Abstract
Germfree rats were monoassociated with either a toxin-producing strain of Clostridium difficile (Tox+) or a variant of this strain (ToxR) which produced much less toxin (1/10,000) in vivo and in vitro. Monoassociation of germfree rats with C. difficile Tox+ resulted in mortality (17%) and in pathology to the small and large intestines, livers, and lungs. Cecal filtrates from the Tox+-monoassociated rats were cytotoxic for tissue culture cells. The cytotoxicity of cecal filtrates could be blocked by sera from Tox+-monoassociated rats. Monoassociation of rats with C. difficile ToxR resulted in no deaths or pathology, and much less toxin was detected in the cecal filtrates of these animals than in those of rats colonized with the Tox+ strain. This gnotobiotic model may be useful for investigating the etiology, prophylaxis, therapy, and exacerbation of C. difficile-induced pseudomembranous colitis.
Full text
PDF



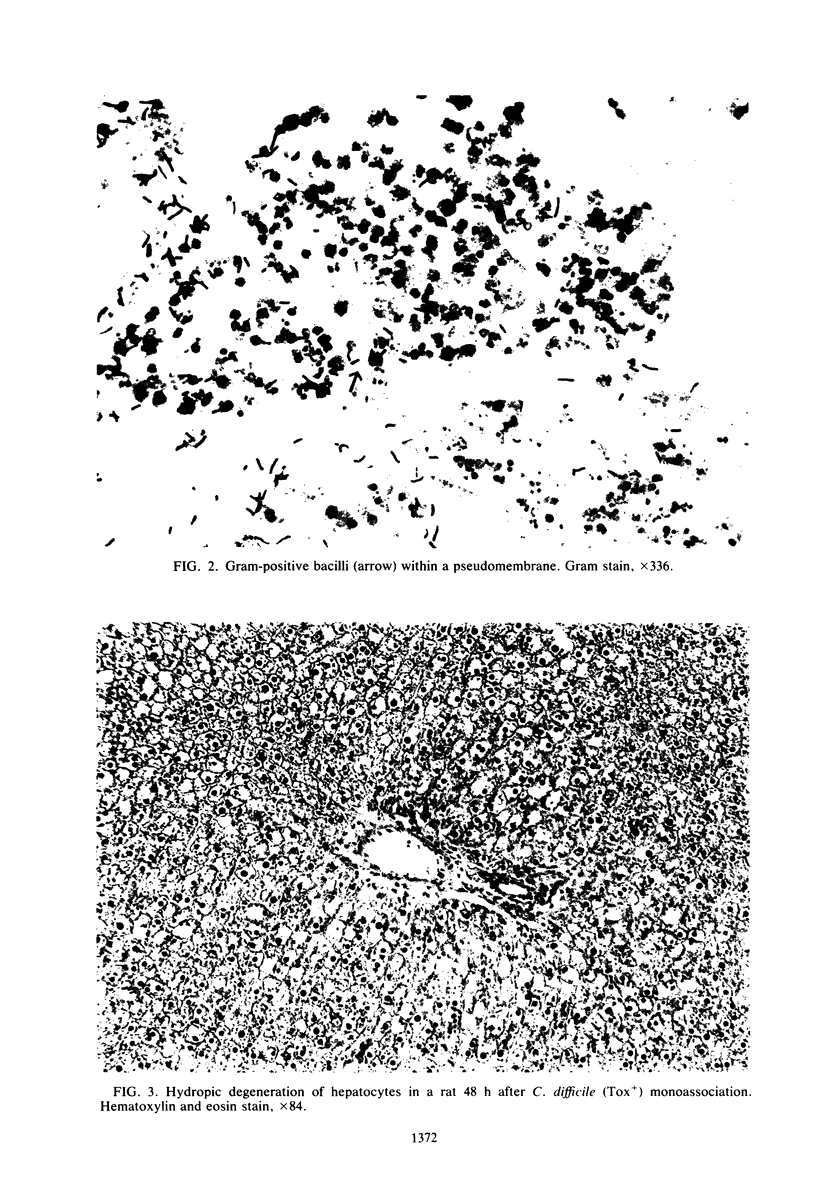

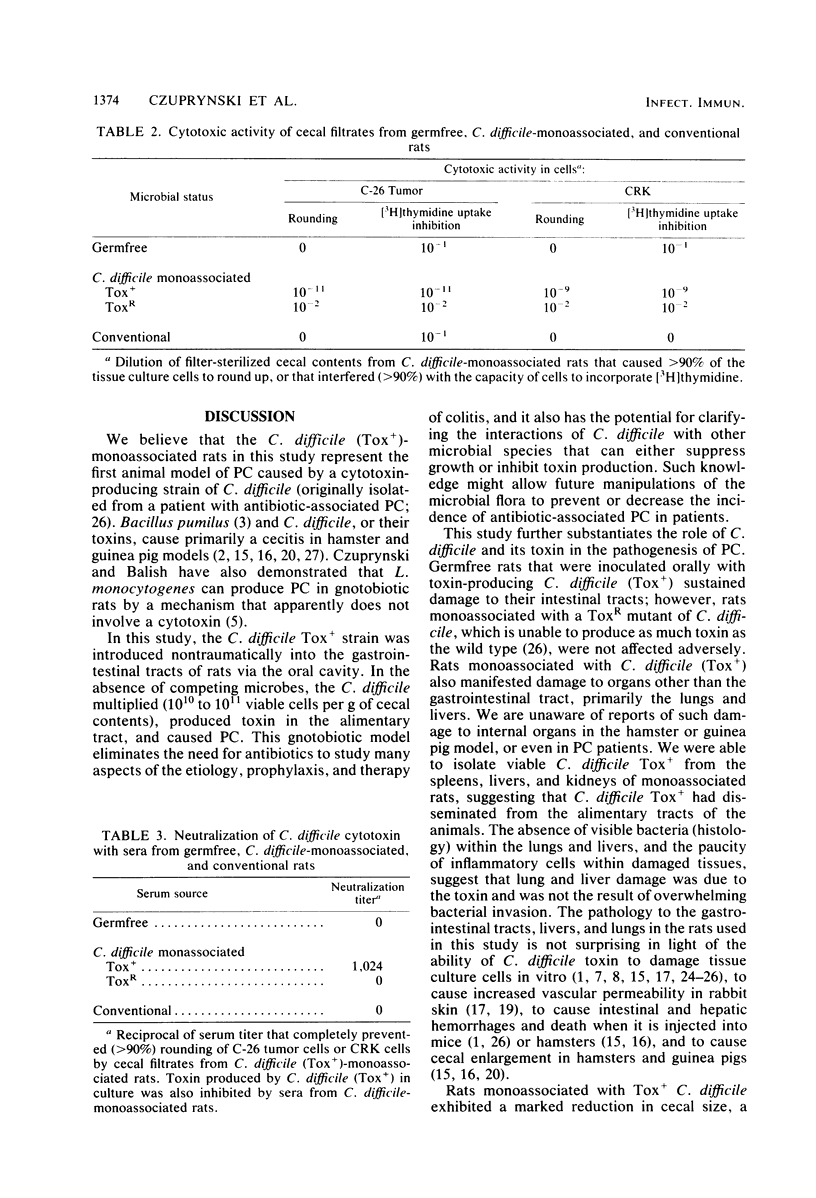


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartlett J. G., Chang T. W., Gurwith M., Gorbach S. L., Onderdonk A. B. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978 Mar 9;298(10):531–534. doi: 10.1056/NEJM197803092981003. [DOI] [PubMed] [Google Scholar]
- Brophy P. F., Knoop F. C. Bacillus pumilus in the induction of clindamycin-associated enterocolitis in guinea pigs. Infect Immun. 1982 Jan;35(1):289–295. doi: 10.1128/iai.35.1.289-295.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czuprynski C. J., Balish E. Pathogenesis of Listeria monocytogenes for gnotobiotic rats. Infect Immun. 1981 Apr;32(1):323–331. doi: 10.1128/iai.32.1.323-331.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabard J., Dubos F., Martinet L., Ducluzeau R. Experimental reproduction of neonatal diarrhea in young gnotobiotic hares simultaneously associated with Clostridium difficile and other Clostridium strains. Infect Immun. 1979 Apr;24(1):7–11. doi: 10.1128/iai.24.1.7-11.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donta S. T., Shaffer S. J. Effects of Clostridium difficile toxin on tissue-cultured cells. J Infect Dis. 1980 Feb;141(2):218–222. doi: 10.1093/infdis/141.2.218. [DOI] [PubMed] [Google Scholar]
- Florin I., Thelestam M. Intoxication of cultured human lung fibroblasts with Clostridium difficile toxin. Infect Immun. 1981 Jul;33(1):67–74. doi: 10.1128/iai.33.1.67-74.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbach S. L., Bartlett J. G. Pseudomembranous enterocolitis: a review of its diverse forms. J Infect Dis. 1977 Mar;135 (Suppl):S89–S94. doi: 10.1093/infdis/135.supplement.s89. [DOI] [PubMed] [Google Scholar]
- Gustafsson B. E., Midtvedt T., Strandberg K. Effects of microbial contamination on the cecum enlargement of germfree rats. Scand J Gastroenterol. 1970;5(4):309–314. [PubMed] [Google Scholar]
- Hammarström S., Perlmann P., Gustafsson B. E., Lagercrantz R. Autoantibodies to colon in germfree rats monocontaminated with Clostridium difficile. J Exp Med. 1969 Apr 1;129(4):747–756. doi: 10.1084/jem.129.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keusch G. T., Present D. H. Summary of a workshop on clindamycin colitis. J Infect Dis. 1976 May;133(5):578–587. doi: 10.1093/infdis/133.5.578. [DOI] [PubMed] [Google Scholar]
- Li L. H., Kuentzel S. L., Shugars K. D., Bhuyan B. K. Cytotoxicity of several marketed antibiotics on mammalian cells in culture. J Antibiot (Tokyo) 1977 Jun;30(6):506–512. doi: 10.7164/antibiotics.30.506. [DOI] [PubMed] [Google Scholar]
- Libby J. M., Jortner B. S., Wilkins T. D. Effects of the two toxins of Clostridium difficile in antibiotic-associated cecitis in hamsters. Infect Immun. 1982 May;36(2):822–829. doi: 10.1128/iai.36.2.822-829.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusk R. H., Fekety R., Silva J., Browne R. A., Ringler D. H., Abrams G. D. Clindamycin-induced enterocolitis in hamsters. J Infect Dis. 1978 Apr;137(4):464–475. doi: 10.1093/infdis/137.4.464. [DOI] [PubMed] [Google Scholar]
- Lyerly D. M., Lockwood D. E., Richardson S. H., Wilkins T. D. Biological activities of toxins A and B of Clostridium difficile. Infect Immun. 1982 Mar;35(3):1147–1150. doi: 10.1128/iai.35.3.1147-1150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin G. D., Fekety F. R., Silva J., Jr Antibiotic-induced colitis implication of a toxin neutralised by Clostridium sordellii antitoxin. Lancet. 1977 Nov 26;2(8048):1103–1106. doi: 10.1016/s0140-6736(77)90547-5. [DOI] [PubMed] [Google Scholar]
- Rothman S. W. Presence of Clostridium difficile toxin in guinea pigs with penicillin-associated colitis. Med Microbiol Immunol. 1981;169(3):187–196. doi: 10.1007/BF02123592. [DOI] [PubMed] [Google Scholar]
- SKELLY B. J., TREXLER P. C., TANAMI J. Effect of a Clostridium species upon cecal size of gnotobiotic mice. Proc Soc Exp Biol Med. 1962 Jul;100:455–458. doi: 10.3181/00379727-110-27548. [DOI] [PubMed] [Google Scholar]
- Silva J., Jr, Fekety R. Clostridia and antimicrobial enterocolitis. Annu Rev Med. 1981;32:327–333. doi: 10.1146/annurev.me.32.020181.001551. [DOI] [PubMed] [Google Scholar]
- Steer H. W. The pseudomembranous colitis associated with clindamycin therapy--a viral colitis. Gut. 1975 Sep;16(9):695–706. doi: 10.1136/gut.16.9.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan N. M., Pellett S., Wilkins T. D. Purification and characterization of toxins A and B of Clostridium difficile. Infect Immun. 1982 Mar;35(3):1032–1040. doi: 10.1128/iai.35.3.1032-1040.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesely D. L., Straub K. D., Nolan C. M., Rolfe R. D., Finegold S. M., Monson T. P. Purified Clostridium difficile cytotoxin stimulates guanylate cyclase activity and inhibits adenylate cyclase activity. Infect Immun. 1981 Jul;33(1):285–291. doi: 10.1128/iai.33.1.285-291.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson K. H., Silva J., Fekety F. R. Suppression of Clostridium difficile by normal hamster cecal flora and prevention of antibiotic-associated cecitis. Infect Immun. 1981 Nov;34(2):626–628. doi: 10.1128/iai.34.2.626-628.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]







