Abstract
Cultured cells of Eschscholtzia californica (Californian poppy) respond to a yeast elicitor preparation or Penicillium cyclopium spores with the production of benzophenanthridine alkaloids, which are potent phytoalexins. Confocal pH mapping with the probe carboxy-seminaphthorhodafluor-1-acetoxymethylester revealed characteristic shifts of the pH distribution in challenged cells: within a few minutes after elicitor contact a transient acidification of cytoplasmic and nuclear areas occurred in parallel with an increase of the vacuolar pH. The change of proton concentration in the vacuole and in the extravacuolar area showed a nearly constant relation, indicating an efflux of vacuolar protons into the cytosol. A 10-min treatment with 2 mm butyric or pivalic acid caused a transient acidification of the cytoplasm comparable to that observed after elicitor contact and also induced alkaloid biosynthesis. Experimental depletion of the vacuolar proton pool reversibly prevented both the elicitor-triggered pH shifts and the induction of alkaloid biosynthesis. pH shifts and induction of alkaloid biosynthesis showed a similar dependence on the elicitor concentration. Net efflux of K+, alkalinization of the outer medium, and browning of the cells were evoked only at higher elicitor concentrations. We suggest that transient acidification of the cytoplasm via efflux of vacuolar protons is both a necessary and sufficient step in the signal path toward biosynthesis of benzophenanthridine alkaloids in Californian poppy cells.
The induction of the biosynthesis of phytoalexins by external elicitors is one component of the multifactorial response of plants to pathogens. In plant tissues the production of plant-specific phytoalexins is usually in concert with the widely distributed reactions of the hypersensitive response complex: cross-linking of cell wall proteins, accumulation of phenolics, lignification, and production of antimicrobial exoenzymes (e.g. chitinases) and of various other pathogenesis-related proteins. An integrative signal system obviously coordinates these activities among the invaded cells and their neighbors and at the systemic level (for recent reviews, see Ricci et al., 1993; Ebel and Cosio, 1994; Kombrink and Somssich, 1995). In cultured cells the primary responses evoked by the contact with exogenous elicitors can be grouped into (a) an oxidative burst, i.e. the generation of reactive oxygen species (Apostol et al., 1989; Sutherland, 1991; Levine et al., 1994; Lamb and Dixon, 1997), and (b) perturbations of the cellular ionic balance, i.e. efflux of K+ and Cl−, influx of Ca2+, external alkalinization, and cellular acidification (Mathieu et al., 1991; Bach et al., 1993).
Whereas in distinct species both groups of responses appear to be causally related (Nürnberger et al., 1997), other findings argue that at least in some plants both responses are not necessarily coupled but rather belong to different signal paths that selectively convert signals from elicitor-binding sites at the plasma membrane into the activation of enzymes and the transcription of genes. In particular, the induction of phytoalexin biosynthesis does not generally seem to depend on an oxidative burst, because it can occur without a measurable accumulation of reactive oxygen (Bach et al., 1993) and in some cell lines is not induced by endogenous or external H2O2 or hydroxide radicals (Levine et al., 1994; Castoria et al., 1995). Similar conclusions have been drawn by comparing the effects of native and chemically modified elicitors: for example, the protein part of a glycoprotein from Verticillium dahliae proved to be an inducer of phytoalexin biosynthesis in soybean cells, whereas the carbohydrate moiety retained the ability to trigger an oxidative burst and other elements of the hypersensitive response (Davis et al., 1993).
Elicitor-triggered ionic fluxes (loss of K+, external alkalinization, or influx of Ca2+) have been found to be correlated with the induction of phytoalexin biosynthesis even under conditions in which no oxidative response could be observed (Mathieu et al., 1991; Bach et al., 1993; Bottin et al., 1994). A transient decrease of intracellular pH has been observed repeatedly (Ojalvo et al., 1987; Kneusel et al., 1989; Guern et al., 1992; Mathieu et al., 1996a) among the changes in the ionic composition after elicitor contact, with only a few exceptions (Horn et al., 1992). This raises the question of whether changes in the intracellular pH could be involved in the signal path toward the elicitation of phytoalexin biosynthesis. Until now a causal relationship between distinct changes of intracellular pH and the induction of plant phytoalexin biosynthesis has not been established. Data on pH-dependent activity control of enzymes and transport proteins involved in cellular signaling are not yet abundant for plant cells but are accumulating from a variety of studies on protein kinases (Tognioli and Basso, 1987), ATPases and carrier systems (Guern et al., 1992; Van der Veen et al., 1992), and ion-channel gating (Blatt and Thiel, 1993; Hedrich and Dietrich, 1996). Confocal pH topography with fluorescent probes has proven to be a convenient tool with which to monitor the distribution of pH in individual cells with the resolution of the light microscope. This slightly invasive method allows the examination of defined optical sections and, thus, avoids averaging of signals from superimposed horizontal layers of the cell (Roos, 1992).
In the present paper we report dynamic changes of the intracellular pH distribution observed by confocal pH topography in elicited cells of Californian poppy (Eschscholtzia californica) and their causal relation to the triggering of a phytoalexin biosynthesis.
MATERIALS AND METHODS
Chemicals
CarboxySNARF-1 was purchased from Molecular Probes (Eugene, OR). Valinomycin, Na butyrate, and pivalic (trimethylacetic) acid were purchased from Sigma, and all other chemicals were of analytical grade.
Cell Cultures
Suspension-cultured cells of Californian poppy (Eschscholtzia californica) were grown in a medium according to the method of Linsmaier and Skoog (1965) with the hormones 2,4-D and α-naphthalene acetic acid (1 μm each). Cultivation was performed on a gyrotary shaker (100 rpm) at 24°C in continuous light (approximately 600 lux) in a 7-d growth cycle. Cells from 4-d cultures were used for all experiments, and the pHext was between 3.8 and 4.1.
Determination of Benzophenanthridine Alkaloids
A 1-mL cell suspension (and/or 1 mL of culture liquid) was mixed with 1 mL of 96% (v/v) ethanol containing 1% HCl, extracted for 30 min at 40°C, and centrifuged at 5000 rpm for 10 min. In the supernatant the alkaloids were determined by reading the fluorescence (λEX 460 nm, λEM 570 nm). Fluorescence intensities were converted into alkaloid concentrations via calibration curves obtained with the benzophenanthridine alkaloid sanguinarine dissolved in an analogous mixture of culture liquid, ethanol, and HCl. Fluorescence quenching of extract components was checked and routinely corrected by using added sanguinarine as an internal standard, but usually proved to be negligible.
The assay was validated by HPLC of test extracts using reverse-phase C18 columns (Merck, Darmstadt, Germany) and gradient elution with acetonitrile:water:H3PO4 according to the method of Gundlach (1992), followed by fluorescence detection. The fluorescence spectra of the crude extracts displayed close similarities with the spectrum of a mixture of isolated benzophenanthridines. The increase of fluorescence found after a 20-h treatment with elicitor (1 μg/mL) represented mainly macarpine (55%) and 12-OH chelirubin (25%) identified by electron impact MS (mole peaks at m/z = 392 and m/z = 378); in a typical experiment the content of macarpine increased at a similar rate, as did the fluorescence of the crude extract (macarpine: from 1.5 to 3.6 μg/mL; total fluorescence, calculated as sanguinarine: from 2.7 to 5.7 μg/mL).
Elicitor Preparations
According to the method of Schumacher et al. (1987), 100 g of commercial bakers' yeast (Deutsche Hefewerke “Gold”, Nürnberg, Germany) was suspended in 150 mL of sodium citrate buffer (20 mm, pH 7.0) and autoclaved at 121°C and 1.1 bar for 20 min. After centrifugation at 10,000g for 20 min the supernatant was mixed with ethanol to give a final concentration of 50% (v/v) and stirred gently overnight. The resulting precipitate was again centrifuged at 10,000g for 20 min and the supernatant was subjected to another ethanol precipitation (final concentration, 67% [v/v] ethanol). The precipitate was lyophilized, dissolved in water, and filter sterilized.
The elicitor preparation obtained in this way is a mixture of glycoproteins and contains up to 40% protein (Gundlach, 1992). In a first step toward the isolation of the active principle(s), the crude extract was fractionated by ultrafiltration (Centricon centrifuge tubes, Amicon, Witten, Germany). The majority of the alkaloid-stimulating activity (>60%) appeared in the cutoff range between 30 and 100 kD. This fraction was the supernatant of a 30-kD filtration performed with a 100-kD ultrafiltrate; it was washed twice with the original volume of water and then diluted to the same volume. Elicitor concentrations refer to the dry weight of the crude elicitor preparation.
Spores of Penicillium cyclopium were harvested from 7-d cultures of this fungus on slant agar (for cultivation conditions, see Roos et al., 1996), suspended in water, and autoclaved (121°C and 1.1 bar for 20 min).
Confocal pH Topography
Cells suspended in culture liquid were loaded for 2 to 3 h with 5 μm carboxySNARF-1 (stock solution: 1 mm in DMSO). Portions (10 μL) were spotted to gel discs (made from phosphate-free culture liquid with 2% agarose) fixed on microscopic slides. Effectors were supplied with a microsyringe close to the cell(s) to be examined. DMSO at a final concentration of 0.75% was present in all of the experiments. (This concentration results from the addition of SNARF plus an effector solution [valinomycin in some experiments] and was maintained for reasons of comparability; it did not change the typical pH distribution known from DMSO-free control experiments.)
Perfusion experiments were performed at 0.2 mL/min with the gel discs fixed in a perfusion chamber of 500 μm inner height.
According to the previously described method (Pönitz and Roos, 1994), a confocal laser scanning microscope (Leica, Deerfield, IL) equipped with an argon ion laser was used in dual-channel mode. Wavelength settings for SNARF were γEX = 514 nm; γEM = 583 nm (channel 1); and γEM = > 610 nm (channel 2). Images were obtained with the Nikon fluor objectives 40/1.2 or 63/1.4. Signals from eight frames were scanned simultaneously in both channels and the intensity ratio (channel 1:channel 2) was calculated for each pixel. pH maps were obtained by color coding these intensity ratios according to self-defined look-up tables. The offset of both photomultipliers was fixed to a level that reduced the fluorescence of cells that did not contain SNARF or alkaloids to <1.
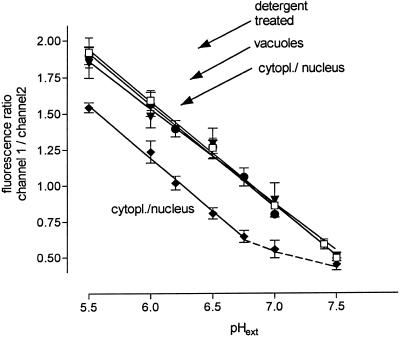
The measured intensity ratios were converted to pH by means of a calibration graph that was obtained with detergent-treated cells. For this purpose SNARF-preloaded cells were resuspended in Mes buffers (60 mm) containing 0.1% CTAB. In these suspensions fluorescence equilibrated rapidly between virtually all parts of the cells and the outer medium, where nearly 90% of the probe was detectable. Extracellular areas of approximately 20 × 20 pixels were scanned and averaged to yield fluorescence ratios at defined pH values. The actual calibration graph (Fig. 1) is linear in the range of pH 5.5 to 7.5 and can be fitted (r2 = 0.998) to the equation pH = 8.22 − (fluorescence ratio/0.715). The ratio-versus-pH curve thus obtained was validated by scanning pH maps of SNARF-preloaded intact cells that were incubated with Mes buffers (40 mm) containing either a membrane-permeable weak base (methylamine) or weak acid (pivalic) plus nigericin and KCl (Fig. 1). Under these conditions fluorescence quotients in the vacuoles or in the cytoplasmic/nuclear region, respectively, responded to changes of pHext with similar sensitivity as the probe liberated from detergent-treated cells.
Figure 1.
Calibration of pH-dependent fluorescence of SNARF. After 2 h of incubation with 5 μm SNARF, cells were filtered and resuspended in Na-Mes or K-Bis-Tris-propane buffers as specified. Stock solutions of CTAB, methylamine, sodium butyrate, or sodium pivalate were titrated to the desired pH with HCl or NaOH and added to the final concentrations, as indicated. Fluorescence images were scanned as described in Methods. Intensity ratios were averaged from six values, each representing the mean of an area of nearly 50 × 50 pixels. All solid lines were obtained via linear regression. □, Cells (100 mg fresh weight/mL) in Mes, 60 mm, plus 0.1% CTAB. pH maps were scanned after 10 min in extracellular areas. Linear regression line: slope = −0.715 ± 0.013, r2 = 0.998. In a parallel experiment, cells received 5 μg/mL yeast elicitor 15 min before CTAB. The resulting data points did not significantly differ from those of the elicitor-free experiment and are omitted from the graph for reasons of clarity. •, Cells (20 mg fresh weight/mL) in Mes, 40 mm, plus methylamine, 40 mm. pH maps were scanned after 30 to 40 min in vacuolar areas. Linear regression line: slope = −0.708 ± 0.038, r2 = 0.988. ♦, Cells (20 mg fresh weight/mL) in Mes, 40 mm, plus sodium butyrate, 10 mm. pH maps were scanned after 20 to 30 min in cytoplasmic/nuclear areas. Linear regression line (in the pH range from 5.5 to 6.75): slope = −0.735 ± 0.034, r2 = 0.994. ▾, Cells (20 mg fresh weight/mL) in K-Bis-Tris-propane, 40 mm, KCl, 100 mm, nigericin, 10 μm, plus the following concentrations of pivalate (estimated to give 0.5 mm undissociated acid): pH 5.5, 2 mm; pH 6.0, 5.5 mm; pH 6.5, 16 mm; and pH 7.0, 50 mm. pH maps of cytoplasmic/nuclear areas were scanned after 60 to 80 min. Linear regression line: slope = −0.656 ± 0.043, r2 = 0.988. All slopes are not significantly different at the 95% level.
The variance of fluorescence ratios obtained at the same pH was lowest at high fluorescence levels but did not severely increase as long as the intensities of areas to be examined could be kept between 20 and 240 on a scale of 0 to 255. Those (few) cells that showed more divergent extremes of fluorescence were not used for pH topography. Fading of fluorescence signals between two consecutive scanning periods was 1% to 3% in both channels and did not significantly influence the calibration graph.
Measurement of K+ Concentrations in the Outer Medium
Cells were filtered through a 200-mesh nylon filter and resuspended (100 mg fresh weight/mL) in one-half of the concentrated culture liquid with KNO3 and NH4NO3 replaced by equimolar amounts of NaNO3; the remaining K+ content was near 1 mm. At 5 or 10 min, samples of 2 mL were taken with a syringe and rapidly passed through cotton filters. The filtrates were diluted 1:100 with water, and the K+ content was analyzed by atomic emission spectrometry (model 929AA spectrometer, Unicam Analytical Systems, Cambridge, UK) at γ = 766.5 nm. The burner was fueled with a stoichiometric mixture of air and acetylene. Calibration of emission intensity to the K+ concentration was done with KNO3 solutions in the range from 10 to 100 μm. Extracellular pH was measured with a standard glass electrode (Hamilton, Reno, NV).
Treatment of Cells with Butyric or Pivalic Acid
Cell were harvested on a 200-mesh nylon filter and resuspended in one-half of the concentrated culture liquid that had been adjusted to pH 3.8 with HCl. Five-milliliter samples received the desired concentration of sodium butyrate or sodium pivalate from a 100 mm stock solution adjusted to the same pH. (At pH 3.8, >90% of either butyrate or pivalate is present in the protonated form; pKa = 4.83 and 5.05, respectively.) After 10 min the cells were collected on a nylon filter, and washed with and resuspended in half-concentrated culture liquid.
RESULTS
Elicitor-Triggered Formation of Phytoalexins
Cultured cells of Californian poppy respond to fungal elicitors with the production of benzophenanthridine alkaloids (Schumacher et al., 1987) via a jasmonate-controlled pathway (Gundlach et al., 1992; Müller et al., 1993). These alkaloids have been reported to act as potent phytoalexins, i.e. they inhibit the growth of bacteria and fungi (Dzink and Socransky, 1985; Gundlach, 1992), because of their antimicrotubule properties and their intercalation into nucleic acids (Wolff and Knipling, 1993).
In our experiments a glycoprotein preparation from bakers' yeast (prepared according to the method of Gundlach [1992]) was used to elicit the formation of the benzophenanthridine alkaloids, which were quantified by their characteristic fluorescence. At the elicitor concentrations used (0.1–5 μg/mL) the newly produced alkaloids appeared mainly in the outer medium, as seen by a comparison of alkaloid contents of cell suspensions and of the culture filtrates. The base level of alkaloids originally present in the culture increased 2- to 3.5-fold within 24 h after addition of near-saturating concentrations (around 1 μg/mL) of elicitor. In the absence of elicitor the alkaloid content increased by nearly 40% during this period (Table I). No correlation existed between the initial alkaloid content of the control culture and the increase evoked by elicitor treatment. Elicitor concentrations ≤ 2 μg/mL did not reduce the growth rate of the challenged cultures; at 5 μg/mL the growth was reduced by 15% at most. Accordingly, the accumulation of the fluorescent probes SNARF or 5- (and 6-)carboxy-2,7-dichlorofluorescein used as viability indicators (see below) reported no cytotoxic effects of ≤2 μg/mL elicitor (used either as a crude preparation or an ultrafiltrate of 30–100 kD).
Table I.
Comparison of cytoplasmic acidification, K+ efflux, and appearance of benzophenanthridine alkaloids in suspension cultures of Californian poppy
| Treatment | Acidification of Cytoplasm/Nucleus | K+ Loss Initial Rate | Alkaloid Production per 24 h |
|---|---|---|---|
| maximum pH shift | μm min−1 g−1 dry wt | %, normalizeda | |
| Control | 0 | 0 | 15 (6) |
| Yeast elicitor (30–100 kD) | |||
| 1 μg/mL | 0.55 (5) | 0.02 (5) | 100 (6) |
| 3 μg/mL | 0.60 (4) | 3.1 (5) | 114 (5) |
| Valinomycin 1 μm | <0.1 (3) | 2.4 (5) | 19 (4) |
| Valinomycin 5 μm | 0.40 (2) | 3.2 (2) | 40 (2) |
| Valinomycin 10 μm | 1.05 (3) | 4.5 (4) | 93 (3) |
| Butyric acid (2 mm, 10 min) | 0.35 (4) | 0.0 (3) | 62 (5) |
| Pivalic acid (2 mm, 10 min) | 0.40 (2) | n.d.b | 70 (3) |
Effectors were added to the growth medium of 4- or 5-d cultures (external pH = 3.8–4.0). Each figure is averaged from three determinations with the same batch of cell suspensions. The sd values were as follows: alkaloid content, 6% to 9%, cytoplasmic pH, 0.03 unit; and K+ loss, 10% to 12%. Independent experiments with different batches of cell suspensions (numbers in parentheses) yielded similar results.
The highest variability was found with the alkaloid production of different batches, the extremes of which were as follows: initial content, 70 to 120 μg/g; increase per 24 h (control), 30 to 54 μg/g; increase per 24 h (1 μg elicitor/mL), 210 to 360 μg/g. To keep the data on alkaloid contents comparable, figures are normalized to the alkaloid production evoked by 1 μg/mL crude elicitor, which is set to 100%.
n.d., Not determined.
In addition to the alkaloid phytoalexins, brown phenolic substances accumulated in cultures treated with yeast elicitor in concentrations greater than 3 μg/mL; the latter response is a common feature of many plant tissues and cultured cells (see Castoria et al., 1994, and refs. cited therein). The addition of catalase (0.5 μkat/mL) together with the elicitor completely prevented the browning reaction (measured as A360 of cellular extracts obtained with 96% [v/v] ethanol containing 0.5 n KOH), which may indicate that this response is mediated by H2O2 or related oxidants. The rate of alkaloid formation was not diminished by catalase treatment. Formation of benzophenanthridines triggered by low elicitor concentrations or by artificial acidification (see below) was not accompanied by a browning response. Thus, induction of alkaloid biosynthesis and accumulation of oxidants appear not to be causally related.
SNARF as a Probe for the Confocal pH Imaging of Californian Poppy Cells
The pH probe carboxySNARF-1 (pKa = 7.5) liberated intracellularly from its acetoxymethylester accumulates in Californian poppy cells in sufficient amounts to allow the mapping of intracellular pH by ratio confocal fluorescence images. Several experiments were performed to determine whether this method could truly reflect the pH distribution in intact cells.
At the applied concentration of 5 μm, SNARF exerted no significant toxicity: the growth rate of 4-d cultures was not changed after a 4-h treatment with this compound and subsequent resuspension in the original culture liquid.
SNARF did not accumulate in cells damaged by detergents (0.05% Triton X-100, 0.05% CTAB, or 5 μg/mL digitonin) or completely leaked out of preloaded cells after such treatments. Similar effects were seen with the viability probe 5- (and 6-)carboxy-2,7-dichlorofluorescein diacetate, which likewise is hydrolyzed by intracellular esterases and stains cells with intact plasma membranes. Thus, high SNARF fluorescence appears to be indicative of intact membrane properties. Using this criterion, about 15% of cells of a 4-d culture were found to display compromised membrane permeability. Nearly all of these cells were stained by genuine benzophenanthridine alkaloids (present in control cultures; see above), as indicated by their red-orange nuclear fluorescence. A 4-h incubation with SNARF did not increase the number of these alkaloid-stained cells nor did it increase the total amount of alkaloids (determined fluorimetrically after correcting for the fluorescence added by SNARF). Thus, cellular accumulation of alkaloids and of the pH probe were mutually exclusive and the pH probe itself did not evoke alkaloid formation.
To prove whether the SNARF fluorescence displayed the same pH sensitivity in vacuolar and cytoplasmic areas, the equilibration of pH between these compartments and the outer medium was attempted by using membrane-permeable weak acids or bases at different pHext values. The fluorescence ratio of vacuolar areas showed a linear dependence on the pH of external buffers that contained 40 mm methylamine in the pH range between 5.5 and 7.0. (Fig. 1), whereas the ratios of cytoplasmic and nuclear fluorescence did not show significant changes. This finding corresponds to recent data reported by Brauer et al. (1997), who found a close relationship between the pH of root-hair vacuoles and that of extracellular buffers containing ammonia or methylamine. As seen in Figure 1, the ratio-versus-pH curve nearly coincides with the calibration curve obtained after the detergent treatment of SNARF-preloaded cells, indicating a similar pH dependence of SNARF present in the vacuole or in extracellular buffers.
Because permeable acids (butyric or pivalic) caused a rapid acidification of cytoplasmic/nuclear areas (see Fig. 7), we tried to exploit this effect for a calibration of cytoplasmic/nuclear fluorescence to pH. Incubation of SNARF-preloaded cells with Mes buffers of different pH containing 5 to 15 mm butyrate resulted in lower fluorescence ratios compared with methylamine-treated vacuoles or detergent-treated cells; however, the ratio-versus-pH curves showed a similar slope once pHext was below a distinct value (Fig. 1). Because the parallel distance between these curves diminished with increasing butyrate (data not shown), this pointed to an incomplete equilibration of cytoplasmic and pHext attributable to limiting concentrations of permeable acid. Consequently, incubation with buffers containing similar concentrations of undissociated, i.e. permeant, butyric or pivalic acid (calculated and adjusted for the given pH), caused the ratio-versus-pH curves to closely approach those of detergent-treated cells, and statistically identical fluorescence ratios were finally reached if, in addition, nigericin and high K+ were present to dissipate K+ gradients (Fig. 1). Whereas not all cells remained intact during the necessary 1- to 2-h incubation (compromised cells were identified as described above), most of the SNARF-accumulating cells approached fluorescence ratios in their cytoplasmic areas that did not significantly differ from those of detergent-treated cells or of vacuolar areas of methylamine-treated cells. Therefore, it appears justified to assume that the fluorescence ratios measured either in the cytoplasm or in the vacuole are based on a similar pH dependence of SNARF and, thus, the same calibration graph could be applied to convert vacuolar and cytoplasmic fluorescence ratios to pH (see Methods).
Figure 7.
Changes of intracellular pH caused by a 10-min treatment with butyric acid. Cell samples were perfused with one-half-concentrated, phosphate-free culture liquid at pHext = 3.8. After a 15-min period of adaptation in which neither pHext nor cytoplasmic/nuclear pH changed significantly, the first image was scanned (t = 0) and the perfusion medium was replaced by 2 mm sodium butyrate in one-half-concentrated, phosphate-free culture liquid pH adjusted to 3.8 with HCl. After another 10 min the perfusion liquid was changed back to the first, butyrate-free medium.
Earlier reports from animal cells suggested shifts of the spectral properties and of the Km of intracellular compared with extracellular SNARF (Owen, 1992), whereas more recent data attribute this phenomenon to a contaminant present in some commercial samples of SNARF (Yassine et al., 1997). In our plant cells no such systematic deviations were observed, but they cannot be excluded completely because of the higher variance of the cytoplasmic versus the extracellular calibration graph (Fig. 1). (It should be noted that all nonvacuolar organelles and proteins of Californian poppy cells are concentrated within a relatively small volume; see Fig. 2.) However, the observed pH sensitivity of cytoplasmic and vacuolar SNARF fluorescence is sufficient to monitor and to compare pH changes of ≤0.15 unit in both compartments.
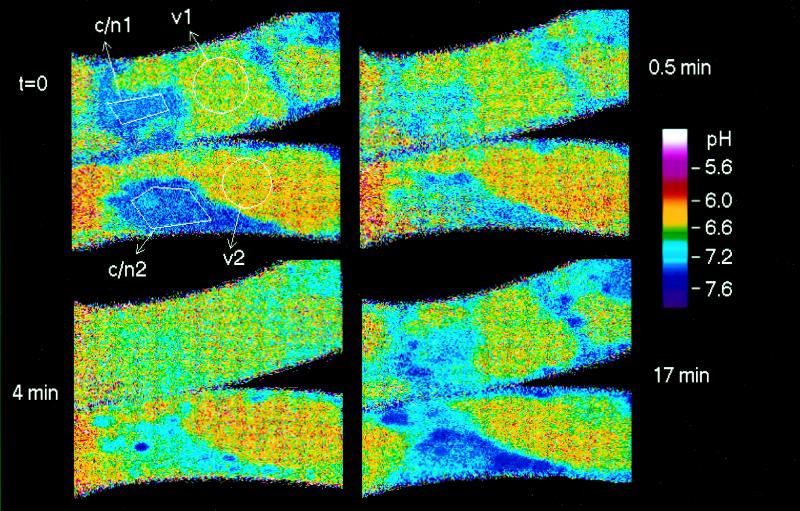
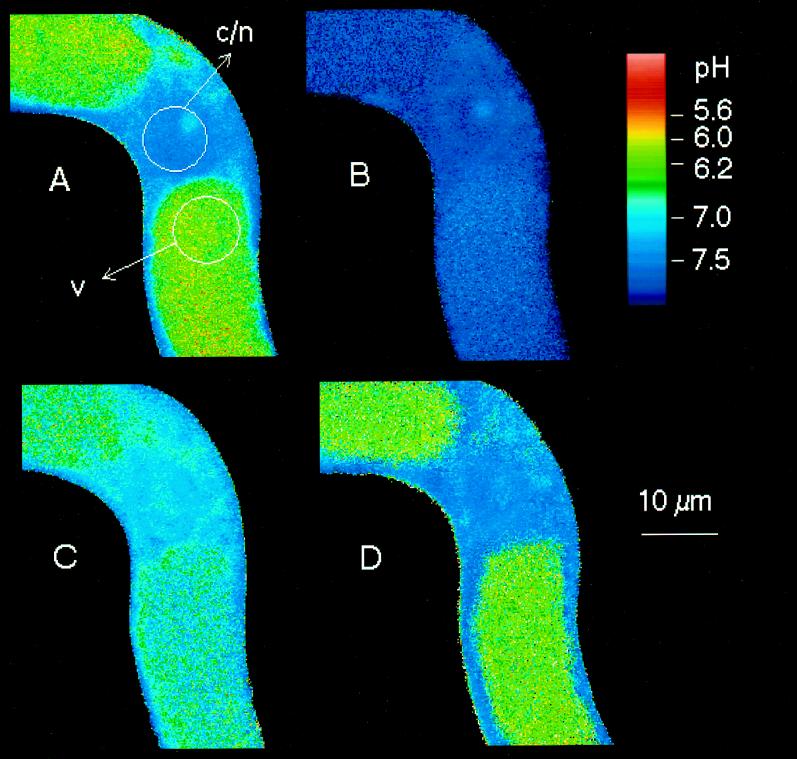
Figure 2.
pH distribution in control cells. Top row, Fluorescence images acquired from channel 2 (left) and channel 1 (right) at the start of the experiment (t = 0), reported in black and white. The averaged intensities (on a scale ranging from 0 to 255) are as follows: channel 1 (right), vacuolar areas 48 to 60, cytoplasmic/nuclear area 150; channel 2 (left), vacuolar areas 25 to 30, cytoplasmic/nuclear area 190; and background, 0.9 to 1.3. Middle row, left, pH map derived by ratioing the above intensity images (channel 1/channel 2). The next two maps were obtained at the times indicated after the start of the first scanning period. This and other micrographs (Figs. 3, 4, 7, and 9) show confocal pH maps of Californian poppy cell strings. The maps display color-coded intensity ratios of individual pixels that are related to pH according to the calibration graph specified in Methods. Two color codes are used: the scale applied to Figures 2, 3, and 9 covers the full range of measurable pH with three colors of varied intensities; and in Figures 4 and 7 more different colors with fewer intensity steps are used to emphasize pH differences between 5.8 and 7.0. Arrows point to analyzed areas within vacuoles (v), cytoplasm (c), or cytoplasmic/nuclear regions (c/n). The presented pH maps are selected from a series of usually six to eight maps scanned within 40 to 60 min. Numbers refer to Figure 5, where the complete pH traces obtained from the selected areas are displayed.
Elicitor treatment neither changed the pH sensitivity of SNARF (Fig. 1, legend) nor caused a rapid decay of this probe: the SNARF fluorescence measured in ethanolic extracts (80%, v/v) of preloaded cells did not decrease after 30 min of elicitor treatment. In this respect, SNARF appeared more stable than pyranine dyes, which have been found to be destroyed by oxidative processes triggered during elicitation of soybean cells (Apostol et al., 1989).
During the period of microscopic examination the scatter of the fluorescence ratio of individual pixels (statistical noise caused by the photomultiplier detectors) remained constant for at least 45 min and then increased slowly because of the loss of fluorescence intensity via leakage of the probe and photobleaching.
In cells of a 4-d culture the mean cytoplasmic pH was in the range of 7.1 to 7.4 (Fig. 2). Usually, the pH in the main part of the nucleus did not differ significantly from that of the surrounding cytoplasm, whereas a distinct region located in the center of the nucleus (probably the nucleolus) frequently exhibited a slightly more acidic pH (Fig. 3, t = 0). The organelles in the cytoplasm (the shape of which was not routinely resolved) usually accumulated different amounts of the probe but did not show significant pH differences (Fig. 2). The vacuolar pH varied markedly between different cells and ranged from 5.5 (lower detection limit) to 6.3. The pH distribution of individual control cells remained sufficiently stable, with a range of variation < 0.2 unit (cytoplasm) and < 0.1 unit (vacuole) for at least 50 min of microscopic observation, including 10 image-scanning periods (Fig. 2).
Figure 3.
Elicitor-triggered changes of intracellular pH in a cell string. Partially purified (1 μg/mL) yeast elicitor (30–100 kD) was added immediately after the first image was scanned. Times are in minutes after addition of elicitor.
Local pH Shifts in Elicited Cells
A series of pH maps of individual cells show dynamic changes triggered by the contact with yeast elicitor (Figs. 3 and 4). Although the velocity and extent of these pH shifts varied between individual cells, two phases were typically observed:
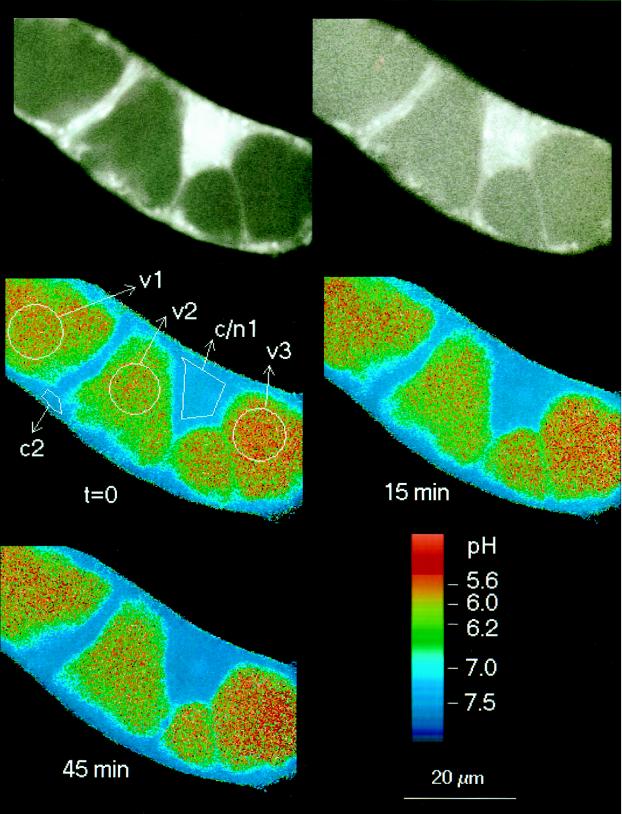
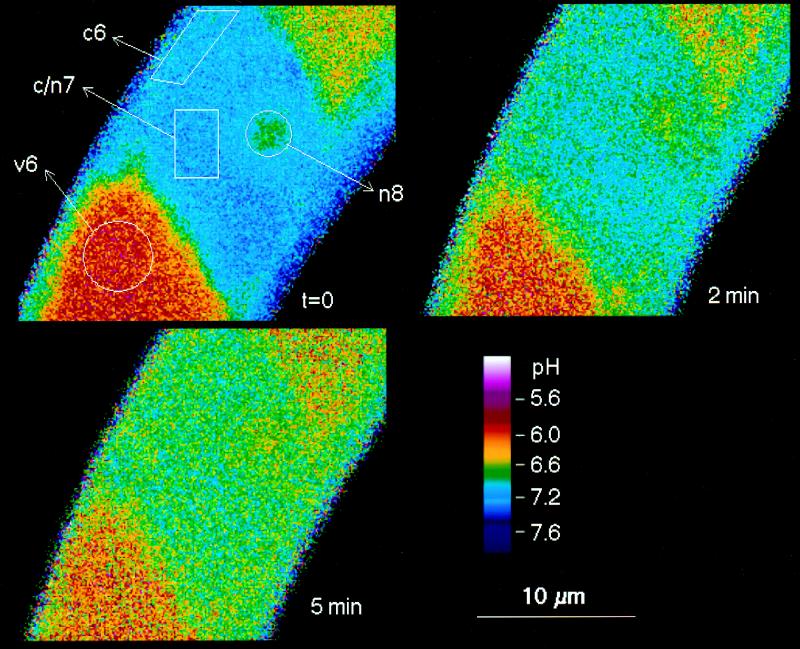
Figure 4.
Detailed pH maps of a single cell during the initial phase of elicitor-triggered pH changes. In addition to vacuolar and cytoplasmic/nuclear areas, a region around the nucleolus is marked (n8). Times are in minutes after the addition of 5 μg/mL crude yeast elicitor.
(a) An acidification of the cytoplasm and of the nuclear region by 0.3 to 0.7 pH unit in parallel with an increase of the vacuolar pH by 0.2 to 0.4 pH unit. These changes usually occurred between 2 and 10 min after elicitor contact. The decrease of pH occurred in parallel in the cytoplasm and in the nucleus (Figs. 3 and 4).
(b) The cytoplasmic/nuclear pH returned toward neutrality and the vacuolar pH toward more acidic values (10–40 min after elicitor contact). As a result, the original pH difference between cytoplasm and vacuole tended to be restored. Although the starting levels of cytoplasmic and vacuolar pH were not completely reestablished in most experiments, a major part of the changes proved to be transient. (Although less likely, it cannot be ruled out that a complete restoration of the original pH gradient is hampered by putative injuries sustained on the cellular pH control system, which might occur during extended periods of microscopic examination [>45 min].)
Conidiospores of the mold P. cyclopium used as a “natural” elicitor caused similar changes of the intracellular pH distribution, as did the yeast preparation, but only after a lag phase of 5 to 15 min (Fig. 5). Both living and dead (autoclaved) spores were active. This response occurred with high sensitivity: pH shifts were still detectable in the presence of 2 × 105 spores/mL (approximately 1 spore per eight Californian poppy cells).
Figure 5.
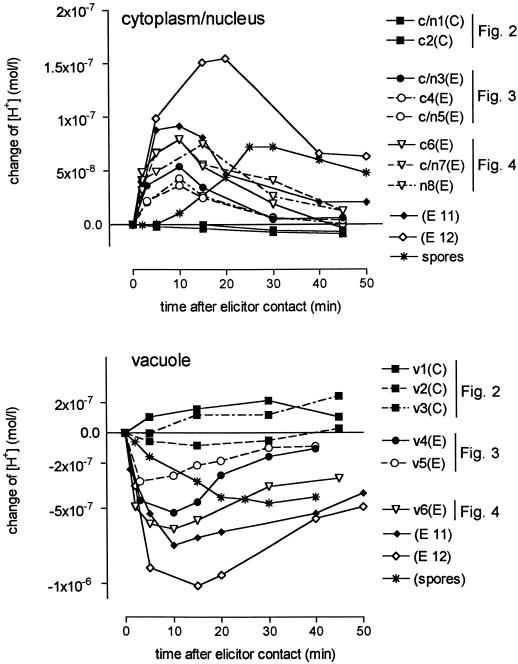
A collection of pH traces measured in control and elicitor-treated cells. y axis, Increase of proton concentration compared with time 0, i.e. the time of adding elicitor or the start of the first scanning period (control cells). Some of the data are derived from the pH maps of Figures 2, 3, and 4. This is indicated together with the corresponding area numbers. Analyzed areas of the same cell are represented by similar symbols, e.g. c/n3 and v4. (E), Elicitor treated; (C), control cell; c, cytoplasmic area; c/n, cytoplasmic area including the nucleus; and n, nucleus. Elicitor concentrations: ▪ (control), no elicitor; •, 1 μg/mL purified yeast elicitor, 30 to 100 kD; ▿, ♦, and ⋄, 5 μg/mL crude yeast elicitor; ✽, conidiospores of P. cyclopium (autoclaved), 6 × 105/mL.
The percentage of living (i.e. SNARF-accumulating) cells that reacted to either yeast elicitor or spores of P. cyclopium with detectable pH changes can be roughly estimated as 80%; this figure results from 35 series of pH maps obtained with individual cells or cell strings from different batches of the Californian poppy cell culture. Because the transient acidification is usually spread over the whole cytoplasmic/nuclear area, it is likely that most if not all cytoplasmic organelles take part in these changes.
Figure 5 summarizes the dynamics of cytoplasmic/nuclear and vacuolar pH values in elicited cells by comparing the data of independent experiments. The measured pH changes were corrected for the different starting pH values of vacuolar and cytoplasmic/nuclear areas and plotted as changes of proton concentrations. By comparing such changes between the vacuole and the cytoplasmic/nuclear region of the same cell (these are marked by similar symbols) both a parallel time course and a rough correlation between the magnitudes of proton loss from the vacuole and of acidification of cytoplasmic/nuclear areas become apparent; the maximum loss of vacuolar proton concentration amounts to approximately 8 to 10 times the maximum increase of protons in the extravacuolar areas of the same cell. Hence, at a given elicitor concentration the extent of cytosolic acidification depends on the actual difference of pH between cytoplasm and vacuole (Fig. 5, traces ▿, ♦, and ⋄).
Relationship of Intracellular pH Shifts to the Elicitation of Alkaloid Production
Dependence on the Elicitor Concentration
The extent of the intracellular pH shifts and the increase of alkaloid formation showed a similar dependence on the elicitor concentration; both effects strongly increased in the range from 0.1 to 1 μg/mL and showed little increments at higher elicitor concentrations (Fig. 6). This correlation was not changed if a 30- to 100-kD fraction obtained by ultrafiltration was used instead of the crude elicitor preparation.
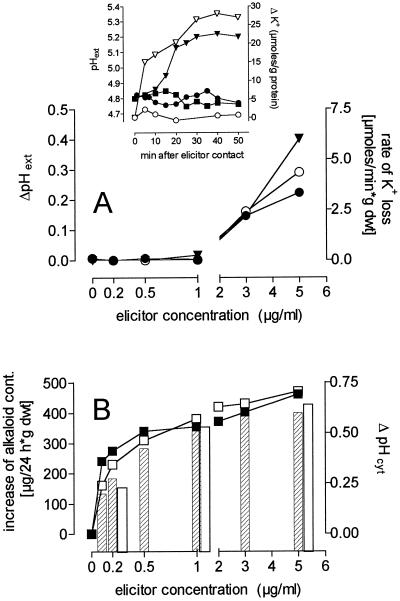
Figure 6.
Concentration dependence of elicitor-triggered effects. The effects of the crude elicitor preparation (closed symbols and hatched columns) and of the 30- to 100-kD elicitor fraction (open symbols and open columns) are demonstrated with respect to the following criteria: A, The maximum increase of pH of the growth medium seen within 50 min (▾, left ordinate) and the initial rate of increase of external K+ (•, ○, right ordinate; data were obtained by nonlinear regression of efflux curves such as those shown in the inset). The changes of external K+ content and pHext (if any) of elicitor-free control cultures were subtracted from the measured data. Inset, pHext and changes of the K+ concentration in the outer medium of elicited cell suspensions; pHext (left ordinate): ▪, control; •, elicitor 1 μg/mL; ▾, elicitor 5 μg/mL; external K+ content (right ordinate): ○, elicitor 1 μg/mL; ▿ elicitor 5 μg/mL. Data from elicitor-free control suspensions were subtracted. B, The maximum decrease of cytosolic pH (pHcyt) seen within 30 min (columns, right ordinate) and the increase of total alkaloid content within 24 h (▪, □, left ordinate). Each data point is averaged from three to four measurements, and sd values are as follows: pHcyt, 0.03 unit; alkaloid content, 6% to 9%; pHext, 0.05 unit; K+ content, 10% to 17%. The alkaloid formation of elicitor-free control suspensions was subtracted. Three independent experiments with 5-d cell suspensions yielded similar results. dwt, Dry weight.
An Artificially Triggered Decrease of Cytoplasmic pH Can Increase Alkaloid Production
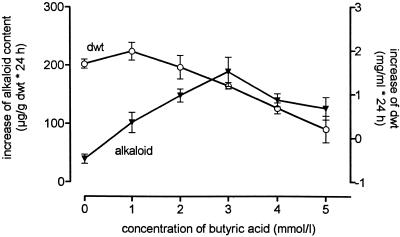
The data presented above raise the question of whether a decrease of the cytoplasmic pH alone is sufficient to produce a signal for the induction of alkaloid biosynthesis. By using a 10-min treatment with butyric acid (a membrane-permeable acid that has frequently been used to experimentally decrease the intracellular pH of various cell types; see Reid et al., 1989; Guern et al., 1992) we were able to evoke a transient acidification of the cytoplasm (Fig. 7). Under optimized conditions (2 mm butyrate, pHext = 3.8) this treatment triggered the formation of benzophenanthridine alkaloids without a significant inhibition of the growth rate of the cells (Fig. 8). Similar findings were obtained with pivalic (trimethylacetic) acid (Table I). These data imply a similar relation of cytoplasmic acidification and an increase of alkaloid production, as found with the yeast elicitor, but a detailed analysis of artificial acidification and its effects on the induction of alkaloid biosynthesis has yet to be done. The present data indicate that cytoplasmic acidification alone is sufficient to induce the biosynthesis of benzophenanthridine alkaloids in the cell strain used.
Figure 8.
Changes of alkaloid content and growth rate after treatment with butyric acid. Four-day cell suspensions with pHext = 3.8 were treated with different concentrations of sodium butyrate as described in Methods. Alkaloid content (▾) and dry weight (○, dwt) were assayed after 24 h. All data are differences between 0 and 24 h. sd values are given for n = 5.
Depleting the Vacuolar Proton Pool Prevents the Effect of Elicitors
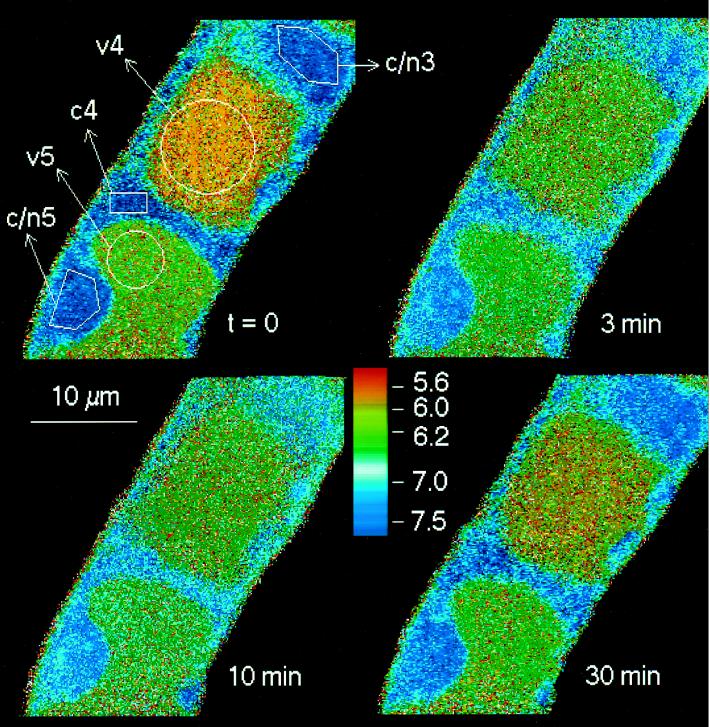
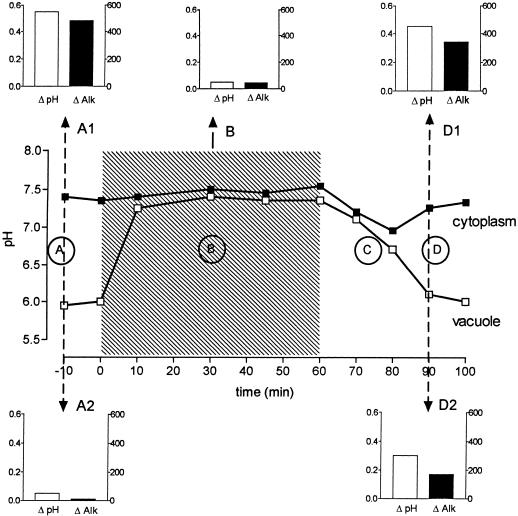
In an alternative approach we designed experimental conditions that prevented elicitor-triggered cytoplasmic acidification and investigated the effect of such treatment on the elicitation process. For this purpose we took advantage of an acid-extrusion mechanism that is induced by adjusting the pHext of cell suspensions to near-neutral pH. Preliminary experiments had shown that exposure to strong buffers, e.g. 80 mm Hepes, pH 7.4, led to an almost complete loss of vacuolar acidity within 30 to 40 min; this effect proved to be reversible at 40 min after resuspension in normal culture liquid. If the nonmetabolized ammonium analog methylammonium (80 mm) was present at the same pHext (pKa of CH3NH2 = 10.4; hence, the concentration of the membrane-permeable, unprotonated methylamine at pH 7.4 was around 80 μm), the cells reacted even faster: within 10 min the vacuolar acidity disappeared almost completely and the cytosolic pH stayed at 7.4 to 7.5. In a perfusion chamber, this steady state could be maintained for more than 60 min; the original pH distribution was restored within 25 to 30 min after the removal of methylamine (Fig. 9).
Figure 9.
Reversible loss of vacuolar acidity by treatment with methylamine at pHext = 7.4. Cell samples from 5-d suspensions were perfused with original culture liquid (pHext = 4.0) for 10 min. Immediately after the first image (map A, time 0) was scanned, the perfusion medium was replaced by one-half-concentrated, phosphate-free culture liquid containing 80 mm methylamine and pH adjusted to 7.4 with HCl. After 60 min the perfusion liquid was changed for one-half-concentrated, phosphate-free culture liquid and the perfusion was continued for another 30 min. The selected pH maps were obtained at time 0, i.e. the beginning of methylamine treatment (A); at 20 min, i.e. during methylamine treatment (B); at 80 min, i.e. 20 min after the end of methylamine treatment (C); and at 100 min, i.e. 40 min after the end of methylamine treatment (D).
Experiments including methylamine treatment of different durations were performed in parallel with individual cell strings perfused under the confocal microscope and cell suspensions in identical culture media. A typical set of experiments is summarized in Figure 10. During the period of clamping the intracellular pH close to 7.4, the addition of elicitor did not cause any detectable cytosolic or nuclear acidification, and the increase of alkaloid production seen after 24 h in the same cell suspension reached ≤10% of an elicited standard culture (subgraph B, similar findings in four of four experiments).
Figure 10.
pH distribution and effects of yeast elicitor as affected by methylamine treatment. Similar to the pH-mapping experiment described in Figure 9, cells from 5-d cultures were washed with and resuspended (approximately 70 mg fresh weight/mL) in 100 mL of one-half-concentrated, phosphate-free culture liquid. After 10 min (time 0) the medium was replaced with the same liquid containing in addition 80 mm methylamine pH adjusted to 7.4. After 60 min cells were washed twice with and resuspended in one-half-concentrated, phosphate-free culture liquid. At the times indicated by arrows, two 10-mL samples were withdrawn and one of them received 1 μg/mL purified yeast elicitor (30–100 kD). After another 30 min both the elicited and the unelicited samples were washed twice with and resuspended in one-half-concentrated, phosphate-free culture liquid. All suspensions were shaken at 24°C and 200 rpm. Alkaloid content and dry weight were assayed for 20 h after the last replacement. Main panel, pH traces of the cytoplasmic/nuclear area (▪) and the vacuolar area (□) marked in the pH maps of Figure 9; letters refer to the corresponding time points. Hatched area, Period of methylamine treatment. Subgraphs, top row, Changes of cytoplasmic pH and alkaloid content caused by 1 μg/mL elicitor; left ordinates, change in pH (units); right ordinates, change in alkaloid (μg/g dry weight). Subgraphs, bottom row, Changes of cytoplasmic pH and alkaloid content in the elicitor-free samples; left ordinates, change in pH (units); right ordinates change in alkaloid (μg/g dry weight). The increase of alkaloid content of unelicited suspensions was subtracted from that of elicitor-treated cultures.
After a recovery period of 20 to 30 min in normal culture liquid the cells responded to elicitor contact with both pH shifts and enhanced alkaloid production (Fig. 10, subgraph D1) that were within the limits of a standard eliciting experiment (Fig. 10, subgraph A1). Hence, the ability to elicit alkaloid biosynthesis under normal culture conditions appears to require a minimum pH gradient between the vacuole and the cytoplasm.
The pH traces shown in Figure 10 reveal a phase in which the cells generated substantial amounts of new acidity (trace C): during the recovery period after methylamine treatment, the pH of the cytoplasmic/nuclear area decreased transiently by around 0.3 unit in parallel with the vacuolar pH (see Fig. 9, map C); in the subsequent phase cytosolic protons disappeared and the pH distribution between the vacuole and the cytoplasm approached the control level (see Fig. 9, map D). The transient acidification of cytoplasmic/nuclear areas under such conditions was close to the level produced by normal cells after contact with 0.2 μg/mL yeast elicitor. If pH change is an inducing signal for alkaloid biosynthesis, this recovery process must be expected to lead to increased alkaloid production. This was indeed the case, as shown by a comparison between cell suspensions that had received a 1-h methylamine treatment and control suspensions (Fig. 10, subgraphs A2 and D2). According to the range of alkaloid-inducing pH shifts outlined in Figure 6, the pH stimulus derived from the recovery mechanism after methylamine treatment (around 0.3 unit) would not be saturating, and hence the response can be expected to increase further by the subsequent addition of elicitor (Fig. 10, subgraphs D2 and D1: the sum of alkaloid data of both graphs equals the total increase of alkaloid content found in a culture elicited immediately after recovery from methylamine treatment).
Efflux of K+ Is Not Instrumental to pH Shifts and Induction of Alkaloid Production
An efflux of K+ is one of the early responses of plant cells to elicitors (e.g. Mathieu et al., 1991). In some plant cells it shows close relations to other events of the hypersensitive reaction (Nürnberger et al., 1994). In the Californian poppy cell suspensions used in this study a significant increase of external K+ was measurable only at elicitor concentrations > 1 μg/mL, whereas shifts of the intracellular pH and stimulation of alkaloid biosynthesis were detected at one-tenth of this concentration (Fig. 6). Thus, K+ efflux was elicited with lower sensitivity than the elicitor-triggered pH shifts; this finding is in agreement with data that argue against an involvement of K+ efflux in the triggering of alkaloid biosynthesis:
(a) The butyric acid treatment described above that induced alkaloid biosynthesis (see Figs. 7 and 8) did not evoke a measurable loss of K+ (Table I).
(b) The K+ ionophore valinomycin at concentrations around 1 μm triggered a significant efflux of K+ but did not significantly influence alkaloid formation (Table I). Only small pH shifts (<0.2 unit) were observed at this valinomycin concentration. Valinomycin concentrations ≥ 5 μm led to an increase of alkaloid production (Table I). This elicitor-like effect was preceded by strong pH shifts (acidification of the cytoplasm and parallel increase of the pH in vacuolar regions) that appeared immediately after contact with this compound and were not reversible within 1 h even by replacing the external medium with ionophore-free culture liquid (Table I).
The last findings suggest that the loss of cellular K+ is not a necessary step in the induction of alkaloid biosynthesis. (They do not, of course, exclude the possibility that a small efflux of K+, which would escape the experimental detection because of rapid reuptake, might contribute to the elicitor-triggered signaling.) The loss of K+ above a critical rate causes strong cytosolic acidification, which is likely to mediate the effect of high valinomycin concentrations on alkaloid biosynthesis. The pH shifts triggered by valinomycin evoked a significantly weaker response in alkaloid formation compared with pH shifts of similar magnitude triggered by either yeast elicitor or butyric acid (Table I). Similar findings were obtained with 5 μm nigericin, which caused a strong cytoplasmic acidification but only moderate increases of alkaloid content and cytotoxic effects, as suggested by a gradual loss of preaccumulated SNARF (data not shown). This argues for an inhibiting effect of a strong K+ loss on the alkaloid-inducing and/or -producing pathway.
External Alkalinization Is Not Involved in the Elicitation of Alkaloid Biosynthesis
The alkalinization of the outer medium is another well-known early response of plant cell cultures to elicitor contact (Mathieu et al., 1991, 1996a; Nürnberger et al., 1994; Granado et al., 1995). In our cell culture pHext typically increased from 3.8 to 4.2 for 30 min after the addition of 5 μg/mL yeast elicitor. However, this effect was completely absent if elicitor concentrations around 1 μg/mL were used, i.e. concentrations that nearly saturated both the pH and the alkaloid response. This finding showed an obvious similarity to the dependence of the K+ efflux on the elicitor concentration (Fig. 6).
Furthermore, even at high elicitor concentrations the external alkalinization was observed only if the pHext was acidic. In media buffered to pH 7.4 with a broad range of buffer strengths (5–60 mm Hepes or Mes), we never observed an alkalinization after elicitor contact. In all cases the cells responded to the exposure to near-neutral buffers with an acidification of the medium that was not influenced by the presence of elicitor. For instance, 10 min after resuspending cells (100 mg fresh weight/mL) in one-half-concentrated culture liquid containing 50 mm Mes, pH 7.4, the pHext decreased to 7.0. Therefore, it appears that an influx of external protons is required neither for the acidification of the cytoplasm nor for the elicitation of alkaloid biosynthesis. This corresponds to the findings of Granado et al. (1995), who qualified the external alkalinization as a nonspecific response of plant cells to fungal pathogens.
Even in the presence of strong external buffers at pH 7.4, cytoplasmic acidification and subsequent increase of alkaloid biosynthesis could be triggered by elicitor contact as long as the vacuole maintained a minimum pH difference toward the cytosol of approximately 0.4 to 0.5 unit, as established by parallel pH mapping of cells perfused with the same medium. (Such a pH difference was found, e.g. 30 min after addition of 50 mm Mes, pH 7.4, and 30 min after adding methylamine and sodium butyrate, each 40 mm, buffered to pH 7.4, with HCl; data not shown.)
DISCUSSION
The data presented indicate a close correlation between the elicitation of benzophenanthridine alkaloid biosynthesis and shifts of the intracellular pH distribution in cell suspensions of Californian poppy.
Various stimuli that induced overproduction of alkaloids share the ability to evoke a transient acidification of the cytoplasm and the nucleus: a yeast elicitor preparation, conidia of P. cyclopium, treatment with butyric or pivalic acid, and restoration of cellular acid production after depletion of vacuolar acidity.
Under these conditions the degree of the cytoplasmic/nuclear acidification correlated with the amount of overproduced alkaloids with a saturating level of pH shift of around 0.6 unit. Furthermore, the reversible depletion of the vacuolar proton pool by external buffers or methylamine prevented the effects of the yeast elicitor both on the cytoplasmic pH and on the alkaloid production. Thus, cytosolic acidification appears to be both a necessary and a sufficient signal for the induction of alkaloid biosynthesis in this cell-suspension culture.
It remains to be demonstrated whether the compound(s) responsible for the pH shift and the induction of alkaloid biosynthesis are identical, because an individual elicitor compound that causes an alkaloid response of Californian poppy has not yet been purified to homogeneity from our yeast preparation. Nevertheless, the correlations described above, the similar dependence on the elicitor concentration of pH-shifting and alkaloid-inducing activities, and the fact that partial purification via ultrafiltration did not change the relation between these activities argue that the ability to affect the cytosolic pH determines the alkaloid-inducing activity of the elicitor preparation.
The effects of the yeast elicitor preparation on intracellular pH were transient in nature (at least partially); cytotoxic effects of the elicitor preparation could be fairly excluded up to near-saturating concentrations, as suggested by the lack of effect on growth rate and the accumulation of viability probes. Tentative experiments have shown that the elicitation of alkaloid biosynthesis requires the presence of metabolizable carbon sources in the medium (G. Leschke and W. Roos, unpublished data). Hence, the observed pH shifts and the increase of alkaloid biosynthesis found under our experimental conditions probably represent activities of intact and metabolizing rather than compromised cells.
The production of benzophenanthridine alkaloids could be triggered independently of a net efflux of K+, alkalinization of the outer medium, and formation of polyphenols (browning). The latter reaction proved to depend on the liberation of H2O2, as suggested by its disappearance in the presence of catalase. Because these reactions are known as signals or components of the hypersensitive response or other, widely distributed defense reactions of plant cells, it seems justified to assume that the elicitation of alkaloid formation in the Californian poppy cell culture is a specific defense response to low elicitor concentrations that are subcritical for the elicitation of other defense mechanisms, including the complex hypersensitive reaction, which usually leads to cell death. The ability to trigger a specific phytoalexin-producing pathway before an all-or-nothing response such as the hypersensitive reaction appears to be advantageous, because it would enable a “flexible response” without an inseparable link to local cell death. The alkaloids produced by Californian poppy cells are good candidates for such a defense reaction, because they are nontoxic for intact, producing cells. Further work is required to substantiate this idea, especially with regard to the individual elicitor molecule(s) involved and the metabolic fate of the produced alkaloids.
The transient decrease of the cytoplasmic pH is an elicitor-triggered signal that the Californian poppy cell culture has in common with some other well-investigated plant cells (Kneusel et al., 1989; Mathieu et al., 1991, 1996a, 1996b; Kuchitsu et al., 1997). However, the origin of the extra protons appearing in the cytoplasm appears to be different: in Californian poppy cells challenged by low concentrations (≤1 μg/mL) of yeast elicitor, the acidification of the cytoplasmic/nuclear area involves a net influx of protons from the vacuolar pool. This is indicated by the corresponding time course of proton loss from the vacuole and increase of cytoplasmic protons and the observed correlation between the magnitudes of both changes, which implies that 8 to 10 protons are lost from the vacuole for every extra proton appearing in the cytoplasmic/nuclear space. Such a relation points to the proton gradient between the cytosol and the vacuole as a driving force of elicitor-triggered acidification of the cytoplasm. Because no net influx of external protons contributes to the acidification process (see above), the metabolism of acids can be considered as a possible additional, but so far speculative, source of cytoplasmic acidification.
In contrast, the intracellular acidification that precedes the hypersensitive response is likely to include the influx of protons from the outer medium, as suggested by its concomitant alkalinization. In a recent study a close relationship of cytoplasmic acidification and extracellular alkalinization was established for tobacco cells challenged with distinct oligogalacturonides (Mathieu et al., 1996b). These data correspond well with the changes of the pHext of our Californian poppy cell suspensions challenged with ≤5 μg/mL yeast elicitor (see Fig. 6). (Because in the former study pH changes were monitored by the uptake of [14C]benzoic acid, a permeable acid that is mainly trapped in the cytoplasm, parallel changes of the vacuolar pH [if any] probably would have gone undetected.) Using in vivo 31P-NMR spectroscopy, Kuchitsu et al. (1997) found cytoplasmic acidification in cultured rice cells triggered by N-acetylchitooligosaccharides. This effect correlated with external alkalinization and efflux of K+, depending on the chain length of the chitin fragments, but no concomitant changes of the vacuolar pH were observed.
The search for a mechanism that relays the elicitor signal received at the plasma membrane to the vacuole of Californian poppy cells is a goal of further work in this laboratory. Our present data would be consistent with an inhibition of the vacuolar ATPase caused after elicitor contact. Tentative experiments have shown that bafilomycin, a specific inhibitor of the vacuolar ATPase, is able to cause both cytoplasmic acidification and increased alkaloid biosynthesis. Further work is required to substantiate whether such an inhibiting effect is really exerted by the yeast elicitor. Nevertheless, the use of the vacuolar reservoir of H+ rather than extracellular acidity in a signaling mechanism appears straightforward from the point of view of pathogen defense, because it would keep the responsiveness of the cell independent of the environmental pH and its possible perturbations.
Although all of our experiments were conducted in the presence of 1 mm external Ca2+, changes of the intracellular availability of this ion in relation to the signaling effects of intracellular pH deserve further interest. In Sanguinaria canadensis, the elicitation of benzophenanthridine alkaloids proved to depend on an external source of Ca2+ (Mahady and Beecher, 1994).
ACKNOWLEDGMENTS
We thank Prof. Dr. M.H. Zenk (University of Munich) for providing us with the Californian poppy cell culture and for his helpful suggestions in the initial phase of the work. The help of Dr. Florian Helbig (this department) with the HPLC separation of the alkaloids and of Tewabech Minelek and Undine Staab (both from this department) with the corroboration of the catalase and butyric acid treatment is gratefully acknowledged.
Abbreviations:
- CTAB
cetyltrimethylammonium bromide
- pHext
external pH
- SNARF
carboxy-seminaphthorhodafluor-1-acetoxymethylester
Footnotes
The work was funded by the Deutsche Forschungsgemeinschaft (no. SFB 363) and supported by the Fonds der Chemischen Industrie.
LITERATURE CITED
- Apostol I, Heinstein PF, Low PS. Rapid stimulation of an oxidative burst during elicitation of cultured plant cells. Plant Physiol. 1989;90:109–116. doi: 10.1104/pp.90.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach M, Schnitzler JP, Seitz HU. Elicitor-induced changes in Ca2+influx, K+ efflux, and 4-hydroxybenzoic acid synthesis in protoplasts of Daucus carota L. Plant Physiol. 1993;103:407–412. doi: 10.1104/pp.103.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt MR, Thiel G. Hormonal control of ion channel gating. Annu Rev Plant Physiol. 1993;44:543–567. [Google Scholar]
- Bottin A, Veronesi C, Pontier D, Esquerre-Tugayé MT, Blein JP, Rusterucci C, Ricci P. Differential responses of tobacco cells to elicitors from two Phytophthora species. Plant Physiol Biochem. 1994;32:373–378. [Google Scholar]
- Brauer D, Uknalis J, Triana R, Shu-I T. Effects of external pH and ammonium on vacuolar pH in maize root cells. Plant Physiol Biochem. 1997;35:31–39. doi: 10.1104/pp.113.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castoria R, Altamura MM, Fabbri AA, Tomassi M, Fanelli C. Interrelationships between browning and phytoalexin accumulation elicited by arachidonic acid. J Plant Physiol. 1995;145:209–214. [Google Scholar]
- Davis D, Merida J, Legendre L, Low PS, Heinstein P. Independent elicitation of the oxidative burst and phytoalexin formation in cultured plant cells. Phytochemistry. 1993;32:607–611. [Google Scholar]
- Dzink JL, Socransky SS. Comparative in vitro activity of sanguinarine against oral microbial isolates. Antimicrob Agents Chemother. 1985;27:663–665. doi: 10.1128/aac.27.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebel J, Cosio EG. Elicitors of plant defense response. Int Rev Cytol. 1994;448:1–36. [Google Scholar]
- Granado J, Felix G, Boller T. Perception of fungal sterols in plants. Subnanomolar concentrations of ergosterol elicit extracellular alkalinization in tomato cells. Plant Physiol. 1995;107:485–490. doi: 10.1104/pp.107.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guern J, Mathieu Y, Thomine S, Jouanneau JP, Beloeil JC. Plant cells counteract cytoplasmic pH changes but likely use these pH changes as secondary messages in signal perception. Curr Top Plant Biochem Physiol. 1992;11:249–269. [Google Scholar]
- Gundlach H (1992) Das Abwehrsystem der Pflanzen: Reinigung eines Elicitors und Induktion von Sekundärstoffen in pflanzlichen Zellkulturen. PhD thesis. University of Munich, Germany
- Gundlach H, Müller MJ, Kutchan TM, Zenk MH. Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proc Natl Acad Sci USA. 1992;89:2389–2393. doi: 10.1073/pnas.89.6.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R, Dietrich P. Plant K+ channels: similarity and diversity. Bot Acta. 1996;105:94–101. [Google Scholar]
- Horn MA, Meadows RP, Apostol I, Jones CR, Gorenstein DG, Heinstein PF, Low PS. Effect of elicitation and changes in extracellular pH on the cytoplasmic and vacuolar pH of suspension-cultured soybean cells. Plant Physiol. 1992;98:680–686. doi: 10.1104/pp.98.2.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneusel RE, Matern U, Nicolay K. Formation of trans-4-coumaryl-CoA by Zn2+-dependent enzymes in cultured plant cells and its activation by an elicitor-induced pH shift. Arch Biochem Biophys. 1989;269:455–462. doi: 10.1016/0003-9861(89)90129-x. [DOI] [PubMed] [Google Scholar]
- Kombrink E, Somssich IE. Defense responses of plants to pathogens. Adv Bot Res. 1995;21:1–34. [Google Scholar]
- Kuchitsu K, Yazaki Y, Sakano K, Shibuya N. Transient cytoplasmic pH change and ion fluxes through the plasma membrane in suspension-cultured rice cells triggered by N-acetylchitooligosaccharide elicitor. Plant Cell Physiol. 1997;38:1012–1018. [Google Scholar]
- Lamb C, Dixon RA. The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:251–275. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon RA, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Linsmaier EM, Skoog F. Organic growth factor requirements of tobacco tissue cultures. Physiol Plant. 1965;18:100–127. [Google Scholar]
- Mahady GB, Beecher CWW. Elicitor-stimulated benzophenanthridine alkaloid biosynthesis in bloodroot suspension cultures mediated by calcium. Phytochemistry. 1994;37:415–419. [Google Scholar]
- Mathieu Y, Kurkdjian A, Xia H, Guern J, Koller A, Spiro MD, O'Neill M, Albersheim P, Darvill A. Membrane responses induced by oligogalacturonides in suspension-cultured tobacco cells. Plant J. 1991;1:333–343. doi: 10.1046/j.1365-313X.1991.t01-10-00999.x. [DOI] [PubMed] [Google Scholar]
- Mathieu Y, Lapous D, Thomine S, Lauriére C, Guern J. Cytoplasmic acidification as an early phosphorylation dependent response of tobacco cells to elicitors. Planta. 1996a;199:416–424. [Google Scholar]
- Mathieu Y, Sanchez FJ, Droillard M-J, Lapous D, Lauriére C, Guern J. Involvement of protein phosphorylation in the early steps of transduction of the oligogalacturonide signal in tobacco cells. Plant Physiol Biochem. 1996b;34:399–408. [Google Scholar]
- Müller MJ, Brodschelm W, Spannagl E, Zenk MH. Signalling in the elicitation process is mediated through the octadecanoid pathway leading to jasmonic acid. Proc Natl Acad Sci USA. 1993;90:7490–7494. doi: 10.1073/pnas.90.16.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nennstiel TD, Jabs T, Sacks W, Nürnberger R, Hahlbrock K, Scheel D. High affinity binding of a fungal oligopeptide elicitor to parsley plasma membranes triggers multiple defense responses. Cell. 1994;78:449–460. doi: 10.1016/0092-8674(94)90423-5. [DOI] [PubMed] [Google Scholar]
- Nürnberger T, Wirtz W, Nennstiel D, Hahlbrock K, Jabs T, Zimermann S, Scheel D. Signal perception and intracellular signal transduction in plant pathogen defense. J Recept Signal Transduct Res. 1997;17:127–136. doi: 10.3109/10799899709036598. [DOI] [PubMed] [Google Scholar]
- Ojalvo I, Rokem JS, Navon G, Goldberg I. 31P NMR study of elicitor treated Phaseolus vulgaris cell suspension cultures. Plant Physiol. 1987;85:716–719. doi: 10.1104/pp.85.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen C (1992) Comparison of spectrum-shifting intracellular pH probes 5′ (and 6′)-carboxy-10-dimethylamino-3-hydroxyspiro[7h-benzo[c]xanthene-7,1′(3′h)-isobenzofuran]-3′-one and 2′,7′-biscarboxyethyl-5(and 6)-carboxyfluorescein. Anal Biochem 204 65–71 [DOI] [PubMed]
- Pönitz J, Roos W. A glucose-activated electron transfer system in the plasma membrane stimulates the H+-ATPase in Penicillium cyclopium. J Bacteriol. 1994;176:5429–5438. doi: 10.1128/jb.176.17.5429-5438.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid RJ, Smith FA, Whittington J. Control of intracellular pH in Chara corallina during uptake of weak acid. J Exp Bot. 1989;40:883–891. [Google Scholar]
- Ricci P, Panabieres F, Bonnet P, Maia N, Ponchet M, Devergne JC, Marais A, Cardin L, Milat M, Blein JP (1993) Proteinaceous elicitors of plant defense responses. In B Fritig, M Legrand, eds, Mechanisms of Plant Defense Responses. Kluwer Academic Publishers, The Netherlands, pp 121–135
- Roos W. Confocal pH-topography of plant cells: acidic layers in the peripheral cytoplasm and the apoplast. Bot Acta. 1992;105:253–259. [Google Scholar]
- Roos W, Schulze R, Steighardt J. Dynamic compartmentation of vacuolar amino acids in Penicillium cyclopium. J Biol Chem. 1996;272:15849–15855. doi: 10.1074/jbc.272.25.15849. [DOI] [PubMed] [Google Scholar]
- Schumacher HM, Gundlach H, Fiedler F, Zenk MH. Elicitation of benzophenanthridine alkaloid synthesis in Eschscholtzia cell cultures. Plant Cell Rep. 1987;6:410–413. doi: 10.1007/BF00272770. [DOI] [PubMed] [Google Scholar]
- Sutherland MW. The generation of oxygen radicals during host plant responses to infection. Physiol Mol Plant Pathol. 1991;39:79–93. [Google Scholar]
- Tognioli L, Basso L. The fusicoccin-stimulated phosphorylation of a 33 kDa polypeptide in cells of Acer pseudoplatanus as influenced by extracellular and intracellular pH. Plant Cell Environ. 1987;10:233–239. [Google Scholar]
- Van der Veen R, Heimovaara-Dijkstra S, Wang M. The cytosolic alkalinization mediated by abscisic acid is necessary, but not sufficient for abscisic acid-induced gene expression in barley aleurone protoplasts. Plant Physiol. 1992;100:699–705. doi: 10.1104/pp.100.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J, Knipling L. Antimicrotubule properties of benzophenanthridine alkaloids. Biochemistry. 1993;32:13334–13339. doi: 10.1021/bi00211a047. [DOI] [PubMed] [Google Scholar]
- Yassine M, Salmon JM, Vigo J, Viallet P. Carboxy-SNARF-1 as a pHi fluoroprobe: discrepancies between conventional and intracellular data do not result from protein interactions. J Photochem Photobiol. 1997;37:18–25. [Google Scholar]