Abstract
Purpose
Percutaneous in situ contouring is based on bilateral bending of rods on the spine, thus increasing lordosis at the fracture. It was analyzed if this technique would provide a better reduction than prone positioning and how sagittal alignment would behave.
Methods
Twenty-nine patients were operated using in situ contouring and selective anterior fusion for non-neurologic A2, A3 or B2 fractures. Clinical results were assessed prospectively using visual analog scale (VAS) and Oswestry Disability Index (ODI). The radiographic deformity correction was measured by sagittal index and regional kyphosis. Sagittal balance was assessed using kyphosis, lordosis, T9 tilt, pelvic incidence, pelvic tilt and sacral slope. Posterior wall fragment reduction was evaluated by computed tomography.
Results
After 2 years, VAS and ODI were comparable to the status prior to the accident. The sagittal index was 19.7° preoperatively, 5.3° after prone positioning and −1.1° after in situ contouring (p < 0.001). The loss of correction was 2.4°, mainly during the first 3 months. Similar observations were made for regional kyphosis. The sagittal spino-pelvic alignment was stable postoperatively. A preoperative canal obstruction ≥50 % was observed in 16 patients, and the fragments migrated anteriorly in all patients.
Conclusions
Percutaneous instrumentation and anterior fusion provides good clinical results. In situ contouring increases lordosis obtained by prone positioning. Anterior column lengthening and ligamentotaxis reduce posterior wall fragments, which decompress the canal without laminectomy. The fusion of anterior defects prevents the loss of correction and provides a stable sagittal profile. The instrumentation may be removed without damaging the paravertebral muscles and loss of correction.
Keywords: Thoracolumbar fracture, Minimally invasive surgery, Percutaneous instrumentation, In situ contouring, Sagittal balance
Introduction
Minimally invasive surgery (MIS) has been used increasingly in the management of thoracolumbar fractures and has become a valuable treatment option [1]. Percutaneous instrumentation techniques avoid paravertebral musculature dissection, facilitating early rehabilitation and representing an advantage over open surgery [2–4]. The minimal access reduces intraoperative blood loss, risk for infection and postoperative pain, and shortens the hospital stay [5]. Although these advantages of MIS are relevant to the patient’s short-term recovery, correction of posttraumatic kyphosis should be as effective as reduction obtained by conventional techniques to provide an adequate sagittal balance and long-term results [6].
Several tools may be used for percutaneous fracture reduction. Prone positioning of the patient eventually combined with traction or bending the table represents the most important factor. Compression-distraction mechanisms of percutaneous instrumentations provide a small additional correction. Bending hyperlordotic rods and coaxing them into the screws will stretch the spine, while increasing lordosis at the fractured level. The use of monoaxial screws adjacent to this level may be helpful when reducing the fracture by providing a 90° connection between the screw and the lordotic rod, thus lengthening the anterior column.
In situ contouring has been described for open surgery [7, 8]. The principle of this technique consists of first giving the rods the shape of the spine, and then to bend the rods inside the patient. This allows correcting the kyphosis at the level of the fractured vertebra and the overlying disc by increasing lordosis and lengthening the anterior column. The fracture will open progressively without excessive stress using a ligamentotaxis mechanism and osseous defects are secondarily grafted through a video-assisted anterior approach (Figs. 1, 2).
Fig. 1.
Principle of in situ contouring, showing the initial position of instrumentation. Arrows indicate the correction mechanism by bending rods (a), which increases lordosis and reduces the fracture by anterior column lengthening and ligamentotaxis. Anterior defect fused by a secondary video-assisted approach (b)
Fig. 2.
Posttraumatic kyphotic deformity (a) reduced by prone positioning (b) and bending the rods in situ (c), which leads to an anterior gap in the cranial disc, secondarily filled with a cage containing bone graft harvested from the fractured vertebra and rib (d)
The purpose of this study was to analyze if this principle was applicable to MIS, if it would provide a better reduction compared to prone positioning, and how the quality of reduction would behave during the first 2 years.
Materials and methods
Institutional review board approval was obtained for this prospective study. Twenty-nine consecutive patients were treated for thoracolumbar fractures using percutaneous in situ contouring at our institution from January to December 2009. The cohort was composed of 17 males and 12 females. The average age at surgery was 46.7 (18–54) years. The fracture levels were located between T11 and L4. The fracture types were A2, A3.2, A3.3 or B2 according to the AO classification [9]. All patients were operated within 72 h, except for four patients who presented a flexible malunion after 6–8 weeks of conservative treatment. The clinical and radiographic follow-up was standardized, using a file for data collection preoperatively, at 6 weeks, 3 months, 6 months, 1 year and 2 years.
During posterior instrumentation, the patient was placed in prone position on a Jackson table. The principle of in situ contouring with MIS remained similar to open surgery, although the surgical techniques were adapted to percutaneous instrumentation. Once the rods were positioned in the screws, bending irons were introduced through small skin incision made for screw placement and fixed to the rods next to the fracture. The bending irons were then gently pushed together on both sides simultaneously under fluoroscopic control, which increased lordosis at the fracture level (Fig. 3). While bending, only the most cranial screw–rod connection was locked. Since polyaxial screws were used, all other implants remained slightly unlocked to prevent the reduction maneuver from modifying the screw position itself without correcting the spinal deformity. This allowed the rod sliding through these screws. The instrumentation of two levels above and below the fracture distributed stress on all implants. Pure titanium rods were used because their elastic modulus would further limit stress at implants while bending and provide a sufficient amount of plastic deformation for correction. Screw migrations were not observed. The anterior column was grafted after 2–3 weeks if the bone defect was important and if part of the reduction occurred in the overlying disc. Iliac crest or a cage was used through an MIS video-assisted anterior approach (Fig. 4). The posterior instrumentation was removed through small incisions after 1–1.5 years, once computed tomography (CT) showed anterior fusion (Fig. 5).
Fig. 3.
Posttraumatic kyphosis of an A2 fracture of L2 measured by sagittal index (SI) and regional kyphosis (RK) on radiographs (a), CT reconstruction (b) and intraoperative reduction maneuver by pushing the bending irons together (c) under fluoroscopic control (d)
Fig. 4.
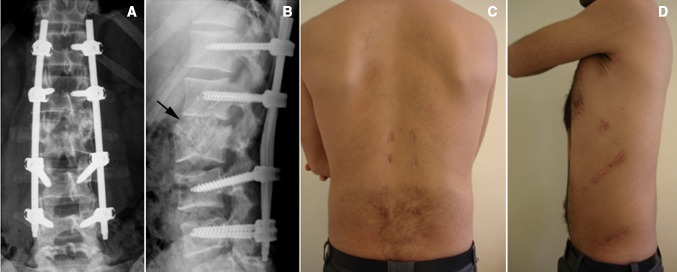
Postoperative radiographs of percutaneous instrumentation from T12 to L4 with selective anterior fusion at L1–L2 (a, b) and clinical aspect of posterior and anterior approaches (c, d) of the same patient as in Fig. 3
Fig. 5.
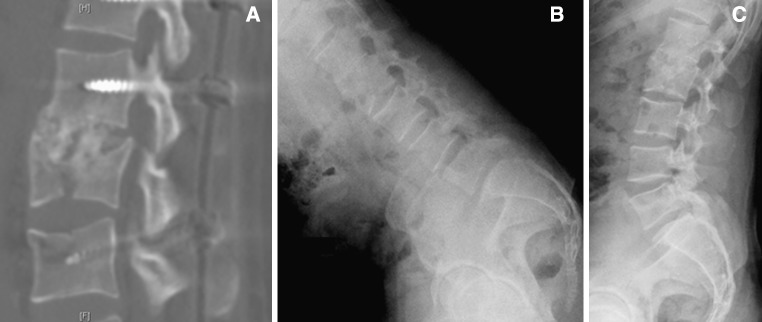
Postoperative CT at 1 year follow-up showing L1–L2 fusion (a) and mobilization of the lumbar spine in flexion–extension after percutaneous removal of the instrumentation (b, c) of the same patient as in Fig. 3
Clinical data were assessed using the visual analog scale (VAS) from 1 to 10 for back pain and the Oswestry Disability Index (ODI). Preoperative values were considered prior to the accident and evaluated retrospectively by the patient at the initial follow-up. Postoperative values were assessed at each follow-up visit.
The deformity at the fracture was measured preoperatively on lateral supine radiographs and postoperatively on standing radiographs using regional kyphosis (RK) and the sagittal index (SI). The SI was normalized to zero with regard to segmental kyphosis or lordosis depending on the level. The angle between the caudal endplate of the fractured vertebra and the caudal endplate of the vertebra above was considered (Fig. 3). At thoracic levels 5° was subtracted, at T12–L1 the direct measurement was considered, and at lumbar levels 10° was added [10]. The SI was additionally measured intraoperatively on lateral fluoroscopy before and after instrumentation.
Global sagittal balance and spino-pelvic alignment were measured postoperatively on lateral full spine radiographs using kyphosis T4–T12, lordosis L1–S1, T9 tilt, pelvic incidence, pelvic tilt and sacral slope [11].
Axial and sagittal CT images were compared before and after fracture reduction prior to anterior grafting, to check the amount of fragment reduction in the canal. Measurements were not found sufficiently reliable. Therefore a simplified classification was used, dividing the posterior wall displacement into ≥50 or <50 % (Fig. 6).
Fig. 6.

Preoperative CT (a, c) and postoperative control after fracture reduction (b, d) showing posterior wall displacement and the effect of increasing lordosis by in situ contouring, which pulls the fragments anteriorly and decompresses the canal
Statistical evaluation was performed with SPSS 18.0.0. The Wilcoxon test was used for the analysis of VAS, ODI and radiographic measurements. The significance level was set at 0.05.
Results
Clinical results
As demonstrated in Table 1, there was no significant VAS difference between the status prior to the accident and after 2 years (p = 0.194). At 6 weeks follow-up, the average VAS score was 0.8 points above the preoperative value (p = 0.039). It decreased by 0.6 points between 6 weeks and 3 months (p = 0.007) and by 0.6 points between the first and second year (p < 0.001).
Table 1.
Visual analog scale (VAS) for back pain and Oswestry Disability Index (ODI) preoperatively before fracture occurred and at postoperative follow-up
| Preoperative | 6 weeks | 3 months | 6 months | 1 year | 2 years | |
|---|---|---|---|---|---|---|
| VAS | ||||||
| Average ± SD | 0.7 ± 1.3 | 1.5 ± 1.3 | 0.9 ± 1.1 | 0.9 ± 1.1 | 0.9 ± 1.1 | 0.3 ± 0.7 |
| Range | 0–5 | 0–4 | 0–4 | 0–4 | 0–4 | 0–2 |
| ODI | ||||||
| Average ± SD | 6.2 ± 8.9 | 23.2 ± 15.8 | 18.7 ± 12.7 | 16.4 ± 13.7 | 13.1 ± 13.2 | 5.9 ± 8.5 |
| Range | 0–32 | 0–56 | 0–50 | 0–46 | 0–38 | 0–26 |
The ODI score was comparable preoperatively and after 2 years, showing no significant difference (p = 0.848). The score increased by 17.0 after 6 weeks compared to the preoperative status (p < 0.001). It decreased by 4.5 between 6 weeks and 3 months (p = 0.007), by 3.3 between 6 months and 1 year (p = 0.018), and by 7.2 between the first and second year (p = 0.001).
Segmental kyphosis correction
Prone positioning of the patient led to an average SI decrease of 14.4° (p < 0.001). In situ contouring provided an additional decrease of 6.4° on fluoroscopic images (p < 0.001), thus obtaining an initial correction of 20.8° (p < 0.001). An average increase of 2.4° was observed between the last intraoperative value and follow-up at 2 years (p < 0.001). Between the operation and 6 weeks, the SI increased by 1.0° (p = 0.002) and by 0.7° between 6 weeks and 3 months (p = 0.016). The variations between the third month and second year were not significant (Table 2).
Table 2.
Sagittal index and regional kyphosis preoperatively before fracture occurred and at postoperative follow-up
| Preop | Pronea | Bendinga | 6 weeks | 3 months | 6 months | 1 year | 2 years | |
|---|---|---|---|---|---|---|---|---|
| Sagittal index | ||||||||
| Average ± SD | 19.7 ± 3.4 | 5.3 ± 2.3 | −1.1 ± 2.0 | −0.1 ± 2.0 | 0.6 ± 2.0 | 0.9 ± 2.2 | 1.0 ± 1.8 | 1.3 ± 2.1 |
| Range | 16 to 27 | 0 to 11 | −5 to 2 | −5 to 4 | −5 to 4 | −4 to 5 | −2 to 4 | −4 to 5 |
| Regional kyphosis | ||||||||
| Average ± SD | 15.3 ± 8.6 | – | – | −1.0 ± 9.0 | −0.8 ± 8.7 | −0.6 ± 8.7 | −0.4 ± 8.7 | 0.7 ± 8.5 |
| Range | −18 to 30 | – | – | −26 to 16 | −28 to 15 | −27 to 16 | −27 to 15 | −25 to 16 |
aIntraoperative measurements on fluoroscopy in prone position without instrumentation and after bending of rods, not assessed for regional kyphosis
An average RK decrease of 16.3° was measured after 6 weeks compared to the preoperative angle (p < 0.001). RK was not assessed intraoperatively on fluoroscopy because strictly lateral endplate projections of the vertebrae above and below the fracture were not clearly obtained in all patients. An increase of 1.7° was measured after 2 years (p = 0.043). Table 2 demonstrates that small increases occurred progressively, although they were not statistically significant.
Global sagittal balance
Table 3 shows that the sagittal spinal balance measured by kyphosis, lordosis and T9 tilt remained stable from 6 weeks to 2 years. The same applied to pelvic incidence and dynamic spino-pelvic parameters such as pelvic tilt and sacral slope. None of these angles varied significantly during the postoperative period.
Table 3.
Global sagittal balance of the spine and pelvis at postoperative follow-up
| 6 weeks | 3 months | 6 months | 1 year | 2 years | |
|---|---|---|---|---|---|
| Kyphosis T4–T12 | |||||
| Average ± SD | 37.0 ± 7.7 | 37.4 ± 9.4 | 36.6 ± 8.9 | 37.5 ± 8.3 | 36.5 ± 8.5 |
| Range | 20 to 51 | 20 to 58 | 17 to 52 | 21 to 54 | 20 to 58 |
| Lordosis L1–S1 | |||||
| Average ± SD | −50.1 ± −8.5 | −50.6 ± −9.4 | −48.7 ± −12.3 | −50.2 ± −9.1 | −49.0 ± −12.5 |
| Range | −26 to −64 | −30 to −70 | −24 to −66 | −22 to −69 | −24 to −69 |
| T9 tilt | |||||
| Average ± SD | −11.7 ± −4.1 | −11.7 ± −3.8 | −11.7 ± −4.0 | −11.3 ± −3.6 | −11.8 ± −4.1 |
| Range | −5 to −24 | −4 to −18 | −3 to −22 | −4 to −17 | −3 to −24 |
| Pelvic incidence | |||||
| Average ± SD | 53.7 ± 8.3 | 52.5 ± 8.5 | 52.9 ± 7.4 | 52.7 ± 7.1 | 53.0 ± 7.3 |
| Range | 37 to 72 | 30 to 68 | 40 to 72 | 38 to 70 | 38 to 70 |
| Pelvic tilt | |||||
| Average ± SD | 17.8 ± 6.0 | 18.7 ± 6.1 | 17.3 ± 5.9 | 17.8 ± 5.5 | 17.5 ± 6.0 |
| Range | 5 to 32 | 3 to 32 | 5 to 27 | 7 to 30 | 5 to 30 |
| Sacral slope | |||||
| Average ± SD | 34.1 ± 7.1 | 33.6 ± 7.3 | 34.0 ± 5.8 | 34.3 ± 6.0 | 33.8 ± 6.1 |
| Range | 22 to 50 | 15 to 48 | 24 to 44 | 22 to 48 | 22 to 48 |
Spinal canal decompression
A preoperative posterior wall displacement ≥50 % was observed on CT in 16 out of 29 patients. In all 16 cases, the postoperative CT evidenced an anterior reduction of posterior wall fragments with a remaining displacement <50 % as shown in Fig. 6. None of the patients presented neurological symptoms at any time.
Discussion
Thoracolumbar fractures leading to major kyphosis with a potential compromise of the spinal canal are usually treated surgically. There is still no consensus on the primary management of these fractures, and it is not clear if an MIS or classic open reduction should be preferred for severe deformities in non-neurological trauma. Although the reduced invasiveness seems attractive at short term, tools used with percutaneous instrumentation should enable to entirely correct the kyphosis to provide an adequate sagittal balance at long term. The concept of in situ contouring had been experienced with open surgery for the last 20 years, providing good clinical results and radiographic correction [7]. This technique has been adapted to the requirements of MIS, which offers the advantage of sparing the paravertebral musculature compared to open surgery [2, 12].
Percutaneous procedures seem to keep the level of postoperative pain relatively low after a few days, which may be beneficial for the patient’s mobilization and rehabilitation. Fuentes et al. [5] and Blondel et al. [13] reported on patients treated by percutaneous instrumentation and kyphoplasty for A3 fractures. The average VAS score was 1.1 at the time of discharge between the third and seventh postoperative day. Palmisani et al. [14] also included A2, B and C type lesions treated by percutaneous instrumentation only, and reported an average VAS score of 1.9 at the 14.2 months follow-up. The results of Pelegri et al. [15] were similar with an average VAS of 1.6 after 17 months, and an average ODI of 16. On comparing our results with these studies, it appeared that the VAS was similar at short term and the pain level was relatively stable between the third month and the first year, whereas the ODI constantly decreased during the first postoperative year, probably indicating the effect of physiotherapy and the patient’s increasing confidence. VAS and ODI further improved between the first and the second year and reached a level that was almost comparable to the status prior to the accident. This improvement occurred after percutaneous removal of the instrumentation, thus permitting a remobilization of unfused levels and strengthening of the paravertebral musculature. When considering open surgery, the Spine Study Group of the German Association of Trauma Surgery prospectively analyzed 733 thoracolumbar fractures and reported that single posterior procedures would lead to a better VAS score after 2 years, compared to combined posterior and anterior procedures [16]. This may be biased by the fact that patients requiring an anterior approach usually present with more severe fractures with an anterior column defect. Although our study does not compare MIS and open procedures, the percutaneous approach may probably be beneficial, when combined with an MIS anterior video-assisted approach in a second step [17].
Sagittal deformity corrections by short segment percutaneous instrumentation with polyaxial screws ranged between 6.3° and 7.9° for local kyphosis (LK) and between 6.3° and 15.1° for RK [6, 14, 15, 18]. Palmisani et al. [14] showed that the use of monoaxial screws improved the initial reduction. A loss of correction was noticed in all of these studies, ranging between 1.1° and 2.8° for LK and between 2.5° and 4.5° for RK. Wild et al. [6] pointed out that the loss of correction was highest within the first year after implant removal with an average increase of 2.1° for LK and 6.8° for RK. The additional use of kyphoplasty may improve the initial correction in recent A3.1, A3.2 and A3.3 fractures. Cement augmentation combined with percutaneous instrumentation may prevent the secondary loss of correction by strengthening the anterior column. Nevertheless, a loss ranging between 2.0° and 3.3° was reported essentially during the first 3 months [5, 13, 19].
Eck [20] combined percutaneous instrumentation and MIS corpectomy for a lumbar burst fracture. This approach is interesting for anterior defects that need to be grafted to avoid loss of correction and implant failure. A selective anterior fusion, focused on the defect, was performed secondarily in our patients. However, the first step of opening the fracture by anterior column lengthening and recreating a lordosis through posterior instrumentation provided the initial correction. The SI in the present study demonstrated that proper prone positioning remained crucial, since this simple maneuver would provide the major amount of correction by placing the spine into lordosis. In situ contouring provided an additional lordosis increase of 6.4°, thus obtaining a slight overcorrection. The loss of correction of 2.4° for SI and 1.7° for RK mainly occurred within the first 3 months, which represents the period of anterior graft fusion, where a certain elasticity of the anterior column may remain. After consolidation, no loss of correction was observed after implant removal. When comparing percutaneous with open in situ contouring, the correction seems comparable [7]. Logroscino et al. [21] hypothesized that percutaneous multilevel constructs would better maintain the reduction without anterior graft, but the German multicenter study [16] showed that a combined procedure would provide a better sagittal correction and a lower loss of correction compared to posterior instrumentation only. Although short segment posterior fixation is currently used, Altay et al. [22] showed that longer constructs may be beneficial in burst fractures with a load sharing score of 7 or more. In situ contouring was performed with longer constructs because the stress would be distributed on multiple levels, which made the technique safe since no screw migration was observed. Rod contouring with longer constructs further allowed a good global sagittal balance of the thoracolumbar spine and pelvis.
In situ contouring led to a posterior wall fragment reduction by increasing lordosis and unfolding the compressive apex at the fracture. Anterior column lengthening further leads to a ligamentotaxis effect, which pulls the fragments anteriorly out of the canal. The internal AO fixator provides a fragment reduction by ligamentotaxis using a parallel distraction and a pivot movement of the screws [23]. The reduction mechanism is slightly different from in situ contouring, which works by initial lordosis increase, but both techniques stretch the anterior spine. Posterior wall fragments could be reduced using ligamentotaxis through percutaneous in situ contouring. Remodeling of the spinal canal may be observed after thoracolumbar burst fractures [24, 25]. Leferink et al. [23] stressed the importance of intraoperative reduction because this would trigger the amount of remodeling over time. The present study indicates that there is no need for a laminectomy if neurological signs are absent. However, if a deficit is present, an open procedure would be recommended instead of MIS.
Conclusion
Percutaneous instrumentation and selective anterior grafting provides good clinical results. In situ contouring allows safe and efficient fracture reduction which enhances lordosis obtained by prone positioning. Anterior column lengthening and ligamentotaxis reduce posterior wall fragments. The anterior graft prevents the loss of correction and provides a stable sagittal profile. After fusion, the instrumentation may be removed without damaging the paravertebral muscles.
Conflict of interest
None.
References
- 1.Ringel F, Stoffel M, Stüer C, Meyer B. Minimally invasive transmuscular pedicle screw fixation of the thoracic and lumbar spine. Neurosurgery. 2006;59:361–367. doi: 10.1227/01.NEU.0000223505.07815.74. [DOI] [PubMed] [Google Scholar]
- 2.Grass R, Biewener A, Dickopf A, Rammelt S, Heineck J, Zwipp H. Percutaneous dorsal versus open instrumentation for fractures of the thoracolumbar border. A comparative, prospective study. Unfallchirurg. 2006;109:297–305. doi: 10.1007/s00113-005-1037-6. [DOI] [PubMed] [Google Scholar]
- 3.Rampersaud YR, Annand N, Dekutoski MB. Use of minimally invasive surgical techniques in the management of thoracolumbar trauma: current concepts. Spine. 2006;31(Suppl 1):S96–S102. doi: 10.1097/01.brs.0000218250.51148.5b. [DOI] [PubMed] [Google Scholar]
- 4.Charles YP, Zairi F, Vincent C, Fuentes S, Bronsard N, Court C, Huec JC. Minimally invasive posterior surgery for thoracolumbar fractures. New trends to decrease muscle damage. Eur J Orthop Surg Traumatol. 2012;22:1–7. doi: 10.1007/s00590-011-0781-2. [DOI] [Google Scholar]
- 5.Fuentes S, Blondel B, Metellus P, Gaudart J, Adetchessi T, Dufour H. Percutaneous kyphoplasty and pedicle screw fixation for the management of thoraco-lumbar burst fractures. Eur Spine J. 2010;19:1281–1287. doi: 10.1007/s00586-010-1444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wild MH, Glees M, Plieschnegger C, Wenda K. Five-year follow-up examination after purely minimally invasive percutaneously and conventionally treated patients. Arch Orthop Trauma Surg. 2007;127:335–343. doi: 10.1007/s00402-006-0264-9. [DOI] [PubMed] [Google Scholar]
- 7.Steib JP, Aoui M, Mitulescu A, Bogorin J, Chiffolot X, Cognet JM, Simon P. Thoracolumbar fractures surgically treated by “in situ contouring”. Eur Spine J. 2006;15:1823–1832. doi: 10.1007/s00586-006-0161-5. [DOI] [PubMed] [Google Scholar]
- 8.Steib JP, Charles YP, Aoui M. In situ contouring technique in the treatment of thoracolumbar fractures. Eur Spine J. 2010;19(Suppl 1):S66–S68. doi: 10.1007/s00586-009-1119-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magerl F, Aebi M, Gertzbein SD, Harms J, Nazarian S. A comprehensive classification of thoracic and lumbar injuries. Eur Spine J. 1994;3:184–201. doi: 10.1007/BF02221591. [DOI] [PubMed] [Google Scholar]
- 10.Farcy JP, Weidenbaum M, Glassman SD. Sagittal index in management of thoracolumbar fractures. Spine. 1990;15:958–965. doi: 10.1097/00007632-199009000-00022. [DOI] [PubMed] [Google Scholar]
- 11.Roussouly P, Nnadi C. Sagittal plane deformity: an overview of interpretation and management. Eur Spine J. 2010;19:1824–1836. doi: 10.1007/s00586-010-1476-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim DY, Lee SH, Chung SK, Lee HY. Comparison of multifidus muscle atrophy and trunk extension muscle strength: percutaneous versus open pedicle screw fixation. Spine. 2005;30:123–129. doi: 10.1097/01.brs.0000157172.00635.3a. [DOI] [PubMed] [Google Scholar]
- 13.Blondel B, Fuentes S, Pech-Gourg G, Adetchessi T, Tropiano P, Dufour H. Percutaneous management of thoracolumbar burst fractures: evolution of techniques and strategy. Orthop Traumatol Surg Res. 2011;97:527–532. doi: 10.1016/j.otsr.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Palmisani M, Gasbarrini A, Brodano GB, Iure F, Cappuccio M, Boriani L, Amendola L, Boriani S. Minimally invasive percutaneous fixation in the treatment of thoracic and lumbar spine fractures. Eur Spine J. 2009;18(Suppl 1):S71–S74. doi: 10.1007/s00586-009-0989-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pelegri C, Benchikh El Fegoun A, Winter M, Brassart N, Bronsard N, Hovorka I, Peretti F. Percutaneous osteosynthesis of lumbar and thoracolumbar spine fractures without neurological deficit: surgical technique and preliminary results. Rev Chir Orthop Reparatrice Appar Mot. 2008;94:456–463. doi: 10.1016/j.rco.2008.03.035. [DOI] [PubMed] [Google Scholar]
- 16.Reinhold M, Knop C, Beisse R, Audigé L, Kandziora F, Pizanis A, Pranzl R, Gercek E, Schultheiss M, Weckbach A, Bühren V, Blauth M. Operative treatment of 733 patients with acute thoracolumbar spinal injuries: comprehensive results from the second, prospective, internet-based multicenter study of the Spine study group of the German Association of Trauma Surgery. Eur Spine J. 2010;19:1657–1676. doi: 10.1007/s00586-010-1451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burgos J, Rapariz JM, Gonzalez-Herranz P. Anterior endoscopic approach to the thoracolumbar spine. Spine. 1998;23:2427–2431. doi: 10.1097/00007632-199811150-00013. [DOI] [PubMed] [Google Scholar]
- 18.Ni WF, Huang YX, Chi YL, Xu HZ, Lin Y, Wang XY, Huang QS, Mao FM. Percutaneous pedicle screw fixation for neurologic intact thoracolumbar burst fractures. J Spinal Disord Tech. 2010;23:530–537. doi: 10.1097/BSD.0b013e3181c72d4c. [DOI] [PubMed] [Google Scholar]
- 19.Rahamimov N, Mulla H, Shani A, Freiman S. Percutaneous augmented instrumentation of unstable thoracolumbar burst fractures. Eur Spine J. 2011 doi: 10.1007/s00586-011-2106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eck JC. Minimal invasive corpectomy and posterior stabilization for lumbar burst fracture. Spine J. 2011;11:904–908. doi: 10.1016/j.spinee.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 21.Logroscino CA, Proietti L, Tamburrelli FC. Minimally invasive spine stabilisation with long implants. Eur Spine J. 2009;18(Suppl 1):S75–S81. doi: 10.1007/s00586-009-0995-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altay M, Ozkurt B, Aktekin CN, Ozturk AM, Dogan O, Tabak AY. Treatment of unstable thoracolumbar junction burst fractures with short- or long-segment posterior fixation in magerl type a fractures. Eur Spine J. 2007;16:1145–1155. doi: 10.1007/s00586-007-0310-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leferink VJ, Nijboer JM, Zimmerman KW, Veldhuis EF, Vergert EM, Duis HJ. Burst fractures of the thoracolumbar spine: changes of the spinal canal during operative treatment and follow-up. Eur Spine J. 2003;12:255–260. doi: 10.1007/s00586-002-0499-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dai LY. Remodeling of the spinal canal after thoracolumbar burst fractures. Clin Orthop Relat Res. 2001;382:119–123. doi: 10.1097/00003086-200101000-00018. [DOI] [PubMed] [Google Scholar]
- 25.Mueller LA, Degreif J, Schmidt R, Pfander D, Forst R, Rommens PM, Mueller LP, Rudig L. Ultrasound-guided spinal fracture repositioning, ligamentotaxis, and remodeling after thoracolumbar burst fractures. Spine. 2006;31:E739–E747. doi: 10.1097/01.brs.0000237012.83128.80. [DOI] [PubMed] [Google Scholar]