Abstract
Cyanamide (CA) has been reported as a natural compound produced by hairy vetch (Vicia villosa Roth.) and it was shown also to be an allelochemical, responsible for strong allelopathic potential in this species. CA phytotoxicity has been demonstrated on various plant species, but to date little is known about its mode of action at cellular level. Treatment of tomato (Solanum lycopersicum L.) roots with CA (1.2 mM) resulted in inhibition of growth accompanied by alterations in cell division, and imbalance of plant hormone (ethylene and auxin) homeostasis. Moreover, the phytotoxic effect of CA was also manifested by modifications in expansin gene expression, especially in expansins responsible for cell wall remodeling after the cytokinesis (LeEXPA9, LeEXPA18). Based on these results the phytotoxic activity of CA on growth of roots of tomato seedlings is likely due to alterations associated with cell division.
Electronic supplementary material
The online version of this article (doi:10.1007/s00425-012-1722-y) contains supplementary material, which is available to authorized users.
Keywords: Allelopathy, Auxin, Ethylene, Mitosis, Phytotoxicity
Introduction
Cyanamide (CA), bioactive secondary plant product was first identified in hairy vetch (Vicia villosa subs. varia Roth) (Kamo et al. 2003). This leguminous plant is widely used as a winter cover crop for prevention of water evaporation and as a source of nitrogen when used as a green manure. However, hairy vetch inhibits the growth of various weed species, e.g. white star (Ipomoea lacunosa L.), barnyard grass (Echinochloa crus-galli L.), prickly sida (Sida spinosa L.) and hairy crabgrass (Digitaria sanguinalis L.) (Hoffman et al. 1993; Fujii 2001; Reddy and Koger 2004), and is used for weed suppression in Japanese crop production. Identification of phytotoxic compounds synthesized in hairy vetch seedlings pointed to CA as the key component. Cyanamide is present in all organs of hairy vetch, including seeds (inside endosperm), but is most abundant in plant shoots (approx. 444 mg kg−1 FW) (Kamo et al. 2003). Apart from hairy vetch, CA was also isolated from bird vetch (V. cracca L.) and black locust (Robinia pseudoacacia L.) (Kamo et al. 2006, 2008).
Nowadays, the term “phytotoxicity” is used to distinguish allelopathy (as phenomenon occurring in natural environment), from laboratory studies on phytotoxic compounds. Therefore, phytotoxicity refers to allelopathic interactions studied under controlled laboratory conditions, using plant extracts, phytochemicals isolated from plant tissue, collected from exudates or even synthetic compounds—identical to natural ones (Macias et al. 2003). Controlled laboratory experiments are design to reduce the impact of other biotic and abiotic factors.
One of the first visible effect of action of phytotoxins is restriction in seed germination or/and seedlings growth (for review see Gniazdowska and Bogatek 2005). Unfortunately, most studies describe only the visual symptoms or morphological impacts of phytotoxins, without detailed analysis of their physiological or biochemical mode of action. It should be noted that delay of seed germination or plant growth by phytotoxins, is often the final result of specific inhibition of key enzyme sites. Moreover, it should be pointed out, that mode of action of the specific phytotoxin in each plant organ (root, hycopotyl, leaves) may be different. Additionally, as described earlier most phytotoxins may be characterized by multidirectional mode of action (Gniazdowska and Bogatek 2005). Cinnamic acid, one of the phenolic compounds, induces membrane depolarization and inhibits absorption of some ions, e.g. phosphate, potassium, nitrate, and magnesium (Einhelling 2004). Moreover, it stimulates ROS generation, lipid peroxidation and inhibits catalase and peroxidase activity. Cinnamic acid alters also auxin biosynthesis and modifies photosynthesis and respiration rate (Einhelling 2004).
Cell division and cell elongation in seedling root are essential processes responsible for root growth. Cell division provides new cells that subsequently reside within the elongation zone, where cell enlargement occurs (Cosgrove 1997). Inhibition of mitosis leads to reduced root length, even without any effects on cell enlargement. A variety of phytotoxins act as inhibitors of division of root tip cells (Ding et al. 2008; Sánchez-Moreiras et al. 2008). Both physiological processes, mentioned above are controlled by phytohormones such as auxins and ethylene (Etheridge et al. 2005; Cho et al. 2007). A distinctive hormonal balance is required for undisturbed root growth. It is known that ethylene has the ability to inhibit root growth, but ethylene-regulated root growth is dependent on auxin transport. It was noted that alterations in any component of auxin transport and signal transduction result in impacts upon ethylene synthesis and signaling pathway and vice versa (Swarup et al. 2007). Briefly, increasing auxin concentration results in local ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC) biosynthesis in root tip and ethylene diffusion and accumulation in elongation zone. Moreover, increasing ethylene concentration inhibits polar auxin transport in the root, resulting in its accumulation in the elongation zone (Smalle and Van Der Straeten 1997). Enhanced concentration of auxins and ethylene emission in the elongation zone results in inhibition of cell growth. However, cell enlargement in roots may not only be affected by altered plant hormone homeostasis, but may also be due to alterations in cell wall flexibility. Expansins are proteins that influence physical properties of cell walls (Cosgrove 2000) by cutting hydrogen bonds between cellulose and hemicellulose. Expansins also take part in cell wall remodeling after cytokinesis (Reinhardt et al. 1998; Vogler et al. 2003).
The objective of the present study was to investigate the physiological effects of CA on tomato (Solanum lycopersicum L.) root growth. The root is of special interest as it is a primary organ exposed to direct influence of potential phytotoxins that may be found in the soil. Our preliminary studies indicated that tomato may be used as an excellent model to investigate the mode of action of CA mostly due to its convenience in cytological observation and well known sequences of expansin genes. Moreover, previously we demonstrated that CA inhibited growth of onion (Allium cepa L.) roots in a dose-dependent manner (Soltys et al. 2011). Experiments conducted on tomato showed that CA-induced restriction in root growth was concentration (0.8–2 mM) dependent (Sołtys et al. 2010). Therefore, in our investigations we have chosen 1.2 mM CA concentration, as that one required for 50 % inhibition of root growth. We performed experiments to study two general aspects of root growth: cell division in root tips and cell enlargement in the elongation zone. Based on experimental findings, we hypothesize that inhibition of tomato root growth by CA is mainly due to disturbance of cell division in root tips as well as imbalances in phytohormone levels in developing roots, which result in altered expansin gene expression.
Materials and methods
Plant material
Tomato (Solanum lycopersicum L. cv. Malinowy Ożarowski) seeds were obtained from Horticultural Seed Company and Nursery “PNOS Ożarów” Ożarów Mazowiecki, Poland. Seeds germinated in water in darkness at 20 °C for 36 h. After radical protrusion (day 0) seedlings of equal size (5 mm root length) were transferred to distilled water (control) or aqueous CA-containing solution (0.8, 1.2, 2 mM). Culture of tomato seedlings was carried out in Petri dishes (ø 18 cm, 30 seedlings per dish) for 7 days in darkness at 20 °C. Length of seedling roots was determined after 7 days. In each of 5 replications, 40 seedling roots were measured.
Recovery effect after CA treatment
After 1 or 3 days of CA (1.2 mM) treatment, seedlings were transferred into water-moistened Petri dishes containing saturated filter paper. Length of seedling roots was measured after additional 5 days recovery following treatment. Data were expressed as % of control (non-treated) plants. Experimental treatments were replicated four times and in each experiment, 25 roots were measured.
Mitotic index
The mitotic index was determined using the carbol fuchsin staining method. After 1 or 3 days of CA (1.2 mM) treatment, distal fragments of roots (1 cm long) were cut off and fixed in Carnoy’s fixative (glacial acetic acid : ethanol, 3:1, w/v) for 24 h. Then, roots were washed three times for 15 min in 70 % ethanol and hydrolyzed in 1 M HCl at 60 °C, stained with carbol fuchsin (0.3 % basic fuchsin, 5 % phenol in distilled water, w/v) and transferred onto the slide. Root tips (2 mm long) were cut off and immersed in a drop of water. Frequencies of each mitotic phase were calculated by examining 1,000 cells. Results were expressed as %. The mitotic index was observed under light microscope Olympus AX70 PROVIS using programs View Finder and Studio Lite (Olympus).
Length of root cells
After 3 days of culture in water or 1.2 mM CA solution, 8-mm-long segments of tomato roots (including root tip) were cut off and fixed in Karnovsky fixative (5 % glutaraldehyde, 4 % paraformaldehyde and 0.2 M sodium cacodylate, v/v) for 4 h at 4 °C. After rinsing in cacodylate buffer (0.2 M sodium cacodylate) root segments were dehydrated in an ethanol series (10, 20, 30, 40, 50, 60, 70, 80, 90 % for 10 min each) and embedded in epon resin 812 (Fluka) with propylene oxide in proportion 0:1, 1:3, 1:1 and 3:1 and left for propylene oxide evaporation. The embedded roots were separated into four (1–4) 2-mm-long segments and poured with epon resin and polymerized for 24 h at 60 °C. The samples were sectioned longitudinally and 3-μm-thick sections were transferred to slides. Each 2-mm-long root segments were conventionally divided into 3 or 4 sections. Length of cells in each section was measured. Photographs of root segments were provided using an Olympus AX70 PROVIS microscope with the programs View Finder and Studio Lite (Olympus). Length of cells was counted using the AnalySIS (SIS) program. Experiments were repeated two times with five replications each.
Concentration of IAA
Indole-3-acetic acid (IAA) was determined by competitive enzyme-linked immunosorbent assay (ELISA) modified after Weiler et al. (1981). Freshly cut roots (0.2 g) from 0, 1, 2 and 3-days-old control and CA (1.2 mM) treated seedlings were homogenized in liquid nitrogen. Extraction of IAA was done according to Marcussen et al. (1988), using 80 % ethanol with butyl-hydroxy-toluene as antioxidant. The obtained extract was centrifuged at 15,000g for 15 min at 4 °C. The supernatant was left for total ethanol evaporation and then was suspended in TBS buffer (50 mM Tris, 150 mM NaCl, 1 mM MgCl2). Probes were methylated using diazomethane and diluted in PBS buffer (0.8 % NaCl, 0.02 % KCl, 0.02 % K2HPO4, 8.1 mM Na2HPO4). ELISA plates were filled with rat anti-mouse antibodies (25 μg ml−1) and incubated for 18 h at 4 °C. Next plates were rinsed with distilled water, and IAA extracts (50 μl) were added for each well and incubated for 1 h at 4 °C. After the incubation time, 50 μl of tracer (IAA conjugated with alkaline phosphatase, Agrisera) diluted 1:1,650 with TBS-G buffer (0.1 % gelatin in TBS buffer) was added to each well and incubated 3 h at 4 °C, then rinsed with distilled water. For enzymatic reaction, substrate for alkaline phosphatase, p-nitrophenol sodium phosphate (Sigma) was added and incubated for 1 h at 37 °C. IAA concentration was measured at 405 nm with referential wave 605 nm (Dynatech MR 5000). IAA determination was performed in three biological and four technical repetitions.
Ethylene emission
Roots of 0, 1, 2, 3-days-old seedlings cultured in water or 1.2 mM CA solution were cut off (about 0.5 g), placed in 15 ml glass flask with 0.2 ml of water or 1 mM 1-aminocyclopropano-1-carboxylic acid (ACC) and left for 20 min. Then flasks were tightly closed and roots were incubated for 3 h at 30 °C. Ethylene content in the gas phase (1 cm3) was detected using gas chromatograph 330 Girdel France (Supelco-HayeSep 80/100 column, FID detector, temperature of injection/detection 120/150 °C). Histograms were analyzed using the HP CORE Chem Station computer program. The actual (-ACC) ethylene emission was detected without exogenous ACC, the potential ethylene emission (+ACC) was measured in the presence of 1 mM ACC. For each experiment three biological and four technical repetitions were performed.
Expansin genes expression
Total RNA was isolated from roots of 0, 1, 2, 3-days-old seedlings grown in water or 1.2 mM CA solution. The samples were homogenized in liquid nitrogen and RNA was isolated according to the modified Chomczyński method (Rybka et al. 2009). Specific primers for expansin genes were used according to Fudali et al. (2008) and validated using Primer 3 release 1.1.0 program. Reverse transcription (RT) was made using RevertAid™ kit (Fermentas) according to manufacturer’s instruction. The RT reaction mixture (10 μl) consisted of 1× RT buffer, 1 mM of dNTP mix, 5 μM of random primers (oligo(dt)primer), 10 U of RNasin, 10 U reverse transcriptase and 0.02 μg total RNA. The cDNA was used for several polymerase chain reactions (PCR) with specific expansin primers: LeEXPA1, LeEXPA2, LeEXPA4, LeEXPA5, LeEXPA8, LeEXPA9 or LeEXPA18 (Fudali et al. 2008). The PCR mixture (10 μl) contained 1× Tag buffer, MgCl2 (1.75 mM), 0.2 mM of each dNTP, primers (0.25 μM each), Taq polymerase (0.01 U, Fermentas), cDNA as a negative control (water was applied in this step). For each expansin primer specific PCR conditions and number of cycles (30) were adjusted. As a positive control, PCR with 18S rRNA primers was performed for each repetition. The PCR products were checked using electrophoresis in 1.5 % agarose/TBE gels (100 mM Tris–HCl, 83 mM boric acid, 1 mM EDTA, pH 8.4) containing 0.5 μg ml−1 ethidium bromide (Midi Horizontal Electrophoresis Unit Set, Sigma). All reactions were performed using GeneAmp® PCR System 9700 cycler (Applied Biosystems) and visualized using transilluminator UV (Gel Logic 200, Molecular Imaging System). The relative transcript level was counted in relation to control using Kodak Molecular Imaging Software. For each expansin gene analysis, two biological and four technical repetitions were performed.
Statistics
Data were analyzed using the STATISTICA 9.1 software. Normality of the distribution and homogeneity of variance was checked using Kolmogorov–Smirnov and Levene’s test, respectively. All showed normal distribution and similar variances, and they fulfilled conditions for use ANOVA. However, due to high sample size, mean values were computed and mean differences were calculated using Tukey’s studentized range test in the following experiments: length of roots, length of roots after recovery phenomenon and length of cells in each root zone. Standard error (SE) and coefficient intervals (CI) were also provided to indicate the variations associated with the particular mean values.
Results
CA inhibits tomato root growth
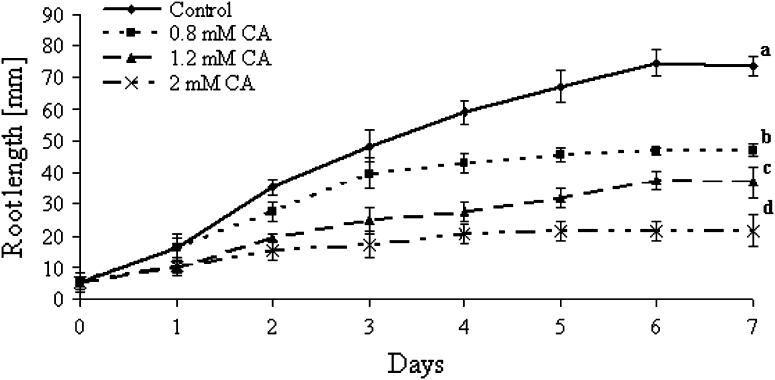
Roots of control (untreated) tomato seedling continued growth during the entire culture period (Fig. 1). After 7 days the length of roots increased from 5 to 72 mm. Roots of seedlings treated with CA were much shorter than control roots. The inhibitory effect of CA treatment was dose dependent (Fig. 2). At the end of the culture period, roots treated with 0.8 mM CA reached approximately 47, 35 mm with 1.2 mM CA, and were only 22 mm long after treatment with 2 mM CA. Morphological (visible) differences between control and CA-treated roots were noticed after 3 days of culture. Roots had brown discoloration in the zone between root and hypocotyl and were light-brown along the entire length. The inhibitory effect of 1.2 mM CA on root growth increased as the experiment progressed. Roots treated with CA for 3 days were 50 % shorter in comparison to the control (Fig. 1). Therefore, in further tests the impact of CA on tomato root growth was determined only using the solution of the allelochemical at 1.2 mM concentration. Elongation growth of CA-treated roots was arrested after 3 days, as a consequence root length of older seedlings did not differ significantly from length of roots exposed to CA for only 3 days (Fig. 1). Consequently, all other tests were conducted only during the 3 days following experimental initiation.
Fig. 1.
Length of roots of tomato seedlings cultured 7 days in water or CA solution at various concentration (0.8–2 mM) (mean ± SE, n = 200). Different letters indicate significant differences by Tukey’s test (P < 0.05)
Fig. 2.
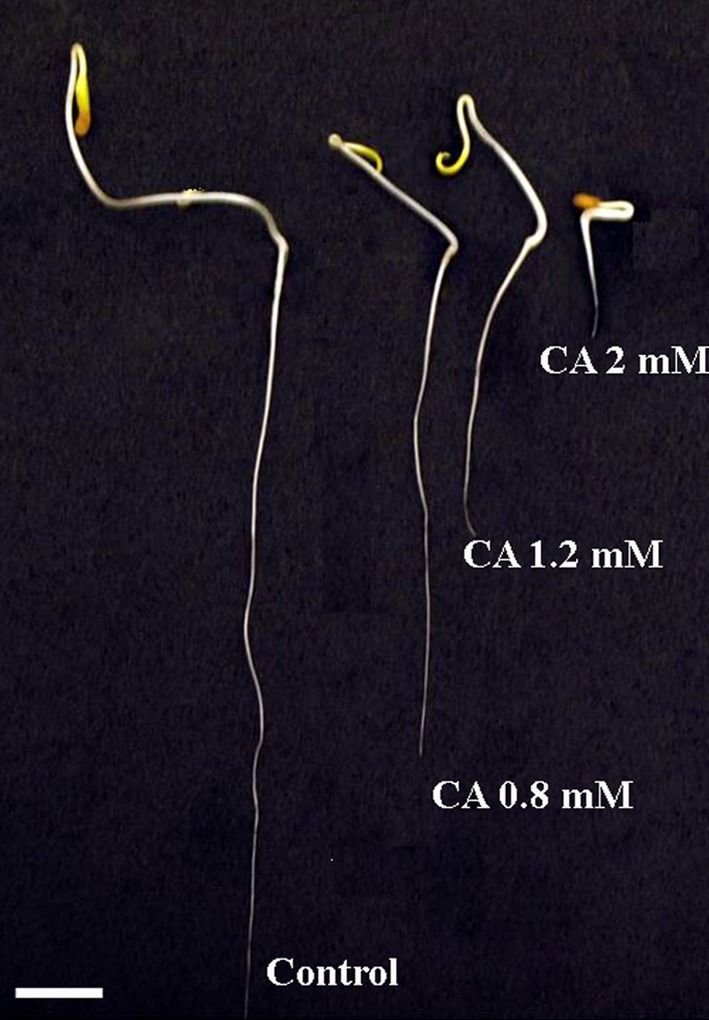
Tomato seedlings grown 7 days in water (control) or CA aqueous solutions (0.8; 1.2 and 2 mM). Scale bar 4.5 cm
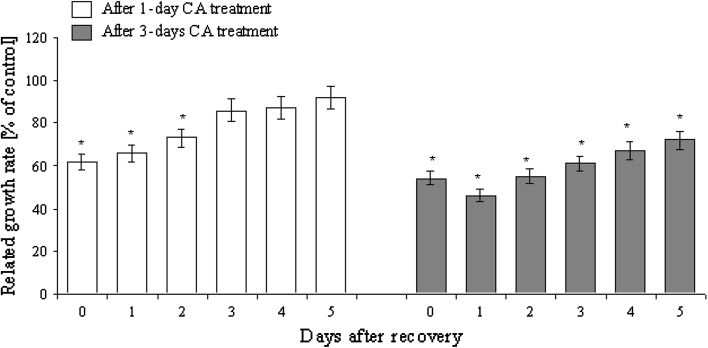
The recovery phenomenon of CA (1.2 mM) treatment was time-dependent (Fig. 3). Effects of short-term (1 day) tomato seedling treatment with CA were fully reversible. After 5 days of recovery, roots reached 90 % of length of control plants (differences were statistically insignificant). Treatment with CA for 3 days led to irreversible alterations in root growth. After the first day of recovery, roots were nearly twofold shorter than the control. After additional 4 days root growth was detected, but treated roots did not reach the length of control roots (were 25 % shorter) (Fig. 3).
Fig. 3.
Length of roots (expressed in % of length of control) of tomato seedlings treated for 1 or 3 days with 1.2 mM CA and after 5 days of recovery in water (mean ± SE, n = 100). *Significant differences by Tukey’s test (P < 0.05)
CA modifies root tip cells division
The mitotic index in root tip cells of control seedlings was constant over the course of the experiment (Table 1). After 1, as well as 3 days of culture, percentage of dividing cells was unchanged and was 2.5 %. Similarly, no alterations in proportion of each phases of mitosis were detected in cells of CA (1.2 mM) treated root tips after short-term (1 day) exposure to phytotoxin. However, prolonged (3 days) CA treatment induced modifications in proportion of phases of mitosis in root tip cells. Two phases of mitosis: prophase and metaphase were inhibited in about 40 % in relation to control. This led to a decrease in percentage of dividing cells, from 2.60 to 1.74 % during 3 days of CA treatment (Table 1).
Table 1.
Mitotic index in root tip cells of control and CA (1.2 mM) treated tomato seedlings after 1 or 3–days of culture (mean values ± SE, n = 10)
| Phases of mitosis | Culture conditions | Dividing cells (%) | |
|---|---|---|---|
| Days of culture | |||
| 1 | 3 | ||
| Prophase | Control | 0.79 ± 0.12Aa | 0.80 ± 0.10Aa |
| CA | 1.01 ± 0.12Aa | 0.55 ± 0.10Bb | |
| Metaphase | Control | 0.82 ± 0.12Aa | 0.81 ± 0.09Aa |
| CA | 0.74 ± 0.10Aa | 0.50 ± 0.13Bb | |
| Anaphase | Control | 0.49 ± 0.10Aa | 0.50 ± 0.08Aa |
| CA | 0.51 ± 0.06Aa | 0.43 ± 0.11Aa | |
| Telophase | Control | 0.40 ± 0.04Aa | 0.40 ± 0.05Aa |
| CA | 0.34 ± 0.05Aa | 0.26 ± 0.08Bb | |
| Cells in division (%) | Control | 2.50 ± 0.28Aa | 2.54 ± 0.23Aa |
| CA | 2.60 ± 0.21Aa | 1.74 ± 0.36Bb | |
Frequencies of each mitotic phase were calculated for 1,000 cells. Results were expressed as %. Various statistical analyses were performed for each mitotic index component. Values for particular culture conditions followed by different capital letter (in rows) are significantly different at P < 0.05 (ANOVA test). Values for particular treatment (in columns) followed by different small letter are significantly different at P < 0.05 (ANOVA test)
CA induces alterations in length of root segments
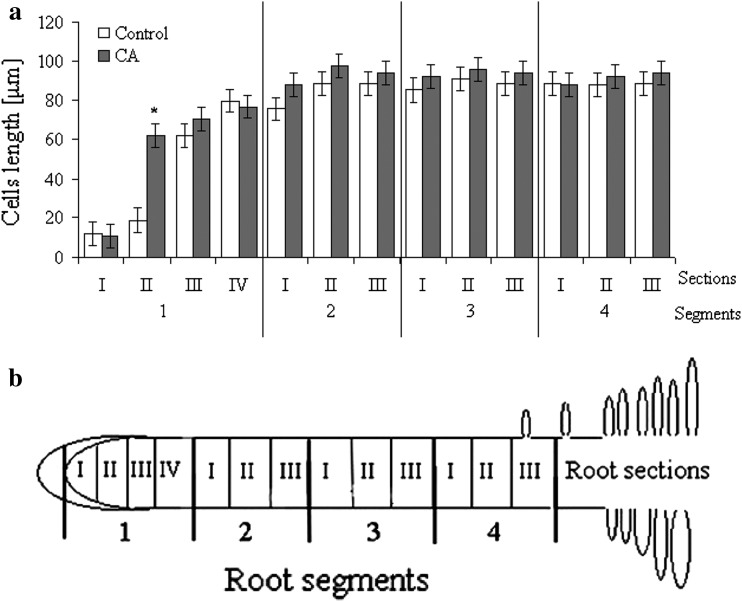
In control tomato roots the length of cells in distal segments (2–4) did not differ significantly. It was approximately 80 μm long in all sections of 2–4 segments (Fig. 4a). The greatest differences in cell length were detected in segment 1 (Fig. 4b). Cells in the two first apical parts (I and II) in segment 1 were less than 20 μm long. Cells in part III and IV of segment 1 were 62 and 80 μm long, respectively. Three-day-long treatment of tomato roots with 1.2 mM CA resulted in modification of cell length in part II of segment 1. Cells of this part of CA-treated roots were threefold longer as compared to control, and their length (approx. 60 μm) was similar to length of cells in part III, segment 1 of control roots. No significant differences in cell length of CA-treated and control plants were observed in other root segments.
Fig. 4.
a Length of cells in various root segments of seedlings after 3 days of culture in water (control) or 1.2 mM CA solution (mean ± SE, n = 100). *Significant differences by Tukey’s test (P < 0.05). b Scheme of root divided into segments (1–4) and parts (I–IV) used for calculation of cell length
CA alters plant hormones homeostasis in roots
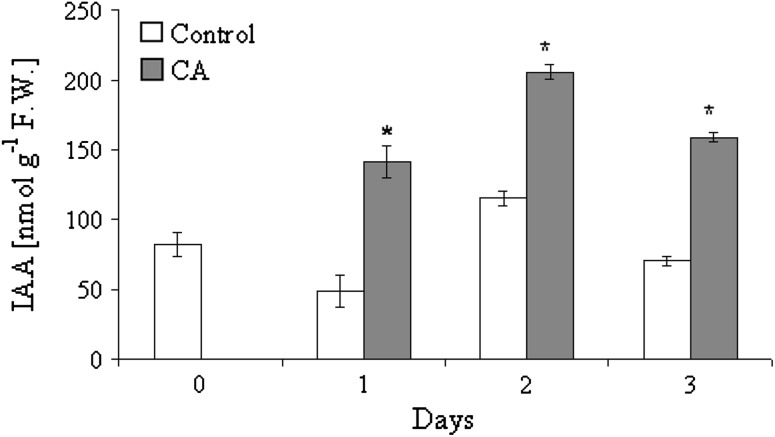
Concentration of IAA in control roots fluctuated (rise and fall down) during 3 days of culture (Fig. 5) and varied from 45 to 110 nmol g−1 FW. Treatment with 1.2 mM CA significantly increased IAA concentration in roots, which was threefold higher than that in controls after 1 day, and almost doubled after 2–3 days (Fig. 5). The actual ethylene emission (−ACC) was lowest (0.13 μmol g−1 FW h−1) from control, untreated roots at the beginning of the experiment (Table 2). It increased almost sixfold (to the value 0.75 μmol g−1 FW h−1) within 1 day of culture and then decreased twice after additional 2 days. Fluctuation in ethylene emission from CA (1.2 mM) treated roots was similar to those observed in control plants, although rates of ethylene emission were significantly higher (for about 50 %) (Table 2). The highest ethylene emission from CA-treated roots was noted after 1 day of experiment, similarly as it was detected in control roots. Nevertheless, the most pronounced effect of CA on actual ethylene emission was observed after 3 days. Potential ethylene emission (+ACC) from control roots decreased continuously during culture period. The highest ethylene emission was noted at the beginning of the experiment, and then it declined sixfold at the end of the culture. CA increased the potential ethylene emission from roots of tomato seedling. After the first day of culture CA-treated roots emitted 36 % more ethylene than control ones. This effect was more pronounced in prolonged CA treatment and finally, potential ethylene emission from CA-treated root was 211 % higher than in control roots (Table 2). No ethylene emission was detected from pure CA solution and after incubation of CA with ACC (data not shown).
Fig. 5.
IAA concentration in roots of control and CA-treated tomato seedlings (mean ± SE, n = 12). *Significant differences by ANOVA test P < 0.05
Table 2.
Ethylene emission from root of control and CA-treated tomato seedlings (mean values ± SE, n = 12)
| Days | Ethylene emission (μmol g−1 FW h−1) | |||
|---|---|---|---|---|
| −ACC | +ACC | |||
| Control | CA | Control | CA | |
| 0 | 0.13 ± 0.01 | – | 19.56 ± 1.66 | – |
| 1 | 0.75 ± 0.06a | 0.98 ± 0.06b | 10.50 ± 0.81a | 14.34 ± 0.81b |
| 2 | 0.27 ± 0.02a | 0.34 ± 0.01b | 4.29 ± 1.73a | 12.01 ± 1.73b |
| 3 | 0.31 ± 0.02a | 0.48 ± 0.04b | 3.14 ± 0.14a | 9.76 ± 0.68b |
Values for particular culture conditions (in rows, means ± CI) followed by different small letter are significantly different at P < 0.05 (ANOVA test)
CA modifies expression of expansin gene
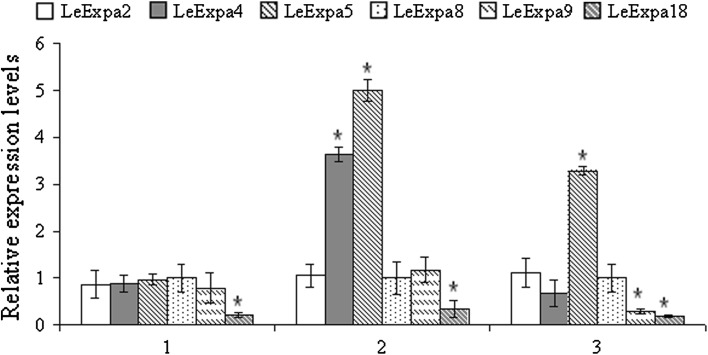
The expression of the LeEXPA gene in control and CA-treated tomato seedlings during 3 days of culture is shown in Fig. 6. Among all analyzed genes, two (LeEXPA2 and LeEXPA8) had unchangeable transcript abundance (bands of the same intensity) in all days of culture both in control and CA-treated plants (Fig. 6; Supplemental data Fig. S1). Expansins, LeEXPA4 and LeEXPA5 were found to be expressed at low level in control roots after 2 and 3 days of experiment, while in CA-treated roots LeEXPA4 and LeEXPA5 transcripts were still observed till the third day of culture. Expression pattern of some other expansin genes were also modified by 1.2 mM CA. Two of them (LeEXPA9 and LeEXPA18) were of special interest. Expression of LeEXPA18 was strongly down-regulated in relation to control, already after 1 day of culture, while LeEXPA9 after 3 days.
Fig. 6.
Relative transcript level of expansin gene. Value 1 means expression level equal to the control (mean ± SE, n = 8). *Significant differences by ANOVA test (P < 0.05)
Discussion
Many natural compounds derived from plant secondary metabolism are highly phytotoxic. It has been demonstrated that some natural products with high phytotoxic activity can induce severe alteration in plant growth due to induction of programmed cell death (PCD) (Ding et al. 2007; Keller et al. 2008) and restriction in cell division (Kuraś et al. 2006; Soltys et al. 2011; Teerarak et al. 2012).
We demonstrated that CA induced 50 % inhibition of root growth at quite high doses (1.2 mM), similarly as a lot of phytotoxins acting as plant growth inhibitors at high concentrations. Coumarin at a concentration of 1.3 mM affected onion root growth (Kupidlowska et al. 1994). Cineole, camphor and β-pinene (1 mM) inhibited turnip (Brassica rapa L. subsp. campestris) germination (Nishida et al. 2005). Benzoxazolin-2(3H)-one (BOA) led to 50 % inhibition of lettuce (Lactuca sativa L.) root growth at a concentration of 0.9 mM (Sánchez-Moreiras et al. 2008).
Our data show cellular alterations in the roots tip of tomato after treatment with CA (Fig. 4a). Moreover, we have shown that CA-induced inhibition of root growth was dose dependent (Fig. 1). This study validated also other data on CA phytotoxicity, e.g. treatment of lettuce with 10 μM CA inhibited root growth in about 40 % (Kamo et al. 2003), and onion roots exposed to CA 2 and 6 mM solutions were shortened in 30 and 60 %, respectively (Soltys et al. 2011). Similar restriction in root growth was also observed for other phytotoxins, e.g., cinnamic acid (Ding et al. 2007), chalcone (Díaz-Tielas et al. 2012), sorgoleone (Czarnota et al. 2003).
We investigated the reversible (recovery) effect of CA phytotoxicity after tomato seedlings treatment with CA and then post-incubation in pure water (Fig. 3). Three-day-long tomato culture in CA solution induced irreversible changes in root growth. Only roots treated with CA for 1 day reached the length of control roots after recovery, so we can conclude that prolonged exposition to CA provokes permanent perturbation in plant metabolism and structure. Moreover, we suspect that cellular mechanisms of CA detoxification were insufficiently expressed even after recovery. Unfortunately, recovery phenomenon after seedling treatment with phytotoxins is still poorly understood. However, data presented are in agreement with our previous studies on onion roots (Soltys et al. 2011). Only 1-day-long CA (2 mM) treatment of onion roots was fully reversible by bulbs transfer into distilled water (Soltys et al. 2011).
Inhibition of root growth by phytotoxins may be an effect of disturbances in cells division and differentiation. We demonstrated that CA may act as inhibitor of mitosis (Table 1). It decreased the percentage of dividing cells and frequencies of all phases of mitosis, apart from anaphases. The main reason for inhibition of mitosis after CA treatment is still not clear. Our former research showed that CA in higher concentration (10 mM) led to inhibition of mitosis in onion roots by disruption of the mitotic spindle organization and disturbances in chromosome condensation and organization in prophases and telophases (Soltys et al. 2011). However, it was also suggested that CA may arrest cell cycle in G2/M checkpoint (Soltys et al. 2011). Such an effect of other phytotoxins has been reported for water extract of cat’s claw (Uncaria tomentosa Willd.; 4 mg ml−1) which decreased the mitotic index in onion cells (Kuraś et al. 2006). Inhibition of cell division was observed in lettuce treated with BOA (1 mM; Sánchez-Moreiras et al. 2008), bean (Pisum sativum L.) exposed to sorgoleone (0.1 mM; Gattás-Hallak et al. 1999) or cockspur grass grown in extract of chinese perfume plant (Aglaia odorata Lour.; Teerarak et al. 2012). Leukamenin E induced disorder in chromosome morphology during mitosis in lettuce root tip cells (Ding et al. 2008).
In root tips, two types of cell proliferation occur: proliferative and quantal. In the first, after mitosis, daughter cells do not change their destination and still form the quiescent center, while cells under quantal cycle enter the differentiation zone (Gahan and Rana 1985). Inhibition of mitosis caused by CA decreases the number of cells under mitosis that may lead to shrinkage of the root tip, observed in our experiment (Fig. 4a). Root tips of CA-treated seedlings were approx. 50 % shorter than control ones. Altered root growth may also be an effect of disorder in cell differentiation in the elongation zone or in the transitional fragments of root tip and differentiation zones. In CA-treated roots dissimilarities/variations in cell length has been observed only in the first 2-mm-long segment, while in distal segments length of cells in control and treated roots remained equal. It suggests a shift in the root zones, but length of cells in part II of segment 1 is the same as in part III of segment 1. It might indicate that shortening of root tips correlates rather to earlier differentiation of cells after the end of mitosis, than to changes in cell length. Additionally, vacuolization, as the first sign of cell differentiation starts in plerom and periblem of treated roots earlier than in control ones (data not shown). Comparable effects have been observed in tobacco (Nicotiana tabacum L.) after treatment with cineol (440 μM; Yoshimura et al. 2011). Cineol influenced divisions in root tips, but did not affect the length of root cells. Moreover, monoterpenes (camphor, cineol, α-pinene and β-pinene) isolated from salvia (Salvia leucophylla L.) affected turnip root growth, although did not contribute to alterations in cells size (length and wide) (Nishida et al. 2005). On the other hand, inhibition of maize (Zea mays L.) root growth by hydroxycoumarin was due to nonsynchronized frequencies of cell division in vascular cylinder and cortex (Kupidlowska et al. 1994).
Cyanamide-dependent modification in root growth may be secondary effect of alterations in phytohormone balance. Both auxins and ethylene are responsible for correct root tip organization and root growth, e.g., for cell growth an inhibition of the local IAA concentration by ethylene is required (Swarup et al. 2007; Stepanova et al. 2007).
It is known that auxins are not sufficient, but important factors regulating cell division, differentiation and balance between the number of cells under mitosis and cells that enter differentiation, or post-division differentiation. Auxins control the cell cycle at early stages: at G1/S checkpoint and S phase before DNA replication starts. In our study after CA treatment in tomato roots an increased concentration of IAA and emission of ethylene was observed (Fig. 5; Table 2). Such variation in concentrations of phytohormones was detected in lettuce treated with leukamenin E and resulted in inhibition of root growth. Increasing IAA concentration led to overexpression of WEI2/7, the gene encoding antranilan synthase, an enzyme which catalyzes the synthesis of tryptophane—IAA’s precursor (Ding et al. 2008).
In our tests ethylene emission was higher in CA-treated roots. Measurement of ethylene emission with exogenous ACC relates to activity of ACC oxidase (ACO) that oxidized ACC into ethylene. Therefore, we assume that ACO activity is not affected by CA treatment and its activity is limited by substrate (ACC) availability. Moreover, it is possible, that ACO activity is stimulated by CA since the actual ethylene emission was enhanced by the phytotoxin. Our data are in agreement with the previously observed inhibition of A. thaliana cell growth in the root elongation zone, formation of root hairs and root thickening as a result of disorder in ethylene synthesis in (Le et al. 2001). It also confirms the involvement of ethylene in the plant reaction to phytotoxicity, since in oats (Avena sativa L.) the PCD has been induced after stimulation of ethylene emission by victorin (Yao et al. 2002).
Cells leaving root tip enlarge several doses due to increasing ploidy by endoreduplication and cell wall arrangement (Perrot-Rechenmann 2010). High turgor pressure is a main factor establishing cell wall reorganization and enlargement. Expansins are proteins that are mainly responsible for cell wall flexibility. They cut hydrogen bonds between cellulose and hemicelluloses molecules resulting in cell wall loosening. In tomato roots treated with CA, strong decrease of expression of LeEXPA9 and LeEXPA18 was observed (Fig. 6). Both of them (LeEXPA9 and LeEXPA18) take part in cell expansion that accompanies cell division and are responsible for proper development of the root tip (Reinhardt et al. 1998; Vogler et al. 2003). It may indicate that the observed reduced value of the mitotic index in tomato roots could be a result of improper cell remodeling elicited by lower expression of expansins (LeEXPA9 and LeEXPA18). On the other hand, the lack of significant changes in cell’s size can be a result of compensation by other expansin genes mainly responsible for cell enlargement, e.g. stable expression of LeExpA2, or even overexpression of LeExpA4 and LeExpA5 in roots treated with CA.
There is no other data about expansin gene expression and protein action under phytotoxic interaction, but their contribution in the regulation of development and growth of plant organs is well known. It was proven that the expression of LeExpA2 occurs in different tomato root zones, hypocotyls, leaves and flowers, but not in meristematic tissues (Reinhardt et al. 1998; Vogler et al. 2003). Moreover, high correlation between transcript level of LeExpA2 and hypocotyl growth was detected (Caderas et al. 2000). On the other hand, high expression of LeExpa4 was shown in seeds (endosperm) of tomato already after 12 h of imbibition (Chen and Bradford 2000). Such data indicate its crucial role in cell wall loosening during seed coat disruption for radicle protrusion. Enhanced expression of expansins LeExpA2 LeExpa4 and LeExpA5 in plant organs characterized by high elongation ability may indicate that expansins play essential roles in the regulation of cells growth.
Conclusions
All these results highlight the mode of action of CA as phytotoxin leading to inhibition of root growth, probably through the alteration in cell division, imbalance of plant hormones (ethylene and auxin) homeostasis and perhaps improper cell wall remodeling under cytokinesis by affecting expansin gene expression.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Authors are greatly thankful to Leslie Weston from Charles Sturt University, Wagga Wagga, Australia, for fruitful discussion and comments during manuscript preparation. Primers for tomato expansin genes were kindly provided by Sylwia Fudali. Authors are also thankful to Kamil Szafranski for help in optimization of qRT-PCR and ELISA. Part of the work was financed by Polish Ministry of Science and Higher Education Grant no NN310 067739.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Abbreviations
- ACC
1-Aminocyclopropane-1-carboxylic acid
- ACO
1-Aminocyclopropane-1-carboxylic acid oxidase
- BOA
Benzoxazolin-2(3H)-one
- CA
Cyanamide
- DW
Dry weight
- ELISA
Enzyme-linked immunosorbent assay
- FW
Fresh weight
- IAA
Indole-3-acetic acid
- PCD
Programmed cell death
- PCR
Polymerase chain reaction
- RT
Reverse transcription
References
- Caderas D, Muster M, Vogler H, Mendel T, Rose JKC, McQueen-Mason S, Kuhlemeier C. Limited correlation between expansin gene expression and elongation growth rate. Plant Physiol. 2000;123:1399–1413. doi: 10.1104/pp.123.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Bradford KJ. Expression of an expansin is associated with endosperm weakening during tomato seed germination. Plant Physiol. 2000;124:1265–1274. doi: 10.1104/pp.124.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho M, Lee OR, Ganguly A, Cho HT. Auxin-signaling: short and long. J Plant Biol. 2007;50:79–89. doi: 10.1007/BF03030615. [DOI] [Google Scholar]
- Cosgrove DJ. Relaxation in high-stress environment: the molecular bases of extensible cell walls and cell enlargement. Plant Cell. 1997;9:1031–1041. doi: 10.1105/tpc.9.7.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ. Loosening of plant cell walls by expansins. Nature. 2000;407:321–326. doi: 10.1038/35030000. [DOI] [PubMed] [Google Scholar]
- Czarnota MA, Paul RN, Weston LA, Duke SO. Anatomy of sorgoleone-secreting root hairs of Sorghum species. Int J Plant Sci. 2003;164:861–866. doi: 10.1086/378661. [DOI] [Google Scholar]
- Díaz-Tielas C, Graña E, Sotelo T, Reigosa MJ, Sánchez-Moreiras AM. The natural compound trans-chalcone induces programmed cell death in Arabidopsis thaliana roots. Plant Cell Environ. 2012 doi: 10.1111/j.1365-3040.2012.02506.x. [DOI] [PubMed] [Google Scholar]
- Ding J, Sun Y, Xiao CL, Shi K, Zhou YH, Yu JQ. Physiological basis of different allelopathic reactions of cucumber and figleaf gourd plants to cinnamic acid. J Exp Bot. 2007;58:3765–3773. doi: 10.1093/jxb/erm227. [DOI] [PubMed] [Google Scholar]
- Ding L, Qi L, Jing H, Li J, Wang W, Wang T. Phytotoxic effects of leukamenin E (an ent-kaurene diterpenoid) on root growth and root hair development in Lactuca sativa L. seedlings. J Chem Ecol. 2008;34:1492–1500. doi: 10.1007/s10886-008-9556-6. [DOI] [PubMed] [Google Scholar]
- Einhelling FA. Mode of allelochemical action of phenolic compounds. In: Macías FA, Galindo JCG, Molinillo J, Cutler HG, editors. Allelopathy. Boca Raton: CRC Press; 2004. pp. 217–233. [Google Scholar]
- Etheridge N, Chen Y-F, Schaller GE. Dissecting the ethylene pathway of Arabidopsis. Brief Funct Genomic Proteomic. 2005;3:372–381. doi: 10.1093/bfgp/3.4.372. [DOI] [PubMed] [Google Scholar]
- Fudali S, Janakowski S, Sobczak M, Griesser M, Grundler FMW, Golinowski W. Two tomato alpha-expansins show distinct spatial and temporal expression patterns during development of nematode-induced syncytia. Physiol Plant. 2008;132:370–383. doi: 10.1111/j.1399-3054.2007.01017.x. [DOI] [PubMed] [Google Scholar]
- Fujii Y. Screening and future exploitation of allelopathic plants as alternative herbicides with special reference to hairy vetch. J Crop Prod. 2001;4:257–275. doi: 10.1300/J144v04n02_09. [DOI] [Google Scholar]
- Gahan PB, Rana MA. The quiescent centre and cell determination in roots of Pisum sativum. Ann Bot. 1985;56:437–442. [Google Scholar]
- Gattás-Hallak AM, Chamma L, Souza D, Souza IF. Effects of sorghum (Sorghum bicolor L.) root exudates on the cell cycle of the bean plant (Phaseolus vulgaris L.) root. Genet Mol Biol. 1999;22:95–99. doi: 10.1590/S1415-47571999000100018. [DOI] [Google Scholar]
- Gniazdowska A, Bogatek R. Alleopathic interaction between plants. Multiside action of allelochemicals. Acta Physiol Plant. 2005;27:395–407. doi: 10.1007/s11738-005-0017-3. [DOI] [Google Scholar]
- Hoffman ML, Regnier EE, Cardina J. Weed and corn (Zea mays) responses to a hairy vetch (Vicia villosa) cover crop. Weed Technol. 1993;7:594–599. [Google Scholar]
- Kamo T, Hiradate S, Fujii Y. First isolation of natural cyanamide as a possible allelochemical from hairy vetch Vicia villosa. J Chem Ecol. 2003;29:275–283. doi: 10.1023/A:1022621709486. [DOI] [PubMed] [Google Scholar]
- Kamo T, Sato M, Kato K, Hiradate S, Nakajima E, Fujii Y, Hirota M. Quantification of cyanamide contents in herbaceous plants. Biosci Biotechnol Biochem. 2006;70:2310–2312. doi: 10.1271/bbb.60171. [DOI] [PubMed] [Google Scholar]
- Kamo T, Endo M, Sato M, Kasahara R, Yamaya H, Hiradate S, Fujii Y, Hirai N, Hirota M. Limited distribution of natural cyanamide in higher plants: occurrence in Vicia villosa subsp. varia, V. cracca, and Robinia pseudo-acacia. Phytochemistry. 2008;69:1166–1172. doi: 10.1016/j.phytochem.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Keller CP, Barkosky RR, Seil JE, Mazurek SA, Grundstad ML. The electrical response of Phaseolus vulgaris roots to abrupt exposure to hydroquinone. Plant Signal Behav. 2008;3:633–640. doi: 10.4161/psb.3.9.5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupidlowska E, Dobrzyńska K, Parys E, Zobel AM. Effect of coumarin and xanthotoxin on mitochondrial structure, oxygen uptake and succinate dehydrogenase activity in onion root cells. J Chem Ecol. 1994;20:2471–2480. doi: 10.1007/BF02036184. [DOI] [PubMed] [Google Scholar]
- Kuraś M, Nowakowska J, Sliwińska E, Pilarski R, Ilasz R, Tykarska T, Zobel A, Gulewicz K. Changes in chromosome structure, mitotic activity and nuclear DNA content from cells of Allium Test induced by bark water extract of Uncaria tomentosa (Willd.) DC. J Ethnopharmacol. 2006;107:211–221. doi: 10.1016/j.jep.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Le J, Vandenbussche F, Van Der Straeten D, Verbelen J-P. In the early response of Arabidopsis roots to ethylene, cell elongation is up and down-regulated and uncoupled from differentiation. Plant Physiol. 2001;125:519–522. doi: 10.1104/pp.125.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macias FA, Marin D, Oliveros-Bastidas A, Varela RM, Simonet AM, Carrera C, Molinillo JMG. Allelopathy as a new strategy for sustainable ecosystems development. Biol Sci Space. 2003;17:18–23. doi: 10.2187/bss.17.18. [DOI] [PubMed] [Google Scholar]
- Marcussen J, Ulvskov P, Olsen CE, Rajagopal R. Preparation and properties of antibodies against indoleacetic acid (IAA)-C5-BSA, a novel ring-coupled IAA antigen, as compared to two other types of IAA-specific antibodies. Plant Physiol. 1988;89:1071–1078. doi: 10.1104/pp.89.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida N, Tamotsu S, Nagata N, Saito C, Sakai A. Allelopathic effects of volatile monoterpenoids produced by Salvia leucophylla: inhibition of cell proliferation and DNA synthesis in the root apical meristem of Brassica campestris seedlings. J Chem Ecol. 2005;31:1187–1203. doi: 10.1007/s10886-005-4256-y. [DOI] [PubMed] [Google Scholar]
- Perrot-Rechenmann C. Cellular responses to auxin: division versus expansion. Cold Spring Harb Perspect Biol. 2010;2:a001446. doi: 10.1101/cshperspect.a001446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy KN, Koger CH. Live and killed hairy vetch cover crop effects on weeds and yield in glyphosate-resistant corn. Weed Technol. 2004;18:835–840. doi: 10.1614/WT-03-228R. [DOI] [Google Scholar]
- Reinhardt D, Wittwer F, Mandel T, Kuhlemeier C. Localized upregulation of a new expansin gene predicts the site of leaf formation in the tomato meristem. Plant Cell. 1998;10:1427–1437. doi: 10.1105/tpc.10.9.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybka K, Rybka Z, Wiśniewska A. Nucleic acids. In: Narwal SS, Bogatek R, Zagdańska BM, Sampietro DA, Vattuone MA, editors. Plant biochemistry. Houston: Studium; 2009. pp. 161–216. [Google Scholar]
- Sánchez-Moreiras AM, Coba de la Peña T, Reigosa MJ. The natural compound benzoxazolin-2(3H)-one selectively retards cell cycle in lettuce root meristems. Phytochemistry. 2008;69:2172–2179. doi: 10.1016/j.phytochem.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Smalle J, Van Der Straeten D. Ethylene and vegetative development. Physiol Plant. 1997;100:593–605. doi: 10.1111/j.1399-3054.1997.tb03065.x. [DOI] [Google Scholar]
- Soltys D, Rudzińska-Langwald A, Kurek W, Gniazdowska A, Sliwinska E, Bogatek R. Cyanamide mode of action during inhibition of onion (Allium cepa L.) root growth involves disturbances in cell division and cytoskeleton formation. Planta. 2011;234:609–621. doi: 10.1007/s00425-011-1429-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sołtys D, Wiśniewska A, Gniazdowska A, Bogatek R. Cyanamide allelopathic action on root growth of tomato (Lycopersicon esculentum) and maize (Zea mays) Acta Soc Bot Pol. 2010;79(Suppl 1):92. [Google Scholar]
- Stepanova AN, Yun J, Likhacheva AV, Alonso JM. Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell. 2007;19:2169–2185. doi: 10.1105/tpc.107.052068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup R, Perry P, Hagenbeek D, Van Der Straeten D, Beemster GTS, Stanberg G, Bhalerano R, Ljung K, Betten MJ. Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. Plant Cell. 2007;19:2186–2196. doi: 10.1105/tpc.107.052100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teerarak M, Charoenying P, Laosinwattana C. Physiological and cellular mechanisms of natural herbicide resource from Aglaia odorata Lour. on bioassay plants. Acta Physiol Plant. 2012 [Google Scholar]
- Vogler H, Caderas D, Mandel T, Kuhlemeier C. Domains of expansin gene expression define growth regions in the shoot apex of tomato. Plant Mol Biol. 2003;53:267–272. doi: 10.1023/B:PLAN.0000006999.48516.be. [DOI] [PubMed] [Google Scholar]
- Weiler EW, Jourdan PS, Conrad W. Levels of indole-3-acetic acid in intact and decapitated coleoptiles as determined by a specific and highly sensitive solid-phase enzyme immunoassay. Planta. 1981;153:561–571. doi: 10.1007/BF00385542. [DOI] [PubMed] [Google Scholar]
- Yao N, Tada Y, Sakamoto M, Nakayashiki H, Park P, Tosa Y, Mayama S. Mitochondrial oxidative burst involved in apoptotic response in oats. Plant J. 2002;30:567–579. doi: 10.1046/j.1365-313X.2002.01314.x. [DOI] [PubMed] [Google Scholar]
- Yoshimura H, Sawai Y, Tamotsu S, Sakai A. 1,8-Cineole inhibits both proliferation and elongation of BY-2 cultured tobacco cells. J Chem Ecol. 2011;37:320–328. doi: 10.1007/s10886-011-9919-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.