Abstract
Introduction
From a clinical point of view, knowledge of customary standing positions among healthy young adolescents is of primary importance. The purpose of this study was to document the correlations between sagittal standing posture parameters in a pre-peak height velocity (pre-PHV) cohort.
Materials and methods
This cohort study included 639 pre-PHV boys (age 12.6 [SD, 0.54] years) and 557 pre-PHV girls (age 10.6 [SD, 0.47] years). Gross body segment orientations and spinopelvic orientation/shape indexes were quantified using a clinical screening protocol. Pearson’s correlation coefficients were determined for all sagittal standing plane alignment parameters, and a postural model was used to analyze the correlations between parameters.
Results
Both at the gross body segment and spinopelvic level, an interdependence was found between postural parameters. No correlations were observed between ‘global’ parameters related to the pelvis, trunk or body anteroposterior translation postures and ‘local’ spinopelvic geometries. A similar pattern and strength of correlations was obtained in pre-PHV boys and girls, except for the reciprocal relationships between the craniovertebral angle and adjacent anatomic segment characteristics and between thoraco-lumbar geometries.
Conclusions
Although the correlation schemes do not imply a causal relationship, the proposed postural model allows conjecture about standing posture to be organized slightly differently in pre-PHV boys and girls. Whereas the standing posture in pre-PHV boys might be organized predominantly according to an ascending mode, bottom-up and top-down organizations appear to coexist in pre-PHV girls.
Keywords: Postural balance, Growth and development, Spine, Pelvis, Clinical protocols
Introduction
Postural change occurs continuously throughout the entire time of ontogenesis, with critical periods at school age and puberty. Large cohort studies analyzing children and adolescents reported reference values of spinal and pelvic sagittal parameters [1–6]. Other studies have characterized the changes in sagittal plane alignment during growth [7, 8]. In order to better understand the spinopelvic balance in normal children and adolescents, Mac-Thiong et al. [9] evaluated the standing lateral radiographs of 341 subjects, aged 3–18 years and proposed a postural model based on the relationship between various morphological, shape, and orientation parameters of the spine and pelvis.
A potential limitation of the majority of these studies [1, 2, 4, 5, 7–9] in characterizing standing postural mechanisms, relates to information on the alignment of the spinopelvic axis in the context of the whole body. External pelvic motion has a critical role in maintaining the balance of the spinopelvic axis: varying rotational position (pelvic tilt) and anteroposterior translation are well documented [10, 11]. However, few attempts have been made to include assessment of the orientation of the lower limbs in space [3, 6, 12–14]. Analogously, there have been few studies including the cervical spine/head regions [3, 6, 12, 15, 16], of which only few were performed in the growing individual [3, 6, 12]. Such information, however, may be essential in pediatric/adolescent subjects as a complex integration must occur between the initial supposed ‘top-down’ or descending mode of postural organization and the later ‘bottom-up’ organization emerging with stance [17, 18]. An additional concern with prior publications involves the fact that numerous arm positions other than simply arms at sides have been adopted. This choice was predominantly made for postural data acquisition in stance, to permit adequate visualization of the spinopelvic orientation. Such altered arm positions may not produce the sagittal spinal alignment that is accurately representative of a subject’s customary standing balance [19].
Research of individual developing adolescents generally forms a major challenge [20, 21]. Puberty is accompanied by major maturational physical alterations in body shape and dimensions, and substantial brain changes [20–23]. A major point not taken into consideration, in many studies, is the maturational difference between boys and girls of similar chronological age. It is well known that girls, on average, mature 2 year earlier than boys [22]. A potential influence of maturation and developmental factors on the postural alignment and spinal geometry has been put forward by several authors [2, 7, 9, 12, 24, 25]. It seems crucial to include the biological state of maturation into the research methodology when dealing with adolescents. Maturity status, however, has rarely been applied in epidemiological research on sagittal posture in healthy adolescents [3, 7, 25].
The objective of this study is to analyze the standing postural mechanisms in healthy young adolescents using clinical methods for quantifying sagittal whole body posture and balance. More specifically, the interactions between the geometric parameters describing gross body segment orientation and spinopelvic profile characteristics are investigated in boys and girls during the same phase of growth, but not necessarily age.
Methods
Subjects
Six hundred thirty-nine boys of mean age 12.6 ± 0.54 years (range 11.4–15.0 years) and 557 girls of mean age 10.6 ± 0.47 years (range 9.6–13.0 years) participated in this study. Boys were first-grade students of mainstream secondary education in the Flemish Community of Belgium; girls were fifth-graders of mainstream primary education.
Children were excluded if they had a history of neurologic conditions, rheumatic disorders, metabolic or endocrine diseases, major congenital anomalies, skeletal disorders, connective tissue disorders, previous spinal fracture or previous spinal surgery. This study was approved by the Ethics Committee of the Ghent University Hospital. All participants and their parents gave written informed consent before the study.
Measurements
Subjects received a clinical screening protocol consisting of sagittal plane posture assessment during habitual standing and anthropometric measurements. Evaluation occurred over a 6-month period from September 2008 to February 2009 and was accomplished at schools and local pupil guidance centers.
Experimental protocol: habitual posture
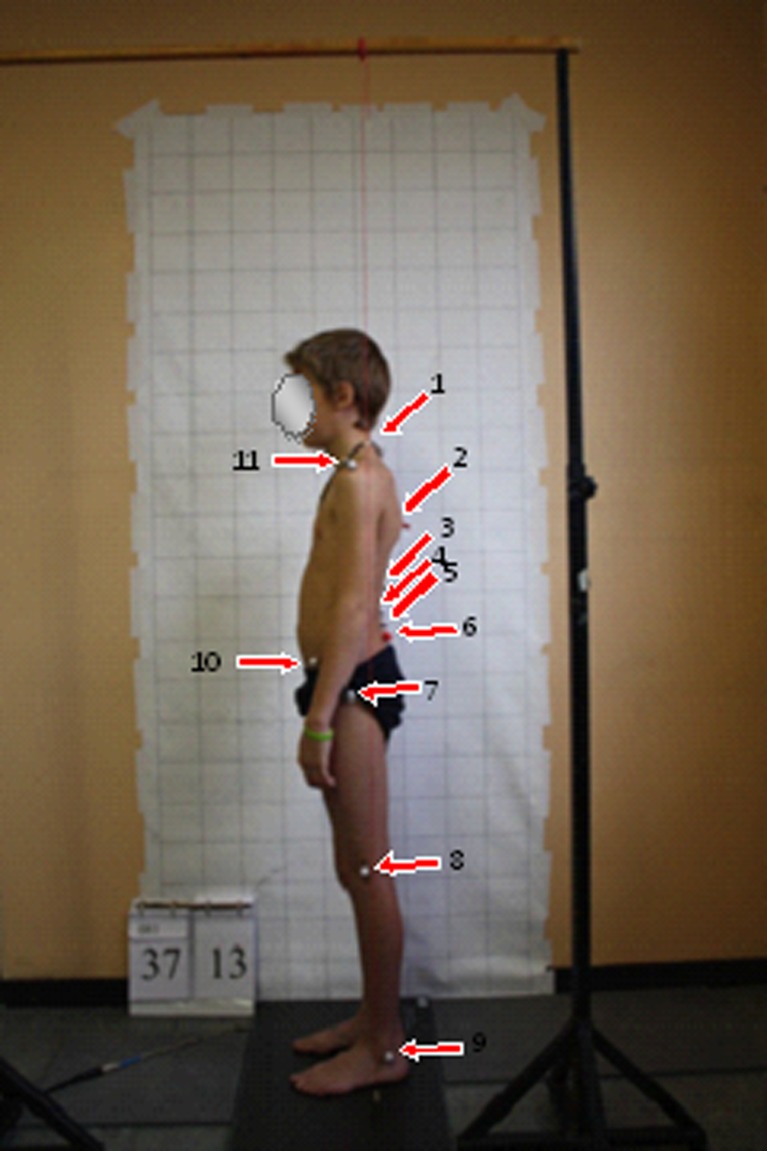
Retro-reflective markers were placed on bony landmarks by one trained examiner (Fig. 1). Participants were instructed to stand in their normal, comfortable relaxed posture, arms resting by the sides, feet shoulder-width apart and equally balanced on both feet. To standardize the head posture, participants viewed a visual target set 1.5 m in front, at eye level. Postural data were obtained after three standing trials, each trial lasting 30 s. Between trials, participants were asked to do some modest walking stationary at the test mark.
Fig. 1.
Placement of reflective markers. 1 spinous process of the 7th cervical vertebra, 2 thoracic apex, 3 inflection point, 4 lumbar apex, 5 spinous process of the 5th lumbar vertebra, 6 posterior superior iliac spine, 7 greater trochanter, 8 lateral femoral condyle, 9 lateral malleolus, 10 anterior superior iliac spine, 11 acromion (most lateral aspect)
Four angular measures describing the orientation of gross body segments with respect to the gravity line were calculated post hoc from digitized photographs of participants (left lateral view) using ImageJ software (National Institutes of Health, Bethesda, MD, USA). These ‘global’ alignment measures were pelvic displacement, trunk lean, body lean, and craniovertebral angle (CVA) (Table 1). In addition, eight ‘local’ spinopelvic features describing the orientation and shape of the pelvis, and lumbar and thoracic spine were assessed (Table 1). The vertebral level of the intercristal line, lumbar apex, inflection point, and thoracic apex were determined by one examiner via visual inspection and palpation. To quantify the sacral inclination, lumbar lordosis, and thoracic kyphosis, a skin-surface electromechanical device, the Spinal Mouse® (Idiag, Voletswil, Switzerland), was used. The Spinal Mouse system is a hand-held, computer-assisted electromechanical device housing accelerometers which records distance and changes of inclination with regard to the plumb line as it is rolled along the length of the spine. This information is then used to calculate the relative positions of the sacrum and vertebral bodies of the underlying bony spinal column using an intelligent, recursive algorithm. Pelvic tilt was measured using the Pro 3600 Digital Inclinometer (SPI-Tronic; Penn Tool Co, Maplewood, NJ, USA) mounted on a caliper. For more detailed methods, see Dolphens et al. [3].
Table 1.
Parameters of ‘global’ body segment orientation and ‘local’ spinopelvic postural alignment
| Parameter | Definition |
|---|---|
| Global alignment | |
| Pelvic displacement | Angle between vertical line and line joining greater trochanter to lateral malleolus; positive when greater trochanter is anterior to the lateral malleolus |
| Trunk lean | Angle between vertical line and line joining spinous process C7 to greater trochanter; positive when spinous process C7 is posterior to the greater trochanter |
| Body lean | Angle between vertical line and line joining spinous process C7 to lateral malleolus; positive when spinous process C7 is posterior to the lateral malleolus |
| Craniovertebral angle | Angle between horizontal line and line joining C7 spinous process to tragus of ear; a small angle indicates more forward head position |
| Spinopelvic alignment | |
| Pelvic tilt | Angle between horizontal line and line joining anterior superior iliac spine (ASIS) and posterior superior iliac spine (PSIS); positive when ASIS is inferior to PSIS |
| Sacral inclination | Sacral angle with respect to the vertical; positive when tilted forward with respect to vertical line |
| Pelvic height | Vertebral level of the intercristal line |
| Lumbar apex | Vertebral level of lumbar curve apex |
| Lumbar lordosis | Sum of segmental angles of appropriate vertebral sections with the ‘lumbar’ segment of the spine located between the inflection point and the L5–S1 interspace; negative when in lordosis |
| Inflection point | Vertebral level where the spine transitions from lordosis to kyphosis |
| Thoracic apex | Vertebral level of thoracic curve apex |
| Thoracic kyphosis | Sum of segmental angles of appropriate vertebral sections with the ‘thoracic’ segment of the spine located between the C7–T1 interspace and the inflection point; positive when in kyphosis |
All postural parameters in degrees except for inflection point, thoracic apex, lumbar apex, and pelvic height (vertebral level)
Anthropometric measurements
Four anthropometric variables (chronological age, stature, sitting height, and body mass) were measured along the guidelines recommended by the Saskatchewan Childhood Growth and Development Research Group (B. Mirwald, personal communication 2007-15-11) and were used in gender-specific regression equations [26] to predict maturity offset, i.e., time before or after peak height velocity (PHV):
Maturity offset in boys = −9.236 + 0.0002708 × leg length and sitting height interaction −0.001663 × age and leg length interaction + 0.007216 × age and sitting height interaction + 0.02292 × weight by height ratio, where R = 0.94, R2 = 0.89, and standard error of estimate (SEE) = 0.59.
Maturity offset in girls = −9.376 + 0.0001882 × leg length and sitting height interaction + 0.0022 × age and leg length interaction + 0.005841 × age and sitting height interaction −0.002658 × age and weight interaction + 0.07693 × weight by height ratio, where R = 0.94, R2 = 0.89, and SEE = 0.57.
Statistical analysis
The data were analyzed using PASW Statistics v18.0 (Chicago: SPSS Inc., 2009). In addition to descriptive statistics, comparisons between genders were performed using independent samples T test. Evaluation of clinical relevance of the results of each of the variables when comparing genders was performed based on distribution-based methods using the effect size (ES) and minimal important difference (MID). A description of these concepts and methods to calculate the clinical relevance of study results, can be found elsewhere [27].
The following formulae were used to calculate the ES and MID, respectively:
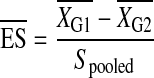 |
where XG1 = mean group 1; XG2 = mean group 2; Spooled = pooled standard deviation.
To calculate the pooled standard deviation, the following formula was used: 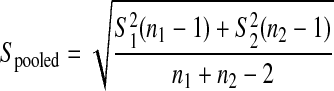 , where S1 = standard deviation group 1; S2 = standard deviation group 2; n1 = sample size for group 1; n2 = sample size for group 2.
, where S1 = standard deviation group 1; S2 = standard deviation group 2; n1 = sample size for group 1; n2 = sample size for group 2.
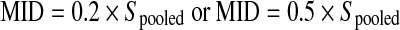 |
Within each gender, relationships between the geometric parameters were assessed using Pearson’s correlation coefficients. The level of significance was set to 0.01 due to the high number of statistical tests performed. In accordance with the suggestions from Cohen [28], statistically significant correlation coefficients were considered clinically significant only if ≥0.3.
Results
The response rate was 82.4 % for boys and 85.6 % for girls. Samples were representative for youth in Flanders regarding educational networks and levels. Predicted years from PHV, a maturational benchmark, were 1.2 ± 0.71 and 1.2 ± 0.59 pre-PHV in boys and girls, respectively. A total of 93.6 % of the boys and 96.2 % of the girls were classified as pre-PHV.
Mean and standard deviation for all postural parameters are presented in Table 2. Also included in Table 2 are the results from the independent samples T test and values for clinical relevance based on ES and MID. Statistically significant differences were found between genders in all postural parameters, except CVA and pelvic displacement angle; non-clinically relevant differences between genders were demonstrated for all parameters, except for body lean angle and thoracic kyphosis (potentially clinically relevant results).
Table 2.
Mean, standard deviation, and comparison between genders of ‘global’ body segment orientation and ‘local’ spinopelvic postural alignment for pre-PHV boys (n = 639) and girls (n = 557), including clinical relevance assessment
| Parameter | Boys | Girls | Mean difference | t | 95 % CI for mean difference | Pooled SD | ES | Interpretation ES | MID (0.2) = 0.2 × pooled SD | MID (0.5) = 0.5 × pooled SD | Final decision clinical relevance |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Global alignment parameters | |||||||||||
| Pelvic displacement angle (deg) | 4.9 ± 1.94 | 4.5 ± 1.90 | 0.4 | 2.38‡ | 0 to 0.5 | 1.92 | 0.21 | SES | 0.4 | 1.0 | NCR |
| Trunk lean angle (deg) | 6.6 ± 2.68 | 7.5 ± 2.55 | −0.9 | −2.94† | −0.8 to −0.2 | 2.62 | −0.34 | SES | 0.5 | 1.3 | NCR |
| Body lean angle (deg) | 0.0 ± 1.12 | 0.6 ± 1.12 | −0.6 | −5.56* | −0.5 to −0.2 | 1.12 | −0.54 | MES | 0.2 | 0.6 | PCR |
| Craniovertebral angle (deg) | 53.8 ± 5.80 | 54.1 ± 5.21 | −0.3 | −0.81 | −0.9 to 0.4 | 5.54 | −0.04 | SES | 1.1 | 2.8 | NCR |
| Spinopelvic parameters | |||||||||||
| Pelvic tilt (deg) | 12.3 ± 4.12 | 13.2 ± 4.22 | −0.9 | −3.61* | −1.3 to −0.4 | 4.17 | −0.22 | SES | 0.8 | 2.1 | NCR |
| Sacral inclination (deg) | 17.7 ± 6.04 | 19.7 ± 5.69 | −2.0 | −6.03* | −2.7 to −1.4 | 5.88 | −0.34 | SES | 1.2 | 2.9 | NCR |
| Pelvic height | L3 level 15.0 ± 0.76 |
Just above L3 level 14.8 ± 0.81 |
0.2 | 3.65* | 0.1 to 0.3 | 0.78 | 0.26 | SES | 0.2 | 0.4 | NCR |
| Lumbar apex | Just above L3 level 14.9 ± 0.78 |
Just above L3 level 14.7 ± 0.81 |
0.2 | 4.93* | 0.1 to 0.3 | 0.79 | 0.25 | SES | 0.2 | 0.4 | NCR |
| Lumbar lordosis (deg) | −28.9 ± 6.89 | −30.7 ± 7.18 | 1.8 | 4.27* | 0.9 to 2.6 | 7.03 | 0.26 | SES | 1.4 | 3.5 | NCR |
| Inflection point | Just below T12 level 12.3 ± 0.91 |
Just below T12 level 12.2 ± 0.93 |
0.1 | 3.17† | 0.1 to 0.3 | 0.92 | 0.11 | SES | 0.2 | 0.5 | NCR |
| Thoracic apex | T6–T7 interspace 6.4 ± 0.81 |
T6–T7 interspace 6.3 ± 0.86 |
0.1 | 3.85* | 0.1 to 0.3 | 0.83 | 0.12 | SES | 0.2 | 0.4 | NCR |
| Thoracic kyphosis (deg) | 34.2 ± 9.94 | 29.1 ± 10.23 | 4.1 | 8.95* | 4.1 to 6.4 | 10.08 | 0.51 | MES | 2.0 | 5.0 | PCR |
Values for boys and girls are mean ± SD. All postural parameters are in degrees except for pelvic height, lumbar apex, inflection point, and thoracic apex (vertebral level). Vertebral level of the clinical identifiable points of interest was coded as follows: 1 for T1 level, 2 for T2 level,…, 13 for L1 level,…, 17 for L5 level. When a point of interest was, e.g. at the L2–L3 interspace, level was coded as 14.5
Criteria for scoring are based on those proposed by Armijo-Olivo et al. [27]: when both the ES are moderate and the mean difference between groups is higher than both MIDs, then scored as CR. If ES is moderate and one of the MIDs is accomplished, it is scored PCR. If ES is small and one of the MID is accomplished, it is scored NCR. If both (ES and MID) are not accomplished, then it is scored NCR
Effect sizes are described analoguous to Armijo-Olivo et al. [27]: SES 0.20 (0–0.39), MES 0.50 (0.4–0.79), LES ≥ 0.80
PHV peak height velocity, CI confidence interval, SD standard deviation, ES effect size, SES small effect size, MES medium effect size, LEF large effect size, MID minimal important difference, NCR not clinically relevant, PCR, potentially clinically relevant, CR clinically relevant
* Significant with P < 0.001 using independent samples T test
†Significant with P < 0.005 using independent samples T test
‡Significant with P < 0.05 using independent samples T test
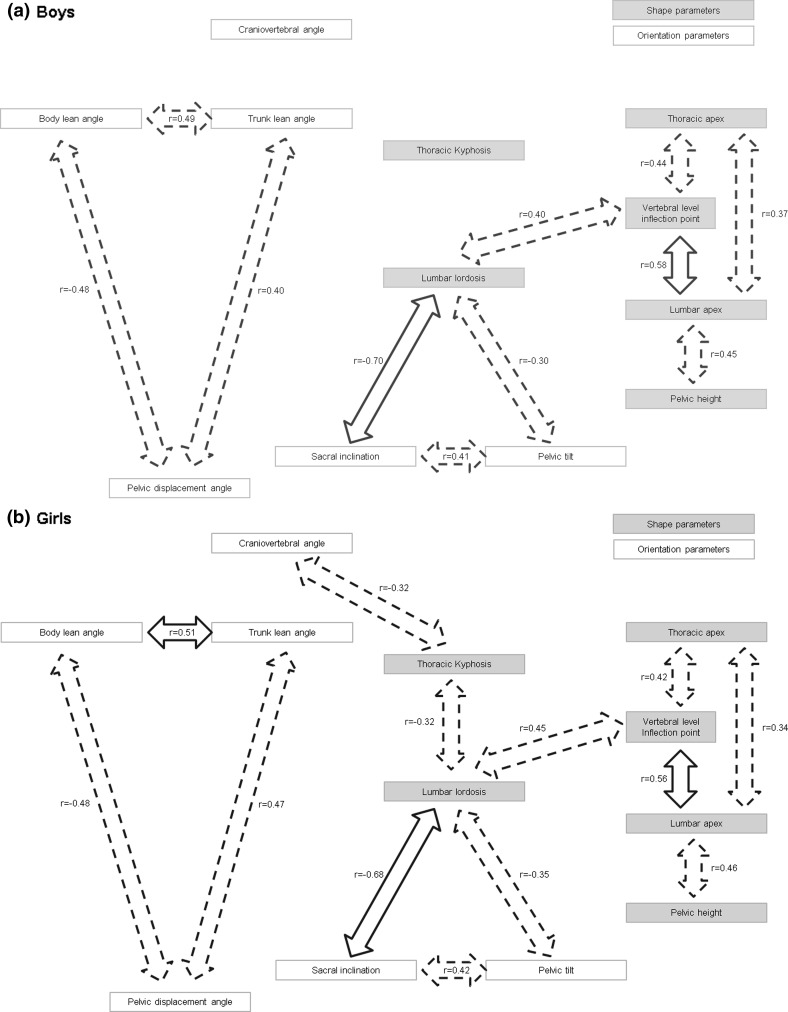
The statistical correlations between the geometric parameters of gross body segment orientation and specific spinopelvic alignment are listed in Table 3. Analogous to previous work [9, 15], a postural model was developed to present the results of the correlation study and to better understand the interactions between all parameters. Since the current study not only investigates sagittal spinopelvic axis parameters, but also the orientation of gross body segments with respect to the gravity line, and sagittal plane posture is assessed clinically as opposed to radiographically, the original models [9, 15] were adapted. ‘Global’ parameters were considered to be orientation parameters; ‘local’ spinopelvic parameters were separated in two conceptual groups: orientation and shape. Using the same template, the postural model was applied to boys (Fig. 2a) and girls (Fig. 2b), separately. Statistically (P < 0.01) and clinically (r ≥ 0.3) significant correlation coefficients were used to implement the proposed postural model.
Table 3.
The statistical correlations between the global and local geometric parameters determined using the Pearson’s correlation coefficient
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | (11) | (12) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pelvic displacement angle (1) | ||||||||||||
| Boys | 0.396* | −0.480* | NS | −0.123† | NS | NS | NS | NS | NS | NS | NS | |
| Girls | 0.472* | −0.477* | NS | NS | −0.127‡ | NS | 0.120‡ | NS | 0.152* | NS | NS | |
| Trunk lean angle (2) | ||||||||||||
| Boys | 0.489* | −0.150* | −0.151* | −0.195* | −0.165* | NS | NS | NS | −0.104‡ | NS | ||
| Girls | 0.511* | NS | NS | −0.126† | −0.201* | NS | NS | NS | NS | NS | ||
| Body lean angle (3) | ||||||||||||
| Boys | −0.183* | NS | −0.107‡ | NS | NS | NS | NS | NS | NS | |||
| Girls | NS | NS | NS | −0.202* | −0.137* | −0.115‡ | −0.113‡ | −0.128† | NS | |||
| Craniovertebral angle (4) | ||||||||||||
| Boys | NS | 0.133* | NS | NS | NS | NS | −0.107‡ | −0.218* | ||||
| Girls | NS | 0.172* | NS | NS | NS | NS | NS | −0.319* | ||||
| Pelvic tilt (5) | ||||||||||||
| Boys | 0.407* | 0.103‡ | NS | −0.304* | −0.117† | NS | NS | |||||
| Girls | 0.418* | 0.127† | NS | −0.348* | −0.165* | −0.223* | NS | |||||
| Sacral inclination (6) | ||||||||||||
| Boys | 0.150* | NS | −0.699* | −0.124† | NS | −0.219* | ||||||
| Girls | 0.156* | −0.124† | −0.683* | −0.218* | −0.240* | −0.201* | ||||||
| Pelvic height (7) | ||||||||||||
| Boys | 0.454* | NS | 0.216* | 0.268* | NS | |||||||
| Girls | 0.461* | NS | 0.193* | 0.189* | NS | |||||||
| Lumbar apex (8) | ||||||||||||
| Boys | 0.170* | 0.583* | 0.373* | 0.102‡ | ||||||||
| Girls | 0.189* | 0.555* | 0.336* | NS | ||||||||
| Lumbar lordosis (9) | ||||||||||||
| Boys | 0.395* | 0.184* | −0.242* | |||||||||
| Girls | 0.453* | 0.253* | −0.316* | |||||||||
| Inflection point (10) | ||||||||||||
| Boys | 0.441* | NS | ||||||||||
| Girls | 0.417* | NS | ||||||||||
| Thoracic apex (11) | ||||||||||||
| Boys | 0.136* | |||||||||||
| Girls | 0.153* | |||||||||||
| Thoracic kyphosis (12) | ||||||||||||
| Boys | ||||||||||||
| Girls | ||||||||||||
NS not significant
* Significant with P < 0.001 (Pearson test)
†Significant with P < 0.005 (Pearson test)
‡Significant with P < 0.01 (Pearson test)
Fig. 2.
Overview of statistically (P < 0.01) and clinically (r ≥ 0.3) significant correlations between parameters of adjacent ‘overall’ and ‘spinopelvic’ anatomic regions in (a) pre-PHV boys, and (b) pre-PHV girls. Moderate (0.3 ≤ r < 0.5) and strong (r ≥ 0.5) correlations are shown in dotted and full arrows, respectively. Weak correlations (0.1 ≤ r < 0.3) are not included in the figure
Regarding the gross body segment orientation parameters, significant linear relationships were found between the pelvic displacement, trunk lean, and body lean angle with moderate (0.3 ≤ r < 0.5) to strong (r ≥ 0.5) correlations between these ‘global’ alignment characteristics in both genders. CVA was weakly related to trunk lean and body lean in boys (−0.1 ≤ r < −0.3, P < 0.001), but not in girls. There were no or at most weak correlations between the ‘global’ parameters and the ‘local’ spinopelvic geometric measures with the exception of the statistically significant moderate correlation between the CVA and thoracic kyphosis in girls (−0.3 ≤ r < −0.5, P < 0.001).
Within the spinopelvic axis, many significant linear correlations were found between pairs of parameters with varying correlation levels. Regarding the shape parameters corresponding to the vertebral level of pre-defined spinopelvic landmarks (pelvic height, lumbar apex, inflection point, thoracic apex), moderate to strong correlations were observed between each single parameter at all anatomic levels (pelvic, lumbar, and thoracic segment), and between adjacent anatomic areas (r ≥ 0.3, P < 0.001). Shape parameters corresponding to the magnitude of the thoracic and lumbar curvatures (thoracic kyphosis and lumbar lordosis) were moderately correlated in girls (−0.3 ≤ r < −0.5, P < 0.001), while they were only weakly related in boys (−0.1 ≤ r < −0.3, P < 0.001). No clinically significant correlations were evident between the spinopelvic shape ‘extensiveness’ and ‘magnitude’ parameters, except in the lumbar region (lordosis vs. inflection point: 0.3 ≤ r < 0.5, P < 0.001). Moderate correlations were also found between the sacropelvic orientation parameters (sacral inclination and pelvic tilt) (0.3 ≤ r < 0.5, P < 0.001), and between the sacropelvic orientation parameters and the degree of lumbar lordosis (r ≤ −0.3, P < 0.001) in both genders.
Discussion
To better appreciate the sagittal alignment in a normal young adolescent population, this study assessed the habitual standing posture in a representative cohort of Flemish boys and girls using a screening protocol with clinical applicability. Human body posture was represented by a series of three solid links representing three major body segments (lower limbs, trunk, and head), and by several local shape/orientation indices within the spinopelvic axis. A key aspect in the current study design was the recruitment of the target population according to a common maturational landmark, the age of attainment of PHV, yielding a developmental age baseline as opposed to a chronological baseline.
The most important finding of this study was that the pattern and strength of correlations is similar between pre-PHV boys and girls, implying a similar interdependence between postural parameters both at the gross body segment and spinopelvic level. More specifically, regarding the ‘global’ level (i.e. the orientation of superimposed body segments with respect to the external world) the results obtained demonstrate moderate to strong correlations between the pelvic displacement, trunk lean, and body lean angle, presumably to keep the Center of Gravity (COG) projection inside a physiological range within the supporting area [10, 13, 17].
In terms of local shape/orientation indices within the spinopelvic axis, analyzes revealed numerous correlations. However, the most notable finding was that the linear correlations were stronger between posture variables at the lumbopelvic region, and weaker at the thoracic level and between the thoracic and lumbar areas, which is consistent with previous reports among normal children [4, 29] and adults [15] using radiography. Furthermore, an interdependence was demonstrated between the adjacent shape parameters corresponding to the vertebral level of pre-defined spinopelvic landmarks (i.e. position of the apex of thoracic and lumbar curves, position of the inflection point, and iliac height), whereas no clinically significant correlation was found between these spinopelvic ‘extensiveness’ parameters and curve ‘magnitude’ parameters, except in the lumbar region (position of the inflection point vs. total degrees of included lordosis). This observation underlines that both ‘magnitude’ (i.e. degrees of kyphosis and lordosis) and ‘extensiveness’ characteristics might be essential to depict spinopelvic shape. However, relatively few reports have been published in which top and limit spinopelvic landmarks were quantified [2–4, 30–32].
The current results further convey that global and local postural alignment are relatively independent since in this study, no or at most weak correlations could be established between the anteroposterior translation postures of the pelvis, trunk, and body with the specific spinopelvic geometric parameters.
Some interesting discrepancies were found when comparing sexes. This in regard to the reciprocal relationships including the orientation of the cervical spine/head unit with respect to the gravity vector (measured as the CVA). The correlation between CVA and thoracic kyphosis was stronger in girls than in boys (moderate vs. weak correlation), and girls as opposed to boys lacked a statistically significant relationship between the CVA and trunk lean angle, and between the CVA and body lean angle. Although there have been no reports published yet on gender-specific correlation patterns between body segments for comparison, one might suggest that the CVA could either serve as a predominantly ‘global’ (pre-PHV boys) or ‘local’ (pre-PHV girls) parameter. In addition, the interruption of the correlations between the CVA and the other gross body segment orientation parameters in girls taken together with the accessory clinically significant correlations observed in girls between cervico-thoracic and thoracolumbar geometries, could give rise to the suggestion that standing posture is organized differently in pre-PHV boys and girls. More specifically, our results hint that standing posture in pre-PHV boys may be organized predominantly according to a bottom-up mode, whereas in pre-PHV girls both bottom-up and top-down organizations appear to coexist in static standing postures. Nonetheless, the proposed correlation schemes do not imply a causal relationship and more investigation is needed to corroborate the suggested difference in postural strategy in young adolescent boys and girls. In this respect, future research should also include boys and girls of similar chronological age within preconceived phases of growth.
This is the first clinical study to systematically quantify the sagittal full-body posture in terms of gross body segment orientation and local spinopelvic shape/orientation parameters within a young adolescent population. Hence, comparison with existing literature remains impeded. The correlation patterns reported above provide a more complete picture of the geometric interaction between body segments and suggest opportunities for innovative, stratified postural subgrouping strategies. Such information could be used as an aid in further research on posture and its clinical importance. Although the observed statistical differences in discrete geometric parameters between genders may be clinically questionable when a general pre-PHV population is considered, these authors opted for performing analyzes on the relationships between the geometric parameters in each sex, separately. The use of mixed sex groups as in the original postural model in normal pediatric subjects [9] might not be appropriate since interference of sex, age, or maturity related differences with the correlation patterns cannot be ruled out.
Study limitations
Overall, the correlations found in the current study were relatively low. Our correlation coefficients (0.10 < r < 0.70) obtained with skin surface measurements were yet consistent with those published by Mac-Thiong et al. [9] in a normal pediatric population (0.14 < r < 0.68) using radiography. The failure to report kinetic data on sagittal balance is a recognized second limitation of this study. Due to the technical difficulties with the plantar pressure measurement platform used, information on the position of the COG projection could not be incorporated. A third limitation regards the particular profile of the current study sample with regard to biological/chronological age. Although the recruitment of boys and girls according to a developmental age baseline constitutes a limitation to the interpretation regarding sex or age related differences in the correlation patterns found, a first attempt was at least made to control for maturity. Concurrently, the authors recognize the importance of follow-up evaluations to allow for consideration of both chronological age and maturity status. Furthermore, these authors acknowledge that there is considerable inter-individual variation in timing, tempo, and sequence of biological changes during the growth spurt, also within each gender [22], which was not taken into account. Finally, the cervical spine indeed was not assessed as thoroughly as were the thoraco-lumbo-pelvic regions.
Conclusions
From a clinical point of view, knowledge of customary standing positions is of primary importance. This study documents the sagittal standing balance in normal pre-PHV subjects, and describes a scheme of correlations in the framework of the segmental theory of postural organization. Generally, the pattern and strength of correlations was similar between both genders, showing a similar interdependence between orientation and shape parameters at both the gross body segment and spinopelvic level. Some interesting discrepancies between sexes were found regarding the reciprocal relationships between the CVA and adjacent segments and between some thoraco-lumbar geometries, suggesting that pre-PHV boys and girls may use a different postural strategy to organize their body segments. Whereas the orientation of the cervical spine/head unit in space (measured as the CVA) appears to fulfill a mainly ‘global’ role in pre-PHV boys, it rather adopts a ‘local’ character in pre-PHV girls. From a developmental perspective, standing posture in pre-PHV boys might be organized mainly along an ascending strategy, whereas an articulated operation between bottom-up and top-down organizations could be suggested in pre-PHV girls.
Acknowledgments
The authors would like to thank all the participants and their parents to take part in this study. Thanks are also due to the staff members of schools and pupil guidance centers for their collaboration in the acquisition of the data and for the use of their facilities during the investigation.
Conflict of interest
The authors declare no conflict of interest. The authors had full control of all primary data.
References
- 1.Mac-Thiong JM, Berthonnaud E, Dimar J, Betz R, Labelle H. Sagittal alignment of the spine and pelvis during growth. Spine. 2004;29:1642–1647. doi: 10.1097/01.BRS.0000132312.78469.7B. [DOI] [PubMed] [Google Scholar]
- 2.Cil A, Yazici M, Uzumcugil A, et al. The evolution of sagittal segmental alignment of the spine during childhood. Spine. 2004;30:93–100. [PubMed] [Google Scholar]
- 3.Dolphens M, Cagnie B, Coorevits P et al (2011) Sagittal standing posture and its association with spinal pain: a school-based epidemiological study of 1196 Flemish adolescents before age at peak height velocity. Spine [Epub ahead of print] [DOI] [PubMed]
- 4.Vedantam R, Lenke L, Keeney J, Bridwell K. Comparison of standing sagittal spinal alignment in asymptomatic adolescents and adults. Spine. 1998;23:211–215. doi: 10.1097/00007632-199801150-00012. [DOI] [PubMed] [Google Scholar]
- 5.Boseker E, Moe J, Winter R, Koop S. Determination of “normal” thoracic kyphosis: a roentgenographic study of 121 “normal” children. J Pediatr Orthop. 2000;20:796–798. doi: 10.1097/01241398-200011000-00019. [DOI] [PubMed] [Google Scholar]
- 6.Lafond D, Descarreaux M, Normand M, Harrison D. Postural development in school children: a cross-sectional study. Chiropr Osteopat. 2007;15:1. doi: 10.1186/1746-1340-15-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poussa M, Heliövaara M, Seitsamo J, Könönen M, Hurmerinta K, Nissinen M. Development of spinal posture in a cohort of children from the age of 11 to 22 years. Eur Spine J. 2005;14:738–742. doi: 10.1007/s00586-004-0701-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Widhe T. Spine: posture, mobility and pain. A longitudinal study from childhood to adolescence. Eur Spine J. 2001;10:118–123. doi: 10.1007/s005860000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mac-Thiong JM, Labelle H, Berthonnaud E, Betz R, Roussouly P. Sagittal spinopelvic balance in normal children and adolescents. Eur Spine J. 2007;16:227–234. doi: 10.1007/s00586-005-0013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwab F, Lafage V, Patel A, Farcy JP. Sagittal plane considerations and the pelvis in the adult patient. Spine. 2009;34:1828–1833. doi: 10.1097/BRS.0b013e3181a13c08. [DOI] [PubMed] [Google Scholar]
- 11.McGregor A, Hukins D. Lower limb involvement in spinal function and low back pain. J Back Musculoskelet Rehabil. 2009;22:219–222. doi: 10.3233/BMR-2009-0239. [DOI] [PubMed] [Google Scholar]
- 12.McEvoy MP, Grimmer K. Reliability of upright posture measurements in primary school children. BMC Musculoskelet Disord. 2005;6:35. doi: 10.1186/1471-2474-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lafage V, Schwab F, Skalli W, et al. Standing sagittal balance and sagittal plane deformity. Analysis of spinopelvic and gravity line parameters. Spine. 2008;33:1572–1578. doi: 10.1097/BRS.0b013e31817886a2. [DOI] [PubMed] [Google Scholar]
- 14.Normand M, Descarreaux M, Harrison D, et al. Three dimensional evaluation of posture in standing with the PosturePrint: an intra- and inter-examiner reliability study. Chiropr Osteopat. 2007;15:15. doi: 10.1186/1746-1340-15-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berthonnaud E, Dimnet J, Roussouly P, Labelle H. Analysis of the sagittal balance of the spine and pelvis using shape and orientation parameters. J Spinal Disord Tech. 2005;18:40–47. doi: 10.1097/01.bsd.0000117542.88865.77. [DOI] [PubMed] [Google Scholar]
- 16.Kuo YL, Tully EA, Galea MP. Video analysis of sagital spinal posture in healthy young and older adults. J Manipulative Physiol Ther. 2009;32:210–215. doi: 10.1016/j.jmpt.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Massion J. Postural control systems in developmental perspective. Neurosci Biobehav Rev. 1998;22:465–472. doi: 10.1016/S0149-7634(97)00031-6. [DOI] [PubMed] [Google Scholar]
- 18.Assaiante C, Amblard B. An ontogenetic model for the sensorimotor organization of balance control in humans. Hum Mov Sci. 1995;14:13–43. doi: 10.1016/0167-9457(94)00048-J. [DOI] [Google Scholar]
- 19.Marks M, Stanfor C, Newton P. Which lateral radiographic positioning technique provides the most reliable and functional representation of a patient’s sagittal balance? Spine. 2009;34:949–954. doi: 10.1097/BRS.0b013e318199650a. [DOI] [PubMed] [Google Scholar]
- 20.Busscher I, Kingma I, Bruin R, et al. Predicting the growth velocity in the individual child: validation of a new growth model. Eur Spine J. 2012;21:71–76. doi: 10.1007/s00586-011-1845-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Busscher I, Gerver WJM, Kingma I, et al. The growth of different body length dimensions is not predictive for the peak growth velocity of sitting height in the individual child. Eur Spine J. 2011;20:791–797. doi: 10.1007/s00586-010-1584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malina RM, Bouchard C, Bar-Or O. Growth, maturation, and physical activity. Champaign: Human Kinetics; 2004. [Google Scholar]
- 23.Patton GC, Viner R. Pubertal transitions in health. Lancet. 2007;369:1130–1139. doi: 10.1016/S0140-6736(07)60366-3. [DOI] [PubMed] [Google Scholar]
- 24.Grivas TB, Vasiliadis ES, Koufopoulos G, et al. Study of trunk asymmetry in normal children and adolescents. Scoliosis. 2006;1:19. doi: 10.1186/1748-7161-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shultz S, Nguyen A, Schmitz R. Differences in lower extremity anatomical and postural characteristics in males and females between maturation groups. J Orthop Sports Phys Ther. 2008;38:137–149. doi: 10.2519/jospt.2008.2645. [DOI] [PubMed] [Google Scholar]
- 26.Mirwald RL, Baxter-Jones AD, Bailey DA, et al. An assessment of maturity from anthropometric measurements. Med Sci Sports Exerc. 2002;34:689–694. doi: 10.1097/00005768-200204000-00020. [DOI] [PubMed] [Google Scholar]
- 27.Armijo-Olivo S, Warren S, Fuentes J, Magee D. Clinical relevance vs. statistical significance: using neck outcomes in patients with temporomandibular disorders as an example. Man Ther. 2011;16:563–572. doi: 10.1016/j.math.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Cohen J. A power primer. Psychol Bull. 1992;112(1):155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 29.Mac-Thiong J, Labelle H, Charlebois M, Huot M, Guise J. Sagittal plane analysis of the spine and pelvis in adolescent idiopathic scoliosis according to the coronal curve type. Spine. 2003;28:1404–1409. doi: 10.1097/01.BRS.0000067118.60199.D1. [DOI] [PubMed] [Google Scholar]
- 30.Vaz G, Roussouly P, Berthonnaud E, et al. Sagittal morphology and equilibrium of pelvis and spine. Eur Spine J. 2002;11:80–87. doi: 10.1007/s005860000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roussouly P, Gollogly S, Berthonnaud E, Dimnet J. Classification of the normal variation in the sagittal alignment of the human lumbar spine and pelvis in the standing position. Spine. 2005;30:346–353. doi: 10.1097/01.brs.0000152379.54463.65. [DOI] [PubMed] [Google Scholar]
- 32.Boyle J, Milne N, Singer K. Influence of age on cervicothoracic spinal curvature: an ex vivo radiographic survey. Clin Biomech. 2002;17:361–367. doi: 10.1016/S0268-0033(02)00030-X. [DOI] [PubMed] [Google Scholar]