Abstract
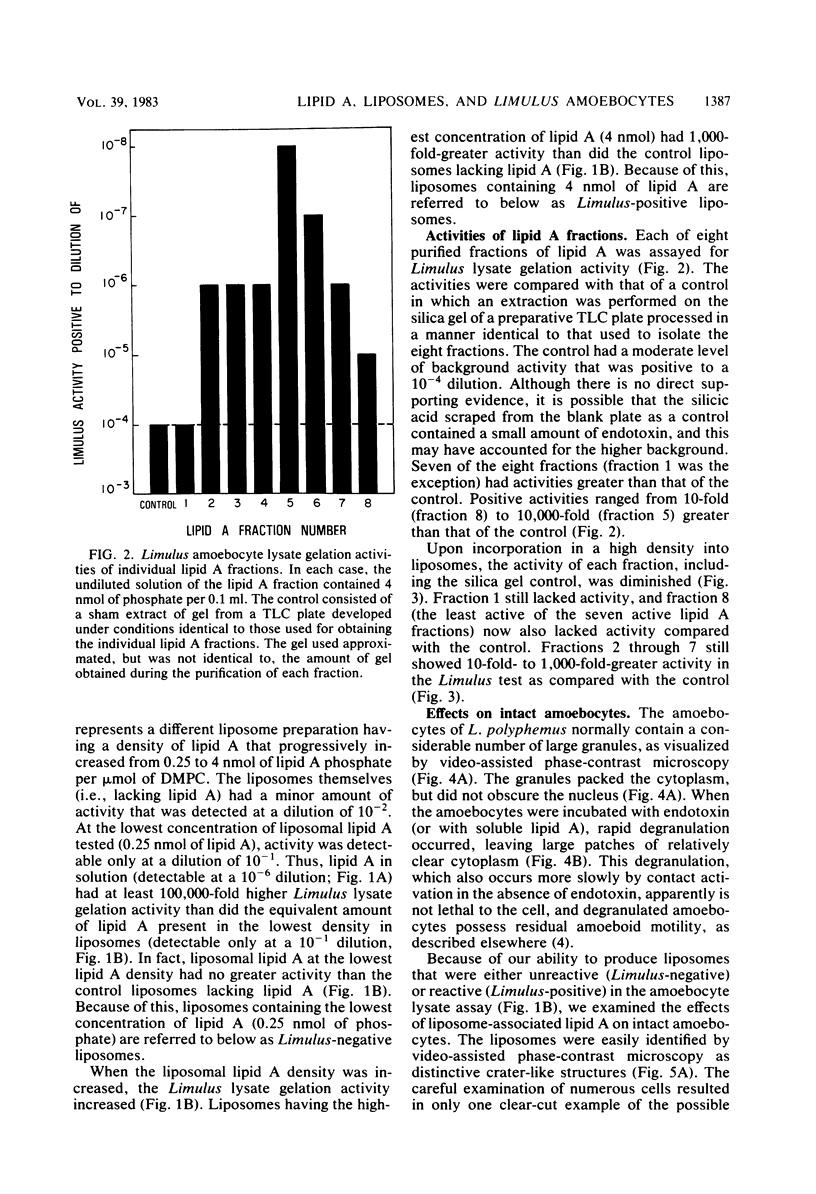
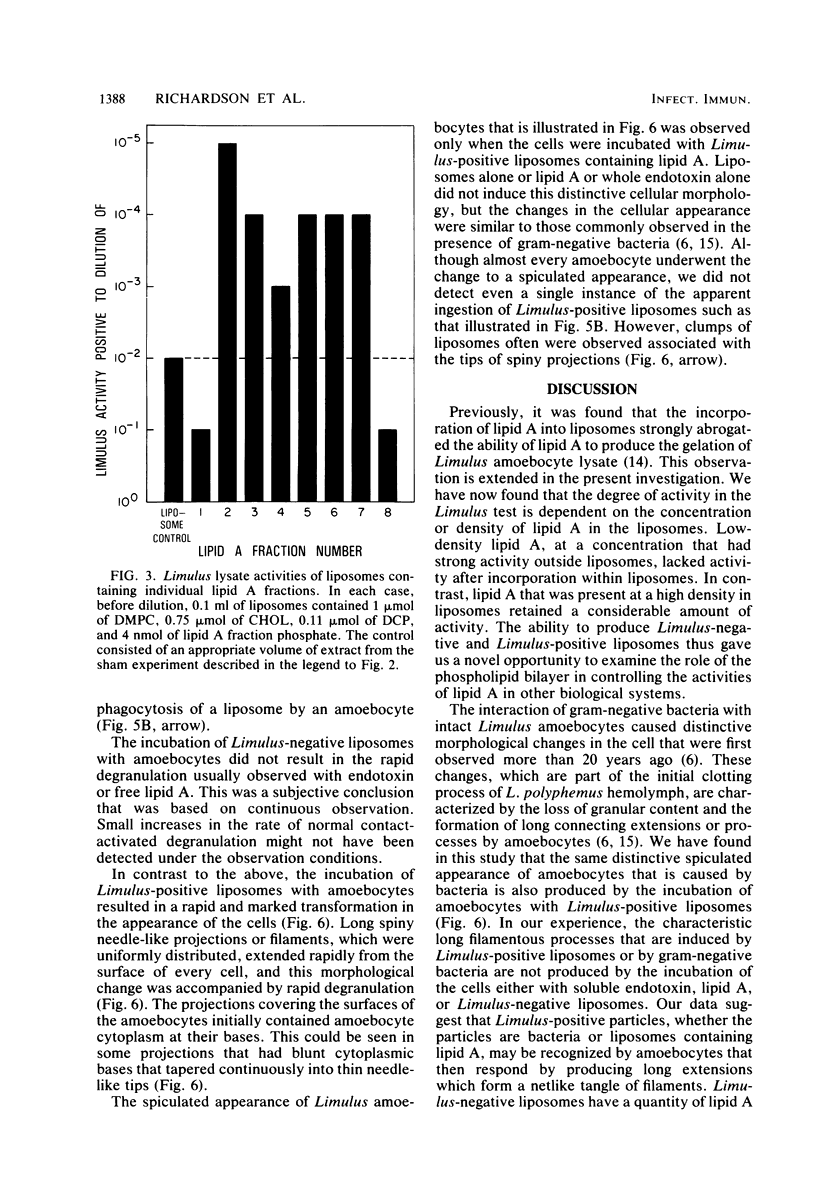
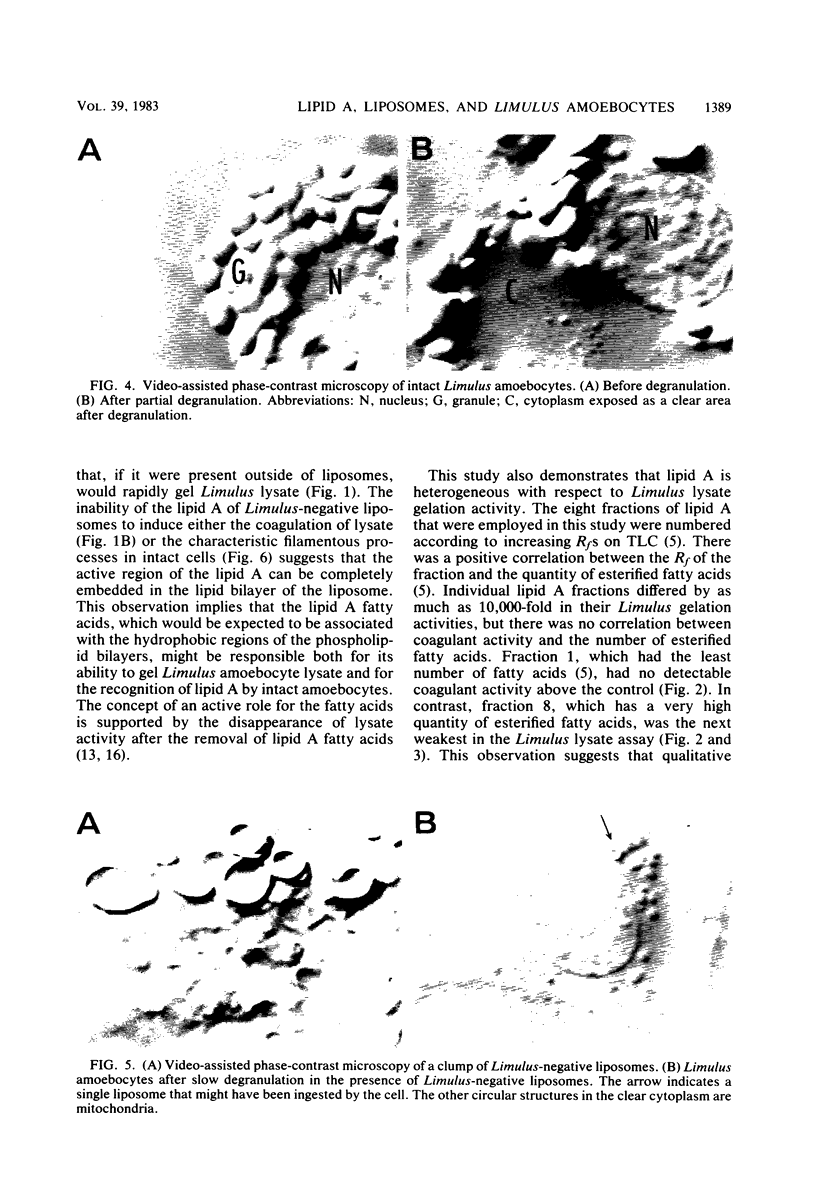
Lipid A or lipid A fractions and liposomes containing lipid A were tested for the ability to gel Limulus amoebocyte lysates and for effects on intact Limulus amoebocytes. Liposomes having a relatively low concentration of lipid A did not produce coagulation of lysate and were designated as Limulus-negative, but liposomes having a high concentration of lipid A were Limulus-positive. Limulus-negative liposomes had no effect on intact amoebocytes. Limulus-positive liposomes caused a striking transformation in the appearance of amoebocytes in that the cells sent out long filamentous extensions that formed a tangled network of processes between cells. The filamentous projections were similar to those that have been previously observed in the presence of gram-negative bacteria. We conclude that amoebocytes have the ability to recognize Limulus-positive liposomes, but the lack of activation of Limulus lysate or the absence of amoebocyte recognition does not prove the absence of liposomal lipid A. We also found that individual lipid A fractions were heterogeneous in their ability to gel lysate. Of eight fractions tested, one (fraction 1) had no detectable activity above the background, and the seven others had activity that ranged from 10-fold to 10,000-fold above the background. The heterogeneity of lipid A fractions detected in assays with amoebocyte lysate was consistent with the finding of heterogeneity in other functional assays of lipid A fractions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. D., Allen N. S., Travis J. L. Video-enhanced contrast, differential interference contrast (AVEC-DIC) microscopy: a new method capable of analyzing microtubule-related motility in the reticulopodial network of Allogromia laticollaris. Cell Motil. 1981;1(3):291–302. doi: 10.1002/cm.970010303. [DOI] [PubMed] [Google Scholar]
- Alving C. R., Banerji B., Shiba T., Kotani S., Clements J. D., Richards R. L. Liposomes as vehicles for vaccines. Prog Clin Biol Res. 1980;47:339–355. [PubMed] [Google Scholar]
- Armstrong P. B. Adhesion and spreading of Limulus blood cells on artificial surfaces. J Cell Sci. 1980 Aug;44:243–262. doi: 10.1242/jcs.44.1.243. [DOI] [PubMed] [Google Scholar]
- BANG F. B. A bacterial disease of Limulus polyphemus. Bull Johns Hopkins Hosp. 1956 May;98(5):325–351. [PubMed] [Google Scholar]
- Banerji B., Alving C. R. Lipid A from endotoxin: antigenic activities of purified fractions in liposomes. J Immunol. 1979 Dec;123(6):2558–2562. [PubMed] [Google Scholar]
- Harris N. S., Feinstein R. A new limulus assay for the detection of endotoxin. J Trauma. 1977 Sep;17(9):714–718. doi: 10.1097/00005373-197709000-00008. [DOI] [PubMed] [Google Scholar]
- LEVIN J., BANG F. B. THE ROLE OF ENDOTOXIN IN THE EXTRACELLULAR COAGULATION OF LIMULUS BLOOD. Bull Johns Hopkins Hosp. 1964 Sep;115:265–274. [PubMed] [Google Scholar]
- Levin J., Bang F. B. Clottable protein in Limulus; its localization and kinetics of its coagulation by endotoxin. Thromb Diath Haemorrh. 1968 Mar 31;19(1):186–197. [PubMed] [Google Scholar]
- Levin J., Poore T. E., Young N. S., Margolis S., Zauber N. P., Townes A. S., Bell W. R. Gram-negative sepsis: detection of endotoxemia with the limulus test. With studies of associated changes in blood coagulation, serum lipids, and complement. Ann Intern Med. 1972 Jan;76(1):1–7. doi: 10.7326/0003-4819-76-1-1. [DOI] [PubMed] [Google Scholar]
- Nandan R., Brown D. R. An improved in vitro pyrogen test: to detect picograms of endotoxin contamination in intravenous fluids using limulus amoebocyte lysate. J Lab Clin Med. 1977 Apr;89(4):910–918. [PubMed] [Google Scholar]
- Niwa M., Hiramatsu T., Waguri O. The gelation reaction between horseshoe crab amoebocytes and endotoxins studied by the quantitative clot protein method. Jpn J Med Sci Biol. 1974 Apr;27(2):108–111. [PubMed] [Google Scholar]
- Ramsey R. B., Hamner M. B., Alving B. M., Finlayson J. S., Alving C. R., Evatt B. L. Effects of lipid A and liposomes containing lipid A on platelet and fibrinogen production in rabbits. Blood. 1980 Aug;56(2):307–310. [PubMed] [Google Scholar]
- Yin E. T., Galanos C., Kinsky S., Bradshaw R. A., Wessler S., Lüderitz O., Sarmiento M. E. Picogram-sensitive assay for endotoxin: gelation of Limulus polyphemus blood cell lysate induced by purified lipopolysaccharides and lipid A from Gram-negative bacteria. Biochim Biophys Acta. 1972 Jan 28;261(1):284–289. doi: 10.1016/0304-4165(72)90340-6. [DOI] [PubMed] [Google Scholar]






