Abstract
Lamellar Ichthyosis (LI) is a form of congenital ichthyosis that is caused by mutations in the TGM1 gene that encodes for the transglutaminase 1 (TG1) enzyme. Functional inactivation of TG1 could be due to mutations, deletion or insertions. In this study, we have screened 16 patients affected by LI and found six new mutations: two transition/transversion (R37G, V112A), two nonsense mutations and two putative splice site both leading to a premature stop codon. The mutations are localized in exons 2 (N-terminal domain), 5, 11 (central catalytic domain), and none is located in the two beta-barrel C-terminal domains. In conclusion, this study expands the current knowledge on TGM1 mutation spectrum, increasing the characterization of mutations would provide more accurate prenatal genetic counselling for parents at-risk individuals.
Keywords: transglutaminase 1, keratinocytes, ichthyosis, differentiation, mutation
The epidermis is a multi-layered, stratified epithelium that provides a physical barrier for the organism, protecting it from pathogens and dehydration.1 The epidermis is continuously regenerated by terminally differentiated keratinocytes2, 3, 4, 5, 6, 7 that migrate from the inner basal layer (proliferative compartment) to the outer cornified layer (terminal differentiated compartment): this process is known as cornification, or formation of the cornified envelope. This requires a complex balance between the proliferating compartment, the differentiation compartment and the cornified envelope, which exert its protective barrier function in the epidermis. In addition to this terminal differentiation, another type of cell death occurs in the skin, namely programmed cell death or apoptosis.8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22 Apoptosis is a developmental remodelling programme and a defensive, organized self-destruction of the cell in reaction to severe damage.
In several genetic disorders, such as ichthyosis, the corneification process is altered due to mutations in key components of the keratinocyte differentiation machinery. Autosomal recessive congenital ichthyosis (ARCI) is a rare heterogeneous keratinization disorder of the skin. According to the results of the ‘First Ichthyosis Consensus Conference' in Sorèze in 2009, in which the nomenclature and classification of inherited ichthyoses has been revised, the disease is clinically and genetically identified including harlequin ichthyosis, lamellar ichthyosis (LI), non-bullous congenital ichthyosiformis and congenital ichthyosiformis.23 Valquist24 recently also speculated on a new umbrella term for ARCI disease represented by noticeable phenotypic changing in early childhood and mild, non-LI/non-EI skin symptoms remaining into adulthood.
There are seven loci associated to ARCI with five causative genes identified.25 These genes code for transglutaminase 1 (TGM1),26, 27 the adenosine triphosphate binding cassette 12A,28, 29 lipoxygenases (ALOXE3, ALOX12B)30 and ichtyin (ichtyin or NIPAL4).31, 32 Patients with the most severe types of ichthyosis show a congenital hyperkeratosis with scales covering a large part of the body's surface. In the LI patients is often present at birth a collodion membrane, covering the neonate, and consisting of shiny taut skin that eventually dries and peels away, leading to the development of large, thick brown scales. Patients with LI can be either homozygous for single mutations or heterozygous for two different mutations at the same locus.
The first gene associated to the disease has been the TGM1 at chromosome 14q11.2.33, 34 The TGM1 gene encodes transglutaminase 1 (TG1), which is a member of a class of enzymes that form Nɛ-(γ-glutamyl)lysine or mono- or bis(γ-glutamyl)spermidine isopeptide bond cross-links between proteins, and it is a calcium-dependent enzyme.1, 35 The TG1 enzyme is synthesized as an 817 residue polypeptide (90 kDa) and is modified by myristoyl and palitoyl adducts near the N-terminus of the protein. It is expressed in the upper spinous and granular layers beneath the stratum corneum. TG1 is an enzyme important in the formation of the cornified cell envelope, responsible for barrier function in stratified squamous epithelia, by the cross-linking of a variety of structural proteins including desmosomal proteins, involucrin, the small proline-rich proteins, loricrin and trichohyalin.1, 36, 37, 38 It is also reported to crosslink hydroxiceramide during the formation of cornified cell envelope, specifically, omega-hydroxyceramides covalently linked by ester bonds to cornified envelope proteins, most abundantly to involucrin.37 The native full-length TG1 protein is proteolysed at two sites during maturation and the three components remain associated in the active membrane-bound form.38 The domain spanning from Met-1 to Met-109 represents the pro-peptide portion of the protein, at the end of which is located the most important cleavage site, its cleavage increase the activity of the enzyme >100 times, respect to the full-length protein.39 The three-dimensional computerized homology model of TG1, based on the factor XIIIa40 crystal structure, revealed that the enzyme operate essentially as a dimer. Each monomer has four distinct domains: the amino-terminal β-sandwich domain (domain 1) from residues Met-109 to Phe-247, codified by exons 2, 3 and 4; the transamidation catalytic core domain (domain 2) from the Ile-255 to Pro-559 (containing the catalytic triade Cys-377; His 336; Asn-459) and two carboxy-terminal β-barrel domains, which include Glu-577 to Arg-687 (domain 3) and Leu-694 to Gly-800 (domain 4), respectively. Mutations in the TGM1 gene cause defect in the intercellular lipid layers in the stratum corneum,41, 42, 43, 26, 27 leading to defective barrier function of the stratum corneum resulting in the ichthyosis phenotype seen in LI patients44 and in the TG1 knockout mice.45 The mutations so far described in the literature are mainly localized not only in the catalytic cysteine or in its surrounding region, but also in the N-terminal domain and in the interface between these two domains, suggesting a functional conformational change occurring during catalysis. Mutations in the two N-terminal beta-barrel domains are less frequent, but still present.
The typical clinical features of LI are large, dark grey or large brownish thick scales, covering the entire body surface, including the face. Palmoplantar keratoderma is frequently seen, whereas the hair and the teeth appear normal. Skin manifestations rarely improve with the age. Light microscopy of lesional skin from patients with TGM1-dependent LI show marked hyperkeratosis. The granular layer is normal or mildly increased in thickness. However, in the milder form of LI, the large, dark, lamellar scales can be seen only at the restricted body sites including the trunk, lower legs, upper arms and foreheads. Sometimes the skin on the face and the extremities appears normal and hyperkeratosis is not seen on either the palms or soles. In these milder forms, thin, white to grey scales are sometimes seen on the neck, the extremities and the rest of the body surface.33
In this study, we have investigated 60 LI patients and families of Italian origin by mutation analysis of the complete coding sequences of TGM1 gene. We found 22 mutations, distributed in 17 patients (26% of the total LI patients investigated), among them, 6 novel and 15 previously described. Some of these mutations are well described and characterized and represent a wide spectrum of characteristic mutations found in LI associated to TG1.
Results
Clinical and genetic analysis
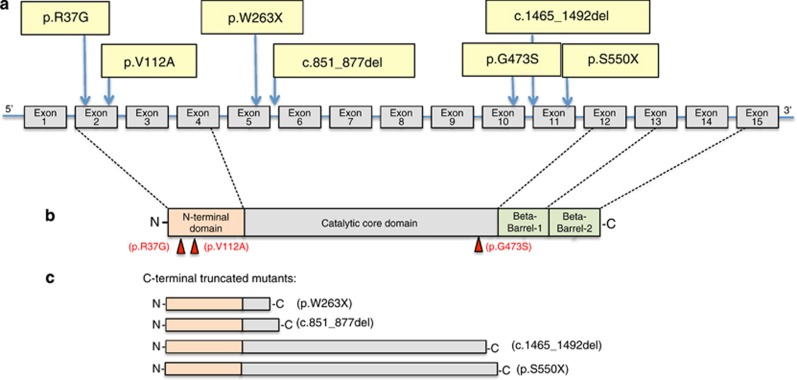
We have screened 60 patients that were diagnosed as LI. Large parts of the probands showed the classical LI phenotypes with large brown and thick scales covering the entire body surface, born as collodion baby (patient 1, 2, 3, 4, 6, 7, 8, 9, 8, 10, 11, 12, 13, 14,15, 16, 17), even if a wide gradient in disease severity had been observed, as described in Table 1. The results of the genetics analysis in Ichthyosis patients are also represented in Table 1. The sequence analysis of the TG1 patients showed the presence of new and known mutations in TGM1 gene in about 26% of them, thus confirming the heterogeneity of genetic inheritance of the disease. Regarding the mutations, they can be classified into three different subgroups: missense mutation leading to amino-acid substitutions, nonsense mutations causing the transition of a codifying codon into a stop codon and insertion/deletion mutations, that modify the reading frame of the coding region and/or the donor/acceptor splice sites. Regarding the new mutations found in homozygousity in patients (see below), they cannot be considered sporadic ‘de novo' mutations, because there is no report of consanguinity in the family. Probably, the mutated alleles are present in very low frequency in the areas in which these patients reside. In the first group we found eight mutations caused by single-nucleotide transition/transversion, two of them are new mutations never described up to now. The new mutations found are: the R37G, found in patient 9 and located in exon 2, codifying for the pro-peptide region and the V112A found in patient 11 located at the beginning of the N-terminal beta-sandwich domain (Figure 1). The other known mutations of the first type found in the IL patients analysed are: G473S found in patient 1 and located in exon 10, codifying the end of the catalytic core domain;45 R142H (patient 4), located in exon 3;26 S272P (patient 5) in exon 5;47 V383M in exon 7.48 E520G (patient 8) located in the exon11;47 R315H (patient 10), located in the exon 6,49 V379L (patient 17), located in exon 7;26 see Table 1 for further details. Among the nonsense mutations, we found four single-nucleotide transitions, all giving rise to stop codons and truncated proteins, the already known R54X (patient 2), R348X (patient 17), and the two new mutations W263X (patient 3) and S550X (patient 11). The first mutation, R54X truncates the enzyme in the second exon, presumably in this case there is the absence of a translation product due to the reduced size of the mRNA. In W263X mutant the enzyme ends at the level of exon 5, and the R348X in exon 7, both resulting in the loss of catalytic triade. The S550X mutants is also C-terminal truncated, it contains the active site but lacks the beta-barrel 1 and 2 domains, important for TG1 activity (Figure 1). In addition, in patient 9 we found an heterozygous mutation analysing the RNA. We discovered the deletion c.1465_1492del giving rise to the Y489X nonsense mutation again generating a truncated enzyme lacking the beta-barrel 1 and 2 domains. In patient 6, we also found a deletion mutation in the last 26 residues of intron 5 (c.851_877del), leading to the insertion of the residual part of the intron 5 in the coding sequence, resulting in the formation of a premature stop codon (Figure 1). It is interesting to note from this study that the most frequent mutation found in Italian IL patients bearing TG1 mutations is splicing error giving rise to intron insertion and consequent frame shift and premature stop codon formation (homozygous mutation in patients 12–16 and heterozygous mutation in patients 7).
Table 1. TGM1 mutations associated with LI, found in this study.
| Patienta | Mutation | Type | Exon (E) intron(I) | Nucleotides substitution | Amino-acid substitution | Collodion baby | LI | CIE | Scale features, other | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | G473S | HOMO | 10 | GGC→AGC | Gly473Ser | Yes | Yes | Small, thin, whitish atopic dermatitis | 45 | |
| 2 | R54X | HOMO | 2 | CGA→TGA | STOP codon | Yes | Yes | Large, thick, grey to brown, adherent, ectropion, severe phenotype | 49 | |
| 3 | W263X | HOMO | 5 | TGG→TAG | STOP codon | Yes | Yes | Large, thick, dark, adherent | NEW | |
| 4 | R142H | HOMO | 3 | CGC→CAC | Arg142His | Yes | Yes | Large, adherent, yellow-brownish ectropion | 26 | |
| 5 | S272P | HOMO | 5 | TCT→CCT | Ser272Pro | Not known | Yes | Large, thick, dark brown, adherent, ectropion, alopecia | 47 | |
| 6 | c.851_877del not found | HETERO | I 5 | - | STOP codon | Yes | Yes | Small, whitish to light brown | NEW | |
| 7 | V383M splicing error | HOMO HOMO | 7 I5/E6 | GAG→ATG AG→GG | Val382Met Insertion of intron 5 | Yes | Yes | large, thin, brownish, adherent affecting the head, nape, back, hands and feet, mild phenotype. | 26, 48 | |
| 8 | E520G; splicing error | HOMO HOMO | 11 13 | GAG→GGG G→A | Glu520Gly STOP codon | Yes | Yes | Large, adherent, brownish, severe ectropion | 47 | |
| 9 | R37G c.1465_1492del Y489X | HETERO HETERO | 2 E10/11 | AGA→GGA | Arg37Gly STOP codon | Yes | Yes | Large, adherent, yellowish, mild ectropion | NEW NEW | |
| 10 | R315H | HOMO | 6 | CGT→CAT | Arg315His | Yes | Yes | Large on the back; small, thin, yellowish on the limbs | 49 | |
| 11 | V112A S550X | HETERO | 2 11 | GTG→GCG TCA→TGA | Val112Ala STOP codon | Yes | Yes | Large and adherent on the scalp; small, thin, yellowish, adherent on the trunk, minimal ectropion. | NEW NEW | |
| 12 | Splicing error | HOMO | I5/E6 | AG→GG | Insertion of intron 5 | Yes | Yes | Large, adherent, yellow-brownish, ectropion, severe phenotype | 26 | |
| 13 | Splicing error | HOMO | I5/E6 | AG→GG | Insertion of intron 5 | Not known | Yes | Large, whitish to grey, adherent, erythema of the face | 26 | |
| 14 | Splicing error | HOMO | AG→GG | Insertion of intron 5 | Yes | Yes | Large, adherent, brownish, mild ectropion | 26 | ||
| 15 | Splicing error | HOMO | I5/E6 | AG→GG | Insertion of intron 5 | Yes | Yes | Small to medium size, yellowish, more evident on the limbs, mild ecctropion. Note: mild erythema | 26 | |
| 16 | Splicing error | HOMO | I5/E6 | AG→GG | Insertion of intron 5 | Yes | Yes | Large, adherent, dark | 26 | |
| 17 | V379L R348X | HETERO | E7 | CGA→TGA GTC→CTC | Val379L STOP codon | Yes | Yes | Large, thick, yellowish, adherent on limbs, thinner and smaller on trunk, mild/severe | 26 |
Abbreviations: LI, lamellar ichthyosis; CIE, congenital ichthyosiform erythroderma.
Mutations reported in the present study (novel) are in bold.
All patients analysed were European, Caucasian race, except patient 9 (African).
Figure 1.
Novel TGM1 mutations associated with lamellar icthyosis. (a) Novel TGM1 mutations reported in this study and their localization in the gene structure. (b) Single amino-acid substitutions are indicated in relationship to the TG1 domains. (c) Missense mutations generated truncated enzyme lacking beta-barrel 1 and 2 domains and catalityc domain (p.W263X, c.851_877del).
Conclusion
Several previous studies have attempted to demonstrate the existence of genotype–phenotype correlations between mutation in TG1 and clinical and/or ultra structural findings of the patient skins. However, there is a strong variability in clinical features of patients with the same mutation and there is not a clear association between the TG1 domain affected by the mutation and the severity of the phenotype. Our work, together with other studies, contribute to the identification of a large number of mutations with the aim to simplify the challenge in confirming the clinical diagnosis of patients affected by LI. Despite the genetic heterogeneity of this disease, TG1 has been the causative gene identified most often25, 46 and consequently the first gene to be analysed to screen for mutations, expanding the mutations spectrum will facilitate the understanding of genetic, molecular and pathophysiological aspect of LI. In addition, although the mutations found are distributed along all the gene sequence, we can observe among italian patients a clear founder effect regarding the intron5/exon6 splice site mutation. Our results could enhance our understanding in the pathogenesis of icthyosis and will be useful for new therapeutic approach also for the phenotypic shifts of ARCI.
Materials and Methods
Collection of clinical material and DNA isolation
Genomic DNA samples were extracted from patient blood and from the other family members according to standard procedures.50 This work was approved in the institutional ethics committee, at IDI-IRCCS, Istituto Dermopatico dell'Immacolata, Rome. The authors obtained informed consent from patients who provided their specimens.
PCR and RT-PCR amplification of TGM1 and DNA sequencing
Reverse transcription was performed using the Superscript-II reverse transcriptase (Life Technologies Ltd, Paisley, UK), with 100 ng of total RNA using 10 pmol of oligo dT primers, buffer and enzyme concentrations according to the manufacturer instructions. The entire coding region of the TGM1 gene was PCR amplified using 0.4 μℳ of primer 5′-CTCCCTCCCACATAAGTCAC-3′, for (+) strand and 5′-TAGCATCTGTTCCCCCAGTGCAAGTGAAG-3′, for (−) strand, designed from the published cDNA sequences. PCR fragments were resolved on 0.8% agarose gel (TAE), extracted and purified using the Qiaex II extraction kit (Qiagen, Valencia, CA, USA). Approximately 100 ng of purified template DNA was automatically sequenced with the BigDye Termination Reaction Kit (Applied Biosystem, Carlsbad, CA, USA) on an ABI-PRISM 377 DNA sequencer (Applied Biosystem), using internal primers. In patients for whom the RNA was not available, the coding region has been amplified using intronic primers flanking exons.
Acknowledgments
This work has been supported by Telethon Grant GGP06048, to EC and partially supported by IDI-IRCCS (RF06 c.73, RF07 c.57, RF08 c.15, RF07 c.57) to GM and EC.
Glossary
- TG1
transglutaminase 1
- TGM1
gene encoding for TG1
- LI
lamellar ichtyosis
The authors declare no conflict of interest.
Footnotes
Edited by KA Knight
References
- Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol. 2005;6:328–340. doi: 10.1038/nrm1619. [DOI] [PubMed] [Google Scholar]
- Alameda P, Fernández-Aceñero MJ, Moreno-Maldonado R, Navarro M, Quintana R, Page A, et al. CYLD regulates keratinocyte differentiation and skin cancer progression in humans. Cell Death Dis. 2011;2:e208. doi: 10.1038/cddis.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T, Miyazaki M, Hakuno F, Takahashi S, Chida K. PKCη promotes a proliferation to differentiation switch in keratinocytes via upregulation of p27Kip1 mRNA through suppression of JNK/c-Jun signaling under stress conditions. Cell Death Dis. 2011;2:e157. doi: 10.1038/cddis.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton CE, Johnson KN, Mays DM, Boehnke K, Shyr Y, Boukamp P, et al. Novel p63 target genes involved in paracrine signaling and keratinocyte differentiation. Cell Death Dis. 2010;1:e74. doi: 10.1038/cddis.2010.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub WE, Weber TA, Schäfer B, Candi E, Durst F, Ou HD, et al. The C-terminus of p63 contains multiple regulatory elements with different functions. Cell Death Dis. 2010;1:e5. doi: 10.1038/cddis.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer C, Lu X. Epithelial cell polarity: a major gatekeeper against cancer. Cell Death Differ. 2011;18:1470–1477. doi: 10.1038/cdd.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi R, Fuchs E. MicroRNA-mediated control in the skin. Cell Death Differ. 2010;17:229–235. doi: 10.1038/cdd.2009.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober AM, Legewie S, Pforr C, Fricker N, Eils R, Krammer PH, et al. Caspase-8 activity has an essential role in CD95/Fas-mediated MAPK activation. Cell Death Dis. 2011;6:e212. doi: 10.1038/cddis.2011.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badmann A, Keough A, Kaufmann T, Bouillet P, Brunner T, Corazza N. Role of TRAIL and the pro-apoptotic Bcl-2 homolog Bim in acetaminophen-induced liver damage. Cell Death Dis. 2011;2:e171. doi: 10.1038/cddis.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yivgi-Ohana N, Eifer M, Addadi Y, Neeman M, Gross A. Utilizing mitochondrial events as biomarkers for imaging apoptosis. Cell Death Dis. 2011;2:e166. doi: 10.1038/cddis.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati A, Graziano V, De Laurenzi V, Pascale M, Turco MC. BAG3: a multifaceted protein that regulates major cell pathways. Cell Death Dis. 2011;2:e141. doi: 10.1038/cddis.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graber TE, Holcik M. Distinct roles for the cellular inhibitors of apoptosis proteins 1 and 2. Cell Death Dis. 2011;2:e135. doi: 10.1038/cddis.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JP, Betin VM, Weir H, Shelmani GM, Moss DK, Lane JD. Caspase cleavage of the Golgi stacking factor GRASP65 is required for Fas/CD95-mediated apoptosis. Cell Death Dis. 2010;1:e8. doi: 10.1038/cddis.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker M, O'Prey J, Tolkovsky AM, Ryan KM. Phosphorylation of Puma modulates its apoptotic function by regulating protein stability. Cell Death Dis. 2010;1:e59. doi: 10.1038/cddis.2010.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KS, Wong CH, Huang YF, Li HY. Survivin withdrawal by nuclear export failure as a physiological switch to commit cells to apoptosis. Cell Death Dis. 2010;1:e57. doi: 10.1038/cddis.2010.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan L, Sebastià J, Tuffy LP, Spring A, Lichawska A, Devocelle M, et al. XIAP impairs Smac release from the mitochondria during apoptosis. Cell Death Dis. 2010;1:e49. doi: 10.1038/cddis.2010.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson V, Gudmundsdottir K, Luong P, Leung KY, Knebel A, Basu S. JNK phosphorylates Yes-associated protein (YAP) to regulate apoptosis. Cell Death Dis. 2010;1:e29. doi: 10.1038/cddis.2010.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G, Fillinger J, Sittadjody S, Avila J, Burd R, Limesand K. IGF1 activates cell cycle arrest following irradiation by reducing binding of ΔNp63 to the p21 promoter. Cell Death Dis. 2010;1:e50. doi: 10.1038/cddis.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G, Verkhratsky A. Long-term (trophic) purinergic signalling: purinoceptors control cell proliferation, differentiation and death. Cell Death Dis. 2010;1:e9. doi: 10.1038/cddis.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight RA, Melino G. Cell death in disease: from 2010 onwards. Cell Death Dis. 2011;2:e202. doi: 10.1038/cddis.2011.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melino G. p63 is a suppressor of tumorigenesis and metastasis interacting with mutant p53. Cell Death Differ. 2011;18:1487–99. doi: 10.1038/cdd.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geering B, Simon HU. Peculiarities of cell death mechanisms in neutrophils. Cell Death Differ. 2011;18:1457–69. doi: 10.1038/cdd.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oji V, Tadini G, Akiyama M, Blanchet Bardon C, Bodemer C, et al. Revised nomenclature and classification of inherited ichthyoses: results of the First Ichthyosis Consensus Conference in Soreze 2009. J Am Acad Dermatol. 2010;63:607–641. doi: 10.1016/j.jaad.2009.11.020. [DOI] [PubMed] [Google Scholar]
- Vahlquist A, Bygum A, Gånemo A, Virtanen M, Hellström-Pigg M, Strauss G, et al. Genotypic and clinical spectrum of self-improving collodion ichthyosis: ALOX12B, ALOXE3, and TGM1 mutations in Scandinavian patients. J Invest Dermatol. 2010;130:438–43. doi: 10.1038/jid.2009.346. [DOI] [PubMed] [Google Scholar]
- Akiyama M. Harlequin ichthyosis and other autosomal recessive congenital ichthyoses: the underlying genetic defects and pathomechanisms. J Dermatol Sci. 2006;42:83–89. doi: 10.1016/j.jdermsci.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Huber M, Rettler I, Bernasconi K, Frenk E, Lavrijsen SP, Ponec M, et al. Mutations of keratinocyte transglutaminase in lamellar ichthyosis. Science. 1995;267:525–528. doi: 10.1126/science.7824952. [DOI] [PubMed] [Google Scholar]
- Russell LJ, DiGiovanna JJ, Rogers GR, Steinert PM, Hashem N, Compton JG, et al. Mutations in the gene for transglutaminase 1 in autosomal recessive lamellar ichthyosis. Nat Genet. 1995;9:279–283. doi: 10.1038/ng0395-279. [DOI] [PubMed] [Google Scholar]
- Annilo T, Shulenin S, Chen ZQ, Arnould I, Prades C, Lemoine C, et al. Identification and characterization of a novel ABCA subfamily member, ABCA12, located in the lamellar ichthyosis region on 2q34. Cytogenet Genome Res. 2002;98:169–176. doi: 10.1159/000069811. [DOI] [PubMed] [Google Scholar]
- Lefevre C, Audebert S, Jobard F, Bouadjar B, Lakhdar H, Boughdene-Stambouli O, et al. Mutations in the transporter ABCA12 are associated with lamellar ichthyosis type 2. Hum Mol Genet. 2003;12:2369–2378. doi: 10.1093/hmg/ddg235. [DOI] [PubMed] [Google Scholar]
- Jobard F, Lefevre C, Karaduman A, Blanchet-Bardon C, Emre S, Weissenbach J, et al. Lipoxygenase-3 (ALOXE3) and 12(R)-lipoxygenase (ALOX12B) are mutated in non-bullous congenital ichthyosiform erythroderma (NCIE) linked to chromosome 17p13.1. Hum Mol Genet. 2002;11:107–113. doi: 10.1093/hmg/11.1.107. [DOI] [PubMed] [Google Scholar]
- Lefevre C, Bouadjar B, Karaduman A, Jobard F, Saker S, Ozguc M, et al. Mutations in ichthyin a new gene on chromosome 5q33 in a new form of autosomal recessive congenital ichthyosis. Hum Mol Genet. 2004;13:2473–2482. doi: 10.1093/hmg/ddh263. [DOI] [PubMed] [Google Scholar]
- Williams ML, Elias PM. Heterogeneity in autosomal recessive ichthyosis. Clinical and biochemical differentiation of lamellar ichthyosis and nonbullous congenital ichthyosiform erythroderma. Arch Dermatol. 1985;121:477–488. doi: 10.1001/archderm.121.4.477. [DOI] [PubMed] [Google Scholar]
- Laiho E, Niemi KM, Ignatius J, Kere J, Palotie A, Saarialho-Kere U. Clinical and morphological correlations for transglutaminase 1 gene mutations in autosomal recessive congenital ichthyosis. Eur J Hum Genet. 1999;7:625–632. doi: 10.1038/sj.ejhg.5200353. [DOI] [PubMed] [Google Scholar]
- Russell LJ, DiGiovanna JJ, Hashem N, Compton JG, Bale SJ. Linkage of autosomal recessive lamellar ichthyosis to chromosome 14q. Am J Hum Genet. 1994;55:1146–1152. [PMC free article] [PubMed] [Google Scholar]
- Kim IG, McBride OW, Wang M, Kim SY, Idler WW, Steinert PM. Structure and organization of the human transglutaminase 1 gene. J Biol Chem. 1992;267:7710–7717. [PubMed] [Google Scholar]
- Marekov LN, Steinert PM. Ceramides are bound to structural proteins of the human foreskin epidermal cornified cell envelope. J Biol Chem. 1998;273:17763–17770. doi: 10.1074/jbc.273.28.17763. [DOI] [PubMed] [Google Scholar]
- Nemes Z, Marekov LN, Fesus L, Steinert PM. A novel function for transglutaminase 1: attachment of long-chain omega-hydroxyceramides to involucrin by ester bond formation. Proc Natl Acad Sci USA. 1999;96:8402–8407. doi: 10.1073/pnas.96.15.8402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert PM, Kim SY, Chung SI, Marekov LN. The transglutaminase 1 enzyme is variably acylated by myristate and palmitate during differentiation in epidermal keratinocytes. J Biol Chem. 1996;271:26242–26250. doi: 10.1074/jbc.271.42.26242. [DOI] [PubMed] [Google Scholar]
- Kim SY, Chung SI, Steinert PM. Highly active soluble processed forms of the transglutaminase 1 enzyme in epidermal keratinocytes. J Biol Chem. 1995;270:18026–18035. doi: 10.1074/jbc.270.30.18026. [DOI] [PubMed] [Google Scholar]
- Yee VC, Pedersen LC, Le Trong I, Bishop PD, Stenkamp RE, Teller DC. Three-dimensional structure of a transglutaminase: human blood coagulation factor XIII. Proc Natl Acad Sci USA. 1994;91:7296–7300. doi: 10.1073/pnas.91.15.7296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candi E, Melino G, Lahm A, Ceci R, Rossi A, Kim IG, et al. Transglutaminase 1 mutations in lamellar ichthyosis. Loss of activity due to failure of activation by proteolytic processing. J Biol Chem. 1998;273:13693–13702. doi: 10.1074/jbc.273.22.13693. [DOI] [PubMed] [Google Scholar]
- Candi E, Melino G, Mei G, Tarcsa E, Chung SI, Marekov LN, et al. Biochemical, structural, and transglutaminase substrate properties of human loricrin, the major epidermal cornified cell envelope protein. J Biol Chem. 1995;270:26382–26390. doi: 10.1074/jbc.270.44.26382. [DOI] [PubMed] [Google Scholar]
- Candi E, Tarcsa E, Idler WW, Kartasova T, Marekov LN, Steinert PM. Transglutaminase cross-linking properties of the small proline-rich 1 family of cornified cell envelope proteins. Integration with loricrin. J Biol Chem. 1999;274:7226–7237. doi: 10.1074/jbc.274.11.7226. [DOI] [PubMed] [Google Scholar]
- Elias PM, Schmuth M, Uchida Y, Rice RH, Behne M, Crumrine D, et al. Basis for the permeability barrier abnormality in lamellar ichthyosis. Exp Dermatol. 2002;11:248–256. doi: 10.1034/j.1600-0625.2001.110308.x. [DOI] [PubMed] [Google Scholar]
- Huber M, Yee VC, Burri N, Vikerfors E, Lavrijsen AP, Paller AS, et al. Consequences of seven novel mutations on the expression and structure of keratinocyte transglutaminase. J Biol Chem. 1997;272:21018–26. doi: 10.1074/jbc.272.34.21018. [DOI] [PubMed] [Google Scholar]
- Kuramoto N, Takizawa T, Takizawa T, Matsuki M, Morioka H, Robinson JM, et al. Development of ichthyosiform skin compensates for defective permeability barrier function in mice lacking transglutaminase 1. J Clin Invest. 2002;109:243–250. doi: 10.1172/JCI13563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G, Auricchio L, Rescigno G, Paparo F, Rinaldi M, Salvatore F. Transglutaminase 1 gene mutations in Italian patients with autosomal recessive lamellar ichthyosis. J Invest Dermatol. 2001;116:809–12. doi: 10.1046/j.1523-1747.2001.01314.x. [DOI] [PubMed] [Google Scholar]
- Petit E, Huber M, Rochat A, Bodemer C, Teillac-Hamel D, Müh JP, et al. Three novel point mutations in the keratinocyte transglutaminase (TGK) gene in lamellar ichthyosis: significance for mutant transcript level, TGK immunodetection and activity. Eur J Hum Genet. 1997;5:218–28. [PubMed] [Google Scholar]
- Herman ML, Farasat S, Steinbach PJ, Wei MH, Toure O, Fleckman P, et al. Transglutaminase-1 gene mutations in autosomal recessive congenital ichthyosis: summary of mutations (including 23 novel) and modeling of TGase-1. Hum Mutat. 2009;30:537–47. doi: 10.1002/humu.20952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Russel D. Cold Stprig Harbor. Cold Spring Harbor Laboratory Press: New York; 2001. [Google Scholar]