Abstract
BACKGROUND AND PURPOSE
5-HT6 receptors are abundant in the hippocampus, nucleus accumbens and striatum, supporting their role in learning and memory. Selective 5-HT6 receptor antagonists produce pro-cognitive effects in several learning and memory paradigms while 5-HT6 receptor agonists have been found to enhance and impair memory.
EXPERIMENTAL APPROACH
The conditioned emotion response (CER) paradigm was validated in rats. Then we examined the effect of the 5-HT6 receptor antagonist, EMD 386088 (10 mg·kg−1, i.p.), and agonists, E-6801 (2.5 mg·kg−1, i.p.) and EMD 386088 (5 mg·kg−1, i.p.) on CER-induced behaviour either alone or after induction of memory impairment by the muscarinic receptor antagonist, scopolamine (0.3 mg·kg−1, i.p) or the NMDA receptor antagonist, MK-801 (0.1 mg·kg−1, i.p).
KEY RESULTS
Pairing unavoidable foot shocks with a light and tone cue during CER training induced a robust freezing response, providing a quantitative index of contextual memory when the rat was returned to the shock chamber 24 h later. Pretreatment (−20 min pre-training) with scopolamine or MK-801 reduced contextual freezing 24 h after CER training, showing production of memory impairment. Immediate post-training administration of 5-HT6 receptor antagonist, SB-270146, and agonists, EMD 386088 and E-6801, had little effect on CER freezing when given alone, but all significantly reversed scopolamine- and MK-801-induced reduction in freezing.
CONCLUSION AND IMPLICATIONS
Both the 5-HT6 receptor agonists and antagonist reversed cholinergic- and glutamatergic-induced deficits in associative learning. These findings support the therapeutic potential of 5-HT6 receptor compounds in the treatment of cognitive dysfunction, such as seen in Alzheimer's disease and schizophrenia.
Keywords: 5-HT6 receptor, SB-271046, EMD 386088, E-6801, conditioned emotional response, fear-motivated learning
Introduction
The 5-hydroxytryptamine6 (5-HT6) receptor is the most recent addition to the 5-HT receptor family, and is almost exclusively expressed within the CNS, with high abundance in areas associated with learning and memory, such as the hippocampus, amygdala and cerebral cortex (Monsma et al., 1993; Ruat et al., 1993; Kohen et al., 2001). 5-HT6 receptor antagonists elicit pro-cognitive effects in several preclinical behavioural tasks thought to have translational relevance to divergent cognitive domains; including novel object recognition (NOR, non-spatial visual learning and memory) (King et al., 2004; Lieben et al., 2005; Hirst et al., 2006; de Bruin et al., 2011), social recognition (social cognition) (Schreiber et al., 2007; Schaffhauser et al., 2009) and the Morris water maze (MWM, spatial visual learning and memory) (Rogers and Hagan, 2001; Woolley et al., 2001; Hirst et al., 2006) and improve extra-dimensional shifts in the attentional set-shifting task (reasoning and problem solving) task (Hatcher et al., 2005) (see reviews by Mitchell and Neumaier, 2005; Fone, 2008; Marsden et al., 2011). In contrast, in passive avoidance and food-motivated conditioned operant tasks (associative learning), 5-HT6 receptor antagonists have relatively little effect when given alone to adult rats, but reverse scopolamine-induced deficits (Bos et al., 2001; Meneses, 2001a; Foley et al., 2004; Schreiber et al., 2007). However, some groups have failed to replicate the pro-cognitive effects of 5-HT6 receptor antagonists possibly because of methodological issues (Russell and Dias, 2002; Lindner et al., 2003; Gravius et al., 2011), discussed in more detail later.
Several groups have also utilized the muscarinic receptor antagonist, scopolamine (cholinergic), and non-competitive NMDA receptor antagonist, dizocilpine (MK-801) (glutamatergic), to impair memory in preclinical behavioural paradigms, and have also shown that 5-HT6 receptor antagonists reverse these drug-induced deficits in NOR, passive avoidance, social recognition, autoshaping associative and MWM learning (Bos et al., 2001; Woolley et al., 2001; Meneses, 2001b; Foley et al., 2004; King et al., 2004; Mitchell et al., 2006; Schreiber et al., 2007). Collectively, these behavioural findings and neurochemical evidence that 5-HT6 receptor antagonists increase cortical and hippocampal ACh and glutamate release (Dawson et al., 2000; Riemer et al., 2003) provide strong evidence that modulation of cholinergic and glutamatergic function contributes to the acute reversal of drug- and time delay-induced impairment of learning and memory produced by 5-HT6 receptor blockade.
Recently, potent selective 5-HT6 receptor agonists have been developed, and paradoxically, these also enhance learning and memory in the attentional set-shifting (Burnham et al., 2010) and NOR tasks (Kendall et al., 2011), but conversely, not in the social recognition paradigms (Loiseau et al., 2008). There is considerable current interest in how both 5-HT6 receptor agonist and antagonist compounds could have acute pro-cognitive effects (Fone, 2008; Marsden et al., 2011). Interestingly, the 5-HT6 receptor interacts with Fyn (a non-receptor protein-tyrosine kinase), Jun activation domain-binding protein 1 (Jab 1, a co-activator of c-Jun-mediated transcription) (Yun et al., 2007; 2010; Riccioni et al., 2011) and possibly also mammalian target of rapamycin (mTOR) (Meffre et al., 2010), a PK involved in the initiation of mRNA translation important for consolidation of memory, as well as Gs (Monsma et al., 1993; Kohen et al., 1996); and differential effects of agonist and antagonist compounds on these signalling pathways could provide one mechanism to explain their apparent paradoxical similar beneficial effect on learning and memory (Codony et al., 2011; Marsden et al., 2011).
Surprisingly little research has been performed on the cognitive effects of 5-HT6 receptor modulation in fear-motivated associative learning using the conditioned emotion response (CER) task, an extremely well-characterized, species conserved, hippocampal, amygdala and cortical-dependent learning and memory task (Anagnostaras et al., 1999; Fanselow, 2000; Sigurdsson et al., 2007). As 5-HT6 receptor compounds are being evaluated in clinical trials to treat cognitive dysfunction (Codony et al., 2011; Liem-Moolenaar et al., 2011; Maher-Edwards et al., 2011), it is also essential that they are tested in a range of preclinical behavioural paradigms that have translational relevance to cognitive domains impaired in common CNS disorders (Young et al., 2009). Associative learning, including long-term memory of emotionally based preference conditioning is impaired in schizophrenia (Rushe et al., 1999; Herbener, 2009) and Alzheimer's disease (Belleville et al., 2008; Hoefer et al., 2008), making it important to evaluate these compounds in a preclinical associative learning task. Therefore, in the present study we analysed the effects of high-affinity selective 5-HT6 receptor full agonists, EMD 386088 (Mattsson et al., 2005; Nikiforuk et al., 2011) and E-6801 (Romero et al., 2006; Kendall et al., 2011) and a well-characterized antagonist, SB-271046 (Bromidge et al., 1999), which has no inverse agonist properties in this paradigm (Routledge et al., 2000). Initially, the CER task was validated to determine the optimal number of conditioned and unconditioned stimulus (CS–US) pairs required to produce a robust association. Following this, the strength of the association formed was evaluated by increasing time between training and test trials, and the speed that memory extinction occurred was observed by repeated re-exposure of the rats to the apparatus without any further US. The effect of acute treatment with SB-271046, EMD 386088 and E-6801 at various time points during the CER paradigm was examined to elucidate the particular process of learning and memory that was affected by the 5-HT6 receptor. Finally, the ability of the 5-HT6 receptor antagonist and agonists to reverse the memory impairment induced with either scopolamine or MK-801 was determined. The present data show that SB-271046, EMD 386088 and E-6801 had little effect on CER when administered alone in healthy adult rats. Interestingly, both 5-HT6 receptor antagonist and agonists reversed cholinergic- and glutamatergic-induced memory impairments in CER when tested 24 h following training and the potential mechanism involved in this action are discussed.
Methods
Animals
Adult male Lister hooded rats (Charles River, Kent, UK or Biomedical Services Unit, University of Nottingham derived from Charles River Stock) were used for all experiments; the total number of rats used was 414. Rats were group housed (3–5) and kept on a 12-h light–dark cycle (07:00–19:00 h) and room temperature (21 ± 2°C) and humidity (55–65%) were kept constant, with food and water available ad libitum. All experiments were performed in accordance with the UK Animals (Scientific Procedures) Act 1986 and approval of local ethical review committee using procedures that were as humane as possible. All studies involving animals are reported in accordance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath et al., 2010).
CER
The CER paradigm utilized an equal-sized two compartment shuttle box [510 (W) × 250 (D) × 240 (H) mm internal; 580 × 360 × 305 mm external, PanLab, LE 916 (Harvard Apparatus Ltd., Holliston, MA, USA)], having one light white-walled and one dark black-walled chamber, separated by a computer-operated door (100 × 100 mm). Within each chamber an independent grid floor was linked to a shock box (PanLab, LE 100-26), a light was connected to the shuttle box control unit (PanLab, LE 900) and a speaker in the back wall between each chamber administered sound to both chambers simultaneously. The position of the rat in the apparatus was detected by weight transducers located below the grid floor in the two chambers. Operation of the shuttle box used the software programme ShutAvoid v.1.8.2. (Harvard Apparatus Ltd). Before each experiment the test arena was thoroughly cleaned with 20% v v-1 ethanol to remove any olfactory cues and all experiments were performed in constant light (125 Lux at floor level in the dark chamber) between 8:00 and 16:00 h.
Optimization of CS–US associations during training
Rats, weighing 325–425 g, were randomly assigned into six treatment groups (n = 7–8 each); receiving 1, 2 or 5 foot shocks with cue or cue alone in the initial validation experiment or no cue or shocks (control) and three shocks with cue in all subsequent drug studies. Rats were left to acclimatize in the behavioural suite, where CER was performed for 1 h before training. Individual rats were placed into the light chamber of the CER apparatus for 30 s free exploration before the intra-chamber door was temporarily opened allowing their spontaneous transfer into the dark chamber, which then automatically closed the door. After a further 30 s of exploration, the rat received 5 s combined light and tone (40 Lux, 89 dB, 3 KHz, CS), and an unavoidable foot shock (1 s, 0.4 mA, US) was delivered in scrambled format through the grid floor during the last second of the CS. Rats remained in the conditioning chamber throughout training and following a 1-min interval, the light, tone and shock were repeated twice for all drug studies or as appropriate to deliver a total of either cue alone or one, three or five CS–US pairings in the validation study. Immediately following the last CS–US pairing (or at the equivalent time in experiments with cue alone or where fewer than three CS–US pairs were delivered), the rat was removed from the apparatus and returned to the home cage. Preliminary studies (data not shown) established that the CS tone was insufficient to induce a startle response or freezing when given alone, but induced freezing when administered alone without any further foot shocks 48 h after conditioning with the CS–US pairing (Jones et al., 2011).
On the test day, 24 h post-training, individual rats were placed directly into the dark chamber for 480 s, but no stimuli were administered. The total cumulative time spent freezing (defined as an absence of any visible movement other than thoracic volume associated with respiration; including ambulatory, stereotypy, or sniffing and vibrissae movement) was recorded manually using a stopwatch by an observer blind to the treatment, who was sat 1 m from the apparatus. The number of bouts of freezing was not recorded but this rarely exceeded two to three further (typically short-duration) bouts once movement was re-initiated. In addition, behaviour was recorded by a custom-made camera located on the top of the dark chamber, using an Ethovision Animal Tracking System software (Noldus, Tracksys Ltd., Nottingham, UK) to permit re-analysis if required.
For all drug studies the CER experimental protocol described earlier with three CS–US pairings during training was used, as this was found to elicit a robust submaximal freezing response in the post-training test trial (which was shortened to 300 s because all rats regained exploration within this time). The weight range of rats utilized across all the studies reported was relatively large. However, in all cases, the weight was balanced across groups in each separate study and heavier rats were only utilized in the pilot optimization and extinction studies. The weight range of rats utilized in the pharmacological studies was relatively small (210–360 g). Furthermore, there was no correlation between body weight and freeze duration in the retention trial when data from all the rats that received three shocks during conditioning and either vehicle or no drug (validation studies) were examined (linear regression; F(1,84)= 0.0747; r2= 0.00089).
Effect of extinction and varying time between training and testing on memory retention in drug-naïve rats
Two separate groups of rats (n = 6–8 per experiment) were employed to determine the effect of extinction with repeated exposure to the context without further CS–US presentation, and secondly, increasing the time between training and the retention test on memory retention in the CER. To test extinction drug-naïve rats, weighing 365–455 g, were sequentially re-exposed to the dark chamber at 24, 48, 72 and 96 h post-training without receiving any further CS–US pairings. Freezing behaviour was recorded during each 300-s test period and would be expected to decrease if relearning occurred. To determine the effect of increased time delay between training and test on memory retention in the CER paradigm without further exposure to the context, drug-naïve rats, weighing 260–560 g, were tested once either 24, 48, 72 or 96 h post-training, and the duration of the freezing response compared to assess the period over which associative learning was retained.
Effect of acute SB-271046 on CER-induced freezing behaviour and pharmacologically-induced memory deficits
Three separate experiments were performed to determine the effects of SB-271046 on memory acquisition, consolidation and retention in CER. To test memory acquisition, rats were randomly assigned into one of four groups (n = 7–8, 230–270 g); receiving vehicle with or without shock or SB-271046 with or without shock. In this study, SB-271046 was administered to rats that received no shock to establish if it had any confounding non-specific (e.g. sedative) adverse effects on CER. To test memory consolidation and retention, two separate groups of rats (weighing 240–300 g, n = 8–9, and 280–310 g, n = 9–11, respectively) received one of three treatments: vehicle with no shock or shock and SB-271046 with shock. On the training day, for memory acquisition and consolidation experiments, each rat received SB-271046 (10 mg·kg−1) or vehicle; 30 min before training or immediately following training, respectively, and to test memory retention, SB-271046 was administered 30 min before the 24-h retention trial.
Two separate experiments analysed the effects of SB-271046 on scopolamine-induced (n = 6–8, 240–300 g) or MK-801-induced (n = 8–10, 240–360 g) cognitive deficits. Saline (1 mL·kg−1), scopolamine (0.3 mg·kg−1, selected on the basis of similar experiments, (Anagnostaras et al., 1999) or MK-801 [0.1 mg·kg−1, based on (Csernansky et al., 2005; Nilsson et al., 2007) ] were administered 20 min before training, then immediately following training rats received either vehicle (0.5% methylcellulose, 3 mL·kg−1) or SB-271046 (10 or 15 mg·kg−1).
Effect of 5-HT6 receptor agonists, EMD 386088 and E-6801, on CER-induced freezing behaviour
Two experiments were performed to establish any effect of the 5-HT6 receptor agonists given alone on memory acquisition and or consolidation. The highest dose of both agonists (EMD 386088 10 mg·kg−1 and E-6801 5 mg·kg−1 or vehicle) was administered 30 min before CER training in the absence or presence of foot shocks to determine any effect on learning or any non-specific confounding effect on behaviour (n = 48, eight per group, 210–240 g). In a separate experiment, EMD 386088 (n = 7–9, 260–325 g, 5 or 10 mg·kg−1) or E-6801 (n = 7–8, 250–310 g, 2.5 or 5 mg·kg−1) were also administered immediately following CER training to determine the effect of 5-HT6 receptor activation on CER-induced memory 24 h post-training. To reduce use of animals, as neither agonist at either pretreatment time had any effect on the 24 h post-training freeze time, the effect of giving the agonist immediately before the test day was not performed. As no pharmacological interaction studies were performed herein administering a drug immediately prior to the retention trial, such studies would also have little relevance to interpretation of the current data.
Four further experiments determined the effect of both EMD 386088 (n = 7–9, 245–310 g) and E-6801 (n = 7–9, 240–305 g) on a scopolamine-induced and MK-801-induced impairment of CER. Using a protocol similar to SB-271046 studies, saline (1 mL·kg−1), scopolamine (0.3 mg·kg−1) or MK-801 (0.1 mg·kg−1) were administered 20 min before training, and immediately following CER vehicle (0.5% methyl cellulose, 3 mL.kg−1), EMD 386088 (5 mg·kg−1) or E-6801 (2.5 mg·kg−1) was administered.
Materials
SB-271046 was provided by GlaxoSmithKline (Harlow, UK), EMD 386088 was purchased from Tocris (Bristol, UK) and E-6801 was a gift from Esteve (Barcelona, Spain). SB-271046 is a high-affinity (pKi= 9) competitive antagonist (Bromidge et al., 1999), and EMD 386088 (Mattsson et al., 2005) and E-6801 (Romero et al., 2006; Kendall et al., 2011) are full agonists (1 and 7 nM affinity, respectively) at the human (h) 5-HT6 receptor expressed in HEK293 cell lines, all three compounds have 20- to 50-fold selectivity over other 5-HT receptors and have all been used before at similar doses to those reported herein for behavioural studies (Routledge et al., 2000; Meneses et al., 2008; Kendall et al., 2011). All 5-HT6 receptor compounds were dissolved in 0.5% methyl cellulose (w v−1) and administered in a volume of 3 mL·kg−1, i.p. Scopolamine hydrobromide and (+)-MK-801 hydrogen maleate, were purchased from Sigma-Aldrich (Dorset, UK), and were dissolved in sterile saline (0.154 M) and administered in a volume of 1 mL·kg−1, i.p. The nomenclature used in this paper conforms to that provided in the Guide to Receptors and Channels (Alexander et al., 2011).
Data and statistical analysis
During each test trial, the total cumulative time spent freezing (s) was recorded in the dark chamber of the apparatus, as an index of fear-motivated contextual learning and memory.
In experiments with equal-balanced group sizes, a between-groups comparison of freezing behaviour was performed using two-way anova followed by a Tukey's post hoc test. To maintain a robust parametric statistical analysis of data where the study design was unbalanced (when inclusion of drug no shock treatment group/s was considered unnecessary and unethical having shown that no non-specific drug-induced behaviours occurred) two separate analyses were performed. Firstly, to confirm whether freezing behaviour required the CS–US association, a Student's t-test was performed between the saline + vehicle no shock- and saline + vehicle shock-treated groups. The saline no shock control group was then omitted from further analysis of the effect and the effect of drug treatment in all groups that received shocks was analysed separately. Dependent on group size, the between-conditions analysis of drug treatment was determined using either a one-way anova with Tukey's post hoc test, or a Student's t-test, on all shock-treated groups. Statistical significance was achieved if P≤ 0.05 and all data are presented as mean ± SEM.
Results
Effects of increasing the CS–US pairings on CER-induced freezing in drug-naïve rats
During the 24-h retention trial, none of the no shock control groups (receiving tone and light cues alone) froze for more than 1% of the total test period when returned to the conditioning chamber, showing that rats did not find the CER test apparatus sufficiently aversive to induce contextual freezing without accompanying US foot shocks. Increasing the number of foot shock pairings caused a progressive increase in the duration of freezing behaviour when the rats were returned to the chamber where CS–US was presented 24 h post-training, such that three and five shock pairs caused freezing for a significantly greater time than a single shock (P≤ 0.001, Table 1). Although no significant difference occurred between the three and five shock pair groups there was still an obvious trend for an increase in freezing duration (Table 1). Three CS–US pairings was chosen for all subsequent studies as it induced a robust, reproducible significant freezing behaviour in the 24 h retention trial, with the potential that this response could be further increased so allowing detection of a pro-cognitive drug enhancement of freezing behaviour, as well as pharmacological attenuation of the response.
Table 1.
The cumulative duration of the conditioned emotional freezing response, induced by returning a rat to a chamber where it had received conditioning, progressively increased with the number of CS–US pairings
| Conditioning | Cumulative freezing response (s, mean ± s. e. mean.) |
|---|---|
| One CS | 1.3 ± 0.6***††† |
| One CS–US | 26.7 ± 5.9***††† |
| Three CS | 2.3 ± 1.6***††† |
| Three CS–US | 221.7 ± 53.7 |
| Five CS | 0.6 ± 0.4***††† |
| Five CS–US | 289.4 ± 30.8 |
The total cumulative time spent freezing (s, mean ± SEM) during a 24 h retention trial (n = 7–8 each group) following training in a chamber where the rat was exposed to either one, three or five light and tone cue (5 s, 40 lux, 89 dB, 3KHz, conditioning stimulus, CS) alone or combined with an unavoidable foot shock (0.4 mA for 1 s, unconditional stimulus, US). ***P < 0.001 versus three CS–US pairs; †††P < 0.001 versus five CS–US pairs (Tukey's post hoc following two-way anova).
Evaluation of extinction and post-training retention of contextual freezing in drug-naïve rats
Extinction of the fear-motivated memory occurred when drug-naïve rats were tested on consecutive days, 24–96 h post-training, such that repeated measures anova showed a significant main effect of test day; F(1,10)= 25.879, P= 0.001 on freezing duration (Table 2). The vehicle shock-treated rats froze significantly longer than the no shock control group (P≤ 0.001, Table 2) in both the 24 and 48h retention trial, but the time spent freezing decreased significantly (P≤ 0.001) from the 24 to 48 h post-training session. A similar progressive decrease in freezing duration occurred in the 72 and 96 h time points, such that there was no longer any significant difference between freezing duration in the shock and no shock-treated groups at either of these extinction time points (Table 2).
Table 2.
Exposure to three unavoidable light, tone and foot-shock pairs in a chamber caused a robust freezing behaviour when rats were returned to the conditioning chamber either (A) sequentially at 24, 48, 72 and 96 h post-training with no further CS–US pairings to examine the process of extinction, or (B) on a single occasion at either 24, 48, 72 or 96 h post-training to determine the duration of retention of learning
| No shock control | Shock | |
|---|---|---|
| (A) Consecutive post-training exposure time (h) | ||
| 24 | 3.5 ± 2.7 | 203.8 ± 40.7*** |
| 48 | 1.9 ± 0.7 | 75.2 ± 15.5*++ |
| 72 | 0.7 ± 0.3 | 27.2 ± 6.1+++ |
| 96 | 2.9 ± 1.6 | 4.6 ± 2.1+++ |
| (B) Single post-training exposure time (h) | ||
| 24 | 5.3 ± 3.2 | 244.7 ± 15.8††† |
| 48 | 0.0 ± 0.0 | 247.9 ± 20.8††† |
| 72 | 2.0 ± 1.2 | 225.8 ± 26.4††† |
| 96 | 4.9 ± 1.6 | 192.2 ± 36.0††† |
Data show total cumulative time spent freezing (s, mean ± SEM, n = 6–8) at the post-training exposure time indicated in separate groups of rats. In (A), shock-conditioned rats froze for significantly longer than non-shocked controls in 24 and 48 h test sessions, ***P≤ 0.001; *P≤ 0.05 Student's t-test versus own no shock control group at that particular exposure time. One-way anova also revealed shock-treated rats underwent extinction (F(3,23)= 16.37, P= 0.001) P≤ 0.001; ++P≤ 0.001 versus a 24 h test point, Tukey's post hoc. In (B), there was no significant difference in freezing time between each separate shock-treated group at each retention trial (one-way anova, F(3,29)= 1.024, P= 0.398) and shock-treated rats froze significantly longer than no shock controls at every exposure time †††P≤ 0.001 versus no shock control (Student's one-tailed t-test).
Importantly, CER induced a robust readily quantifiable freezing behaviour indicative of fear-motivated associative learning and memory that was retained up to 96 h post-training in rats on their first re-exposure to the context where the CS–US was presented. During each of the individual retention trials, the rats that received shock froze significantly longer than the no shock controls for that particular trial (P≤ 0.001, Table 2). Irrespective of the time between training and the following first contextual test, all rats that received three shocks spent an equal time freezing, showing that there was little temporal diminution of contextual freezing over the time period examined and the 24 h post-training period was used for all subsequent pharmacological studies.
Effect of acute SB-271046 on CER-induced freezing behaviour and pharmacologically induced memory deficits
Figure 1A shows that pre-training administration of SB-271046 (10 mg·kg−1 i.p.) significantly attenuated CER-induced freezing behaviour in the 24 h retention trial such that two-way anova showed a significant shock × drug interaction (F(1, 30)= 6.45, P= 0.017). Post hoc analysis showed the vehicle + shock group froze significantly longer than all other groups (P≤ 0.001 vs. both no-shock and P≤ 0.01 vs. SB-271046 + shock), and the SB-271046 + shock group froze significantly longer than both no shock controls (P≤ 0.05, Figure 1A), but froze for less than half the time of vehicle + shock rats, which was significant (P≤ 0.01). In this initial study with SB-271046, the antagonist given alone without shock exposure had absolutely no effect on freezing time compared with the vehicle-treated no shock control, confirming that it had no indirect adverse confounding effect upon CER-induced freezing. To prevent any unnecessary animal experimentation SB-271046 was therefore not re-examined with no shock in further drug-response studies. When SB-271046 was injected immediately following CER training, there was a small but significant reduction in freezing duration compared with the vehicle + shock group (P≤ 0.05, Figure 1B). As with previous experiments, shock treatment induced a robust freezing behaviour significantly (P≤ 0.001) greater than seen in the vehicle + no shock group. In contrast, when SB-271046 was administered 30 min before the retention test (24 h post-conditioning), it had no effect on CER-induced freezing behaviour (Figure 1C) such that vehicle and SB-271046 + shock groups spent an equal time freezing (>70% of the total test period). Analogous to the previous experiments, shock treatment caused a significant freezing response (P≤ 0.001) compared with the no shock-treated group. Previous data suggest that the 5-HT6 receptor antagonist and agonists affect acquisition and/or consolidation, but not retention (King et al., 2004; Kendall et al., 2011), so the current studies were designed to ensure optimal plasma drug concentration during the appropriate test period, but to simultaneously have minimal associated behavioural effects, which could confound interpretation of the paradigm. Thus, as the agonists had no impact on CER-induced freezing (see later) and the antagonist had much less impact on freezing when it was administered immediately after (rather than before) training, in all subsequent pharmacological studies SB-271046 or the 5-HT6 receptor agonists, EMD 386088 and E-6801, were administered immediately following CER training.
Figure 1.
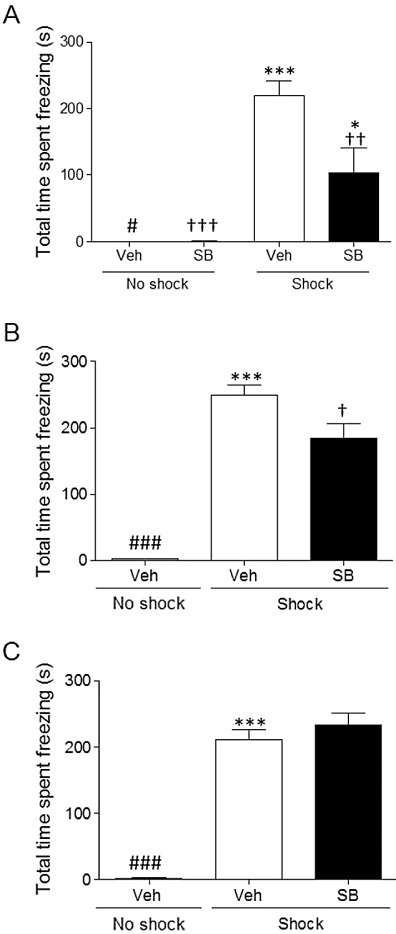
Comparison of the effect of the 5-HT6 receptor antagonist, SB-271046, injected at different stages of learning and memory on a fear-motivated conditioned emotional freezing behaviour (s, mean ± SEM) induced 24-h post-training. Three separate groups of rats received SB-271046 (10 mg·kg−1, i.p.) either: (A) 30 min before training (n = 7–8); (B) immediately after training (n = 8–9); or (C) 30 min before the 24-h retention test in the chamber where the CS–US pairing had been presented (n = 9–11). As there were balanced group sizes in the initial experiment, a two-way anova (shock and drug treatment; F(1,30)= 6.45, P= 0.017) was performed, followed by Tukey's post hoc. As there were only two drug groups in experiments depicted in (B) and (C), Student's t-tests were used to determine independent effects of shock and drug treatment on CER-induced freezing behaviour. ***P≤ 0.001 and *P≤ 0.05 versus own no shock control group, or †††P≤ 0.001; ††P≤ 0.01; †P≤ 0.05 versus vehicle shock group, ###P≤ 0.001; #P≤ 0.05 versus SB-271046 shock group. SB = SB-271046, Veh = vehicle.
As the cholinergic system is involved in learning and memory including fear conditioning (Anagnostaras et al., 1999) the effect of SB-271046 on a scopolamine-induced impairment of CER was examined (Figure 2). Shock treatment induced a significant freezing response (P≤ 0.001, Student's t-test) compared with that in the vehicle no shock control group confirming that robust fear-motivated contextual associative learning had occurred. To enable robust parametric analysis of data to be performed, subsequent analysis was performed on the four groups that received CS–US shock pairings (excluding the no shock control group). anova revealed a significant main effect of drug treatment on freezing duration in shock-treated rats (F(3,30)= 12.422, P= 0.001). Pre-training scopolamine (−20 min) administration attenuated the CER-induced freezing duration (by 72% from control, P < 0.001, Figure 2). Both doses of SB-271046 (10 and 15 mg·kg−1) partially reversed the scopolamine-induced reduction in freezing duration (P≤ 0.05), but these two 5-HT6-treated groups still froze significantly less than the saline + vehicle, shocked rats (P≤ 0.05).
Figure 2.
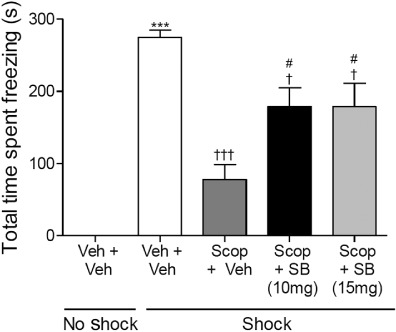
Post-training administration of SB-271046 partially reversed a scopolamine-induced deficit of freezing behaviour (s, mean ± SEM) in a CER paradigm during a 24-h retention trial. Rats were assigned to five groups (n = 6–9), receiving either saline (1 mL·kg−1) or scopolamine (0.3 mg·kg−1) 20 min before training, and either vehicle [0.5% methyl cellulose, (3 mL·kg−1) or SB-271046 (10 or 15 mg·kg−1) ] given immediately following training. Student's t-test was performed to determine the effect of shock on freezing behaviour between vehicle no shock and vehicle shock-treated groups; ***P≤ 0.001. To determine the effect of drug treatment on the CER-induced freezing response, anova was performed on the four remaining groups that received CS–US pairings (F(3,30)= 12.422, P= 0.001) followed by Tukey's post hoc test. †††P≤ 0.001 and †P≤ 0.05 versus vehicle-vehicle shock group, #P≤ 0.05 versus the scopolamine vehicle shock group. SB = SB-271046, Scop = scopolamine, Veh = vehicle. Note none of the rats which did not receive a shock during training froze when re-introduced into the test chamber.
To examine the interaction of the 5-HT6 receptor antagonist with glutamatergic processes involved in CER, freezing duration was attenuated by pretreatment with the NMDA receptor antagonist, MK-801 (Figure 3). Between-conditions analysis revealed an overall main effect of drug treatment on freezing behaviour 24 h post-training (anovaF(2,26)= 4.266, P= 0.026), such that MK-801 administered 20 min before training significantly attenuated freezing behaviour in the 24-h retention trial compared with that seen in the saline + vehicle shock group (P≤ 0.05, Figure 3). Post-training administration of SB-271046 reversed the MK-801-induced impairment (P≤ 0.05) to levels comparable to that in the saline + vehicle shock group.
Figure 3.
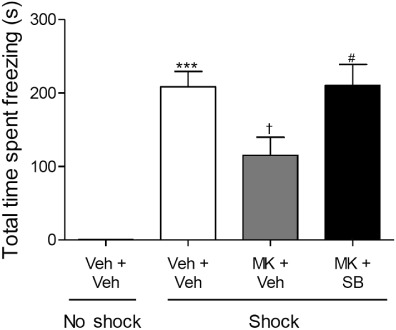
Post-training administration of SB-271046 reversed an MK-801-induced deficit in CER-induced freezing behaviour during the 24-h retention trial of CER (s, mean ± SEM). Rats were assigned into four treatment combination groups (n = 8–10), saline (1 mL·kg−1) or MK-801 (0.1 mg·kg−1) was administered 20 min before training, with vehicle 0.5% methyl cellulose (3 mL·kg−1) or SB-271046 (10 mg·kg−1) immediately following training. Effect of shock on CER-induced freezing was analysed with a Student's t-test between vehicle shock and no shock-treated rats, ***P≤ 0.001 versus vehicle no-shock control group. Between-conditions anova followed by Tukey's post hoc was utilized to determine effect of drug on shock-treated CER-induced freezing responses, F(2,26)= 4.266, P= 0.026, †P≤ 0.05 versus vehicle shock group, #P≤ 0.05 versus MK-801 shock group. MK = MK-801, SB = SB-271046, Veh = vehicle.
Effect of 5-HT6 receptor agonists, EMD 386088 and E-6801, on CER-induced freezing behaviour and pharmacological deficits
Consistent with all previous experiments, in all three 5-HT6 receptor agonist studies the vehicle shock-treated rats froze significantly longer than the no-shock control groups (P≤ 0.001, Student's t-test). When administered before conditioning at the highest dose or immediately post-conditioning (at either dose) neither agonist (EMD 386088 or E-6801) caused any freezing behaviour in non-shocked control rats, nor did they have any effect on CER-induced freezing behaviour compared with vehicle-treated shock-exposed rats in the 24 h retention trial (Figure 4A–C).
Figure 4.
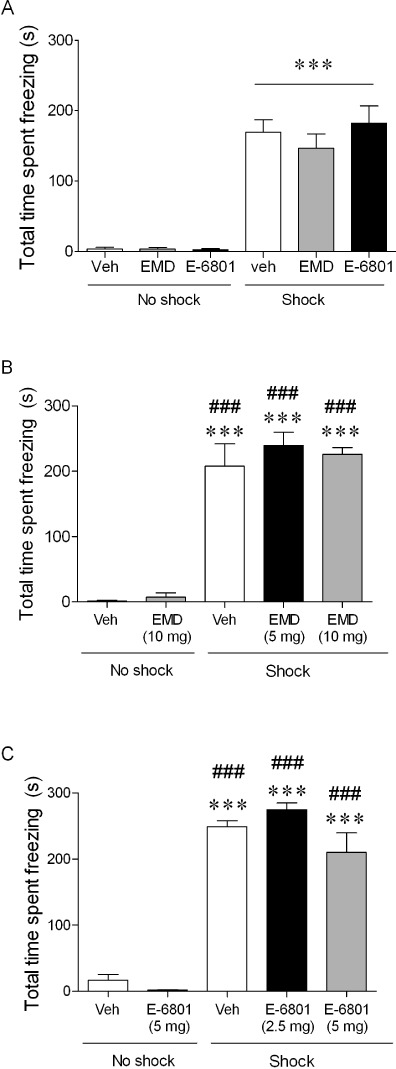
Lack of effect of administration of either 5-HT6 receptor agonist; (A) EMD 386088 (10 mg·kg−1, n = 8 per group) or E-6801 (5 mg·kg−1 i.p., n = 8) or vehicle (3 mL·kg−1 i.p., n = 8) 30 min before training or (B) injecting EMD 386088 (5 or 10 mg·kg−1, n = 7–9) or (C) E-6801 (2.5 or 5 mg·kg−1 i.p., n = 7–8) immediately post-training on the total cumulative CER-induced freezing time (s, mean ± SEM) recorded in the 24-h retention trial in rats receiving no shocks (control) or three shocks during conditioning (as indicated by the bar). ***P≤ 0.001 versus vehicle + no-shock control group and ###P≤ 0.001 versus EMD 386088 (10 mg·kg−1) or E-6801 (5 mg·kg−1) + no shock group, Student's t-test. There were no significant differences in freezing time between any shocked-treated groups (anova). EMD = EMD 386088, Veh = vehicle.
As in the previous experiment with SB-271046, pre-training administration of scopolamine significantly reduced freezing behaviour, in both 5-HT6 receptor agonist studies, compared with that of the saline + vehicle shock-treated group (Figure 5). In both studies, anova showed a main effect of drug treatment on freezing behaviour (F(3,34)= 61.05, P= 0.001, EMD 386088 and F(3,33)= 9.363, P= 0.001, E-6801). However, the reversal of the cholinergic-induced memory impairment was not dose-related, such that in both 5-HT6 receptor agonist studies, the lower dose of each agonist produced a greater reversal of the scopolamine-induced memory deficit. EMD 386088 (5 mg·kg−1) increased freezing duration (by 49%) compared with that in the scopolamine vehicle-treated rats, although this just failed to reach significance (Figure 5A, P= 0.08, Tukey's post hoc following anova of all shock treated groups). E-6801 (2.5 mg·kg−1) produced a comparable reversal of the scopolamine-induced memory deficit (a 44% increase, Figure 5B), but in this agonist group the effect reached significance (Tukey's post hoc, P≤ 0.05).
Figure 5.
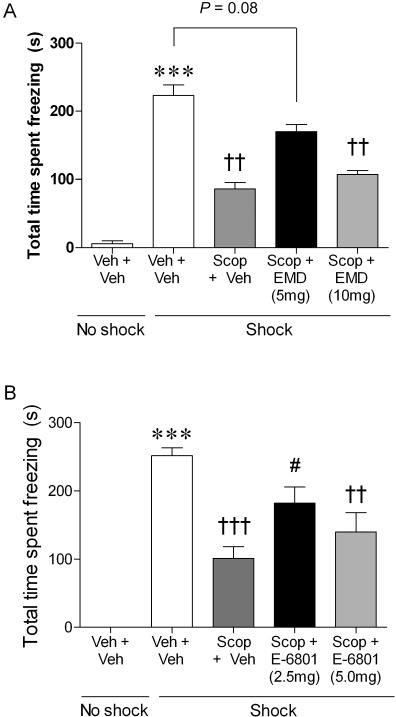
Post-training administration of 5-HT6 receptor agonists, EMD 386088 and E-6801, reversed a pre-training scopolamine-induced memory deficit in the 24 h retention trial. Histograms represent the total cumulative time spent freezing (s, mean ± SEM) in rats treated with either (A) EMD 386088 (5 and 10 mg·kg−1, n = 7–9) or (B) E-6801 (2.5 and 5 mg·kg−1, n = 8–9) following a cholinergic-induced memory impairment produced by administration of scopolamine 20 min before CER training trial. anova on all shock-treated groups showed a main effect of drug treatment on freezing behaviour (F(3,34)= 61.05, P= 0.001, EMD 386088 and F(3,33)= 9.363, P= 0.001 E-6801, respectively). ***P≤ 0.001 versus saline + vehicle + no shock control group, Student's t-test, and †††P≤ 0.001 and ††P≤ 0.01 versus saline + vehicle shock group, #P≤ 0.05 versus scopolamine + vehicle shock group (Tukey's post hoc test). EMD = EMD 386088, Scop = scopolamine, Veh = vehicle.
In the last set of experiments, the effects of post-training administration of the 5-HT6 receptor agonists on a glutamatergic-induced deficit in CER were examined (Figure 6). anova revealed a significant main effect of drug treatment on freezing behaviour in both the EMD 386088 (F(2,21)= 5.794, P= 0.011), and E-6801 (F(2,24)= 7.923, P= 0.003) groups. Post hoc analysis showed that EMD 386088 (5 mg·kg−1) and E-6801 (2.5 mg·kg−1) administered post-training significantly reversed (P≤ 0.05) the attenuation of freezing behaviour induced by pre-training administration of the NMDA receptor antagonist MK-801 (Figure 6). Taken together these results provide strong evidence that activation of the 5-HT6 receptor by two different agonists reversed both a cholinergic- and a glutamatergic-induced memory deficit in a rat fear conditioning-associative learning paradigm.
Figure 6.
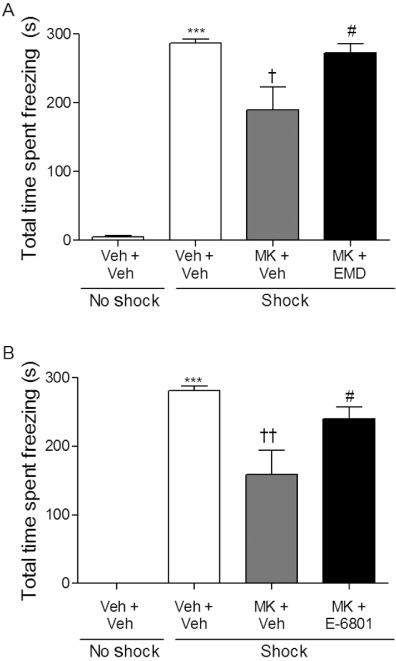
Post-training administration of 5-HT6 receptor agonists, EMD 386088 or E-6801, reversed pre-training MK-801-induced memory deficit during the 24 h retention trial. Total cumulative time spent freezing (s, mean ± SEM) in rats treated with either (A) EMD 386088 (5 mg·kg−1, n = 6–8) or (B) E-6801 (2.5 mg·kg−1, n = 8–9) following a glutamatergic-induced memory impairment produced by administration of MK-801 (0.1 mg·kg−1 i.p.) 20 min before conditioning. anova on all shock-treated rats showed a main effect of both EMD 386088 (F(2,21)= 5.794, P= 0.011), and E-6801 (F(2,24)= 7.923, P= 0.003) on freezing behaviour. ***P≤ 0.001 versus saline + vehicle no-shock control group, Student's t-test, and ††P≤ 0.01 and †P≤ 0.05 versus saline + vehicle shocked group, #P≤ 0.05 versus MK-801 + vehicle shock group (Tukey's post hoc test). EMD = EMD 386088, MK = MK-801, Veh = vehicle.
Discussion
The present studies validated a fear-motivated learning paradigm, which, essential for pharmacological quantification, induced a robust, reproducible, CER readily quantified by measuring the duration of contextual freezing behaviour (time spent immobile). Data were consistent with those from previous studies showing that freezing behaviour is an established index of conditioned fear, amenable to pharmacological studies and parametric analysis (Anagnostaras et al., 1999; Stiedl et al., 2000; Csernansky et al., 2005). The paradigm was then used to assess the stage(s) of learning and memory affected by acute administration of the 5-HT6 receptor antagonist, SB-271046, and agonists, EMD 386088 and E-6801. Subsequently, the ability of SB-271046, EMD 386088 and E-6801 to reverse cholinergic- (scopolamine) and glutamatergic- (MK-801) induced memory deficits in CER was quantified.
Fear conditioning is a robust paradigm for studying learning and memory and many studies show rats form a strong association between a context (CS) and foot shock (US) (Maren and Fanselow, 1997; Sigurdsson et al., 2007). However, rats shocked immediately after placement into a context exhibit little or no freezing on return to that context (Landeira-Fernandez et al., 2006; McHugh and Tonegawa, 2007), a phenomenon called immediate shock deficit, which is prevented if time is allowed to explore the context before shock delivery. Thus, a period of 30 s for exploration of the light compartment occurred before the rat was allowed to transfer to the dark compartment where the shock was received. The current study showed that three CS–US pairings caused reproducible contextual freezing typically lasting for 200–250 s without diminution even when first was tested 96 h post-training. This freezing, as expected, disappeared when further US was not provided during consecutive returns to the training context, as observed previously (Tronson et al., 2008). Other studies have found that rats can remember a fearful context after 16 months (Gale et al., 2004), further supporting the view that CER produces a robust long-term memory suitable for pharmacological analysis.
An array of preclinical studies have shown that 5-HT6 receptor antagonists restore drug-induced, neurodevelopmental- or age-related memory impairment or time-dependent natural forgetting in rats (Woolley et al., 2004; Schreiber et al., 2006; Fone, 2008; Marsden et al., 2011). However, the precise impact of these drugs on learning and memory depends on the task used, the time of antagonist administration and the age of the animal. Relatively few studies have examined the effect of 5-HT6 receptor antagonists on associative learning, such as the CER paradigm used in this study, a form of learning and memory impaired in schizophrenia (Rushe et al., 1999) and Alzheimer's disease (Belleville et al., 2008; Hoefer et al., 2008) making it important to determine whether these compounds affect this behaviour as they are in phase I and II clinical trials as potential adjunct therapy to treat the cognitive impairment in these disorders (Codony et al., 2011; Maher-Edwards et al., 2011). Interestingly, when given alone just before the conditioning, SB-271046 reduced freezing behaviour, inconsistent with the expected improvement in learning and memory, but rather suggesting that it caused memory impairment. Importantly, no reduction in freezing behaviour was seen when the drug was administered immediately before testing 24 h later, but a small reduction also occurred when SB-271046 was administered immediately after conditioning. One possible interpretation of these data is that the 5-HT6 receptor antagonist impairs acquisition and, to some extent, consolidation, but has no effect on retention in this paradigm. However, this is inconsistent with the reversal of time-dependent natural forgetting or drug-induced learning and memory impairment produced by this and many other 5-HT6 receptor antagonists in several cognitive paradigms; including NOR (King et al., 2004), social recognition (Loiseau et al., 2008), attentional set-shifting (Hatcher et al., 2005) and MWM learning (Marcos et al., 2008). It is therefore more likely that the inhibition of freezing behaviour seen when rats are conditioned in the presence of SB-271046 is due to non-specific reduction in the effect of conditioning. Interestingly, 5-HT6 receptor antagonists are anxiolytic in Vogel-punished drinking and elevated plus maze (Wesolowska and Nikiforuk, 2007; Wesolowska et al., 2007), which could reduce the strength of the association between the aversive, nociceptive foot shocks and the context where they are received and hence reduce CER-induced freezing behaviour. However, the 5-HT6 agonists, WAY-181187 and EMD 386088, are also anxiolytic in a schedule-induced polydipsia model of obsessive compulsive disorder (Schechter et al., 2008a) and Vogel conflict and elevated plus maze tests (Nikiforuk et al., 2011), respectively. Yet, in the current study, the agonists did not attenuate freezing when administered before or immediately after training and the antagonist did not attenuate freezing when given before retention, so an acute anxiolytic action is unlikely to explain why only the antagonist decreased contextual freezing when given pre-training. The 5-HT6 antagonist is antinociceptive in a formalin-evoked pain behaviour (Finn et al., 2007), and such an action would reduce the effectiveness of the US–CS association if drug was present during training, but not if given before the retention task (as observed herein). However the effect of 5-HT6 receptor agonists has not been examined in such paradigms.
Therefore, administering SB-271046 before conditioning by attenuating the aversive nature of the foot shocks would reduce the time spent freezing on return to the conditioned environment. In agreement with this hypothesisLindner et al. (2003) also found that SB-271046 attenuated freezing in a very similar two shock CER paradigm when administered before conditioning, but they interpreted this as failure to demonstrate any pro-cognitive effect rather than questioning possible confounding affects. Following validation studies, CER was used herein to determine the stage(s) of learning and memory affected by acute systemic administration of the 5-HT6 receptor antagonist, SB-271046, and agonists, EMD 386088 and E-6801, when administered alone, and then to examine their ability to reverse cholinergic- or glutamatergic-induced memory impairment.
Consistent with the current observations acute administration of 5-HT6 receptor antagonists has little effect in passive avoidance (a fear-motivated behavioural paradigm) but reverses a scopolamine-induced deficit in both young and old rats (Bos et al., 2001; Riemer et al., 2003; Foley et al., 2004), although not all groups have replicated these findings (Lindner et al., 2003; Gravius et al., 2011). In the current study, pre-training scopolamine administration significantly attenuated freezing behaviour, probably due to attenuation of central cholinergic neurotransmission, as no effect was observed with methylscopolamine (data not shown), an antagonist that does not cross the blood–brain barrier. Indeed, many studies have utilized scopolamine to disrupt conditioning and reduce contextual freezing behaviour (Anagnostaras et al., 1999; Wallenstein and Vago, 2001; Lindner et al., 2003; Gravius et al., 2011). The non-competitive NMDA receptor antagonist, MK-801, also induces a memory deficit in several behavioural tasks, including fear conditioning (King et al., 2004; Csernansky et al., 2005) consistent with the current findings. Results obtained after administration of MK-801 at various stages during the learning and memory process suggest it impairs encoding and/or acquisition rather than consolidation or retrieval (Nilsson et al., 2007), so in the current study, this drug was also administered immediately before conditioning. Furthermore, intra-hippocampal administration of the NMDA receptor antagonist, DL-2-amino-phosphonovaleric acid, or the muscarinic receptor antagonist, scopolamine, before fear conditioning attenuates subsequent contextual freezing (Kim et al., 1991; Stiedl et al., 2000; Wallenstein and Vago, 2001) consistent with the involvement of this area in the response. SB-271046 ameliorated the memory deficits induced by both scopolamine and MK-801 in CER, suggesting that blockade of 5-HT6 receptors may reverse cognitive impairment by enhancing both cholinergic and glutamatergic neurotransmission. In agreement with results from the present study, SB-271046 has been shown to reverse MK-801 deficits in spatial learning in the water maze (Marcos et al., 2008), and prevent the Ro-046790-induced improvement in NOR (King et al., 2004). However, in a very recent rat CER study, two 5-HT6 receptor antagonists failed to reverse either scopolamine- or MK-801-induced contextual freezing impairments, but the drugs were administered before conditioning, which may have reduced the effectiveness of the conditioning process, as discussed earlier. 5-HT6 receptor antagonists increase microdialysate ACh and glutamate overflow in both the prefrontal cortex and dorsal hippocampus (Dawson et al., 2000; 2001; Hirst et al., 2006), while activation of 5-HT6 receptors enhances hippocampal GABAergic neurotransmission, as measured using microdialysis (Schechter et al., 2008b) or electrophysiology (West et al., 2009); these areas are known to be involved in fear conditioning and these results are consistent with the current data. However, discrete microinjection of the compounds is required to establish the precise CNS site/s of action in this paradigm.
The original hypothesis was that 5-HT6 receptor agonists would have opposing actions to the antagonists and impair learning and memory (Fone, 2008; King et al., 2008). However, initial preclinical learning and memory experiments proved controversial. The 5-HT6 receptor agonist, WAY 181187, impaired social recognition in normal adult rats (Loiseau et al., 2008), but facilitated memory in the attentional set-shifting paradigm (Burnham et al., 2010). The agonist EMD 386088 impaired performance in the operant autoshaping task (Meneses et al., 2008). Viral vector-induced 5-HT6 receptor overexpression in the dorsal striatum impaired acquisition of food-motivated operant learning, which was reversed by pretreatment with the 5-HT6 receptor antagonist, SB-258585 (Mitchell et al., 2007). In contrast, we recently showed that EMD 386088 and E-6801 both improved natural forgetting and reversed a cholinergic or glutamatergic impairment in NOR in adult rats (Kendall et al., 2011). To date, no studies have evaluated the effects of 5-HT6 receptor agonists on fear-motivated learning, as performed herein. Neither EMD 386088 nor E-6801 given alone altered CER-induced freezing, either when given before or immediately after training, and although no rat froze for >300 s, as the response is near maximal in this protocol, a ceiling effect may have prevented observation of any further enhancement.
Pre-training administration of scopolamine impaired CER in both sets of agonist experiments. When either agonist was administered following training in scopolamine-pretreated rats, freezing increased close to that in vehicle controls (being significant with E-6801 but not quite with EMD 386088), indicating that the agonists restore memory via modulation of cholinergic neurotransmission. This result is consistent with results from our recent NOR studies (Kendall et al., 2011), which showed that E-6801, at identical doses, reversed a scopolamine-induced deficit. Of note, both agonists showed a bell-shaped dose-response curve for the NOR (Kendall et al., 2011), similar to the pattern observed in the present study, where the highest dose of both EMD 386088 and E-6801 failed to reverse the scopolamine-induced deficit in CER. As neither drug produced any locomotor hyperactivity or other marked behaviour (data not shown) at the doses used there is no other obvious explanation for the alteration in freezing behaviour than reversal of amnesia. Both EMD 386088 and E-6801 reversed MK-801-induced memory impairments seen 24 h post-training in the CER. Although the 5-HT6 receptor agonists reverse the cognitive impairment produced by antagonism of the NMDA receptor they may not mediate this by increasing glutamate release, as recent electrophysiological studies suggest 5-HT6 receptor activation inhibits prefrontal cortex and striatal glutamate neurotransmission (Tassone et al., 2011) and attenuates potassium- or sodium azide-enhanced cortical glutamate release in microdialysates (Schechter et al., 2008a). So the precise mechanism by which the 5-HT6 receptor agonists modify glutamate function to reverse the NMDA-induced impairment of contextual freezing is currently unclear.
The current study shows that 5-HT6 receptor antagonists and agonists paradoxically both have little effect on CER freezing when given alone, but reverse the memory deficits caused by modulation of cholinergic or glutamatergic neurotransmission. Preclinical studies investigating the effects of 5-HT6 receptor compounds on anxiety, depression, obesity and feeding behaviour have also shown paradoxical effects with 5-HT6 receptor antagonists and agonists (Woolley et al., 2001; Fisas et al., 2006; Svenningsson et al., 2007; Wesolowska et al., 2007; Heal et al., 2008; Nikiforuk et al., 2011). As previously stated, 5-HT6 receptor antagonists improve drug-induced learning and memory in a variety of preclinical behavioural paradigms, and more recent studies have found that agonists also share this effect (Fone, 2008; Burnham et al., 2010; Kendall et al., 2011), although some groups have found amnesic effects with the agonists (Loiseau et al., 2008; Meneses et al., 2008). From the literature, and the current study, it is clear that antagonists and agonists acting at the 5-HT6 receptor can induce similar behavioural responses, although the underlying mechanisms are unclear. Dual-labelled immunohistochemistry shows that there is little coexistence between 5-HT6 receptors and choline acetyltransferase, and lesions of the cholinergic system using the immunotoxin 192-IgG-saporin has no effect on 5-HT6 receptor mRNA or protein levels (Woolley et al., 2004; Marcos et al., 2006), suggesting that few 5-HT6 receptors are located on cholinergic neurones. In contrast, there appears to be extensive coexistence of the receptor with glutamic acid decarboxylase 67, suggesting extensive localization on GABAergic neurones (Woolley et al., 2004). Therefore, within the hippocampus, agonists may act upon the few 5-HT6 receptors that are located directly on the cholinergic and/or glutamatergic neurones, which receive little tonic 5-hydroxytryptaminergic input, whereas the antagonists may primarily act on 5-HT6 receptors located on upstream inhibitory GABAergic interneurones, receiving active 5-hydroxytryptaminergic input that disinhibits ACh and glutamate release. A recent neurotoxin study (King et al., 2009) showed that reversal of a time-delay induced impairment in learning and memory by the 5-HT6 receptor antagonist, Ro-04 6790, on NOR was abolished by the destruction of midbrain raphe neurones, consistent with its action requiring tonic 5-HT release, but no similar study has been performed with 5-HT6 receptor agonists. Therefore, both agonist and antagonist may enhance glutamate function and ACh neurotransmission in the cortex and/or hippocampus by different loci of action, accounting for their ability to reverse scopolamine- and MK-801-induced deficits in CER. An alternative or additional possibility is that the 5-HT6 receptor antagonists and agonists operate through modulation of distinct intracellular signalling pathways. In vitro studies show that 5-HT6 receptors expressed in cell lines are positively coupled to cAMP production by coupling to Gs (Ruat et al., 1993; Shen et al., 1993; Kohen et al., 1996). However, recent studies show that the human 5-HT6 receptor also interacts with Fyn-tyrosine kinase (Yun et al., 2007), the transcription factor jab1 (Yun et al., 2010) and mTOR (Meffre et al., 2010), a PK involved in the initiation of mRNA translation and thought to be important for consolidation of learning (Myskiw et al., 2008). Furthermore, the 5-HT6 receptor agonist, LY-586713, has been found to increase expression of frontal cortical brain-derived neurotrophic factor and the immediate early gene activity-regulated cytoskeletal-associated, neither of which were not antagonized by 5-HT6 receptor antagonist SB 271046 (De Foubert et al., 2007), consistent with the suggestion that they could regulate different molecular mechanisms. The agonists and antagonists could therefore act differentially on these various pathways in different neuronal populations and ultimately produce similar beneficial effects on learning and memory.
These results provide further evidence for the potential use of 5-HT6 receptor ligands to treat learning and memory deficits, such as those seen in Alzheimer's disease and/or schizophrenia. As both 5-HT6 receptor antagonists and agonists can reverse drug-induced impairment in CER, it will be interesting to determine their respective underlying molecular mechanisms and establish in clinical trials (Upton et al., 2008; Maher-Edwards et al., 2010; 2011) which has the greatest efficacy, if any, in treating cognitive disorders in man.
Acknowledgments
SW was funded by an MRC-DTA studentship, and we would like to thank GlaxoSmithKline for the generous gift of SB-271046, and Laboratorios Dr Esteve for supplying E-6801. The authors would like to thank Dr Xavier Codony and Malcolm Skingle for their assistance with approval of submission of the article.
Glossary
- 5-HT6
5-hydroxytryptamine6
- CER
conditioned emotion response
- CS–US
conditioned stimulus and unconditioned stimulus
- E-6801
(6-chloro-N-(3-(2-(dimethylamino)ethyl)-1H-indol-5-yl)imidazo[2,1-b]thiazole-5-sulfonamide)
- EMD
386088, 5-chloro-2-methyl-3-(1,2,3,6-tetrahydropyridin-4yl)-1H -indole
- Jab1
Jun activation domain-binding protein 1
- MK-801
dizocilpine
- mTOR
mammalian target of rapamycin
- MWM
Morris water maze
- NOR
novel object recognition
- SB-271046
[5-chloro-N-(4-methoxy-3-piperazin-1-yl-phenyl)-3-methyl-2-benzothiophenesulfonamide]
Conflict of interest
There are no conflicts of interest.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th edition. Br J Pharmacol. 2011;164:S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostaras SG, Maren S, Sage JR, Goodrich S, Fanselow MS. Scopolamine and pavlovian fear conditioning in rats: dose-effect analysis. Neuropsychopharmacology. 1999;21:731–744. doi: 10.1016/S0893-133X(99)00083-4. [DOI] [PubMed] [Google Scholar]
- Belleville S, Sylvain-Roy S, de Boysson C, Menard MC. Characterizing the memory changes in persons with mild cognitive impairment. In: Sossin WS, Lacaille JC, Castellucci VF, Belleville S, editors. Essence of Memory. Vol. 169. Amsterdam: Elsevier Science Bv; 2008. pp. 365–375. [DOI] [PubMed] [Google Scholar]
- Bos M, Sleight AJ, Godel T, Martin JR, Riemer C, Stadler H. 5-HT6 receptor antagonists: lead optimisation and biological evaluation of N-aryl and N-heteroaryl 4-amino-benzene sulfonamides. Eur J Med Chem. 2001;36:165–178. doi: 10.1016/s0223-5234(00)01209-5. [DOI] [PubMed] [Google Scholar]
- Bromidge SM, Brown AM, Clarke SE, Dodgson K, Gager T, Grassam HL, et al. 5-chloro-N-(4-methoxy-3-piperazin-1-yl-phenyl)-3-methyl-2- benzothiophenesulfonamide (SB-271046): a potent, selective, and orally bioavailable 5-HT6 receptor antagonist. J Med Chem. 1999;42:202–205. doi: 10.1021/jm980532e. [DOI] [PubMed] [Google Scholar]
- de Bruin NMWJ, Prickaerts J, van Loevezijn A, Venhorst J, de Groote L, Houba P, et al. Two novel 5-HT(6) receptor antagonists ameliorate scopolamine-induced memory deficits in the object recognition and object location tasks in Wistar rats. Neurobiol Learn Mem. 2011;96:392–402. doi: 10.1016/j.nlm.2011.06.015. [DOI] [PubMed] [Google Scholar]
- Burnham KE, Baxter MG, Bainton JR, Southam E, Dawson LA, Bannerman DM, et al. Activation of 5-HT6 receptors facilitates attentional set shifting. Psychopharmacology. 2010;208:13–21. doi: 10.1007/s00213-009-1701-6. [DOI] [PubMed] [Google Scholar]
- Codony X, Miguel Vela J, Javier Ramirez M. 5-HT(6) receptor and cognition. Curr Opin Pharmacol. 2011;11:94–100. doi: 10.1016/j.coph.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Martin M, Shah R, Bertchume A, Colvin J, Dong HX. Cholinesterase inhibitors ameliorate behavioral deficits induced by MK-801 in mice. Neuropsychopharmacology. 2005;30:2135–2143. doi: 10.1038/sj.npp.1300761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson LA, Nguyen HQ, Li P. In vivo effects of the 5-HT6 antagonist SE-271046 on striatal and frontal cortex extracellular concentrations of noradrenaline, dopamine, 5-HT, glutamate and aspartate. Br J Pharmacol. 2000;130:23–26. doi: 10.1038/sj.bjp.0703288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson LA, Nguyen HQ, Li P. The 5-HT6 receptor antagonist SB-271046 selectively enhances excitatory neurotransmission in the rat frontal cortex and hippocampus. Neuropsychopharmacology. 2001;25:662–668. doi: 10.1016/S0893-133X(01)00265-2. [DOI] [PubMed] [Google Scholar]
- De Foubert G, O'Neill MJ, Zetterstrom TSC. Acute onset by 5-HT6-receptor activation on rat brain brain-derived neurotrophic factor and activity-regulated cytoskeletal-associated protein mRNA expression. Neuroscience. 2007;147:778–785. doi: 10.1016/j.neuroscience.2007.04.045. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behav Brain Res. 2000;110:73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- Finn DP, Forte KCF, Beckett SRG, Baxter JA, Ansell L, Marsden CA, et al. The effects of pharmacological blockade of the 5-HT6 receptor on formalin-evoked nociceptive behaviour, locomotor activity and hypothalamo-pituitary-adrenal axis activity in rats. Eur J Pharmacol. 2007;569:59–63. doi: 10.1016/j.ejphar.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Fisas A, Codony X, Romero G, Dordal A, Giraldo J, Merce R, et al. Chronic 5-HT6 receptor modulation by E-6837 induces hypophagia and sustained weight loss in diet-induced obese rats. Br J Pharmacol. 2006;148:973–983. doi: 10.1038/sj.bjp.0706807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley AG, Murphy KJ, Hirst WD, Gallagher HC, Hagan JJ, Upton N, et al. The 5-HT6 receptor antagonist SB-271046 reverses scopolamine-disrupted consolidation of a passive avoidance task and ameliorates spatial task deficits in aged rats. Neuropsychopharmacology. 2004;29:93–100. doi: 10.1038/sj.npp.1300332. [DOI] [PubMed] [Google Scholar]
- Fone KCF. An update on the role of the 5-hydroxytryptamine(6) receptor in cognitive function. Neuropharmacology. 2008;55:1015–1022. doi: 10.1016/j.neuropharm.2008.06.061. [DOI] [PubMed] [Google Scholar]
- Gale GD, Anagnostaras SG, Godsil BP, Mitchell S, Nozawa T, Sage JR, et al. Role of the basolateral amygdala in the storage of fear memories across the adult lifetime of rats. J Neurosci. 2004;24:3810–3815. doi: 10.1523/JNEUROSCI.4100-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravius A, Laszy J, Pietraszek M, Saghy K, Nagel J, Chambon C, et al. Effects of 5-HT6 antagonists, Ro-4368554 and SB-258585, in tests used for the detection of cognitive enhancement and antipsychotic-like activity. Behav Pharmacol. 2011;22:122–135. doi: 10.1097/FBP.0b013e328343d804. [DOI] [PubMed] [Google Scholar]
- Hatcher PD, Brown VJ, Tait DS, Bate S, Overend P, Hagan JJ, et al. 5-HT6 receptor antagonists improve performance in an attentional set shifting task in rats. Psychopharmacology. 2005;181:253–259. doi: 10.1007/s00213-005-2261-z. [DOI] [PubMed] [Google Scholar]
- Heal DJ, Smith SL, Fisas A, Codony X, Buschmann H. Selective 5-HT6 receptor ligands: progress in the development of a novel pharmacological approach to the treatment of obesity and related metabolic disorders. Pharmacol Ther. 2008;117:207–231. doi: 10.1016/j.pharmthera.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Herbener ES. Impairment in long-term retention of preference conditioning in schizophrenia. Biol Psychiatry. 2009;65:1086–1090. doi: 10.1016/j.biopsych.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst WD, Stean TO, Rogers DC, Sunter D, Pugh P, Moss SF, et al. SB-399885 is a potent, selective 5-HT6 receptor antagonist with cognitive enhancing properties in aged rat water maze and novel object recognition models. Eur J Pharmacol. 2006;553:109–119. doi: 10.1016/j.ejphar.2006.09.049. [DOI] [PubMed] [Google Scholar]
- Hoefer M, Allison SC, Schauer GF, Neuhaus JM, Hall J, Dang JN, et al. Fear conditioning in frontotemporal lobar degeneration and Alzheimer's disease. Brain. 2008;131:1646–1657. doi: 10.1093/brain/awn082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CA, Brown AM, Auer DP, Fone KCF. The mGluR2/3 agonist LY379268 reverses post-weaning social isolation-induced recognition memory deficits in the rat. Psychopharmacology. 2011;214:269–283. doi: 10.1007/s00213-010-1931-7. [DOI] [PubMed] [Google Scholar]
- Kendall I, Slotten HA, Codony X, Burgueno J, Pauwels PJ, Vela JM, et al. E-6801, a 5-HT6 receptor agonist, improves recognition memory by combined modulation of cholinergic and glutamatergic neurotransmission in the rat. Psychopharmacology. 2011;213:413–430. doi: 10.1007/s00213-010-1854-3. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Decola JP, Landeirafernandez J, Fanselow MS. N-methyl-D-aspartate receptor antagonist APV blocks acquisition but not experession of fear conditioning. Behav Neurosci. 1991;105:126–133. doi: 10.1037//0735-7044.105.1.126. [DOI] [PubMed] [Google Scholar]
- King M, Spicer C, Sleight A, Marsden C, Fone K. Impact of regional 5-HT depletion on the cognitive enhancing effects of a typical 5-ht(6) receptor antagonist, Ro 04-6790, in the Novel Object Discrimination task. Psychopharmacology. 2009;202:111–123. doi: 10.1007/s00213-008-1334-1. [DOI] [PubMed] [Google Scholar]
- King MV, Sleight AJ, Woolley ML, Topham IA, Marsden CA, Fone KCF. 5-HT6 receptor antagonists reverse delay-dependent deficits in novel object discrimination by enhancing consolidation – an effect sensitive to NMDA receptor antagonism. Neuropharmacology. 2004;47:195–204. doi: 10.1016/j.neuropharm.2004.03.012. [DOI] [PubMed] [Google Scholar]
- King MV, Marsden CA, Fone KCF. A role for the 5-HT1A, 5-HT4 and 5-HT6 receptors in learning and memory. Trends Pharmacol Sci. 2008;29:482–492. doi: 10.1016/j.tips.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Kohen R, Metcalf MA, Khan N, Druck T, Huebner K, Lachowicz JE, et al. Cloning, characterization, and chromosomal localization of a human 5-HT6 serotonin receptor. J Neurochem. 1996;66:47–56. doi: 10.1046/j.1471-4159.1996.66010047.x. [DOI] [PubMed] [Google Scholar]
- Kohen R, Fashingbauer LA, Heidmann DEA, Guthrie CR, Hamblin MW. Cloning of the mouse 5-HT6 serotonin receptor and mutagenesis studies of the third cytoplasmic loop. Mol Brain Res. 2001;90:110–117. doi: 10.1016/s0169-328x(01)00090-0. [DOI] [PubMed] [Google Scholar]
- Landeira-Fernandez J, Kim JJ, DeCola JP, Fanselow MS. Immediate shock deficit in fear conditioning: effects of shock manipulations. Behav Neurosci. 2006;120:873–879. doi: 10.1037/0735-7044.120.4.873. [DOI] [PubMed] [Google Scholar]
- Lieben CKJ, Blokland A, Sik A, Sung E, van Nieuwenhuizen P, Schreiber R. The selective 5-HT6 receptor antagonist Ro4368554 restores memory performance in cholinergic and serotonergic models of memory deficiency in the rat. Neuropsychopharmacology. 2005;30:2169–2179. doi: 10.1038/sj.npp.1300777. [DOI] [PubMed] [Google Scholar]
- Liem-Moolenaar M, Rad M, Zamuner S, Cohen AF, Lemme F, Franson KL, et al. Central nervous system effects of the interaction between risperidone (single dose) and the 5-HT(6) antagonist SB742457 (repeated doses) in healthy men. Br J Clin Pharmacol. 2011;71:907–916. doi: 10.1111/j.1365-2125.2011.03902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner MD, Hodges DB, Hogan JB, Orie AF, Corsa JA, Barten DM, et al. An assessment of the effects of serotonin 6 (5-HT6) receptor antagonists in rodent models of learning. J Pharmacol Exp Ther. 2003;307:682–691. doi: 10.1124/jpet.103.056002. [DOI] [PubMed] [Google Scholar]
- Loiseau F, Dekeyne A, Millan MJ. Pro-cognitive effects of 5-HT6 receptor antagonists in the social recognition procedure in rats: implication of the frontal cortex. Psychopharmacology. 2008;196:93–104. doi: 10.1007/s00213-007-0934-5. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh TJ, Tonegawa S. Spatial exploration is required for the formation of contextual fear memory. Behav Neurosci. 2007;121:335–339. doi: 10.1037/0735-7044.121.2.335. [DOI] [PubMed] [Google Scholar]
- Maher-Edwards G, Zvartau-Hind M, Hunter AJ, Gold M, Hopton G, Jacobs G, et al. Double-blind, controlled phase II study of a 5-HT6 receptor antagonist, SB-742457, in alzheimer's disease. Curr Alzheimer Res. 2010;7:374–385. doi: 10.2174/156720510791383831. [DOI] [PubMed] [Google Scholar]
- Maher-Edwards G, Dixon R, Hunter J, Gold M, Hopton G, Jacobs G, et al. SB-742457 and donepezil in Alzheimer disease: a randomized, placebo-controlled study. Int J Geriatr Psychiatry. 2011;26:536–544. doi: 10.1002/gps.2562. [DOI] [PubMed] [Google Scholar]
- Marcos B, Gil-Bea FJ, Hirst WD, Garcia-Alloza M, Ramirez MJ. Lack of localization of 5-HT6 receptors on cholinergic neurons: implication of multiple neurotransmitter systems in 5-HT6 receptor-mediated acetylcholine release. Eur J Neurosci. 2006;24:1299–1306. doi: 10.1111/j.1460-9568.2006.05003.x. [DOI] [PubMed] [Google Scholar]
- Marcos B, Chuang TT, Gil-Bea FJ, Ramirez MJ. Effects of 5-HT6 receptor antagonism and cholinesterase inhibition in models of cognitive impairment in the rat. Br J Pharmacol. 2008;155:434–440. doi: 10.1038/bjp.2008.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. Electrolytic lesions of the fimbria/fornix, dorsal hippocampus, or entorhinal cortex produce anterograde deficits in contextual fear conditioning in rats. Neurobiol Learn Mem. 1997;67:142–149. doi: 10.1006/nlme.1996.3752. [DOI] [PubMed] [Google Scholar]
- Marsden CA, King MV, Fone KCF. Influence of social isolation in the rat on serotonergic function and memory – relevance to models of schizophrenia and the role of 5-HT(6) receptors. Neuropharmacology. 2011;61:400–407. doi: 10.1016/j.neuropharm.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Mattsson C, Sonesson C, Sandahl A, Greiner HE, Gassen M, Plaschke J, et al. 2-Alkyl-3-(1,2,3,6-tetrahydropyridin-4-yl)-1H-indoles as novel 5-HT6 receptor agonists. Bioorg Med Chem Lett. 2005;15:4230–4234. doi: 10.1016/j.bmcl.2005.06.067. [DOI] [PubMed] [Google Scholar]
- Meffre J, Seveno M, Milian MJ, La Cour CM, Dekeyne A, Bockaert J, et al. Proteomic and functional analysis suggests that 5-HT6 receptors compromise cognition by recruiting mTOR. Schizophr Res. 2010;117:373–374. [Google Scholar]
- Meneses A. Effects of the 5-HT6 receptor antagonist Ro 04-6790 on learning consolidation. Behav Brain Res. 2001a;118:107–110. doi: 10.1016/s0166-4328(00)00316-8. [DOI] [PubMed] [Google Scholar]
- Meneses A. Role of 5-HT6 receptors in memory formation. Drug News Perspect. 2001b;14:396–400. doi: 10.1358/dnp.2001.14.7.660941. [DOI] [PubMed] [Google Scholar]
- Meneses A, Perez-Garcia G, Liy-Salmeron G, Flores-Galvez D, Castillo C, Castillo E. The effects of the 5-HT6 receptor agonist EMD and the 5-HT7 receptor agonist AS19 on memory formation. Behav Brain Res. 2008;195:112–119. doi: 10.1016/j.bbr.2007.11.023. [DOI] [PubMed] [Google Scholar]
- Mitchell ES, Neumaier JF. 5-HT6 receptors: a novel target for cognitive enhancement. Pharmacol Ther. 2005;108:320–333. doi: 10.1016/j.pharmthera.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Mitchell ES, Hoplight BJ, Lear SP, Neumaier JF. BGC20-761, a novel tryptamine analog, enhances memory consolidation and reverses scopolamine-induced memory deficit in social and visuospatial memory tasks through a 5-HT6 receptor-mediated mechanism. Neuropharmacology. 2006;50:412–420. doi: 10.1016/j.neuropharm.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Mitchell ES, Sexton T, Neumaier JF. Increased expression of 5-HT6 receptors in the rat dorsomedial striatum impairs instrumental learning. Neuropsychopharmacology. 2007;32:1520–1530. doi: 10.1038/sj.npp.1301284. [DOI] [PubMed] [Google Scholar]
- Monsma FJ, Shen Y, Ward RP, Hamblin MW, Sibley DR. Cloning and expression of a novel serotonin receptor with high-affinity for tricyclic psychotropic-drugs. Mol Pharmacol. 1993;43:320–327. [PubMed] [Google Scholar]
- Myskiw JC, Rossato JI, Bevilaqua LRM, Medina JH, Izquierdo I, Cammarota M. On the participation of mTOR in recognition memory. Neurobiol Learn Mem. 2008;89:338–351. doi: 10.1016/j.nlm.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Nikiforuk A, Kos T, Wesolowska A. The 5-HT6 receptor agonist EMD 386088 produces antidepressant and anxiolytic effects in rats after intrahippocampal administration. Psychopharmacology. 2011;217:411–418. doi: 10.1007/s00213-011-2297-1. [DOI] [PubMed] [Google Scholar]
- Nilsson M, Hansson S, Carlsson A, Carlsson ML. Differential effects of the N-methyl-D-aspartate receptor antagonist MK-801 on different stages of object recognition memory in mice. Neuroscience. 2007;149:123–130. doi: 10.1016/j.neuroscience.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Riccioni T, Bordi F, Minetti P, Spadoni G, Yun HM, Im BH, et al. ST1936 stimulates cAMP, Ca2+, ERK1/2 and Fyn kinase through a full activation of cloned human 5-HT(6) receptors. Eur J Pharmacol. 2011;661:8–14. doi: 10.1016/j.ejphar.2011.04.028. [DOI] [PubMed] [Google Scholar]
- Riemer C, Borroni E, Levet-Trafit B, Martin JR, Poli S, Porter RHP, et al. Influence of the 5-HT6 receptor on acetylcholine release in the cortex: pharmacological characterization of 4-(2-bromo-6- pyrrolidin-1-ylpyridine-4-sulfonyl)phenylamine, a potent and selective 5-HT6 receptor antagonist. J Med Chem. 2003;46:1273–1276. doi: 10.1021/jm021085c. [DOI] [PubMed] [Google Scholar]
- Rogers DC, Hagan JJ. 5-HT6 receptor antagonists enhance retention of a water maze task in the rat. Psychopharmacology. 2001;158:114–119. doi: 10.1007/s002130100840. [DOI] [PubMed] [Google Scholar]
- Romero G, Sanchez E, Pujol M, Perez P, Codony X, Holenz J, et al. Efficacy of selective 5-HT6 receptor ligands determined by monitoring 5-HT6 receptor-mediated cAMP signaling pathways. Br J Pharmacol. 2006;148:1133–1143. doi: 10.1038/sj.bjp.0706827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routledge C, Bromidge SM, Moss SF, Price GW, Hirst W, Newman H, et al. Characterization of SB-271046: a potent, selective and orally active 5-HT6 receptor antagonist. Br J Pharmacol. 2000;130:1606–1612. doi: 10.1038/sj.bjp.0703457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruat M, Traiffort E, Arrang JM, Tardivellacombe J, Diaz J, Leurs R, et al. A novel rat serotonin (5-HT6) receptor – molecular cloning, localization and stimulation of cAMP accumulation. Biochem Biophys Res Commun. 1993;193:268–276. doi: 10.1006/bbrc.1993.1619. [DOI] [PubMed] [Google Scholar]
- Rushe TM, Woodruff PWR, Murray RM, Morris RG. Episodic memory and learning in patients with chronic schizophrenia. Schizophr Res. 1999;35:85–96. doi: 10.1016/s0920-9964(98)00117-0. [DOI] [PubMed] [Google Scholar]
- Russell MGN, Dias R. Memories are made of this (perhaps): a review of serotonin 5-HT6 receptor ligands and their biological functions. Curr Top Med Chem. 2002;2:643–654. doi: 10.2174/1568026023393877. [DOI] [PubMed] [Google Scholar]
- Schaffhauser H, Mathiasen JR, DiCamillo A, Huffman MJ, Lu LD, McKenna BA, et al. Dimebolin is a 5-HT6 antagonist with acute cognition enhancing activities. Biochem Pharmacol. 2009;78:1035–1042. doi: 10.1016/j.bcp.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Schechter LE, Lin Q, Smith DL, Zhang G, Shan Q, Platt B, et al. Neuropharmacological profile of novel and selective 5-HT6 receptor agonists: WAY-181187 and WAY-208466. Neuropsychopharmacology. 2008a;33:1323–1335. doi: 10.1038/sj.npp.1301503. [DOI] [PubMed] [Google Scholar]
- Schechter LE, Lin Q, Smith DL, Zhang G, Shan Q, Platt B, et al. Neuropharmacological profile of novel and selective 5-HT(6) receptor agonists: WAY-181187 and WAY-208466. Neuropsychopharmacology. 2008b;33:1323–1335. doi: 10.1038/sj.npp.1301503. [DOI] [PubMed] [Google Scholar]
- Schreiber R, Sleight AJ, Woolley ML. 5-HT6 receptors as targets for the treatment of cognitive deficits in schizophrenia. Serotonin Receptors: From Molecular Pharmacology to Human Therapeutics. 2006. pp. 495–515.
- Schreiber R, Vivian J, Hedley L, Szczepanski K, Secchi RL, Zuzow M, et al. Effects of the novel 5-HT6 receptor antagonist RO4368554 in rat models for cognition and sensorimotor gating. Eur Neuropsychopharmacol. 2007;17:277–288. doi: 10.1016/j.euroneuro.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Shen Y, Monsma FJ, Metcalf MA, Jose PA, Hamblin MW, Sibley DR. Molecular-cloning and expression of a 5-hydroxytryptamine7 serotonin receptor subtype. J Biol Chem. 1993;268:18200–18204. [PubMed] [Google Scholar]
- Sigurdsson T, Doyere V, Cain CK, LeDoux JE. Long-term potentiation in the amygdala: a cellular mechanism of fear learning and memory. Neuropharmacology. 2007;52:215–227. doi: 10.1016/j.neuropharm.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Stiedl O, Birkenfeld K, Palve M, Spiess J. Impairment of conditioned contextual fear of C57BL/6J mice by intracerebral injections of the NMDA receptor antagonist APV. Behav Brain Res. 2000;116:157–168. doi: 10.1016/s0166-4328(00)00269-2. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Tzavara ET, Qi HS, Carruthers R, Witkin JM, Nomikos GG, et al. Biochemical and behavioral evidence for antidepressant-like effects of 5-HT6 receptor stimulation. J Neurosci. 2007;27:4201–4209. doi: 10.1523/JNEUROSCI.3110-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassone A, Madeo G, Schirinzi T, Vita D, Puglisi F, Ponterio G, et al. Activation of 5-HT6 receptors inhibits corticostriatal glutamatergic transmission. Neuropharmacology. 2011;61:632–637. doi: 10.1016/j.neuropharm.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Schrick C, Fischer A, Sananbenesi F, Pages G, Pouyssegur J, et al. Regulatory mechanisms of fear extinction and depression-like behavior. Neuropsychopharmacology. 2008;33:1570–1583. doi: 10.1038/sj.npp.1301550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton N, Chuang TT, Hunter AJ, Virley DJ. 5-HT6 receptor antagonists as novel cognitive enhancing agents for Alzheimer's disease. Neurother. 2008;5:458–469. doi: 10.1016/j.nurt.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenstein GV, Vago DR. Intrahippocampal scopolamine impairs both acquisition and consolidation of contextual fear conditioning. Neurobiol Learn Mem. 2001;75:245–252. doi: 10.1006/nlme.2001.4005. [DOI] [PubMed] [Google Scholar]
- Wesolowska A, Nikiforuk A. Effects of the brain-penetrant and selective 5-HT6 receptor antagonist SB-399885 in animal models of anxiety and depression. Neuropharmacology. 2007;52:1274–1283. doi: 10.1016/j.neuropharm.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Wesolowska A, Nikiforuk A, Stachowicz K. Anxiolytic-like and antidepressant-like effects produced by the selective 5-HT6 receptor antagonist SB-258585 after intrahippocampal administration to rats. Behav Pharmacol. 2007;18:439–446. doi: 10.1097/FBP.0b013e3282d28f9c. [DOI] [PubMed] [Google Scholar]
- West PJ, Marcy VR, Marino MJ, Schaffhauser H. Activation of the 5-HT6 receptor attenuates long-term potentiation and facilitates GABAergic neurotransmission in the rat hippocampus. Neuroscience. 2009;164:692–701. doi: 10.1016/j.neuroscience.2009.07.061. [DOI] [PubMed] [Google Scholar]
- Woolley ML, Bentley JC, Sleight AJ, Marsden CA, Fone KCF. A role for 5-ht(6) receptors in retention of spatial learning in the Morris water maze. Neuropharmacology. 2001;41:210–219. doi: 10.1016/s0028-3908(01)00056-9. [DOI] [PubMed] [Google Scholar]
- Woolley ML, Marsden CA, Fone KCF. 5-ht6 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3:59–79. doi: 10.2174/1568007043482561. [DOI] [PubMed] [Google Scholar]
- Young JW, Powell SB, Risbrough V, Marston HM, Geyer MA. Using the MATRICS to guide development of a preclinical cognitive test battery for research in schizophrenia. Pharmacol Ther. 2009;122:150–202. doi: 10.1016/j.pharmthera.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun HM, Kim S, Kim HJ, Kostenis E, Kim JI, Seong JY, et al. The novel cellular mechanism of human 5-HT6 receptor through an interaction with Fyn. J Biol Chem. 2007;282:5496–5505. doi: 10.1074/jbc.M606215200. [DOI] [PubMed] [Google Scholar]
- Yun HM, Baik JH, Kang I, Jin C, Rhim H. Physical Interaction of Jab1 with human serotonin 6 G-protein-coupled receptor and their possible roles in cell survival. J Biol Chem. 2010;285:10016–10029. doi: 10.1074/jbc.M109.068759. [DOI] [PMC free article] [PubMed] [Google Scholar]


