Abstract
Objectives
To compare two scanning protocols (free breathing versus breath-hold) for perfusion imaging using dynamic volume computed tomography (CT) and to evaluate their effects on image registration.
Material and methods
Forty patients underwent dynamic volume CT for pancreatic perfusion analysis and were randomly assigned to either a shallow-breathing (I) or breath-hold (II) group. Both dynamic CT protocols consisted of 17 low-dose volumetric scans. Rigid image registration was performed by using the volume with highest aortic attenuation as reference. All other volumes were visually matched with the pancreatic lesion serving as the volumetric region of interest. The overall demand for post-processing per patient was calculated as the median of three-dimensional vector lengths of all volumes in relation to the relative patient origin. The number of volumes not requiring registration was recorded per group.
Results
Registration mismatch for groups I and II was 2.61 mm (SD, 1.57) and 4.95 mm (SD, 2.71), respectively (P < 0.005). Twenty-eight volumes in group I (8.2%) and 47 volumes in group II (14.1%) did not require manual registration (P = 0.014).
Conclusion
Shallow breathing during dynamic volume CT scanning reduces the overall demand for motion correction and thus may be beneficial in perfusion imaging of the pancreas
Main Messages
• Shallow breathing during perfusion CT scanning reduces the overall demand for motion correction.
• Shallow breathing may be beneficial in perfusion imaging of the pancreas.
• Image registration is crucial for CT perfusion imaging.
Keywords: CT perfusion, Image registration, Artefacts
Introduction
Abdominal perfusion imaging using computed tomography (CT) has gained considerable interest in oncology, especially as a tool for tissue characterisation, staging and monitoring treatment response to anti-angiogenesis drugs [1]. The linear relationship between tissue attenuation and iodine concentration has simplified various analysis models and facilitated the understanding of underlying physiological principles. With perfusion imaging representing a well-established evaluation technique in stroke patients [2], CT perfusion has been applied to many areas of the body including the lung, abdominal and pelvic organs, predominantly with oncological indications [3–5]. Lesion characterisation, identification of occult malignancies, provision of prognostic information based on tumour vascularity and monitoring the therapeutic effects predominantly of anti-angiogenic drugs have been the key targets for abdominal perfusion CT [6–9].
The fundamental principle of perfusion CT is based on the temporal changes in tissue attenuation provided the target volume remains stationary in space. Motion during data acquisition in CT inevitably leads to artefacts which jeopardise accurate calculation of perfusion values and parameter maps, particularly in abdominal CT where organ shift, deformation and displacement peak during the respiratory cycle. Most imaging protocols in perfusion CT encompass several acquisitions usually spread over more than 1 min [10]. All perfusion analysis methods have in common that they require “free-of-motion-artefact” data to maintain spatial fidelity. Anatomical mismatch between the multiple acquisitions inescapably results in inaccurate calculation of perfusion values [11]. In fact, patients were excluded from analysis in several studies to avoid incorrect perfusion results [12, 13].
Motion correction therefore plays a key role in abdominal CT perfusion analysis [14] and has been identified as an important factor to ensure reliable perfusion measurements [15, 16]. Some manufacturers have recognised the need for image registration and their perfusion packages include either manual rigid, automatic rigid, or automatic elastic motion correction based on complex algorithms.
The aim of our study was to analyse and compare the effect of multiple breath-hold periods versus continued shallow breathing on motion correction using manual rigid image registration technique.
Materials and methods
Institutional ethics approval for the study was obtained. All patients signed informed consent prior to participation in this trial.
Patients
Forty patients clinically referred for CT perfusion of the pancreas were prospectively enrolled in the study. Reasons for referral were known pancreatic adenocarcinoma (n = 23) or focal pancreatic neoplasm not further specified (n = 17) ranging between 1 and 4 cm in its largest diameter. Inclusion criteria were known focal abnormality of the pancreas, absence of the usual exclusion criteria for CT examinations using intravenous contrast material, patient’s age above 18 years, ability to sign the consent form and the willingness to participate in the study. Patients with chronic lung disease or patients who could not follow breathing instructions (e.g. language barrier, hearing problems) were excluded from participation. Patients’ age, weight and height were taken from the medical charts and documented.
Two imaging protocol groups were defined: a shallow-breathing group (I) and a breath-hold group (II). Patients were randomly assigned to either group. Patients in group I were instructed to breathe as shallowly as possible, patients in group II were given several inspiratory breath-hold commands during the entire CT examination. All patients were prepared 5 min prior to and during the CT examination with oxygen hyperventilation through a mask at a flow rate of 2 l/min in order to facilitate both shallow breathing and multiple breath-holds, respectively. Due to the requirement of a high flow rate of contrast material injection, a 16-gauge peripheral venous catheter was placed in an antecubital vein. Adequacy of the intravenous access route was tested with a 20-ml saline bolus at an injection rate of 8 ml/s.
Imaging
Imaging was performed on a 320-slice dynamic volume CT scanner (Aquilion One, Toshiba Medical Systems, Ottawara, Japan) using 16-cm detector coverage with 0.5-mm primary slice thickness and 0.25-mm reconstruction interval, resulting in 640 axial images per volume. All patients were imaged in supine and feet first position. The scout-view in anterior-posterior and lateral direction was used to define the scan range and reconstruction field of view. A single 16-cm volume was acquired prior to contrast material administration for confirmation of full coverage of the entire pancreas including the focal abnormality. The following image parameters were used for patients in both groups: 100 kV tube voltage, 90 mA tube current and 0.5 s gantry revolving time. A total of 60 ml non-ionic contrast material (370 mg iodine/ml, Ultravist; Bayer-Schering, Berlin, Germany) was injected at a flow rate of 8 ml/s using a dual-head power injector (Nemoto Kyorindo, Tokyo, Japan), followed by a 30-ml saline chaser. A bolus-tracking technique with real-time image reconstruction was applied; as soon as contrast material became visible within the pulmonary artery, the actual scanning protocol was initiated, starting with an inspiratory breath-hold command in group II and silence of the same length in group I.
The scanning protocol consisted of 17 volumetric acquisitions with identical intervals in both groups obtained over a period of 66 s using the tube settings stated above. The sequence in group II was as follows: inspiratory breathing command, nine volumetric scans every 2 s, 3.5 s interval, five volumetric scans every 4 s, expiratory/inspiratory command (9.5 s), two volumetric scans every 10 s, expiratory/inspiratory command (9.5 s), single volumetric scan, expiratory command. The sequence in group I was identical to group II but breath-hold commands were replaced by silence of identical length.
A diagnostic helical scan of abdomen and pelvis was performed after injection of an additional 90 ml intravenous contrast material at a fixed delay of 60 s using 120 kV and tube current modulation (target standard deviation for image noise: 15 HU for 5-mm slices, soft tissue reconstruction kernel).
Post-processing
Reconstructed perfusion CT images were transferred to a commercially available stand-alone workstation (Toshiba Medical Systems) loaded with perfusion CT software. Data were processed by a radiologist with 4 years of experience in CT perfusion and blinded to the patient’s group assignment. After an examination comprising all 17 volumes was loaded, the volume with the highest vascular contrast defined as the examination’s maximum attenuation value in the descending aorta, was determined as the volume of reference for manual rigid volume registration. Knowing that tumour conspicuity would not be the highest on this volume, it served as the volume that is best defined within the series. All remaining volumes were visually matched in all three dimensions, with the target lesion in the pancreas serving as the volume-of-interest. Rotational mismatches were not corrected for because quantification may become difficult in three dimensions and the necessity for better matching was believed to be minimal. Best match was accepted when the differential volume from the volume of reference and the registered volume (i.e. after subtraction) displayed the least difference in anatomical contours.
On a per patient basis, the linear translation (i.e. the shift in space) of each volume to be matched to the volume of reference was called displacement vector. The median of the displacement vectors of all volumes was defined as relative patient origin. The difference between the displacement vector (for each volume) and relative patient origin was defined as registration mismatch vector. The vector length is given in millimetres. The median of registration mismatch vectors was defined as the patient registration shift and given in millimetres. The number of volumes with registration mismatch vector equal to zero (no registration required) was recorded per group.
Following volume registration, perfusion analysis was performed using maximum-slope [17] and Patlak [18] analysis techniques. Values for tumour perfusion and Patlak blood volume before and after volume registration were statistically compared in both groups using the paired t-test. Both the diagnostic helical scans and colour-coded perfusion maps were read during the clinical interpretation process. Radiation dose of both the dynamic and helical scan was calculated from the dose-length products (DLP.e) listed on the patient summary sheet multiplied by a factor of 0.015 [19].
Statistical analysis was performed using WinSTAT plug-in for Excel 2007 (Microsoft office 2007). As equivalence is assumed, median age and body mass index (BMI) were compared between the breathing and breath-hold group using the parameter-free U test with alpha error set to 0.3. The Wilcoxon signed rank sum test with continuity correction was applied to compare the median 3D vector lengths of both groups. Fisher’s exact test—ignoring the potential cluster error in the data—was applied to compare the frequency of volumes in both groups which did not require manual registration. Corrected P values less than 0.05 were assumed to indicate significant differences.
Results
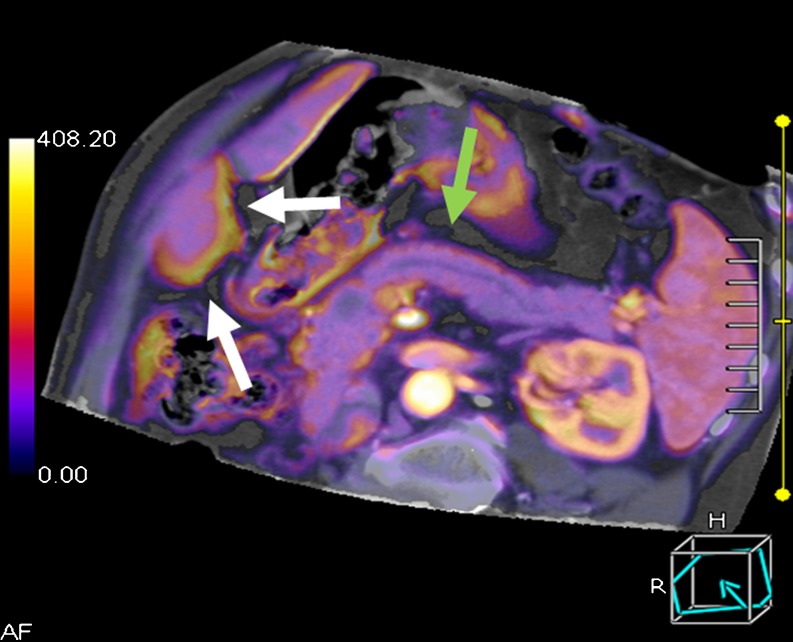
All 40 patients enrolled in this study were successfully imaged, post-processed, analysed and used for statistical evaluation. Despite the relatively high injection rate, none of the patients experienced extravasation of contrast material. Twelve men and eight women were assigned to group I (age ranging from 41 to 72 years, median age/BMI; 55.4 years/22.5 kg/m2) and 13 men and seven women to group II (age ranging between 45 and 76 years, median age/BMI; 54.6 years/23.7 kg/m2) without statistically significant differences between both groups (P = 0.56). Figure 1 exemplifies a colour-coded map of the pancreas generated from maximum slope analysis. The radiation exposure amounted to 9.0 mSv for the entire perfusion CT examination (DLP.e, 600.7 mGy·cm).
Fig. 1.
Colour-coded map of the pancreas with a small pancreatic carcinoma and ductal dilatation calculated based on maximum slope analysis (multiplanar reformation). After image registration, the borders of the pancreas are well defined (green arrows), whereas the lower edge of the liver exhibits registration artefacts (white arrows)
In group I (shallow breathing) mean tumour perfusion values and Patlak blood volume were 0.33 ml·100 ml−1·min−1 and 22.2 ml/100 mg without image registration and 0.31 ml·100 ml−1·min−1 and 21.9 ml/100 mg with image registration, differences were statistically not significant. In group II (breath-hold), tumour perfusion and blood volume were 0.37 ml·100 ml−1·min−1 and 36.7 ml/100 mg without image registration and 0.33 ml·100 ml−1·min−1 and 18.8 ml/100 mg with image registration, the difference was not statistically significant for maximum slope perfusion but significant for Patlak blood volume (P < 0.001).
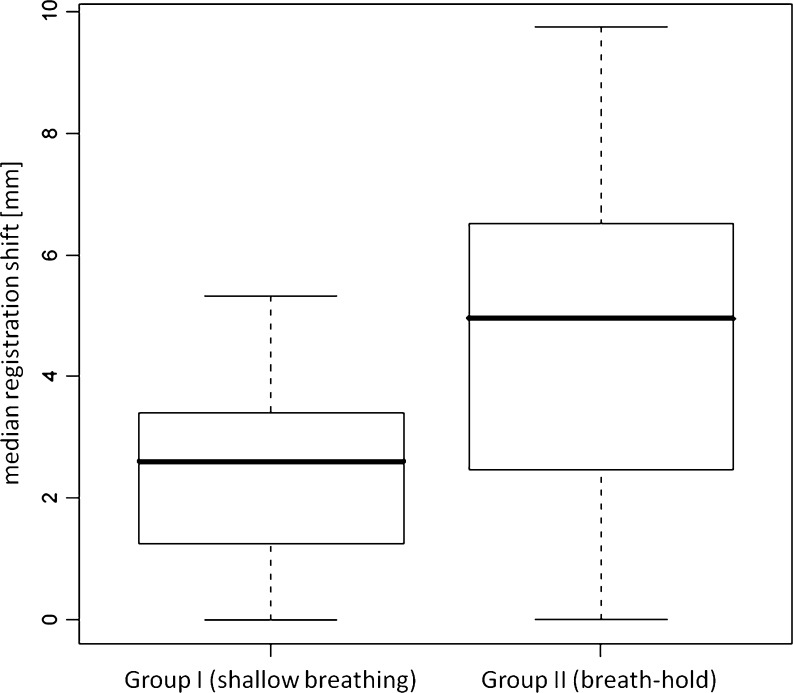
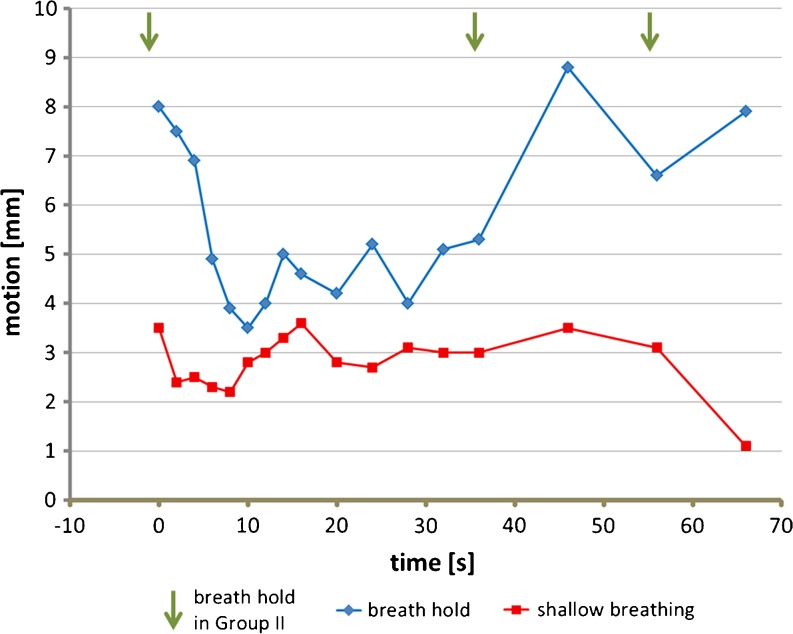
The median registration shift for group I (shallow breathing) and group II was 2.61 mm (SD, 1.57) and 4.95 mm (SD, 2.71), respectively, as demonstrated in Fig. 2. The difference was statistically significant (P < 0.005). Figure 3 illustrates the median registration mismatch vector length for both groups, sequentially plotted over time. Of note, most of motion correction was required in group II in the beginning and after 40 s with the acquisition time point for the first volume set to zero. Conversely, a relatively constant and small amount of motion correction deemed necessary for most of the volumes in group I. Moreover, 8.2% (n = 28) of the volumes in group I and 14.1% (n = 47) in group II did not require any manual registration, the difference was statistically significant (P = 0.014). There was no patient in either group for whom volume registration was not required.
Fig. 2.
Boxplot of the median registration shift in both groups
Fig. 3.
Graphical display of motion correction over time. Time = 0 is set for the first volume
Discussion
We found that shallow breathing during abdominal CT perfusion imaging requires more volumes to be motion corrected in comparison with multiple breath-hold intervals, but the overall amount of motion correction is significantly reduced and, more specifically, large jumps in volume position might be avoided when patients breathe shallowly during data acquisition. Moreover, we found a significant difference in tumour blood volume values before and after volume registration, but only in the group with multiple breath-hold intervals. The larger the positional mismatch between the reference target volume and the source volume to be motion corrected, the higher the risk that the matching process becomes inaccurate and the source volume needs to be excluded from further image analysis, potentially jeopardising the entire perfusion analysis [20].
Evidently, if the perfusion target moves in and out of the axial plane in case of limited anatomical coverage, image registration will be unable to correct for the lack of acquired image information. Volumetric CT scanning mitigates that risk, be it by virtue of shuffle mode scanning with limited detector coverage or by using wide area detector CT as in this study, and enables the comfort of larger anatomical coverage with the potential of extended motion correction in all three dimensions [21].
Currently, there is no general consensus amongst radiologists as to the optimal data acquisition technique for abdominal perfusion examinations. Both methods—constant shallow breathing and multiple breath-hold sequences—have been utilised in previously published trials [22–25]. Shallow breathing, however, is notably preferred over breath-hold sequences in magnetic resonance imaging (MRI) perfusion examinations [26], albeit in conjunction with navigator techniques. Quiet breathing has also been suggested to be preferable for longer CT perfusion protocols in order to minimise breathing artefacts in patients who have difficulties holding their breath, especially in liver perfusion studies where tissue texture renders image registration quite challenging. Adequate coaching of patients prior to CT perfusion imaging is essential and patients should be instructed to withstand the temptation to take a deep breath when experiencing a hot flush from the fast contrast material injection [11].
We randomly assigned participants in our study to either of the two groups and did not find demographic differences between both groups. The patient registration shift was used as a surrogate parameter to assess the amount of manual post-processing required. Although obviously time-consuming, we chose to manually pick the volumes and performed visual three-dimensional translation until both volumes—the reference volume to which all others were matched and the target volume—exhibited the least amount of difference as assessed on a subtraction image. We chose manual rigid registration as opposed to automatic rigid registration in order to be able to measure and quantify the actual volume shift in space. As elastic warping was not available to us on the workstation used (and would be difficult to perform manually), we focused the matching process only to the area of interest, in our case the pancreatic neoplasm.
Image registration in clinical imaging represents a process of matching two or more images of the same scene taken at different times, from different viewpoints or by different modalities. Radiologists in their clinical practice perform imaginary image registration to differentiate abnormalities from norms and this is one of the most essential professional skills. Algorithms, methods and techniques have been developed to automate this spatial transformation computationally. A lot of research efforts have been made in recent years and image registration is slowly becoming routine in radiology and research laboratories. Nevertheless, computer supported image registration is a complex procedure and still has a lot of limitations [27] as most algorithms are solely based of density values, thus leading to better registration of dense structures such as bones and limited registration of soft tissues. Some authors have used an abdominal strap to restrict anterior wall motion [28, 29] but convincing evidence that such restriction reduces artefacts in post-processing is missing. Respiratory gating has been discussed as a potential other option in the future to decrease spatial inconsistency in perfusion data; however, integration into current perfusion protocols might become a challenging task.
All perfusion analysis methods have in common that they require “free-of-motion-artefact” data to maintain spatial fidelity. Be it from breathing or patient movement during data acquisition: when tissue contours mismatch from volume to volume and temporal analysis is performed without motion correction, perfusion values might be significantly influenced by non-perfusion-related density changes and no longer reflect vascular physiology alone [30]. In fact, patients were excluded from analysis in several studies to avoid incorrect perfusion results [12, 13]. With exception for assessment of cerebral perfusion, motion correction has been identified as an important factor to ensure reliable perfusion measurements [15, 16]. Some manufacturers have recognised the need for image registration and their perfusion packages include either manual rigid, automatic rigid or automatic elastic motion correction based on complex algorithms.
There are several potential factors that might have influenced our results.
First, the initial breath-hold command in group II following the bolus tracking sequence might have been too fast-paced and not allowed the patient enough time to take a deep breath and to suspend breathing. The fact that we observed a higher degree of motion correction for the first few volumes supports this assumption. On the other hand, a longer initial breath-hold command would have carried the risk of missing the initial contrast material inflow simply by initiating the scanning process too late. Second, despite patient preparation with oxygen and verbal coaching during the scanning process, we noted that some patients started to move at the end of the first breath-hold period, at times resulting in sharp peaks of breathing excursions. A similar observation has been reported in the literature and found problematic for image registration [20].
The easiest and most obvious solution would be to split long breath-hold periods. However, not only would splitting inevitably increase the overall number of breath-hold periods and breathing instructions, it would also interrupt the time sequence during contrast media inflow and potentially jeopardise sufficient data acquisition at peak arterial attenuation. Nevertheless, if capturing peak arterial attenuation is not deemed crucial for perfusion analysis, i.e. when applying Patlak analysis only, shortening the duration of breath-holds might be a valid option to reduced volume mismatch.
We also have to consider that shallow breathing not only leads to some differences in spatial position between subsequent volumes but it also leads to some degree of motion artefacts within axial slices as every volume is acquired over 500 ms exposure time [31]. We did not assess the degree of motion artefacts within a volume but one could assume minor degradation of image quality in particular regarding spatial resolution and spatial accuracy. Whether or not such image degradation impacts on perfusion results remains to be determined, however.
Age and body mass index could influence the individual’s ability to hold the breath. We found, however, no difference in mean age or body mass index between both groups. The low number of subjects in our study, 20 in each group, must be regarded as a limitation of our study. In order to generalise our conclusion, further validation on larger patient cohorts seems necessary. Another limitation might be the fact that during manual rigid image registration, every volume was visually matched by the same radiologist, who focused on the target lesion. Mistakes could have occurred and would remain undetected. Furthermore, we did not assess intra-individual reproducibility, which would be of interest in future studies.
Lastly, the radiation dose in CT imaging restricts the numbers of perfusion phases potentially desirable for perfusion analysis. Radiation doses ranging from 10 to 27 mSv have been reported for perfusion CT [15, 32]. The number of required volumes, on the other hand, depends on the selected perfusion analysis model. We included both single and dual compartment models (maximum slope and Patlak) and subsequently needed to acquire image data with high initial sampling rates (in order to capture peak arterial attenuation) as well as with slower sampling rates over a longer time span (for Patlak analysis). We limited our perfusion protocol to 17 separate volumes and utilised low-dose scanning parameters for each volume, accumulating 9 mSv on average for the perfusion part of the examination. This dose value compares well to that of a diagnostic helical scan when standard parameters with low image noise would be employed.
In summary, our study results underscore that shallow breathing during CT perfusion imaging of the abdomen reduces the necessity for extensive motion correction during post-processing which may minimise artefacts on parametric images and reduce errors in perfusion value calculation.
References
- 1.Meijerink MR, Cruijsen H, Hoekman K, et al. The use of perfusion CT for the evaluation of therapy combining AZD2171 with gefitinib in cancer patients. Eur Radiol. 2007;17(7):1700–1713. doi: 10.1007/s00330-006-0425-9. [DOI] [PubMed] [Google Scholar]
- 2.Allmendinger AM, Tang ER, Lui YW, Spektor V. Imaging of stroke: Part 1, Perfusion CT–overview of imaging technique, interpretation pearls, and common pitfalls. AJR Am J Roentgenol. 2012;198(1):52–62. doi: 10.2214/AJR.10.7255. [DOI] [PubMed] [Google Scholar]
- 3.Petralia G, Bonello L, Viotti S, Preda L, d'Andrea G, Bellomi M. CT perfusion in oncology: how to do it. Canc Imag. 2010;10:8–19. doi: 10.1102/1470-7330.2010.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohno Y, Koyama H, Matsumoto K, et al. Differentiation of malignant and benign pulmonary nodules with quantitative first-pass 320-detector row perfusion CT versus FDG PET/CT. Radiology. 2011;258(2):599–609. doi: 10.1148/radiol.10100245. [DOI] [PubMed] [Google Scholar]
- 5.Figueiras RG, Goh V, Padhani AR, Naveira AB, Caamano AG, Martin CV. The role of functional imaging in colorectal cancer. AJR Am J Roentgenol. 2010;195(1):54–66. doi: 10.2214/AJR.10.4422. [DOI] [PubMed] [Google Scholar]
- 6.Goh V, Ganeshan B, Nathan P, Juttla JK, Vinayan A, Miles KA. Assessment of response to tyrosine kinase inhibitors in metastatic renal cell cancer: CT texture as a predictive biomarker. Radiology. 2011;261(1):165–171. doi: 10.1148/radiol.11110264. [DOI] [PubMed] [Google Scholar]
- 7.Jiang T, Kambadakone A, Kulkarni NM, Zhu AX, Sahani DV. Monitoring response to antiangiogenic treatment and predicting outcomes in advanced hepatocellular carcinoma using image biomarkers, CT perfusion, tumor density, and tumor size (RECIST) Invest Radiol. 2012;47(1):11–17. doi: 10.1097/RLI.0b013e3182199bb5. [DOI] [PubMed] [Google Scholar]
- 8.Bellomi M, Petralia G, Sonzogni A, Zampino MG, Rocca A. CT perfusion for the monitoring of neoadjuvant chemotherapy and radiation therapy in rectal carcinoma: initial experience. Radiology. 2007;244(2):486–493. doi: 10.1148/radiol.2442061189. [DOI] [PubMed] [Google Scholar]
- 9.Miles KA. Functional CT imaging in oncology. Eur Radiol. 2003;13(Suppl 5):M134–M138. doi: 10.1007/s00330-003-2108-0. [DOI] [PubMed] [Google Scholar]
- 10.Kambadakone AR, Sahani DV. Body perfusion CT: technique, clinical applications, and advances. Radiol Clin North Am. 2009;47(1):161–178. doi: 10.1016/j.rcl.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Miles KA. Perfusion CT for the assessment of tumour vascularity: which protocol? Br J Radiol. 2003;76(Spec No 1):S36–S42. doi: 10.1259/bjr/18486642. [DOI] [PubMed] [Google Scholar]
- 12.Ng CS, Wang X, Faria SC, Lin E, Charnsangavej C, Tannir NM. Perfusion CT in patients with metastatic renal cell carcinoma treated with interferon. AJR Am J Roentgenol. 2010;194(1):166–171. doi: 10.2214/AJR.09.3105. [DOI] [PubMed] [Google Scholar]
- 13.Bize PE, Platon A, Becker CD, Poletti PA. Perfusion measurement in acute pancreatitis using dynamic perfusion MDCT. AJR Am J Roentgenol. 2006;186(1):114–118. doi: 10.2214/AJR.04.1416. [DOI] [PubMed] [Google Scholar]
- 14.Chen CT. Radiologic image registration: old skills and new tools. Acad Radiol. 2003;10(3):239–241. doi: 10.1016/S1076-6332(03)80096-X. [DOI] [PubMed] [Google Scholar]
- 15.Meijerink MR, Waesberghe JH, Weide L, Tol P, Meijer S, Kuijk C. Total-liver-volume perfusion CT using 3-D image fusion to improve detection and characterization of liver metastases. Eur Radiol. 2008;18(10):2345–2354. doi: 10.1007/s00330-008-0996-8. [DOI] [PubMed] [Google Scholar]
- 16.Maintz JB, Viergever MA. A survey of medical image registration. Med Image Anal. 1998;2(1):1–36. doi: 10.1016/S1361-8415(01)80026-8. [DOI] [PubMed] [Google Scholar]
- 17.Dawson P. Functional imaging in CT. Eur J Radiol. 2006;60(3):331–340. doi: 10.1016/j.ejrad.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 18.Patlak CS, Blasberg RG. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J Cereb Blood Flow Metab. 1985;5(4):584–590. doi: 10.1038/jcbfm.1985.87. [DOI] [PubMed] [Google Scholar]
- 19.Valentin J (2007) Managing patient dose in multi-detector computed tomography(MDCT). ICRP Publication 102. Ann ICRP, 37(1):1–79, iii [DOI] [PubMed]
- 20.Zollner FG, Sance R, Rogelj P, et al. Assessment of 3D DCE-MRI of the kidneys using non-rigid image registration and segmentation of voxel time courses. Comput Med Imaging Graph. 2009;33(3):171–181. doi: 10.1016/j.compmedimag.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Kandel S, Kloeters C, Meyer H, Hein P, Hilbig A, Rogalla P. Whole-organ perfusion of the pancreas using dynamic volume CT in patients with primary pancreas carcinoma: acquisition technique, post-processing and initial results. Eur Radiol. 2009;19(11):2641–2646. doi: 10.1007/s00330-009-1453-z. [DOI] [PubMed] [Google Scholar]
- 22.Lu N, Feng XY, Hao SJ, et al. 64-slice CT perfusion imaging of pancreatic adenocarcinoma and mass-forming chronic pancreatitis. Acad Radiol. 2011;18(1):81–88. doi: 10.1016/j.acra.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Sahani DV, Holalkere NS, Mueller PR, Zhu AX. Advanced hepatocellular carcinoma: CT perfusion of liver and tumor tissue–initial experience. Radiology. 2007;243(3):736–743. doi: 10.1148/radiol.2433052020. [DOI] [PubMed] [Google Scholar]
- 24.Ippolito D, Sironi S, Pozzi M, et al. Hepatocellular carcinoma in cirrhotic liver disease: functional computed tomography with perfusion imaging in the assessment of tumor vascularization. Acad Radiol. 2008;15(7):919–927. doi: 10.1016/j.acra.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Squillaci E, Manenti G, Ciccio C, et al. Perfusion-CT monitoring of cryo-ablated renal cells tumors. J Exp Clin Cancer Res. 2009;28:138. doi: 10.1186/1756-9966-28-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sourbron S. Technical aspects of MR perfusion. Eur J Radiol. 2010;76(3):304–313. doi: 10.1016/j.ejrad.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 27.Zitova B. Image registration methods: a survey. Image Vis Comput. 2003;21:977–1000. doi: 10.1016/S0262-8856(03)00137-9. [DOI] [Google Scholar]
- 28.Qiao ZW, Xia CM, Zhu YB, Shi WP, Miao F. First-pass perfusion computed tomography: initial experience in differentiating adrenal adenoma from metastasis. Eur J Radiol. 2010;73(3):657–663. doi: 10.1016/j.ejrad.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Goh V, Bartram C, Halligan S. Effect of intravenous contrast agent volume on colorectal cancer vascular parameters as measured by perfusion computed tomography. Clin Radiol. 2009;64(4):368–372. doi: 10.1016/j.crad.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 30.Park MS, Klotz E, Kim MJ, et al. Perfusion CT: noninvasive surrogate marker for stratification of pancreatic cancer response to concurrent chemo- and radiation therapy. Radiology. 2009;250(1):110–117. doi: 10.1148/radiol.2493080226. [DOI] [PubMed] [Google Scholar]
- 31.Chen GT, Kung JH, Beaudette KP. Artifacts in computed tomography scanning of moving objects. Semin Radiat Oncol. 2004;14(1):19–26. doi: 10.1053/j.semradonc.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Goh V, Halligan S, Taylor SA, Burling D, Bassett P, Bartram CI. Differentiation between diverticulitis and colorectal cancer: quantitative CT perfusion measurements versus morphologic criteria–initial experience. Radiology. 2007;242(2):456–462. doi: 10.1148/radiol.2422051670. [DOI] [PubMed] [Google Scholar]