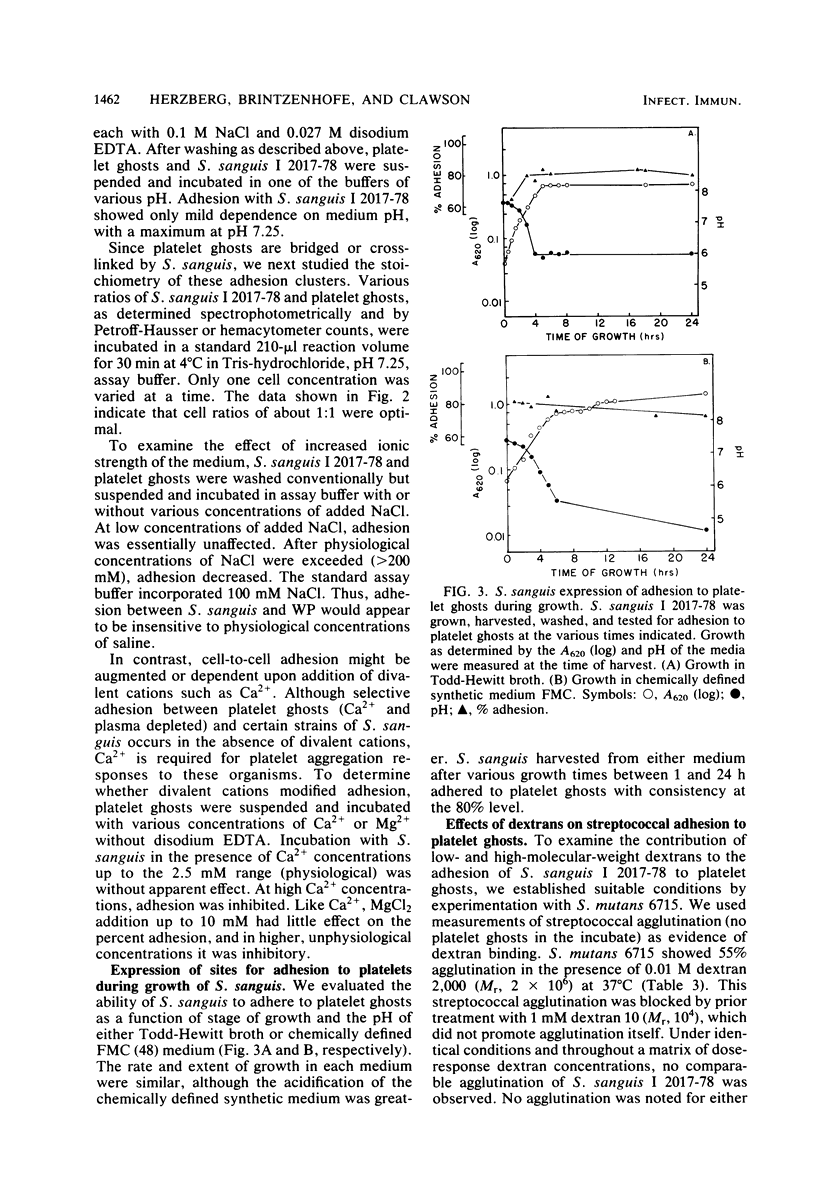
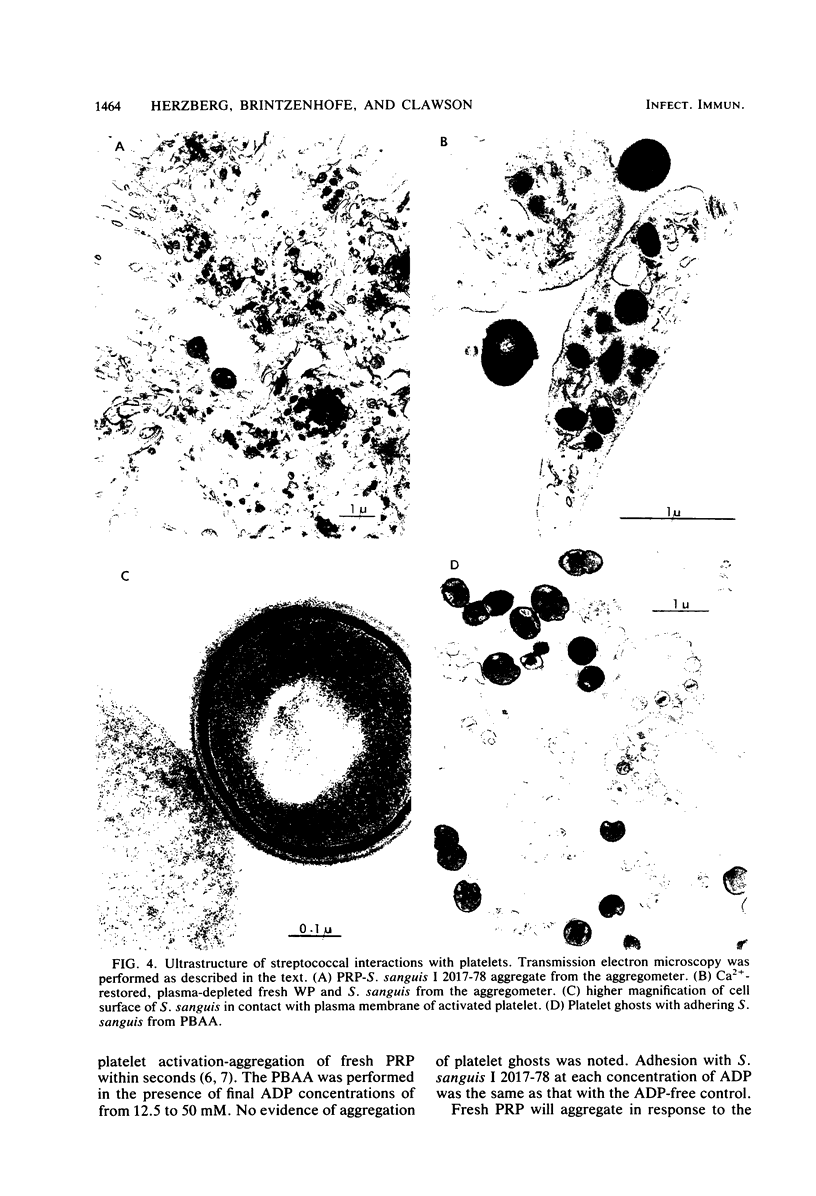
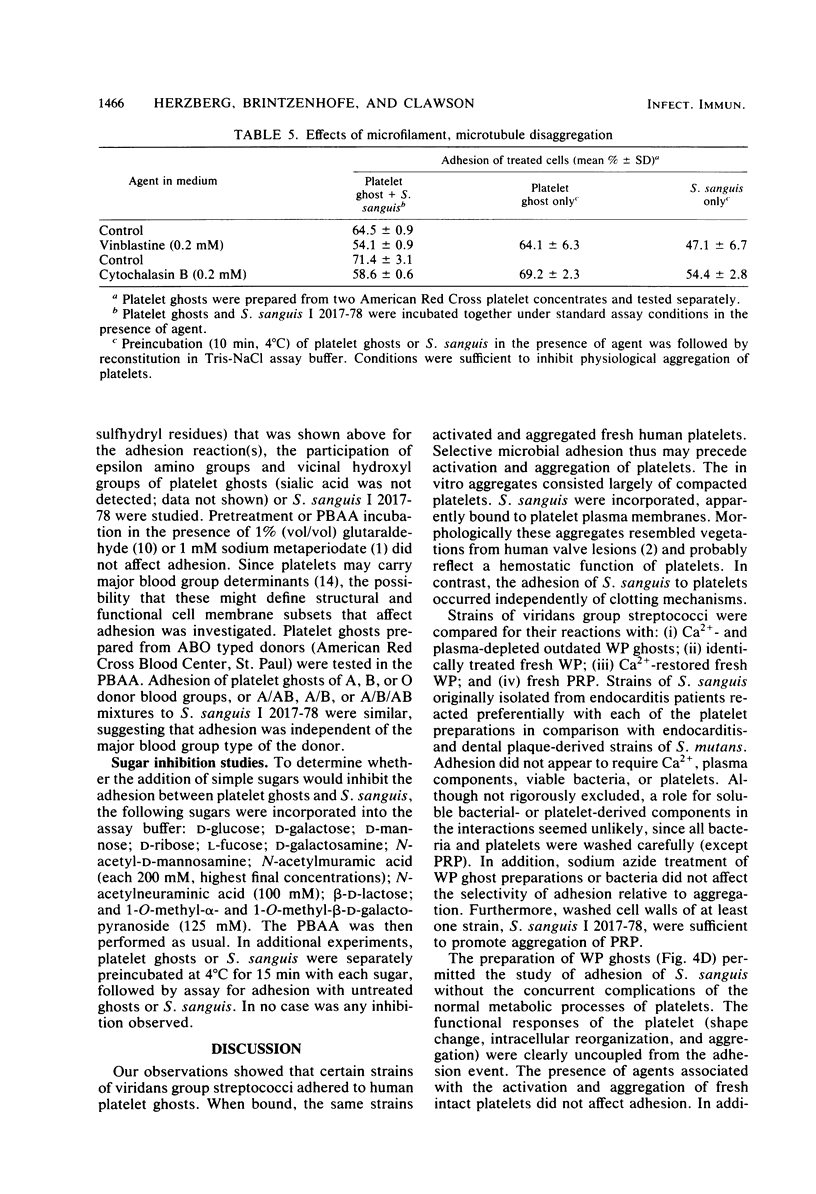
Abstract
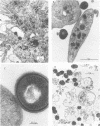
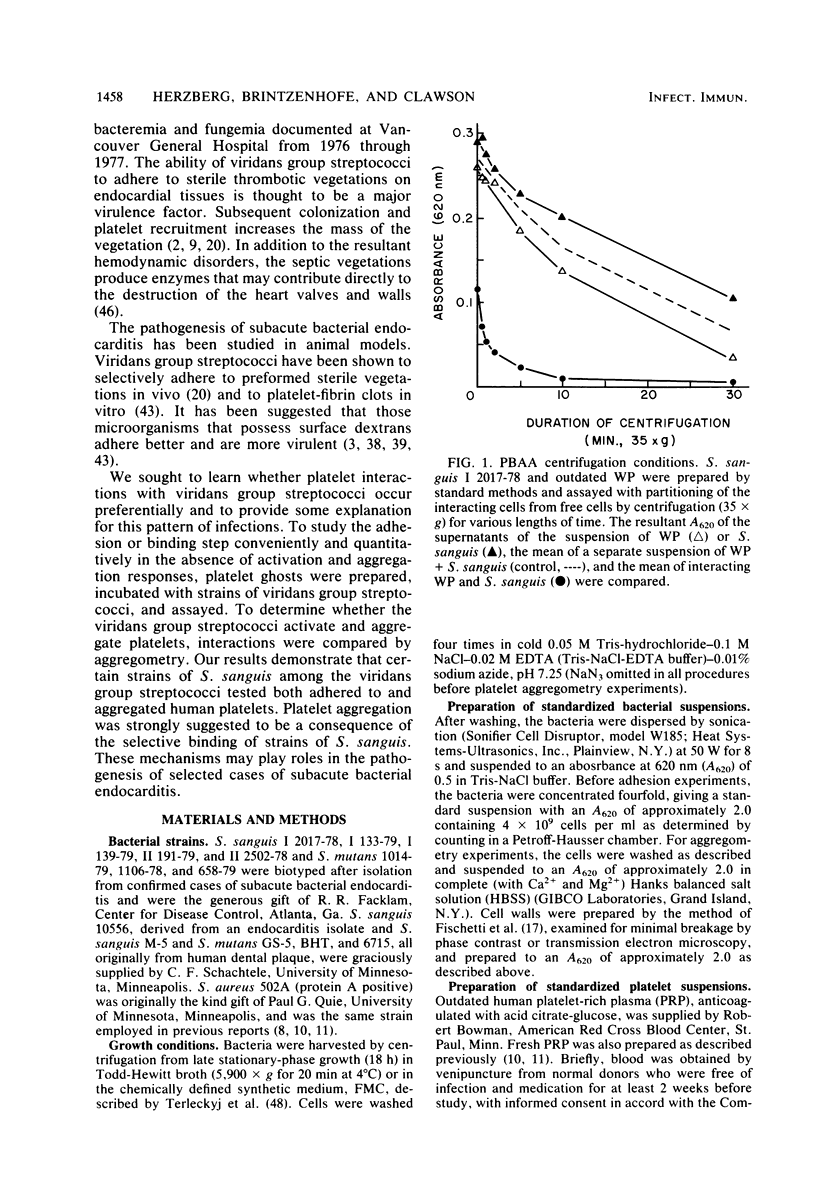
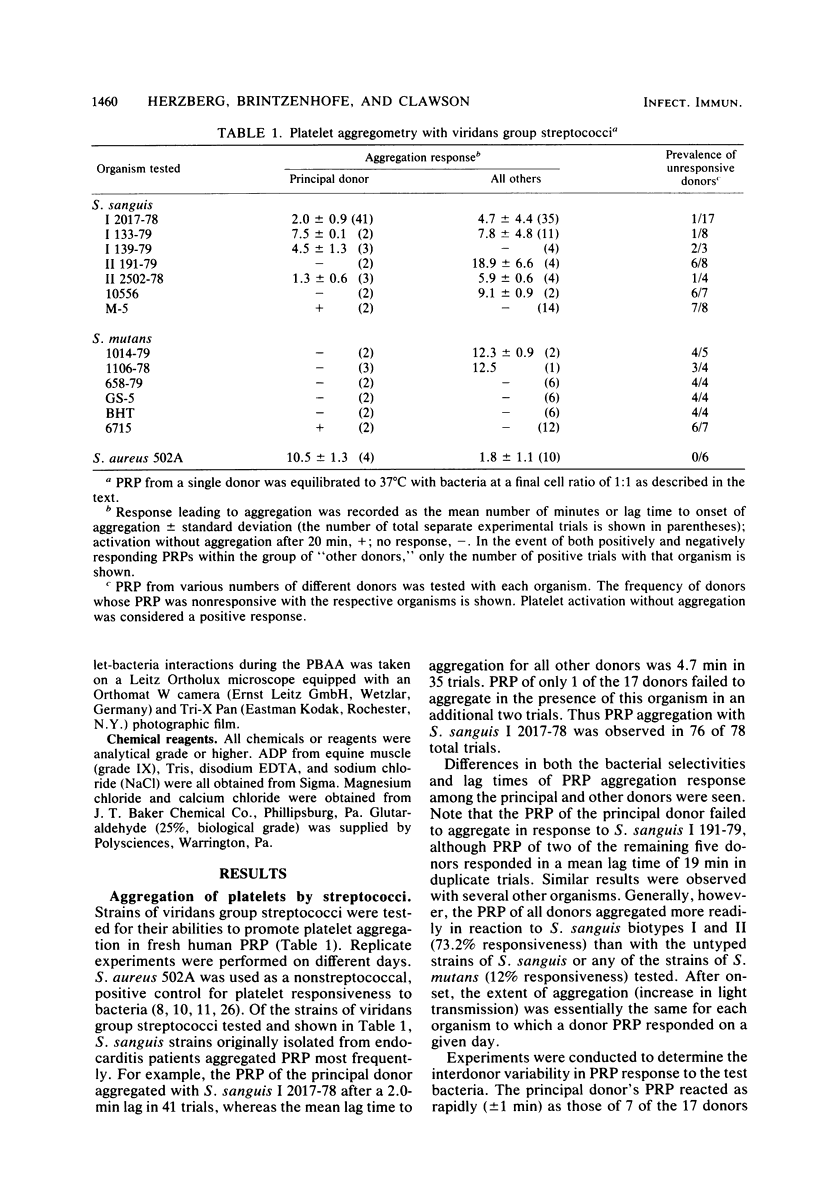
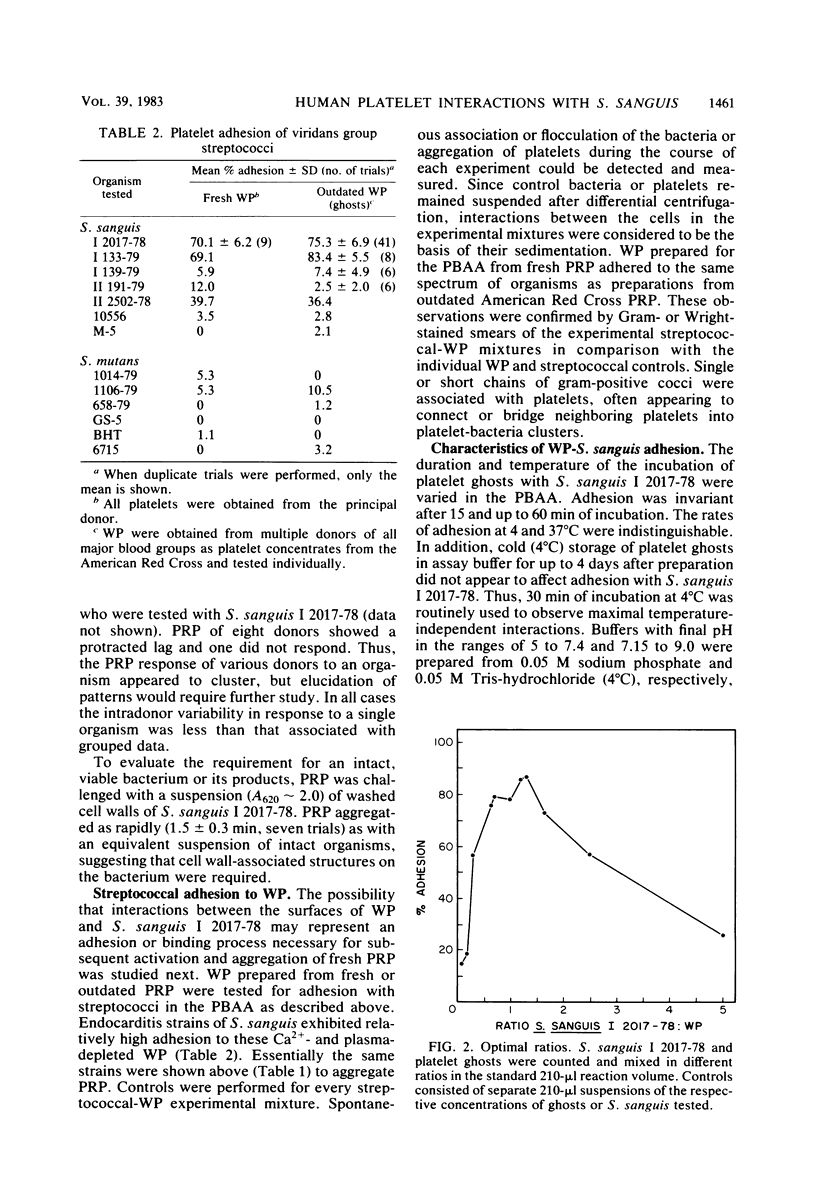
Platelet vegetations or thrombi are common findings in subacute bacterial endocarditis. We investigated the hypothesis that human platelets selectively bind or adhere strains of Streptococcus sanguis and Streptococcus mutans and aggregate, as a result, into an in vitro thrombus. Earlier ultrastructural studies suggested that aggregation of platelets over time by Staphylococcus aureus was preceded in order by adhesion and platelet activation. We uncoupled the adhesion step from activation and aggregation in our studies by incubating streptococci with platelet ghosts in a simple, quantitative assay. Adhesion was shown to be mediated by protease-sensitive components on the streptococci and platelet ghosts rather than cell surface carbohydrates or dextrans, plasma components, or divalent cations. The same streptococci were also studied by standard aggregometry techniques. Platelet-rich plasma was activated and aggregated by certain isolates of S. sanguis. Platelet ghosts bound the same strains selectively under Ca2+- and plasma-depleted conditions. Fresh platelets could activate after washing, but Ca2+ had to be restored. Aggregation required fresh platelets in Ca2+-restored plasma and was inducible by washed streptococcal cell walls. These reactions in the binding and aggregometry assays were confirmed by transmission electron microscopy. Surface microfibrils on intact S. sanguis were identified. These appendages appeared to bind S. sanguis to platelets. The selectivity of adhesion of the various S. sanguis strains to platelet ghosts or Ca2+- and plasma-depleted fresh washed platelets was similar for all donors. Thus, the platelet binding site was expressed widely in the population and was unlikely to be an artifact of membrane aging or preparation. Since selective adhesion of S. sanguis to platelets was apparently required for aggregation, it is suggested that functionally defined receptors for ligands on certain strains of S. sanguis may be present on human platelets. Some differences in the selectivity and rate of the aggregation response were noted among platelet donors, although the meaning of the variability requires further study. Nonetheless, these interactions may contribute to platelet accretion in the initiation and development of vegetative lesions in the subacute bacterial endocarditis.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson L. C., Gahmberg C. G. Surface glycoproteins of human white blood cells. Analysis by surface labeling. Blood. 1978 Jul;52(1):57–67. [PubMed] [Google Scholar]
- Aoki N., Naito K., Yoshida N. Inhibition of platelet aggregation by protease inhibitors. Possible involvement of proteases in platelet aggregation. Blood. 1978 Jul;52(1):1–12. [PubMed] [Google Scholar]
- BORN G. V. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature. 1962 Jun 9;194:927–929. doi: 10.1038/194927b0. [DOI] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Bick R. L. Disseminated intravascular coagulation and related syndromes: etiology, pathophysiology, diagnosis, and management. Am J Hematol. 1978;5(3):265–282. doi: 10.1002/ajh.2830050311. [DOI] [PubMed] [Google Scholar]
- Born G. V., Dearnley R., Foulks J. G., Sharp D. E. Quantification of the morphological reaction of platelets to aggregating agents and of its reversal by aggregation inhibitors. J Physiol. 1978 Jul;280:193–212. doi: 10.1113/jphysiol.1978.sp012380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clawson C. C. Platelet interaction with bacteria. 3. Ultrastructure. Am J Pathol. 1973 Mar;70(3):449–471. [PMC free article] [PubMed] [Google Scholar]
- Clawson C. C., White J. G., Herzberg M. C. Platelet interaction with bacteria. VI. contrasting the role of fibrinogen and fibronectin. Am J Hematol. 1980;9(1):43–53. doi: 10.1002/ajh.2830090106. [DOI] [PubMed] [Google Scholar]
- Clawson C. C., White J. G. Platelet interaction with bacteria. I. Reaction phases and effects of inhibitors. Am J Pathol. 1971 Nov;65(2):367–380. [PMC free article] [PubMed] [Google Scholar]
- Czuprynski C. J., Balish E. Interaction of rat platelets with Listeria monocytogenes. Infect Immun. 1981 Jul;33(1):103–108. doi: 10.1128/iai.33.1.103-108.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durack D. T. Experimental bacterial endocarditis. IV. Structure and evolution of very early lesions. J Pathol. 1975 Feb;115(2):81–89. doi: 10.1002/path.1711150204. [DOI] [PubMed] [Google Scholar]
- Fischetti V. A., Gotschlich E. C., Siviglia G., Zabriskie J. B. Streptococcal M protein extracted by nonionic detergent. I. Properties of the antiphagocytic and type-specific molecules. J Exp Med. 1976 Jul 1;144(1):32–53. doi: 10.1084/jem.144.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. E., Phillips D. R. Inhibition of actin polymerization in blood platelets by cytochalasins. Nature. 1981 Aug 13;292(5824):650–652. doi: 10.1038/292650a0. [DOI] [PubMed] [Google Scholar]
- Francioli P. B., Freedman L. R. Streptococcal infection of endocardial and other intravascular vegetations in rabbits: natural history and effect of dexamethasone. Infect Immun. 1979 May;24(2):483–491. doi: 10.1128/iai.24.2.483-491.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman L. R., Valone J., Jr Experimental infective endocarditis. Prog Cardiovasc Dis. 1979 Nov-Dec;22(3):169–180. doi: 10.1016/0033-0620(79)90021-5. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Fitzgerald R. J. Dextran-induced agglutination of Streptococcus mutans, and its potential role in the formation of microbial dental plaques. J Bacteriol. 1969 May;98(2):341–346. doi: 10.1128/jb.98.2.341-346.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawiger J., Steckley S., Hammond D., Cheng C., Timmons S., Glick A. D., Des Prez R. M. Staphylococci-induced human platelet injury mediated by protein A and immunoglobulin G Fc fragment receptor. J Clin Invest. 1979 Oct;64(4):931–937. doi: 10.1172/JCI109559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney D. M., Chao F. C. Microtubule inhibitors alter the secretion of beta-glucuronidase by human blood platelets: involvement of microtubules in release reaction II. J Cell Physiol. 1978 Jul;96(1):43–52. doi: 10.1002/jcp.1040960106. [DOI] [PubMed] [Google Scholar]
- Kosaki G., Nomura T., Kambayashi J. The mechanism of the inhibitory effect of protease inhibitors on platelet aggregation and cellular synthesis of prostaglandins. I. The effect on the release of arachidonic acid from phospholipids. Thromb Res. 1980 Dec 1;20(5-6):587–598. doi: 10.1016/0049-3848(80)90147-4. [DOI] [PubMed] [Google Scholar]
- Levine M. J., Herzberg M. C., Levine M. S., Ellison S. A., Stinson M. W., Li H. C., van Dyke T. Specificity of salivary-bacterial interactions: role of terminal sialic acid residues in the interaction of salivary glycoproteins with Streptococcus sanguis and Streptococcus mutans. Infect Immun. 1978 Jan;19(1):107–115. doi: 10.1128/iai.19.1.107-115.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljemark W. F., Bloomquist C. G. Isolation of a protein-containing cell surface component from Streptococcus sanguis which affects its adherence to saliva-coated hydroxyapatite. Infect Immun. 1981 Nov;34(2):428–434. doi: 10.1128/iai.34.2.428-434.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B. M., Feinman R. D., Detwiler T. C. Platelet stimulation by thrombin and other proteases. Biochemistry. 1975 Mar 25;14(6):1308–1314. doi: 10.1021/bi00677a032. [DOI] [PubMed] [Google Scholar]
- Murray P. A., Levine M. J., Tabak L. A., Reddy M. S. Specificity of salivary-bacterial interactions: II. Evidence for a lectin on Streptococcus sanguis with specificity for a NeuAc alpha 2, 3Ga1 beta 1, 3Ga1NAc sequence. Biochem Biophys Res Commun. 1982 May 31;106(2):390–396. doi: 10.1016/0006-291x(82)91122-6. [DOI] [PubMed] [Google Scholar]
- Nachman R. L., Ferris B. Studies on the proteins of human platelet membranes. J Biol Chem. 1972 Jul 25;247(14):4468–4475. [PubMed] [Google Scholar]
- Neame P. B., Kelton J. G., Walker I. R., Stewart I. O., Nossel H. L., Hirsh J. Thrombocytopenia in septicemia: the role of disseminated intravascular coagulation. Blood. 1980 Jul;56(1):88–92. [PubMed] [Google Scholar]
- Pelletier L. L., Jr, Coyle M., Petersdorf R. Dextran production as a possible virulence factor in streptococcal endocarditis. Proc Soc Exp Biol Med. 1978 Jul;158(3):415–420. doi: 10.3181/00379727-158-40216. [DOI] [PubMed] [Google Scholar]
- Pfueller S. L., Cosgrove L. J. Staphylococci-induced human platelet injury. 1980 Aug 15-Sep 1Thromb Res. 19(4-5):733–735. doi: 10.1016/0049-3848(80)90048-1. [DOI] [PubMed] [Google Scholar]
- Ramirez-Ronda C. H. Adherence of glucan-positive and glucan-negative streptococcal strains to normal and damaged heart valves. J Clin Invest. 1978 Oct;62(4):805–814. doi: 10.1172/JCI109192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Ronda C. H. Effects of molecular weight of dextran on the adherence of Streptococcus sanguis to damaged heart valves. Infect Immun. 1980 Jul;29(1):1–7. doi: 10.1128/iai.29.1.1-7.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riba A. L., Thakur M. L., Gottschalk A., Andriole V. T., Zaret B. L. Imaging experimental infective endocarditis with indium-111-labeled blood cellular components. Circulation. 1979 Feb;59(2):336–343. doi: 10.1161/01.cir.59.2.336. [DOI] [PubMed] [Google Scholar]
- Roberts F. J. A review of positive blood cultures: identification and source of microorganisms and patterns of sensitivity to antibiotics. Rev Infect Dis. 1980 May-Jun;2(3):329–339. doi: 10.1093/clinids/2.3.329. [DOI] [PubMed] [Google Scholar]
- Roberts R. B., Krieger A. G., Schiller N. L., Gross K. C. Viridans streptococcal endocarditis: the role of various species, including pyridoxal-dependent streptococci. Rev Infect Dis. 1979 Nov-Dec;1(6):955–966. doi: 10.1093/clinids/1.6.955. [DOI] [PubMed] [Google Scholar]
- Scheld W. M., Valone J. A., Sande M. A. Bacterial adherence in the pathogenesis of endocarditis. Interaction of bacterial dextran, platelets, and fibrin. J Clin Invest. 1978 May;61(5):1394–1404. doi: 10.1172/JCI109057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semeraro N., Colucci M., Vermylen J. Complement-dependent and complement-independent interactions of bacterial lipopolysaccharides and mucopeptides with rabbit and human platelets. Thromb Haemost. 1979 Apr 23;41(2):392–406. [PubMed] [Google Scholar]
- Sheth N. K., Kurup V. P., Barron B. A. The role of Aspergillus fumigatus antigens in blood coagulation and platelet function. Microbios. 1980;28(112):91–96. [PubMed] [Google Scholar]
- Straus D. C., Mattingly S. J., Milligan T. W. Production of extracellular material by streptococci associated with subacute bacterial endocarditis. Infect Immun. 1977 Jul;17(1):148–156. doi: 10.1128/iai.17.1.148-156.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak L. A., Levine M. J., Mandel I. D., Ellison S. A. Role of salivary mucins in the protection of the oral cavity. J Oral Pathol. 1982 Feb;11(1):1–17. doi: 10.1111/j.1600-0714.1982.tb00138.x. [DOI] [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wörner P., Patscheke H. Antagonistic effects of N-ethylmaleimide on platelets treated with agents that are known to increase levels of cyclic AMP. Thromb Res. 1981 Jan 1;21(1-2):201–206. doi: 10.1016/0049-3848(84)90050-1. [DOI] [PubMed] [Google Scholar]
- Zimmerman T. S., Spiegelberg H. L. Pneumococcus-induced serotonin release from human platelets. Identification of the participating plasma/serum factor as immunoglobulin. J Clin Invest. 1975 Oct;56(4):828–834. doi: 10.1172/JCI108161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker M. B., Grant R. A. Nonreversible loss of platelet aggregability induced by calcium deprivation. Blood. 1978 Sep;52(3):505–513. [PubMed] [Google Scholar]