) |
| HU-910 | 1400 | 6 | Horvath et al. (2012) |
| Inverse CB2 agonist/antagonist | |||
| AM630 | 5152 | 31.2 | Pertwee (2005) |
| SR144528 | 50.3 to >10 000 | 0.28–5.6 | Pertwee (2005) |
| Inverse CB1 agonist/antagonist | |||
| SR141716A | 1.8–11.8 | 515–13200 | Pertwee (2005) |
| AM251 | 7.5 | 2290 | Pertwee (2005) |
| AM281 | 12 | 4200 | Pertwee (2005) |
| LY320135 | 141 | 14900 | Pertwee (2005) |
Intracellular signalling of CB2
CB2 receptors are GPCRs that exert their biological effects through heterotrimeric Gi/0-proteins (Klein et al., 2003; Howlett, 2005). Coupling to Giα subunits inhibits AC and reduction in cAMP accumulation. This leads to decreased PKA activity and reduced phosphorylation of cAMP response-element binding-protein, thus decreasing gene expression (reviewed in Bosier et al., 2010). In addition, reduction of PKA activity causes decrease in constitutive inhibitory phosphorylation of the MAPK cascade (reviewed in Bosier et al., 2010). Simultaneously, CB2 activation induces members of the MAPK family through Gβγ, which triggers expression of genes (e.g. involved in cell survival, proliferation or stress response) (reviewed in Klein et al., 2003; Howlett, 2005) (Table 2). A majority of genes induced by CB2 activation is associated with nuclear translocation of transcription factor NF-κB in promyelocytic cells HL-60 transfected with the CB2 receptor (Derocq et al., 2000).
Table 2.
Intracellular signalling in vascular and immune cells
| Cell type/tissue | Receptor agonist | Intracellular target | Reference |
|---|---|---|---|
| HL-60 | 2-AG | MEK 1/2 → ERK 1/2, p38, Rho | (Kobayashi et al., 2001; Kishimoto et al., 2003) |
| Jurkat | JWH015 | ERK 1/2 | (Ghosh et al., 2006) |
| Human monocytes | JWH015 | Akt, ERK 1/2 | (Montecucco et al., 2008) |
| Human neutrophils | JWH133 | ERK 1/2 | (Montecucco et al., 2011) |
| Human coronary artery EC | JWH133, HU-308 | NF-κB, Rho | (Rajesh et al., 2007a) |
| Human sinusoidal EC | JWH133, HU-308, HU-910 | NF-κB? | (Batkai et al., 2007; Rajesh et al., 2007b; Horvath et al., 2012) |
| Human brain EC | JWH133, O-1966 | NF-κB? | (Ramirez et al., 2012) |
| Human coronary artery SMC | Ras, p38 MAPK, ERK 1/2, SAPK/JNK, Akt | (Rajesh et al., 2008) | |
| Mouse heart | JWH133 | MEK 1/2 → ERK 1/2, JAK → STAT-3 | (Montecucco et al., 2009) |
HL-60, human promyelocytic leukaemia cell line; MEK, p44/42 MAPK (kinase activating ERK 1/2); SAPK, stress-activated protein kinase; ‘?’ depicts studies providing indirect evidence on the CB2-dependent inhibition of NF-κB activation (e.g. inhibition of the expression of various adhesion molecules).
In contrast to CB1, CB2 receptor activation does not modulate ion channel function (reviewed in Demuth and Molleman, 2006; Bosier et al., 2010). As a result, CB2 receptor-mediated Ca2+ responses are less pronounced (Felder et al., 1995; Zoratti et al., 2003; Rao et al., 2004; Schuehly et al., 2011) than the potent CB1 receptor-mediated effects on Ca2+ fluxes (reviewed in Bosier et al., 2010). In calf pulmonary artery cells, Zoratti et al. (2003) observed CB2-dependent increases in cytosolic Ca2+ via activation of PLC and subsequent release from endoplasmic reticulum stores.
In addition to G-protein-dependent signalling, GPCRs can also mediate G-protein-independent signalling that involves receptor phosphorylation as well as interaction with regulatory proteins mediating receptor internalization or acting as scaffolds to modulate G-protein-mediated signalling (reviewed in Bockaert et al., 2004; Ritter and Hall, 2009; Smith et al., 2010). So far, available data about CB2 receptor modification and trafficking are limited and mainly based on stably CB2 expressing cells. Bouaboula et al. (1999) reported constitutively active, phosphorylated (at serine 352) and internalized CB2 receptor expression at basal levels in stably transfected CHO cells. The effect was sensitive to the CB2 antagonist/inverse agonist SR144528, which blocked phosphorylation and up-regulated CB2 levels at the cell surface. Conversely, CP55,940 agonist treatment resulted in sustained (>8 h) CB2 receptor phosphorylation. This is in accordance with the data from Derocq et al. (2000) reporting CB2 phosphorylation in response to CP55,940 activation in CB2-transfected HL-60 cells, which was maximal after 15 min. In naturally CB2-expressing microglial cells, receptor expression was shown both internalized and at the cell surface in absence of agonist, while agonist stimulation increased receptor internalisation (Carrier et al., 2004). A recent study also provided evidence for marked functional selectivity of cannabinoid receptor internalization, depending on the cannabinoid ligand used (Atwood et al., 2012).
Chronic exposure of CB1-expressing cells to agonists results in a loss of response when cells are subsequently challenged after drug washout (Breivogel et al., 1999). This adaptation to prolonged drug exposure is known as receptor desensitization and occurs in part due to uncoupling of receptors from their downstream G-proteins and/or effectors (Smith et al., 2010). In cell lines stably transfected with CB2, chronic endocannabinoid as well as synthetic agonist treatment resulted in CB2 receptor desensitization and down-regulation, as determined by the capacity to inhibit AC activity and receptor binding assays (Shoemaker et al., 2005). Following internalization in endocytosis vesicles, it is thought that CB2 receptors are resensitized by dephosphorylation which allows receptor recycling to the cell surface (Bouaboula et al., 1999; Grimsey et al., 2011).
Whether the above described mechanisms of CB2 trafficking has physiological relevance in primary vascular and peripheral blood immune cells deserve further investigations.
Intracellular targets of CB2 activation in vascular and immune cells
The regulation of normal vascular endothelial, smooth muscle and cardiomyocyte cell proliferation, survival, growth and differentiation involves extracellular ligands, growth factors and cytokines that bind to cell surface receptors and activate intracellular signal transduction cascades. In cardiovascular diseases, the role of several MAPK signalling pathways has been recognized, including ERK, JNK and p38 MAPK (Muslin, 2008).
A number of in vitro and in vivo studies have demonstrated the capacity of CB2 agonists to interact with signalling pathways induced by other cell surface receptors under pathophysiological/inflammatory conditions, suggesting a cross-talk between individual signal transduction pathways (Table 2). For example, CB2 receptors have been implicated in the modulation of immune cell migration (reviewed in Miller and Stella, 2008). In particular, monocytes treated with the CB2 agonist JWH015 showed significantly reduced chemokine-induced migration, associated with reduced expression of corresponding chemokine receptors CCR2 and CCR1 as well as IFN-γ-induced adhesion molecule ICAM-1 induction (Montecucco et al., 2008). JWH015 also cross-desensitized human monocytes for chemokine-induced migration by its own chemoattractant properties. The underlying pathways involved PI3K/Akt and ERK 1/2, but not p38 MAPK.
There is also evidence for CB2-mediated modulation of endothelial cell responses to the pro-inflammatory cytokine TNF-α. The healthy endothelium which separates blood and vessel wall is a highly selective permeable barrier with anti-adhesive properties. Endothelial injury results in increased permeability and subendothelial lipid accumulation, adhesion molecule up-regulation, release of cytokines and growth factors, and adherence of platelets and monocytes (reviewed in Libby et al., 2011). In human primary coronary artery (Rajesh et al., 2007a), liver sinusoidal (Batkai et al., 2007; Rajesh et al., 2007b; Horvath et al., 2012) and brain endothelial cells (Ramirez et al., 2012) CB2 activation with selective synthetic agonists attenuated the bacterial endotoxin- or TNF-α-induced NF-κB and RhoA activation (Rajesh et al., 2007a), ICAM-1 and VCAM-1 up-regulation (Batkai et al., 2007; Rajesh et al., 2007a,b; Horvath et al., 2012; Ramirez et al., 2012), CCL2 release as well as transendothelial migration and/or adhesion of THP-1 monocytes or neutrophils (Batkai et al., 2007; Rajesh et al., 2007a,b; Horvath et al., 2012).
Vascular smooth muscle cell migration and proliferation are key events during vascular development, in response to injury and atherosclerosis (reviewed in Gerthoffer, 2007). Within the atherosclerotic plaque, smooth muscle cells together with connective tissue encapsulate the necrotic lipid core, thus protecting the plaque from rupturing (reviewed in Lusis, 2000). On the other hand, smooth muscle cells, like macrophages, can express a variety of receptors for lipid uptake and can form foam cells, thereby participating in the cellular accumulation of lipids within plaques. They may also contribute to monocyte recruitment through expression of cytokines and adhesion molecules (reviewed in Doran et al., 2008). In the pathogenesis of restenosis in response to endovascular coronary or peripheral artery interventions, smooth muscle cells play a detrimental role through excessive migration and proliferation, leading to critical reduction in blood flow (reviewed in Ferns and Avades, 2000). In human coronary artery smooth muscle cells, CB2 activation inhibited the TNF-α-induced proliferation and migration via inhibition of TNF-α-induced Ras, p38 MAPK, ERK 1/2, SAPK/JNK and Akt activation (Rajesh et al., 2008).
In vitro role of CB2 in oxidized LDL-induced apoptosis
Elevated levels of plasma cholesterol, in particular low-density lipoprotein (LDL), are recognized as a major cardiovascular risk factor and lead to higher concentrations in the subendothelial intimal space. In the intima, LDL is oxidatively modified by reactive oxygen species (ROS) produced in endothelial cells, resident macrophages or smooth muscle cells. Oxidized LDL may injure the endothelium and play a role in the increased leukocyte adherence (Maier et al., 1996; Vora et al., 1997; Wang et al., 1997; Aikawa et al., 2002). Furthermore, oxidized LDL accumulates in macrophages and triggers macrophage apoptosis within atherosclerotic lesions (reviewed in Lusis, 2000). Apoptosis of macrophages might be beneficial for plaque stability if apoptotic bodies are removed. Indeed, it has been demonstrated that impaired macrophage apoptosis triggers lesion formation in mice (Liu et al., 2005). In advanced lesions, however, apoptosis of macrophage-derived foam cells promotes the formation of a pro-thrombotic central lipid pool whose size correlates with plaque instability. Thus, in advanced lesions, macrophage apoptosis could be considered as a pro-atherogenic factor triggering plaque vulnerability and risk of acute plaque rupture (reviewed in Libby et al., 1996).
There is in vitro evidence for a role of CB2 deficiency in oxidized LDL-induced macrophage apoptosis, which involves modulation of the Akt survival pathway (Freeman-Anderson et al., 2008). The apoptosis rate was significantly reduced in peritoneal macrophages from CB2 knockout mice as compared with wild-type animals. While oxidized LDL inhibited Akt phosphorylation, the effect was impaired in CB2-deficient macrophages. In rat peritoneal macrophages, oxidized LDL dose-dependently induced endocannabinoid levels as well as cannabinoid receptor CB1 and CB2 expression (Jiang et al., 2009). On the other hand, treatment with a synthetic agonist promoted cholesterol accumulation in these cells in a CB1-dependent manner.
Effects of plant-derived or synthetic CB2 agonists in experimental atherosclerosis
The potential implication of CB2 receptors in atherosclerosis, at least in mice, evolved from the initial discovery that the non-selective agonist Δ9-THC was atheroprotective in a CB2-dependent matter (Steffens et al., 2005). The inhibitory effect of Δ9-THC on atherosclerotic plaque progression was reversed by the CB2 antagonist/inverse agonist SR144528. The reduction of plaque size was associated with lower relative plaque macrophage content. In vitro proliferative responses and IFN-γ release were inhibited in splenocytes from Δ9-THC-treated mice, and migration of peritoneal macrophages versus CCL2 was also reduced. CB2 antagonism reversed the anti-migratory effects, and Δ9-THC did not affect migration of CB2−/− macrophages.
In 2010, Zhao et al. provided further evidence for anti-atherosclerotic effects of pharmacological CB2 activation. In ApoE−/− mice fed 8 weeks on high-cholesterol diet, daily i.p. injection with the synthetic non-selective agonist WIN55,212-2 during the last 2 weeks before harvest reduced plaque size, macrophage content and expression of adhesion molecules VCAM-1, ICAM-1 and P-selectin (Zhao et al., 2010b). Pharmacologic CB2 antagonism with AM630 inhibited the atheroprotective effects of WIN55,212-2. In a second study, WIN55,212-2 (administered by daily i.p. injection for 8 weeks before analysis) reduced atherosclerotic plaque formation, lesional macrophage content and mRNA levels of inflammatory markers IL-6, TNF-α and CCL2, as well as NF-κB activation in ApoE−/− mice fed 16 weeks on high-cholesterol diet (Zhao et al., 2010a).
Lessons from experimental atherosclerosis studies employing genetic CB2 deficiency
So far, the postulated role for CB2 in anti-atherosclerotic effects was exclusively based on non-selective agonists combined with pharmacological receptor blockade, which paved the way for further studies investigating the impact of genetic CB2 receptor deficiency in experimental atherosclerosis. Several groups independently studied atherosclerosis development in LDLR−/− or ApoE−/− mouse models, based on double deficiency and/or bone marrow transplantation. Some controversy exists in the reported effects on atherosclerotic plaque size and composition between these studies, which might be only partially explained by the different genetic backgrounds and experimental strategies.
First, Netherland et al. (2010) reported accelerated macrophage and smooth muscle cell infiltration and a less stable plaque phenotype without changes in plaque size in LDLR−/−CB2−/− mice. The CB2−/− strain used for generation of double knockout mice was from Nancy Buckley et al. (2000).
Second, Willecke et al. (2011) claimed that neither genetic deficiency nor activation of CB2 modulated atherogenesis in LDLR−/− mice. In fact, they reported no changes in plaque size but significantly increased macrophage and lipid plaque content in LDLR−/−CB2−/− mice, which were independently generated with CB2−/− mice obtained from Jackson Laboratories (Bar Harbor, ME, USA). Surprisingly, they did not observe any effect on atherosclerotic lesion size, plaque composition or immune cell function in LDLR−/− mice treated with CB2 agonist JWH133. Based on the limited pharmacokinetic data provided in this study, it is difficult to assess if the lack of anti-atherosclerotic effect might be due to insufficient frequency and/or dosage of cannabinoid ligand administration. In an attempt to proof the in vivo efficacy of JWH133 administration, the authors performed additional experiments based on thioglycollate-induced peritonitis and found reduced peritoneal macrophage recruitment in JWH133-treated mice. However, no effect was observed on acute TNF-α-induced systemic cytokine release or leukocyte adhesion marker expression. In vitro flow chamber assays also failed to show inhibitory effects on peritoneal macrophage adhesion to endothelial cells. The latter could also be related to the possibility that peritoneal macrophages and/or endothelial cells were already activated during the handling, possible presence of high levels of endocannabinoids in the serum used to culture cells (Marazzi et al., 2011), as well as to the potential rapid internalization of the CB2 receptors (Atwood et al., 2012) and concomitant activation of CB1 receptors with opposing consequences (reviewed in Pacher, 2009; Pacher and Mechoulam, 2011), by the extremely high concentration of the JWH133 used. In contrast to the above mentioned study, several recent reports using JWH133 and other CB2 agonists in vitro, ex vivo or in vivo, confirmed the anti-inflammatory effects of CB2 activation in endothelium, and its inhibitory effect on monocytes/macrophages and/or leukocyte migration during unrelated pro-inflammatory conditions (Ni et al., 2004; Murikinati et al., 2010; Horvath et al., 2012; Ramirez et al., 2012; Zarruk et al., 2012).
A third study was conducted by Georg Nickenig's group and the CB2−/− mice from Andreas Zimmer (Buckley et al., 2000), based on the ApoE−/− mouse model of atherosclerosis. The authors employed both double deficiency and bone marrow chimeric approach to demonstrate that CB2 expression in both vascular as well as immune cells influences atherosclerosis development (Hoyer et al., 2011). ApoE−/−CB2−/− mice had significantly increased plaque macrophage infiltration and aortic superoxide production, but a non-significant increase in plaque size. Similarly, lethally irradiated ApoE−/− mice reconstituted with CB2−/− bone marrow had increased macrophage infiltration without significant increase in lesion size. On the other hand, treatment with CB2 agonist JWH133 significantly reduced atherosclerotic lesion size, macrophage infiltration, superoxide production and improved vascular endothelium-dependent relaxations ex vivo in isolated aortic ring preparations. Interestingly, the authors further reported some changes in aortic levels of endocannabinoids and related lipid mediators (i.e. reduced 2-AG and increased OEA levels) in CB2−/− mice on C57BL6 wild-type background. Unfortunately, whether similar endocannabinoid levels are detectable in CB2-deficient ApoE−/− mice (on normal chow or high cholesterol diet) remains unclear.
Finally, Delsing et al. studied the effect of immune cell CB2 deficiency in irradiated LDLR−/− mice reconstituted with bone marrow from CB2−/− mice (from Andreas Zimmer). At the level of the aortic arch but not the aortic sinus, the lesional area was significantly increased in CB2−/− bone marrow transplanted mice as compared with mice receiving wild-type bone marrow after 12 weeks of high cholesterol (0.15%) diet (Delsing et al., 2011).
Pharmacological CB2 activation: a new approach for restenosis prevention?
Since the first percutaneous transluminal coronary angioplasty (PTCA) performed in the 1970s (Gruntzig et al., 1979), this intervention has become one of the most common procedures to reopen occluded vessels in coronary artery disease patients. Despite significant advances in this technique based on inflation of a balloon-tipped catheter and the introduction of stents (including drug-eluting stents), restenosis remains the primary limitation of PTCA (reviewed in Bittl, 1996; Douglas, 2007; Finn et al., 2007).
Restenosis is an inflammatory process in response to arterial injury, leading to secretion of cytokines and growth factors, recruitment of inflammatory cells as well as increased migratory, proliferative and secretory responses of vascular smooth muscle cells (reviewed in Ferns and Avades, 2000; Weber et al., 2004; Gerthoffer, 2007). Efforts to limit this constrictive vascular remodelling process have focused on inhibiting smooth muscle cell proliferation and migration, leading to the development of local stent-based delivery of antiproliferative agents such as sirolimus and paclitaxel (reviewed in Finn et al., 2007). Although these drug-eluting stents reduce rates of restenosis compared with bare metal stents, still a significant percentage of higher-risk patients develop in-stent restenosis (reviewed in Douglas, 2007). Moreover, late stent thrombosis due to the lack of complete endothelial repair has emerged as a major safety concern (reviewed in Joner et al., 2006; Finn et al., 2007; Luscher et al., 2007). Therefore, novel strategies should be targeted on restenosis prevention without impairing the arterial healing process.
Since CB2 receptor activation inhibited inflammatory proliferation and migration of vascular smooth muscle cells in vitro (Rajesh et al., 2008), we subsequently investigated the effect of CB2 activation in a mouse model of balloon angioplasty (Molica et al., 2012). As reported in other models of organ injury or inflammation (reviewed in Pacher and Mechoulam, 2011), balloon injury increased vascular CB2 expression in hypercholesterolaemic ApoE−/− mice (Molica et al., 2012). Injured vessels of mice treated with CB2 agonist JWH133 showed reduced intimal and medial thickening, associated with less in situ proliferation, smooth muscle cells and macrophages. Re-endothelialization was not inhibited by treatment with the CB2 agonist, according to CD31 immunostaining. Conversely, CB2 deficiency resulted in increased intima formation compared with wild-type mice, whereas JWH133 did not affect intimal formation in CB2−/− mice. Apoptosis rates assessed by in situ TUNEL staining were significantly higher in the CB2 knockouts. In vitro proliferation rates were significantly increased in CB2−/− smooth muscle cells compared with wild-type cells. Bone marrow-derived CB2−/− macrophages showed enhanced adherence and migration compared with CB2+/+ macrophages. The underlying mechanisms involved increased mRNA levels of adhesion molecule ICAM-1, chemokine receptors CCR1 and CCR5, as well as the pro-inflammatory chemokine CCL2.
Implication of CB2 in myocardial preconditioning based on ex vivo or in vitro models
An implication of the endocannabinoid system in the cardioprotective mechanisms of preconditioning has been initially described in isolated rat heart models (Lagneux and Lamontagne, 2001; Joyeux et al., 2002; Bouchard et al., 2003; Lepicier et al., 2003). However, whether CB1, CB2 or both receptors are involved in these models is controversial; furthermore the clinical relevance of these ex vivo model is limited because of the absence of the important inflammatory response (reviewed in Pacher and Hasko, 2008).
Cardioprotective effects of endocannabinoid-mediated CB2 activation were first reported in LPS-induced preconditioning (Lagneux and Lamontagne, 2001). Perfusion with CB2 antagonist SR144528 abolished the cardioprotective effect of LPS pretreatment, whereas CB1 antagonism with rimonabant had no effect. The implication of NO in CB2-dependent cardioprotection was shown by additional experiments using NOS inhibitor or NO donor respectively. Similarly, blocking of CB2, but not CB1 receptors reversed cardioprotection by heat stress-mediated preconditioning (Joyeux et al., 2002). As to endocannabinoid perfusion-mediated cardioprotection, one study reported that only CB2, but not CB1 (or only partially) reversed the protective effect (Lepicier et al., 2003). A different study, however, reported inhibition of endocannabinoid-mediated cardioprotection by both CB1 and CB2 antagonists (Underdown et al., 2005).
Two studies further investigated the link between endocannabinoid signalling and NO-mediated cardioprotection (Wagner et al., 2006; Lepicier et al., 2007). Both studies suggest the requirement for NO in CB1, but not CB2-mediated cardioprotection. By contrast, in isolated neonatal cardiomyocytes preventive effects of the plant-derived cannabinoid Δ9-THC against hypoxia were dependent on NO production and sensitive to CB2, but not CB1 antagonism (Shmist et al., 2006).
In vivo role of CB2 in myocardial ischaemia/reperfusion injury, importance of inflammatory response
A different experimental approach is based on in vivo myocardial ischaemia/reperfusion in anaesthetized rodents, pretreated with cannabinoid receptor agonists or antagonists. The synthetic cannabinoid HU-210 decreased the incidence of ventricular arrhythmias following ischaemia/reperfusion in rats through activation of CB2 receptors (Krylatov et al., 2001). Treatment with the non-selective agonist WIN55,212-2 before ischaemia significantly reduced the infarct size in mouse hearts (Di Filippo et al., 2004). The CB2 antagonist AM630, but not the CB1 antagonist AM251 abolished the effect of WIN55,212-2.
In a clinically more relevant experimental setting, CB2 activation with JWH133 administered at the end of the ischaemic period significantly reduced the infarct size as compared with vehicle-treated mice (Montecucco et al., 2009). Serum levels of the clinical marker cardiac troponin I, which is released from necrotic cardiomyocytes, were significantly lower in mice treated with JWH133. The infarct size reduction was associated with a decrease in ROS production and neutrophil infiltration into the infarcted myocardium, and activation of cardioprotective signalling pathways (Montecucco et al., 2009). Pre-injection with kinase inhibitors for Akt, ERK 1/2, STAT-3 pathways partially abrogated the JWH133-mediated infarct size reduction. In vitro, JWH133 inhibited TNF-α induced chemotaxis and integrin CD18/CD11b up-regulation on human neutrophils (Montecucco et al., 2009), suggesting a mechanistic explanation that might contribute to the infarct size reduction and potential relevance for human pathology.
In addition, CB2 receptors have been involved in the cardioprotective effects of remote ischaemic preconditioning, which is a protective phenomenon induced by preceding ischaemia in other organs or vascular beds (Hajrasouliha et al., 2008). Systemic pretreatment with CB2 antagonist AM630, but not the CB1 antagonist AM251, abolished the cardioprotective effects of remote preconditioning on infarct size and arrhythmias.
As to a potential clinical relevance for CB2 signalling in myocardial infarction, a large case-control study enrolling 1968 individuals addressed the involvement of the gene encoding CB2, CNR2, in the development of myocardial infarction and several cardiovascular risk factors. In particular, a potential association of genetic variations with the development of myocardial infarction and classic cardiovascular risk factors, including arterial hypertension, obesity, hypercholesterolaemia and diabetes mellitus was investigated (Reinhard et al., 2008). However, none of the 13 investigated single nucleotide polymorphisms in the CNR2 gene was associated with myocardial infarction or any of the investigated risk factors.
CB2 in post-ischaemic repair and heart failure
Acute myocardial infarction leads to necrosis of cardiac myocytes, which induces repair mechanisms that lead to scar formation (Sun, 2009). This process of post-infarction cardiac remodelling involves adaptive changes in shape, size and function of the ventricle, which may ultimately lead to contractile dysfunction and heart failure. Defer et al. (2009) provided substantial evidence for a protective role of CB2 receptors in ischaemic cardiac myocyte cell death, fibrosis and cardiac dysfunction. Hearts of CB2−/− mice had larger infarcts in comparison with wild-type mice and more sustained cell loss 3 days after ischaemia, together with accelerated injury and apoptosis in the non-ischaemic remote myocardium. Furthermore, increased infiltration of macrophages was observed in the infarct-surrounding myocardium of CB2−/− mice. Accelerated cardiac remodelling in CB2−/− post-ischaemic hearts was documented by higher number of spindle-shaped α-smooth muscle actin-positive myofibroblasts. In vitro, CB2−/− cardiomyocytes and fibroblasts were more susceptible to oxidative stress-induced cell death. Long-term effects of cardiac remodelling in CB2−/− hearts involved marked fibrosis, accelerated cardiomyocyte hypertrophy, dilative cardiomyopathy and cardiac dysfunction, as reported 4 weeks post infarction. By contrast, wild-type post-ischaemic hearts developed moderate fibrosis and cardiomyocyte hypertrophy, while cardiac function was preserved.
Heart failure is a complication of many diseases that affect the heart, such as coronary artery disease, arterial hypertension, valvular disease or diabetic cardiomyopathy (Jessup and Brozena, 2003; Boudina and Abel, 2007). Recent findings in humans reported an up-regulation of cardiac CB2 expression and enhanced endocannabinoid AEA and 2-AG levels in patients with chronic heart failure (Weis et al., 2010). Analysis of cannabinoid receptor expression by real-time PCR and immunohistochemistry also revealed a slight but significant down-regulation of cardiac CB1 receptors in chronic heart failure patients. Concerning potential pathophysiological consequences of CB2 up-regulation, the authors speculate that it may have a negative inotropic effect due to reduced cAMP levels that may contribute to ventricular weakening. On the other hand, CB2 receptors might mediate positive inotropic effects through cAMP-independent mechanisms, thus representing a compensatory mechanism to maintain cardiac performance. Moreover, CB2 up-regulation could represent a protective response to counterbalance chronic heart failure-induced structural changes, as shown in mice (Defer et al., 2009).
In vivo role of CB2 in cerebral ischaemia/reperfusion injury
Ischaemic stroke is a leading cause of death in developed countries and acquired adult disability. Increased accumulation of CB2-positive macrophages derived from resident microglia and/or invading monocytes following cerebral ischaemia/reperfusion have previously been reported (Ashton et al., 2007). Numerous recent studies have investigated the role of CB2 receptors by evaluating the effects of various CB2 ligands or knockout mice in experimental models of stroke (models of cerebral ischaemia–reperfusion injury) (Ni et al., 2004; Zhang et al., 2007; 2008; Murikinati et al., 2010; Ramirez et al., 2012). Similarly to the effects observed in myocardial ischaemia/reperfusion injury, CB2 receptor activation limited cerebral infarct size in experimental stroke by attenuating endothelial cell activation, chemokine signalling, inflammatory cell infiltration, glial activation, oxidative/nitrative stress and consequent cell death (Ni et al., 2004; Zhang et al., 2007; 2008; Murikinati et al., 2010; Zarruk et al., 2012). Most of these beneficial effects could be prevented by CB2 receptor antagonist(s) and/or were absent in CB2 knockout mice, which often exhibited enhanced injury indicative of the protective role of endocannabinoid system through CB2 receptors. Principally comparable protection could also be seen in hepatic ischaemia/reperfusion injury models (Batkai et al., 2007; Rajesh et al., 2007b; Horvath et al., 2012), which emphasizes a key role of CB2 receptors in limiting reperfusion damage in general and also provides a strong rationale for development of this promising approach for clinical use.
Interestingly, recent findings further suggest protective effects of the endocannabinoid-related brain compound N-arachidonoyl-l-serine (araS) in traumatic brain injury (Cohen-Yeshurun et al., 2011). Despite its structural similarity to anandamide, araS exhibits very low affinity at CB1 and CB2 receptors (Milman et al., 2006). Nevertheless, treatment with selective CB2 and transient receptor potential vanilloid 1, but not CB1 and GPR55 receptor antagonists reversed the protective effects in traumatic brain injury (Cohen-Yeshurun et al., 2011). This raises an intriguing possibility that certain metabolites of araS may exert direct protective effects on these receptors.
Conclusions
Collectively, several lines of evidence discussed above have established that functional CB2 receptors in cells of the cardiovascular system (e.g. endothelial and vascular smooth muscle cells, cardiomyocytes, fibroblasts and resident immune cells), as well as in infiltrating monocytes/macrophages and leukocytes during various pathological conditions of the cardiovascular system (e.g. atherosclerosis, restenosis, stroke, myocardial infarction and heart failure) may play an important compensatory role in controlling tissue inflammation and injury (Figure 1). In most of the cases, these receptors limit inflammation and associated tissue injury; however, in certain cases or disease states, CB2 receptor activation may also enhance tissue damage (Pacher and Mechoulam, 2011). On the basis of preclinical results, pharmacological modulation of CB2 receptors may hold a unique therapeutic potential in stroke, myocardial infarction and atherosclerosis despite some controversies (Figure 1). Nevertheless, the successfully translation of these promising preclinical results to clinical practice requires a better understanding of the underlying pathology, CB2 pharmacology and signalling, as well as the therapeutic window and long term safety of the use of various CB2 ligands. Finally, there is an urgent need for better, more selective pharmacological tools that are suitable for clinical application.
Figure 1.
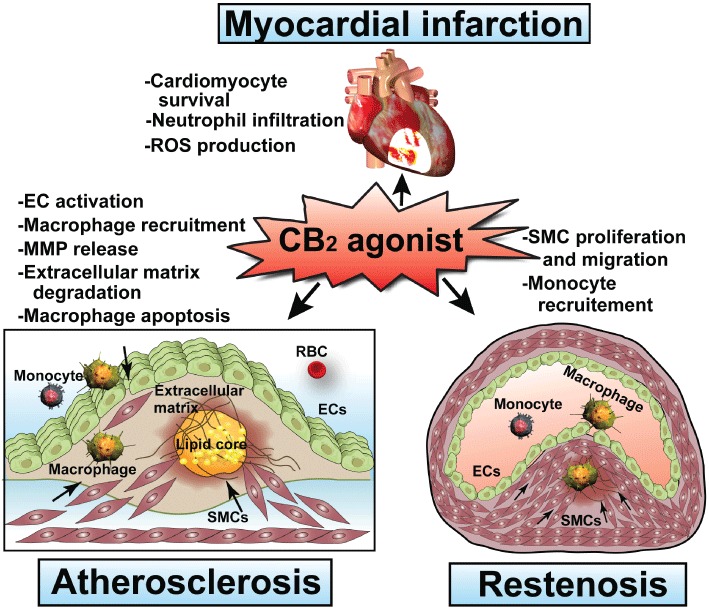
Potential therapeutic targets of CB2 activation in cardiovascular disorders. EC, endothelial cell; RBC, red blood cell; SMC, smooth muscle cells.
Acknowledgments
This publication was supported by grants from the Swiss National Science Foundation (# 310030_130732_1), Bangerter Rhyner, OPO, Novartis and Swiss Life foundation to Dr Steffens and the Intramural Research Program of NIH/NIAAA to Dr Pacher. The authors are indebted to Dr George Kunos for providing key resources and support.
Glossary
- 2-AG
2-arachidonoylglycerol
- Δ9-THC
Δ-9-tetrahydrocannabinol
- AEA
anandamide
- MCP-1/CCL2
monocyte chemotactic protein-1
- OEA
oleoylethanolamine
- ROS
reactive oxygen species
Conflict of interest
None.
References
- Aikawa M, Sugiyama S, Hill CC, Voglic SJ, Rabkin E, Fukumoto Y, et al. Lipid lowering reduces oxidative stress and endothelial cell activation in rabbit atheroma. Circulation. 2002;106:1390–1396. doi: 10.1161/01.cir.0000028465.52694.9b. [DOI] [PubMed] [Google Scholar]
- Ashton JC, Rahman RM, Nair SM, Sutherland BA, Glass M, Appleton I. Cerebral hypoxia-ischemia and middle cerebral artery occlusion induce expression of the cannabinoid CB2 receptor in the brain. Neuroscience Lett. 2007;412:114–117. doi: 10.1016/j.neulet.2006.10.053. [DOI] [PubMed] [Google Scholar]
- Atwood BK, Wager-Miller J, Haskins C, Straiker A, Mackie K. Functional selectivity in CB2 cannabinoid receptor signaling and regulation: implications for the therapeutic potential of CB2 ligands. Mol Pharmacol. 2012;81:250–263. doi: 10.1124/mol.111.074013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barutta F, Piscitelli F, Pinach S, Bruno G, Gambino R, Rastaldi MP, et al. Protective role of cannabinoid receptor type 2 in a mouse model of diabetic nephropathy. Diabetes. 2011;60:2386–2396. doi: 10.2337/db10-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batkai S, Osei-Hyiaman D, Pan H, El-Assal O, Rajesh M, Mukhopadhyay P, et al. Cannabinoid-2 receptor mediates protection against hepatic ischemia/reperfusion injury. FASEB J. 2007;21:1788–1800. doi: 10.1096/fj.06-7451com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittl JA. Advances in coronary angioplasty. N Engl J Med. 1996;335:1290–1302. doi: 10.1056/NEJM199610243351707. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Roussignol G, Becamel C, Gavarini S, Joubert L, Dumuis A, et al. GPCR-interacting proteins (GIPs): nature and functions. Biochem Soc Trans. 2004;32:851–855. doi: 10.1042/BST0320851. [DOI] [PubMed] [Google Scholar]
- Bosier B, Muccioli GG, Hermans E, Lambert DM. Functionally selective cannabinoid receptor signalling: therapeutic implications and opportunities. Biochem Pharmacol. 2010;80:1–12. doi: 10.1016/j.bcp.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Bouaboula M, Dussossoy D, Casellas P. Regulation of peripheral cannabinoid receptor CB2 phosphorylation by the inverse agonist SR 144528. Implications for receptor biological responses. J Biol Chem. 1999;274:20397–20405. doi: 10.1074/jbc.274.29.20397. [DOI] [PubMed] [Google Scholar]
- Bouchard JF, Lepicier P, Lamontagne D. Contribution of endocannabinoids in the endothelial protection afforded by ischemic preconditioning in the isolated rat heart. Life Sci. 2003;72:1859–1870. doi: 10.1016/s0024-3205(02)02474-8. [DOI] [PubMed] [Google Scholar]
- Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–3223. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Vogt LJ, Sim-Selley LJ. Chronic delta9-tetrahydrocannabinol treatment produces a time-dependent loss of cannabinoid receptors and cannabinoid receptor-activated G proteins in rat brain. J Neurochem. 1999;73:2447–2459. doi: 10.1046/j.1471-4159.1999.0732447.x. [DOI] [PubMed] [Google Scholar]
- Buckley NE, McCoy KL, Mezey E, Bonner T, Zimmer A, Felder CC, et al. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB(2) receptor. Eur J Pharmacol. 2000;396:141–149. doi: 10.1016/s0014-2999(00)00211-9. [DOI] [PubMed] [Google Scholar]
- Carrier EJ, Kearn CS, Barkmeier AJ, Breese NM, Yang W, Nithipatikom K, et al. Cultured rat microglial cells synthesize the endocannabinoid 2-arachidonylglycerol, which increases proliferation via a CB2 receptor-dependent mechanism. Mol Pharmacol. 2004;65:999–1007. doi: 10.1124/mol.65.4.999. [DOI] [PubMed] [Google Scholar]
- Cohen-Yeshurun A, Trembovler V, Alexandrovich A, Ryberg E, Greasley PJ, Mechoulam R, et al. N-arachidonoyl-L-serine is neuroprotective after traumatic brain injury by reducing apoptosis. J Cereb Blood Flow Metab. 2011;31:1768–1777. doi: 10.1038/jcbfm.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defer N, Wan J, Souktani R, Escoubet B, Perier M, Caramelle P, et al. The cannabinoid receptor type 2 promotes cardiac myocyte and fibroblast survival and protects against ischemia/reperfusion-induced cardiomyopathy. FASEB J. 2009;23:2120–2130. doi: 10.1096/fj.09-129478. [DOI] [PubMed] [Google Scholar]
- Delsing DJ, Leijten FP, Arts K, van Eenennaam H, Garritsen A, Gijbels MJ, et al. Cannabinoid receptor 2 deficiency in haematopoietic cells aggravates early atherosclerosis in LDL receptor deficient mice. Open Cardiovasc Med J. 2011;5:15–21. doi: 10.2174/1874192401105010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuth DG, Molleman A. Cannabinoid signalling. Life Sci. 2006;78:549–563. doi: 10.1016/j.lfs.2005.05.055. [DOI] [PubMed] [Google Scholar]
- Derocq JM, Jbilo O, Bouaboula M, Segui M, Clere C, Casellas P. Genomic and functional changes induced by the activation of the peripheral cannabinoid receptor CB2 in the promyelocytic cells HL-60. Possible involvement of the CB2 receptor in cell differentiation. J Biol Chem. 2000;275:15621–15628. doi: 10.1074/jbc.275.21.15621. [DOI] [PubMed] [Google Scholar]
- Di Filippo C, Rossi F, Rossi S, D'Amico M. Cannabinoid CB2 receptor activation reduces mouse myocardial ischemia-reperfusion injury: involvement of cytokine/chemokines and PMN. J Leukoc Biol. 2004;75:453–459. doi: 10.1189/jlb.0703303. [DOI] [PubMed] [Google Scholar]
- Doran AC, Meller N, McNamara CA. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:812–819. doi: 10.1161/ATVBAHA.107.159327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas JS., Jr Pharmacologic approaches to restenosis prevention. Am J Cardiol. 2007;100:10K–16K. doi: 10.1016/j.amjcard.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Felder CC, Joyce KE, Briley EM, Mansouri J, Mackie K, Blond O, et al. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol Pharmacol. 1995;48:443–450. [PubMed] [Google Scholar]
- Ferns GA, Avades TY. The mechanisms of coronary restenosis: insights from experimental models. Int J Exp Pathol. 2000;81:63–88. doi: 10.1046/j.1365-2613.2000.00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn AV, Nakazawa G, Joner M, Kolodgie FD, Mont EK, Gold HK, et al. Vascular responses to drug eluting stents: importance of delayed healing. Arterioscler Thromb Vasc Biol. 2007;27:1500–1510. doi: 10.1161/ATVBAHA.107.144220. [DOI] [PubMed] [Google Scholar]
- Freeman-Anderson NE, Pickle TG, Netherland CD, Bales A, Buckley NE, Thewke DP. Cannabinoid (CB2) receptor deficiency reduces the susceptibility of macrophages to oxidized LDL/oxysterol-induced apoptosis. J Lipid Res. 2008;49:2338–2346. doi: 10.1194/jlr.M800105-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, et al. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Gerthoffer WT. Mechanisms of vascular smooth muscle cell migration. Circ Res. 2007;100:607–621. doi: 10.1161/01.RES.0000258492.96097.47. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Preet A, Groopman JE, Ganju RK. Cannabinoid receptor CB(2) modulates the CXCL12/CXCR4-mediated chemotaxis of T lymphocytes. Mol Immunol. 2006;43:2169–2179. doi: 10.1016/j.molimm.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Grimsey NL, Goodfellow CE, Dragunow M, Glass M. Cannabinoid receptor 2 undergoes Rab5-mediated internalization and recycles via a Rab11-dependent pathway. Biochim Biophys Acta. 2011;1813:1554–1560. doi: 10.1016/j.bbamcr.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Gruntzig AR, Senning A, Siegenthaler WE. Nonoperative dilatation of coronary-artery stenosis: percutaneous transluminal coronary angioplasty. N Engl J Med. 1979;301:61–68. doi: 10.1056/NEJM197907123010201. [DOI] [PubMed] [Google Scholar]
- Hajrasouliha AR, Tavakoli S, Ghasemi M, Jabehdar-Maralani P, Sadeghipour H, Ebrahimi F, et al. Endogenous cannabinoids contribute to remote ischemic preconditioning via cannabinoid CB2 receptors in the rat heart. Eur J Pharmacol. 2008;579:246–252. doi: 10.1016/j.ejphar.2007.09.034. [DOI] [PubMed] [Google Scholar]
- Horvath B, Magid L, Mukhopadhyay P, Batkai S, Rajesh M, Park O, et al. A new cannabinoid 2 receptor agonist HU-910 attenuates oxidative stress, inflammation, and cell death associated with hepatic ischemia/reperfusion injury. Br J Pharmacol. 2012;165:2462–2478. doi: 10.1111/j.1476-5381.2011.01381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC. Cannabinoid receptor signaling. Handb Exp Pharmacol. 2005;168:53–79. doi: 10.1007/3-540-26573-2_2. [DOI] [PubMed] [Google Scholar]
- Hoyer FF, Steinmetz M, Zimmer S, Becker A, Lutjohann D, Buchalla R, et al. Atheroprotection via cannabinoid receptor-2 is mediated by circulating and vascular cells in vivo. J Mol Cell Cardiol. 2011;51:1007–1014. doi: 10.1016/j.yjmcc.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- Jiang LS, Pu J, Han ZH, Hu LH, He B. Role of activated endocannabinoid system in regulation of cellular cholesterol metabolism in macrophages. Cardiovasc Res. 2009;81:805–813. doi: 10.1093/cvr/cvn344. [DOI] [PubMed] [Google Scholar]
- Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193–202. doi: 10.1016/j.jacc.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Joyeux M, Arnaud C, Godin-Ribuot D, Demenge P, Lamontagne D, Ribuot C. Endocannabinoids are implicated in the infarct size-reducing effect conferred by heat stress preconditioning in isolated rat hearts. Cardiovasc Res. 2002;55:619–625. doi: 10.1016/s0008-6363(02)00268-7. [DOI] [PubMed] [Google Scholar]
- Kishimoto S, Gokoh M, Oka S, Muramatsu M, Kajiwara T, Waku K, et al. 2-arachidonoylglycerol induces the migration of HL-60 cells differentiated into macrophage-like cells and human peripheral blood monocytes through the cannabinoid CB2 receptor-dependent mechanism. J Biol Chem. 2003;278:24469–24475. doi: 10.1074/jbc.M301359200. [DOI] [PubMed] [Google Scholar]
- Klein TW, Newton C, Larsen K, Lu L, Perkins I, Nong L, et al. The cannabinoid system and immune modulation. J Leukoc Biol. 2003;74:486–496. doi: 10.1189/jlb.0303101. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Arai S, Waku K, Sugiura T. Activation by 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand, of p42/44 mitogen-activated protein kinase in HL-60 cells. J Biochem. 2001;129:665–669. doi: 10.1093/oxfordjournals.jbchem.a002904. [DOI] [PubMed] [Google Scholar]
- Krylatov AV, Ugdyzhekova DS, Bernatskaya NA, Maslov LN, Mekhoulam R, Pertwee RG, et al. Activation of type II cannabinoid receptors improves myocardial tolerance to arrhythmogenic effects of coronary occlusion and reperfusion. Bull Exp Biol Med. 2001;131:523–525. doi: 10.1023/a:1012381914518. [DOI] [PubMed] [Google Scholar]
- Lagneux C, Lamontagne D. Involvement of cannabinoids in the cardioprotection induced by lipopolysaccharide. Br J Pharmacol. 2001;132:793–796. doi: 10.1038/sj.bjp.0703902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepicier P, Bouchard JF, Lagneux C, Lamontagne D. Endocannabinoids protect the rat isolated heart against ischaemia. Br J Pharmacol. 2003;139:805–815. doi: 10.1038/sj.bjp.0705313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepicier P, Lagneux C, Sirois MG, Lamontagne D. Endothelial CB1-receptors limit infarct size through NO formation in rat isolated hearts. Life Sci. 2007;81:1373–1380. doi: 10.1016/j.lfs.2007.08.042. [DOI] [PubMed] [Google Scholar]
- Libby P, Geng YJ, Aikawa M, Schoenbeck U, Mach F, Clinton SK, et al. Macrophages and atherosclerotic plaque stability. Curr Opin Lipidol. 1996;7:330–335. doi: 10.1097/00041433-199610000-00012. [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- Liu J, Thewke DP, Su YR, Linton MF, Fazio S, Sinensky MS. Reduced macrophage apoptosis is associated with accelerated atherosclerosis in low-density lipoprotein receptor-null mice. Arterioscler Thromb Vasc Biol. 2005;25:174–179. doi: 10.1161/01.ATV.0000148548.47755.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher TF, Steffel J, Eberli FR, Joner M, Nakazawa G, Tanner FC, et al. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation. 2007;115:1051–1058. doi: 10.1161/CIRCULATIONAHA.106.675934. [DOI] [PubMed] [Google Scholar]
- Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier JA, Barenghi L, Bradamante S, Pagani F. Induction of human endothelial cell growth by mildly oxidized low density lipoprotein. Atherosclerosis. 1996;123:115–121. doi: 10.1016/0021-9150(95)05793-5. [DOI] [PubMed] [Google Scholar]
- Marazzi J, Kleyer J, Paredes JM, Gertsch J. Endocannabinoid content in fetal bovine sera – unexpected effects on mononuclear cells and osteoclastogenesis. J Immunol Methods. 2011;373:219–228. doi: 10.1016/j.jim.2011.08.021. [DOI] [PubMed] [Google Scholar]
- Miller AM, Stella N. CB2 receptor-mediated migration of immune cells: it can go either way. Br J Pharmacol. 2008;153:299–308. doi: 10.1038/sj.bjp.0707523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milman G, Maor Y, Abu-Lafi S, Horowitz M, Gallily R, Batkai S, et al. N-arachidonoyl L-serine, an endocannabinoid-like brain constituent with vasodilatory properties. Proc Natl Acad Sci U S A. 2006;103:2428–2433. doi: 10.1073/pnas.0510676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molica F, Matter CM, Burger F, Pelli G, Lenglet S, Zimmer A, et al. The cannabinoid receptor CB2 protects against balloon-induced neointima formation. Am J Physiol Heart Circ Physiol. 2012;302:1064–1074. doi: 10.1152/ajpheart.00444.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecucco F, Burger F, Mach F, Steffens S. CB2 cannabinoid receptor agonist JWH-015 modulates human monocyte migration through defined intracellular signaling pathways. Am J Physiol Heart Circ Physiol. 2008;294:H1145–H1155. doi: 10.1152/ajpheart.01328.2007. [DOI] [PubMed] [Google Scholar]
- Montecucco F, Lenglet S, Braunersreuther V, Burger F, Pelli G, Bertolotto M, et al. CB(2) cannabinoid receptor activation is cardioprotective in a mouse model of ischemia/reperfusion. J Mol Cell Cardiol. 2009;46:612–620. doi: 10.1016/j.yjmcc.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Montecucco F, Di Marzo V, da Silva RF, Vuilleumier N, Capettini L, Lenglet S, et al. The activation of the cannabinoid receptor type 2 reduces neutrophilic protease-mediated vulnerability in atherosclerotic plaques. Eur Heart J. 2011;33:846–856. doi: 10.1093/eurheartj/ehr449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay P, Rajesh M, Batkai S, Patel V, Kashiwaya Y, Liaudet L, et al. CB1 cannabinoid receptors promote oxidative stress and cell death in murine models of doxorubicin-induced cardiomyopathy and in human cardiomyocytes. Cardiovasc Res. 2010a;85:773–784. doi: 10.1093/cvr/cvp369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay P, Rajesh M, Pan H, Patel V, Mukhopadhyay B, Batkai S, et al. Cannabinoid-2 receptor limits inflammation, oxidative/nitrosative stress, and cell death in nephropathy. Free Radic Biol Med. 2010b;48:457–467. doi: 10.1016/j.freeradbiomed.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Murikinati S, Juttler E, Keinert T, Ridder DA, Muhammad S, Waibler Z, et al. Activation of cannabinoid 2 receptors protects against cerebral ischemia by inhibiting neutrophil recruitment. FASEB J. 2010;24:788–798. doi: 10.1096/fj.09-141275. [DOI] [PubMed] [Google Scholar]
- Muslin AJ. MAPK signalling in cardiovascular health and disease: molecular mechanisms and therapeutic targets. Clin Sci (Lond) 2008;115:203–218. doi: 10.1042/CS20070430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netherland CD, Pickle TG, Bales A, Thewke DP. Cannabinoid receptor type 2 (CB2) deficiency alters atherosclerotic lesion formation in hyperlipidemic Ldlr-null mice. Atherosclerosis. 2010;213:102–108. doi: 10.1016/j.atherosclerosis.2010.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni X, Geller EB, Eppihimer MJ, Eisenstein TK, Adler MW, Tuma RF. Win 55212-2, a cannabinoid receptor agonist, attenuates leukocyte/endothelial interactions in an experimental autoimmune encephalomyelitis model. Mult Scler. 2004;10:158–164. doi: 10.1191/1352458504ms1009oa. [DOI] [PubMed] [Google Scholar]
- Pacher P. Cannabinoid CB1 receptor antagonists for atherosclerosis and cardiometabolic disorders: new hopes, old concerns? Arterioscler Thromb Vasc Biol. 2009;29:7–9. doi: 10.1161/ATVBAHA.108.178129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Hasko G. Endocannabinoids and cannabinoid receptors in ischaemia-reperfusion injury and preconditioning. Br J Pharmacol. 2008;153:252–262. doi: 10.1038/sj.bjp.0707582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Mechoulam R. Is lipid signaling through cannabinoid 2 receptors part of a protective system? Prog Lipid Res. 2011;50:193–211. doi: 10.1016/j.plipres.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacological actions of cannabinoids. Handb Exp Pharmacol. 2005;168:1–51. doi: 10.1007/3-540-26573-2_1. [DOI] [PubMed] [Google Scholar]
- Rajesh M, Mukhopadhyay P, Batkai S, Hasko G, Liaudet L, Huffman JW, et al. CB2-receptor stimulation attenuates TNF-alpha-induced human endothelial cell activation, transendothelial migration of monocytes, and monocyte-endothelial adhesion. Am J Physiol Heart Circ Physiol. 2007a;293:H2210–H2218. doi: 10.1152/ajpheart.00688.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajesh M, Pan H, Mukhopadhyay P, Batkai S, Osei-Hyiaman D, Hasko G, et al. Cannabinoid-2 receptor agonist HU-308 protects against hepatic ischemia/reperfusion injury by attenuating oxidative stress, inflammatory response, and apoptosis. J Leukoc Biol. 2007b;82:1382–1389. doi: 10.1189/jlb.0307180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajesh M, Mukhopadhyay P, Hasko G, Huffman JW, Mackie K, Pacher P. CB(2) cannabinoid receptor agonists attenuate TNF-alpha-induced human vascular smooth muscle cell proliferation and migration. Br J Pharmacol. 2008;153:347–357. doi: 10.1038/sj.bjp.0707569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez SH, Hasko J, Skuba A, Fan S, Dykstra H, McCormick R, et al. Activation of cannabinoid receptor 2 attenuates leukocyte-endothelial cell interactions and blood-brain barrier dysfunction under inflammatory conditions. J Neurosci. 2012;32:4004–4016. doi: 10.1523/JNEUROSCI.4628-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao GK, Zhang W, Kaminski NE. Cannabinoid receptor-mediated regulation of intracellular calcium by delta(9)-tetrahydrocannabinol in resting T cells. J Leukoc Biol. 2004;75:884–892. doi: 10.1189/jlb.1203638. [DOI] [PubMed] [Google Scholar]
- Reinhard W, Stark K, Neureuther K, Sedlacek K, Fischer M, Baessler A, et al. Common polymorphisms in the cannabinoid CB2 receptor gene (CNR2) are not associated with myocardial infarction and cardiovascular risk factors. Int J Mol Med. 2008;22:165–174. [PubMed] [Google Scholar]
- Ritter SL, Hall RA. Fine-tuning of GPCR activity by receptor-interacting proteins. Nat Rev Mol Cell Biol. 2009;10:819–830. doi: 10.1038/nrm2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuehly W, Paredes JM, Kleyer J, Huefner A, Anavi-Goffer S, Raduner S, et al. Mechanisms of osteoclastogenesis inhibition by a novel class of biphenyl-type cannabinoid CB(2) receptor inverse agonists. Chem Biol. 2011;18:1053–1064. doi: 10.1016/j.chembiol.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Shmist YA, Goncharov I, Eichler M, Shneyvays V, Isaac A, Vogel Z, et al. Delta-9-tetrahydrocannabinol protects cardiac cells from hypoxia via CB2 receptor activation and nitric oxide production. Mol Cell Biochem. 2006;283:75–83. doi: 10.1007/s11010-006-2346-y. [DOI] [PubMed] [Google Scholar]
- Shoemaker JL, Joseph BK, Ruckle MB, Mayeux PR, Prather PL. The endocannabinoid noladin ether acts as a full agonist at human CB2 cannabinoid receptors. J Pharmacol Exp Ther. 2005;314:868–875. doi: 10.1124/jpet.105.085282. [DOI] [PubMed] [Google Scholar]
- Smith TH, Sim-Selley LJ, Selley DE. Cannabinoid CB1 receptor-interacting proteins: novel targets for central nervous system drug discovery? Br J Pharmacol. 2010;160:454–466. doi: 10.1111/j.1476-5381.2010.00777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens S, Veillard NR, Arnaud C, Pelli G, Burger F, Staub C, et al. Low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice. Nature. 2005;434:782–786. doi: 10.1038/nature03389. [DOI] [PubMed] [Google Scholar]
- Sun Y. Myocardial repair/remodelling following infarction: roles of local factors. Cardiovasc Res. 2009;81:482–490. doi: 10.1093/cvr/cvn333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underdown NJ, Hiley CR, Ford WR. Anandamide reduces infarct size in rat isolated hearts subjected to ischaemia-reperfusion by a novel cannabinoid mechanism. Br J Pharmacol. 2005;146:809–816. doi: 10.1038/sj.bjp.0706391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vora DK, Fang ZT, Liva SM, Tyner TR, Parhami F, Watson AD, et al. Induction of P-selectin by oxidized lipoproteins. Separate effects on synthesis and surface expression. Circ Res. 1997;80:810–818. doi: 10.1161/01.res.80.6.810. [DOI] [PubMed] [Google Scholar]
- Wagner JA, Abesser M, Harvey-White J, Ertl G. 2-Arachidonylglycerol acting on CB1 cannabinoid receptors mediates delayed cardioprotection induced by nitric oxide in rat isolated hearts. J Cardiovasc Pharmacol. 2006;47:650–655. doi: 10.1097/01.fjc.0000211752.08949.eb. [DOI] [PubMed] [Google Scholar]
- Wang GP, Deng ZD, Ni J, Qu ZL. Oxidized low density lipoprotein and very low density lipoprotein enhance expression of monocyte chemoattractant protein-1 in rabbit peritoneal exudate macrophages. Atherosclerosis. 1997;133:31–36. doi: 10.1016/s0021-9150(97)00109-3. [DOI] [PubMed] [Google Scholar]
- Weber C, Schober A, Zernecke A. Chemokines: key regulators of mononuclear cell recruitment in atherosclerotic vascular disease. Arterioscler Thromb Vasc Biol. 2004;24:1997–2008. doi: 10.1161/01.ATV.0000142812.03840.6f. [DOI] [PubMed] [Google Scholar]
- Weis F, Beiras-Fernandez A, Sodian R, Kaczmarek I, Reichart B, Beiras A, et al. Substantially altered expression pattern of cannabinoid receptor 2 and activated endocannabinoid system in patients with severe heart failure. J Mol Cell Cardiol. 2010;48:1187–1193. doi: 10.1016/j.yjmcc.2009.10.025. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Beletskaya ID, Ng EW, Dai Z, Crocker PJ, Mahadevan A, et al. Resorcinol derivatives: a novel template for the development of cannabinoid CB(1)/CB(2) and CB(2)-selective agonists. J Pharmacol Exp Ther. 2002;301:679–689. doi: 10.1124/jpet.301.2.679. [DOI] [PubMed] [Google Scholar]
- Willecke F, Zeschky K, Ortiz Rodriguez A, Colberg C, Auwarter V, Kneisel S, et al. Cannabinoid receptor 2 signaling does not modulate atherogenesis in mice. PLoS ONE. 2011;6:e19405. doi: 10.1371/journal.pone.0019405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarruk JG, Fernandez-Lopez D, Garcia-Yebenes I, Garcia-Gutierrez MS, Vivancos J, Nombela F, et al. Cannabinoid type 2 receptor activation downregulates stroke-induced classic and alternative brain macrophage/microglial activation concomitant to neuroprotection. Stroke. 2012;43:211–219. doi: 10.1161/STROKEAHA.111.631044. [DOI] [PubMed] [Google Scholar]
- Zhang M, Martin BR, Adler MW, Razdan RK, Jallo JI, Tuma RF. Cannabinoid CB(2) receptor activation decreases cerebral infarction in a mouse focal ischemia/reperfusion model. J Cereb Blood Flow Metab. 2007;27:1387–1396. doi: 10.1038/sj.jcbfm.9600447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Martin BR, Adler MW, Razdan RK, Ganea D, Tuma RF. Modulation of the balance between cannabinoid CB(1) and CB(2) receptor activation during cerebral ischemic/reperfusion injury. Neuroscience. 2008;152:753–760. doi: 10.1016/j.neuroscience.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Liu Y, Zhang W, Xue J, Wu YZ, Xu W, et al. WIN55212-2 ameliorates atherosclerosis associated with suppression of pro-inflammatory responses in ApoE-knockout mice. Eur J Pharmacol. 2010a;649:285–292. doi: 10.1016/j.ejphar.2010.09.027. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Yuan Z, Liu Y, Xue J, Tian Y, Liu W, et al. Activation of cannabinoid CB2 receptor ameliorates atherosclerosis associated with suppression of adhesion molecules. J Cardiovasc Pharmacol. 2010b;55:292–298. doi: 10.1097/FJC.0b013e3181d2644d. [DOI] [PubMed] [Google Scholar]
- Zoratti C, Kipmen-Korgun D, Osibow K, Malli R, Graier WF. Anandamide initiates Ca(2+) signaling via CB2 receptor linked to phospholipase C in calf pulmonary endothelial cells. Br J Pharmacol. 2003;140:1351–1362. doi: 10.1038/sj.bjp.0705529. [DOI] [PMC free article] [PubMed] [Google Scholar]


