Abstract
Purpose
Signaling pathway stimulation by activating mutations of oncogenes occurs in most melanomas and can provide excellent targets for therapy, but the short-term therapeutic success is limited by intrinsic and acquired resistance. The mitogen-activated protein kinase (MAPK) and PI3 kinase/AKT/mTOR pathways are activated in most cutaneous melanomas. The purpose of this trial was to prospectively evaluate two molecularly-targeted drug combinations in patients with untreated metastatic melanoma.
Patients and Methods
This randomized Phase II study enrolled patients between May 2008 and November 2009 with non-ocular melanoma, no prior systemic chemotherapy, and no history of brain metastasis. Arm A: oral sorafenib 200 mg b.i.d. plus intravenous temsirolimus 25 mg weekly; Arm B: oral sorafenib 400 mg q.a.m., 200 mg q.p.m. daily plus oral tipifarnib 100 mg twice daily, 3 weeks of every 4. The primary objectives were to evaluate progression-free survival (PFS), objective response rate (ORR), and toxicity for the two regimens.
Results
On Arm A (63 evaluable patients), the median PFS was 2.1 months and median overall survival (OS) 7 months. Three patients achieved partial response (PR). Thirty-nine evaluable patients were accrued to Arm B, which closed after first-stage accrual; the median PFS was 1.8 months and OS 7 months, with 1 patient achieving PR.
Conclusion
The combinations of molecularly-targeted agents tested did not demonstrate sufficient activity to justify further use. Newer agents and improved patient selection by characterization of the molecular targets in individual tumors show great promise and should be incorporated into future studies, along with appropriate laboratory correlates.
INTRODUCTION
Melanoma is a molecularly heterogeneous group of tumors that can be characterized by the constitutive activation of one or more pathways essential to acquiring and maintaining the malignant phenotype, including invasion, metastasis, and resistance to apoptosis. Mutations associated with these activated pathways have been characterized for a large fraction of cutaneous, uveal, and mucosal primary melanomas (1). Development of resistance due to upstream oncogenic events such as NRAS activating mutations or activation of receptor tyrosine kinases through overexpression or mutation, thereby turning on other molecular pathways, has hindered the success of single-agent approaches (2, 3). However, the availability of small molecule targeted inhibitors for several molecular pathways important in melanoma has raised the possibility that greater and more durable effects could be achieved with combinations that also have non-overlapping toxicities, allowing their combination at or near full doses (4–6).
The most frequent oncogenic mutation in cutaneous melanoma (about 50% of tumors) is the BRAFV600 substitution, resulting in constitutive activity of the MAPK pathway (7). Melanomas often have mutational, deletional, or epigenetic loss of expression of the phosphatase and tensin homolog (PTEN) tumor suppressor that leads to over-activation of PI3K, AKT and downstream mTOR complexes (8). Melanoma cells lacking these mutations can have both the MAPK and PI3K/AKT pathways activated downstream from mutated, constitutively-activated NRAS, which has been shown to occur in about 15 of melanomas (and does not occur in combination with activating mutations of BRAF) (9). Preclinical observations with combinations of sorafenib and an inhibitor of mTOR showed reduced proliferation of melanoma cell lines and inhibition of tumor growth at primary and lymph node metastatic sites without enhanced toxicity. The observation that neovascularization was also blocked, with resulting tumor necrosis, showed that this approach could target both tumor and its microenvironment (10–12).
We designed this study in 2004 (S0438, ClinicalTrials.gov Identifier NCT00281957) to test the antitumor activity of two pairs of targeted agents in patients with previously untreated metastatic melanoma. Sorafenib, on oral agent which was developed to inhibit RAF kinase, and temsirolimus, an intravenously administered mTOR inhibitor, were combined to provide parallel pathway blockade. Sorafenib and tipifarnib, an oral inhibitor of farnesyl transferase, an enzyme required for activation of RAS, were combined to provide sequential blockade of two steps within the MAPK pathway. Archival tissue was collected for planned correlative analyses of mutations and molecular pathway activation. The trial design provided for accrual sufficient to test each regimen separately in comparable patients, using a two-stage design for each treatment cohort to screen for activity and to ultimately select the combination with the most favorable therapeutic index for further study.
PATIENTS AND METHODS
Patients
Patients were required to have biopsy-proven melanoma of cutaneous, mucosal or unknown primary origin, with measurable metastatic disease and no history of or current central nervous system metastasis. All patients were requested to provide a tissue sample for submission to the SWOG biospecimen repository, where it was earmarked for the NCI Molecular Targeted Combinations study to be analyzed with biospecimens from similar trials of targeted agents in other malignancies. Imaging of all known and suspected tumor sites, including brain, and serum lactate dehydrogenase determination were required at study entry. No prior systemic therapy for metastatic disease was permitted, but any prior adjuvant regimen was allowed if relapse occurred at least 90 days after the last adjuvant therapy, with the exception that prior exposure to any of the protocol drugs or agents of similar mechanism was not allowed. Adequate cardiac, hematologic, hepatic, and renal function and a Zubrod performance status of 0 or 1 were required, as well as recovery from any prior surgery or radiation therapy. Patients with human immunodeficiency virus requiring antiretroviral therapy, patients with CTCAE 3.0 grade ≥2 symptomatic neuropathy, subjects with a history of bleeding or coagulation disturbances, and those requiring full anticoagulation for a recent or recurrent thromboembolic event, were excluded. Patients with other medical co-morbidities such as hypertension, hyperlipidemia, and/or gastrointestinal disorders were required to be stable, permitting adequate assessment of toxicities and compliance with protocol requirements. Patients who at study enrollment or during study participation required any drugs or nutritional supplements known to be strong inducers or inhibitors of selected cytochromes were also excluded.
All study subjects provided their voluntary, written informed consent using a document approved by the institution’s human subject protection committee. The protocol and all amendments were also approved by the Southwest Oncology Group and by regulatory committees at the participating institutions.
Treatment and Monitoring
Therapeutic agents were supplied to the investigational pharmacy at each participating institution by the NCI Cancer Therapy and Evaluation Program. Patients were randomized by the SWOG Statistical Center and assigned to treatment in a non-blinded fashion. Considering the dose-limiting toxicities of the combinations and the recommended Phase II doses emerging from the Phase I trials upon which this trial was based, the doses recommended for Cohort A were sorafenib 200 mg orally twice daily with temsirolimus 25 mg total dose intravenously weekly, continuously. For Cohort B, both drugs were to be given orally twice daily, sorafenib 400 mg plus 200 mg doses continuously and tipifarnib 100 mg doses, for 3 weeks out of every 4 week period. Patients in Cohort A were advised to receive an H1 blocking antihistamine as premedication for potential temsirolimus-associated infusion reactions, Patients on Arm B received daily oral sorafenib, 400 mg in the morning and 200 mg in the evening continuously, plus oral tipifarnib, 100 mg twice daily with food, 3 consecutive weeks out of every 4-week cycle. The list of excluded medications and supplements (strong inducers and inhibitors of hepatic cytochrome P enzymes) as well as those for which “caution must be exercised” (as determined by the NCI Investigational Pharmacy Branch) was provided to the investigational pharmacy at each institution, as well as to participating subjects and their physicians. Dosing for all agents was to be continued until disease progression, protocol-specified toxicities mandating discontinuation of therapy, or patient preference.
Guidelines were provided for the management of mild to moderate toxicities, such as hypertension and mucocutaneous side effects. For higher-grade toxicities, therapy was to be withheld for clinically important grade ≥3 toxicities until resolution to ≤grade 1, at which time resumption of therapy at one or two levels below the prior dose was allowed in patients with stable or responding tumor. Because of partially overlapping toxicities in some categories, any toxicity potentially attributable to both agents (clinician- and principal investigator-assessed) was to be withheld and dose adjustments imposed on both agents at the time of resuming therapy.
Monitoring of toxicity required physical assessments every cycle, toxicity annotations every week, and laboratory analyses, to include thyroid function studies, every two weeks; weekly monitoring of serum cholesterol and triglycerides was required during the first cycle. Tumor assessments were performed after every second 4-week therapy cycle and were to include all sites of measurable disease selected at baseline, plus any clinically suspected sites of progression. Adverse event reporting was performed in accordance with the requirements of the NCI and each institution’s human subjects’ protection policies.
In response to a requirement by the Cancer Therapy Evaluation Program at the time of study approval, archival pretherapy tissue from the primary or a histologically-representative biopsy was collected for correlative analyses of mutations and molecular pathway activation, to be perfomed on samples from this study along with a series of other studies of molecularly targeted combinations.
Statistical Methods
The objectives of this study were to assess the overall response rate (ORR, confirmed complete or partial responses) and four-month progression-free survival (PFS) rate of patients undergoing therapy on each of the two treatment arms, and if both met the predefined activity threshold, to select the more promising regimen for further evaluation. Randomization was stratified by M stage (M1a/b versus M1c). For each arm, further investigation would be considered if either the ORR was ≥20% or the 4-month PFS rate was ≥45%. Based on previous SWOG phase II trials in stage IV melanoma, a regimen would be considered inactive if the ORR was ≤5% and the 4-month PFS rate was ≤25%
An identical two-stage design was used in each arm (13). Initially, 30 eligible patients were to be enrolled. If 8 or fewer patients were alive and progression-free at 4 months and 2 or fewer confirmed responses were documented, then that arm would be permanently closed with the conclusion that the regimen was inactive as defined above. If at least 3 confirmed responses were documented or at least 9 patients were alive without progression at 4 months, an additional 25 patients would be enrolled for a total of 55 patients. If at least 21 of 55 patients were alive and progression-free at 4 months, or at least 8 confirmed responses were documented, this would be considered evidence that the treatment warranted further investigation. For each arm, the false positive rate (declaring the combination worthy of further investigation when the true ORR was 5% and the true 4-month PFS was 25%) was 0.024. If either the true ORR was 20% or the true 4 month PFS rate was 45%, the power to declare the combination worthy of investigation was at least 87%. For both study arms combined, the false positive rate was 0.05 and the power was at least 76%. These calculations assumed that patients with responses and patients whose disease progressed (or who died) prior to 4 months were mutually exclusive.
Response was evaluated using RECIST criteria. PFS was defined as the time from the date of enrollment until the first date of documented disease progression (per RECIST), symptomatic deterioration, or death due to any cause. Patients last known to be alive and progression-free were censored at date of last contact. OS was defined as the time from the date of enrollment until the date of death due to any cause. Patients last known to be alive were censored at date of last contact. Duration of response was defined as the time from first documentation of response until the date of progression. OS and PFS estimates were calculated using the Kaplan-Meier method (14) and 95% confidence intervals (CIs) for medians were constructed using the Brookmeyer and Crowley method (14). Exact binomial CIs were calculated for response outcomes.
RESULTS
The protocol was opened in May 2008 and accrual was completed in November 2009. One hundred nine patients were accrued to this Intergroup trial, 21 patients from institutions in the Eastern Cooperative Oncology Group and the remainder from SWOG institutions. Three patients were ineligible: one due to presence of brain/CNS metastases, one with performance status 2, and one who did not have evidence of measurable disease as defined by RECIST. Four eligible patients did not receive any protocol treatment and were not analyzable for the study endpoints. Sixty-three patients were enrolled on the sorafenib + temsirolimus arm (Arm A). After the first stage of accrual was completed, the sorafenib + tipifarnib arm (Arm B, 39 evaluable patients) did not demonstrate sufficient activity to open the second stage of accrual. Thus, the second stage of accrual to Arm A was no longer randomized. Demographic data for the 102 evaluable patients are shown in Table 1. Patients were well balanced for factors such as gender, performance status, M stage, and primary site.
Table 1.
Demographics and Baseline Characteristics
| Sorafenib + Temsirolimus (n=63)
|
Sorafenib + Tipifarnib (n=39)
|
|||
|---|---|---|---|---|
| AGE | ||||
| Median (Range) | 64.5 | (27–85) | 58 | (28–84) |
| SEX | ||||
| Males | 34 | 54% | 22 | 56% |
| Females | 29 | 46% | 17 | 44% |
| RACE | ||||
| White | 61 | 97% | 39 | 100% |
| Black | 1 | 2% | 0 | 0% |
| Unknown | 1 | 2% | 0 | 0% |
| PERFORMANCE STATUS | ||||
| 0 | 36 | 57% | 21 | 54% |
| 1 | 27 | 43% | 18 | 46% |
| M STAGE | ||||
| M1a/M1b | 25 | 40% | 14 | 36% |
| M1c | 38 | 60% | 25 | 64% |
| SITE(S) OF METASTASES | ||||
| Bone | 12 | 19% | 11 | 28% |
| Liver | 20 | 32% | 12 | 31% |
| Distant Lymph Nodes | 36 | 57% | 21 | 54% |
| Lung | 45 | 71% | 26 | 67% |
| Other visceral | 12 | 19% | 13 | 33% |
| Other non-visceral | 10 | 16% | 7 | 18% |
| PRIMARY TYPE | ||||
| Cutaneous | 46 | 73% | 29 | 74% |
| Mucosal | 6 | 10% | 0 | 0% |
| Unknown primary | 10 | 16% | 9 | 23% |
| Not reported | 1 | 2% | 1 | 3% |
| ELEVATED SERUM LDH | ||||
| No | 34 | 54% | 25 | 61% |
| Yes | 29 | 46% | 16 | 39% |
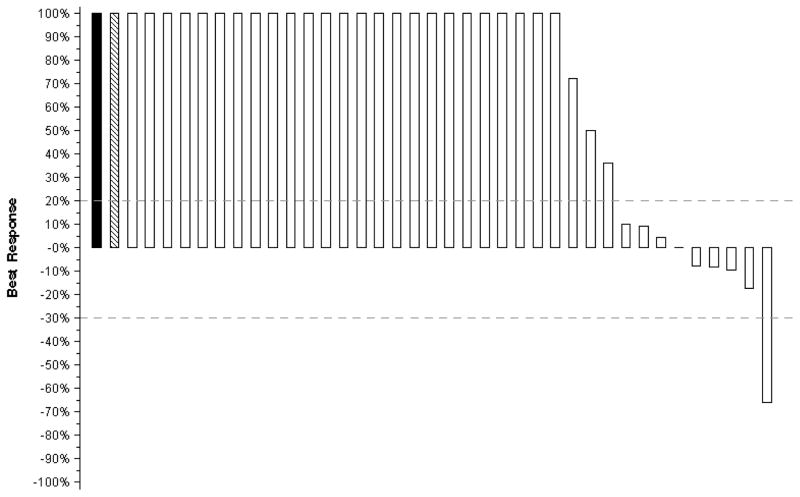
In Arm B, one confirmed partial response (PR) was documented out of 39 patients, for an ORR of 3% (95% CI: 0–13%). One patient who was taken off protocol to begin alternate therapy prior to any response assessments was counted as a non-responder. The duration of the one confirmed PR was 4 months. Arm A had 3 confirmed PRs out of 63 patients (ORR rate 5%, 95% CI: 1–13%)). Three patients who were taken off protocol to begin other therapies prior to any response assessments are counted as non-responders. The duration of the 3 confirmed PRs were 4, 9, and 13 months. Since targeted agents often provide minor regression or disease control over prolonged intervals that may be beneficial to patients, each patient’s best response (i.e., maximum reduction or minimum increase in the sum of longest tumor diameters) is illustrated on a “waterfall” plot, shown in Figures 1 and 2.
Figure 1. Sorafenib plus Temsirolimus (N=63).
Tumor regression in Arm A, Sorafenib plus Temsirolimus. The bars on each plot represent the largest decrease under baseline of the sum of longest diameters of all target measurable lesions, or if no decrease was observed, the smallest increase in the sum of longest diameters of target measurable lesions. Patients whose best response was progression due to new lesions, death (due to disease), or clear worsening of non-measurable disease are represented by a bar showing a 100% increase. In addition, patients whose best response could not be determined due to symptomatic deterioration (cross-hatched bar showing 100% increase) or inadequate assessment (solid bar showing 100% increase) are represented on the far left side of the plot. Dashed lines correspond to RECIST-defined progression and partial response.
Figure 2. Sorafenib plus Tipifarnib (N=39).
Tumor regression in Arm B, Sorafenib plus Tipifarnib
The estimated median PFS on Arm A was 2.1 months (95% CI: 1.9 – 3.3 months), the 4-month estimate was 29% (95% CI: 19% – 41%), and the 6-month estimate was 18% (95% CI: 10% – 29%). On Arm B, the estimated median PFS was 1.8 months (95% CI: 1.7 – 1.9 months), the 4-month estimate was 18% (95% CI: 8% – 31%), and the 6-month estimate was 5% (95% CI: 1% – 15%). Figure 3 shows Kaplan-Meier plots of PFS. The estimated median OS for Arm A was 7 months (95% CI: 5 – 7 months) and the estimated one-year OS was 19% (95% CI: 11% – 30%). On Arm B, the estimated median OS was also 7 months (95% CI: 5 – 11 months), and the estimated one-year OS was 31% (95% CI: 17% – 45%). Figure 4 shows Kaplan-Meier plots of OS.
Figure 3.
Progression-Free Survival
Figure 4.
Overall Survival
Toxicities were reported using CTCAE version 3.0. Selected toxicities for each arm are given in Table 2. Among 63 patients on the arm receiving sorafenib + temsirolimus (Arm A), there were two treatment-related deaths, one due to pneumonitis and the other to pancreatitis, both known adverse effects of temsirolimus and related rapamycin analogues. An additional 4 patients (6%) experienced treatment-related Grade 4 adverse events, one patient with hypokalemia and whole body weakness, one with anorexia and hypophosphatemia, one with left ventricular dysfunction (a reversible fall in ejection fraction in a patient with pre-existing ischemic cardiomyopathy), and one patient who was hospitalized briefly for a severe infusion reaction (bronchospasm and hypoxia). On Arm B, 39 patients were assessed for toxicities related to sorafenib + tipifarnib. One patient experienced a treatment-related Grade 4 toxicity manifest as elevated amylase and lipase.
Table 2.
Toxicities by Treatment Arm
| Sorafenib + Temsirolimus (n=63) | Sorafenib + Tipifarnib (n=39) | ||||
|---|---|---|---|---|---|
| Grade 1–2 N (%) |
Grade 3–4 N (%) |
Grade 1–2 N (%) |
Grade 3–4 N (%) |
||
|
| |||||
| Blood/Bone Marrow | Hemoglobin | 26 (41) | 2 (3) | 3 (8) | - |
| Leukocytes | 15 (24) | - | 1 (3) | - | |
| Neutrophils | 6 (10) | - | - | - | |
| Platelets | 19 (30) | 1 (2) | 3 (8) | - | |
| Cardiac General | Hypertension | 10 (16) | 1 (2) | 6 (15) | 2 (5) |
| Left ventricular systolic dysfunction | - | 1 (2) | - | - | |
| Constitutional symptoms | Fatigue | 35 (56) | 8 (13) | 20 (51) | 2 (5) |
| Dermatology/Skin | Acne | 18 (29) | 3 (5) | 8 (21) | 5 (13) |
| Erythema multiforme | 2 (3) | 1 (2) | 1 (3) | - | |
| Hand-foot | 3 (5) | 2 (3) | 3 (8) | 4 (10) | |
| Pruritus | 7 (11) | 2 (3) | 8 (21) | 1 (3) | |
| Rash | 14 (22) | 4 (6) | 9 (23) | 2 (5) | |
| Gastrointestinal | Anorexia | - | 1 (2) | - | - |
| Dehydration | 3 (5) | 3 (5) | 1 (3) | - | |
| Diarrhea | 14 (22) | 4 (6) | 16 (41) | 1 (3) | |
| Mucositis | 12 (19) | 2 (3) | 5 (13) | - | |
| Nausea | 19 (30) | 3 (5) | 7 (18) | - | |
| Vomiting | 7 (11) | 3 (5) | 2 (5) | - | |
| Hemorrhage/Bleeding | GI Hemorrhage: stomach | - | 1 (2) | - | - |
| Metabolic/Laboratory | ALT | 14 (22) | - | 1 (3) | 1 (3) |
| AST | 15 (24) | - | 1 (3) | 1 (3) | |
| Amylase | - | - | - | 1 (3) | |
| Cholesterol | 25 (40) | - | 7 (18) | - | |
| Hyperglycemia | 14 (22) | 1 (2) | 4 (10) | - | |
| Hypokalemia | - | 1 (2) | - | - | |
| Lipase | - | - | - | 1 (3) | |
| Hypophosphatemia | - | 1 (2) | - | - | |
| Musculoskeletal | Muscle weakness: whole body | - | 1(2) | - | - |
| Neurology | Neurology-other | - | 1 (2) | - | - |
| Pulmonary | Pulmonary-other | - | 1 (2) | - | - |
| Renal/Genitourinary | Renal failure | - | 1 (2) | - | - |
While __ of patients in Cohort A and __ of patients in Cohort B had archival tissue collected and stored in the SWOG ___ in accordance with the Molecularly Targeted Combinations project mandated by the National Cancer Institute, to date no definitive assay plans have been made.
DISCUSSION
The discovery of potential molecular targets for therapy of melanoma has led to a major paradigm shift. Notoriously resistant to cytotoxic agents, melanoma has been targeted primarily by immunotherapy, but while some patients benefit greatly, the great majority do not benefit at all. The most prevalent activating oncogenic mutations in melanoma was that of BRAF V600E/K, in which a single amino acid change leads to constitutive activation of this serine-threonine kinase in the MAP kinase pathway (7). This mutation is found in nearly half of cutaneous melanomas and is the single most common oncogenic mutation in this disease (1). After the recognition of the frequency and significance of this mutation, the first drug targeted against BRAF to undergo broad testing in melanoma was sorafenib, an agent that also inhibits several other cellular kinases. Its single-agent activity in melanoma as well as its ability to enhance the activity of cytotoxic therapy for this disease were very low, even in tumors with the BRAFV600E mutation (16,17). Another common molecular alteration in tumors with BRAF mutation is loss of PTEN activity, by mutation, deletion, or epigenetic silencing. This results in activation of AKT and subsequent proliferative and metabolic advantages favoring tumor viability and resistance to apoptosis (8). Although mTOR inhibitors such as rapamycin can be immunosuppressive, the rapamycin analog temsirolimus showed less immunosuppression and better antitumor activity in animal models. Single-agent temsirolimus was inactive in a Phase II trial of unselected patients with zero or one prior systemic therapy, possibly due in part to Akt activation through loss of a feedback loop (18).
Melanomas that do not have BRAF and PTEN alterations may still feature activation of both the MAPK and the AKT pathways, due to mutational or upstream activation of N-RAS, which promotes signaling down both pathways (9). Inhibition of RAS signaling has been reported with agents that block cellular farnesylation reactions, but the specificity of these drugs and the contribution of this pathway to their cytotoxic effects have not been clearly demonstrated. Inhibition of N-RAS farnesylation by tipifarnib has an IC50 below 10 nM (a concentration that was far exceeded at steady state in patients receiving the identical regimen in the Phase I study upon which our doses were based [6]). However, some Ras isoforms may be alternatively activated, and the overall impact of inhibiting Ras activity melanoma cells that may have one or more additional mutationally-activated pathways has not been well-documented, with the results of limited clinical testing disappointing for both clinical endpoints of antitumor activity and molecular endpoints of MAPK inhibition (19).
We reasoned that despite the low single agent activity of each agent, combinations of molecular pathway blockade could overcome innate resistance or delay acquired resistance due to a second mutation or compensatory pathway activation. Sorafenib plus temsirolimus could potentially block both MAPK and AKT pathways, resulting in cytotoxicity against cells with activation of one or both pathways. Combining sorafenib with tipifarnib would sequentially block two steps in the RAS-RAF-MAPK pathway, effectively overcoming resistance mediated by RAS activation of a bypass pathway in the presence of BRAF inhibition (4–6). It is essential to note that this trial was conceived in 2004 in response to the National Cancer Institute solicitation for molecularly targeted combinations in several solid tumors at a time when assays for target expression in tumor were not available and their role in preselection of patients not yet demonstrated.
The clinical testing of combinations containing an mTOR pathway inhibitor (such as temsirolimus) plus sorafenib, which at the time was believed to be a potent inhibitor of BRAF and the MAPK pathway, required careful attention to combined toxicities that were assessed in phase I trials using a range of doses for each agent, given on their standard schedules. Despite the relative lack of myelosuppression at the recommended doses, other toxicities of the combination from phase I trials included mucocutaneous (skin rash, hand-foot syndrome, diarrhea), constitutional (fatigue) and metabolic (hyperlipidemia, hyperglycemia) disturbances. Some of the reported data suggested a pharmacokinetic interaction between sorafenib and temsirolimus or tipifarnib leading to increased exposures to temsirolimus and to decreased inhibition of farnesyl transferase by tipifarnib in the presence of sorafenib (4–6). The data from these phase I trials demonstrated the ability to achieve serum drug levels associated with in vitro antitumor activity and inhibition of downstream targets of mTOR at the recommended phase II doses, The Phase I/II trial of sorafenib plus temsirolimus for melanoma also suggested the combination might have activity against melanoma: no objective responses were seen but nearly half of patients (10 of 23) experienced stable disease over periods of 2–8 months (4).
Despite the rationale supporting the two combinations tested in this randomized phase II trial, the antitumor effects were disappointing. The sorafenib plus tipifarnib arm (Arm B) failed to meet the criteria for a second stage of accrual, despite significant toxicity. Arm A, sorafenib plus temsirolimus, did meet the criteria for full accrual, but in the end did not meet the prespecified levels of activity to pursue the regimen further. We also compared the observed results for both trial arms to the cumulative results from 70 cooperative group phase II trials in stage IV melanoma summarized in the metaanalysis performed by Korn et al (20). Based on the model presented that meta-analysis, the predicted 6-month PFS and 1-year OS were 16% and 34% for the temsirolimus arm, and 15% and 32% for the tipifarnib arm (the small differences were based on slight differences in patient characteristics between the arms). The observed 6-month PFS (18% and 5%) and 1-year OS (19% and 31%) from both arms were not significantly better than these predicted values—justifying our conclusion that the results show both regimens, even the slightly more active arm A, to be insufficiently active for further study (20).
This study provides a number of lessons for future clinical trials of targeted agent combinations in melanoma. The concepts that formed the basis for this trial remain very much at the forefront of current thinking. But we now recognize that the potency and specificity of the inhibitors used are critical factors for success. It is unclear whether any of the three agents used in these studies hit their target with sufficient potency to inhibit its downstream effect. Furthermore, we must focus our efforts om patients who actually have the targets (mutated oncogenes) that can be inhibited by the agents used. In this case, we need to know the mutational status of BRAF, NRAS, and PTEN and of the degree of constituitive activation of the relevant pathways. Since this study was completed, several new agents (vemurafenib and GSK2118436) have shown remarkable activity in patients with BRAF V600-mutant melanoma, with objective regression in up to 80% of patients and median response durations of approximately 6–7 months (21, 22). Insights into mechanisms of resistance that have emerged very recently will inform the design of future regimens using simultaneous or sequential combinations of small molecule pathway-targeted agents, as well as strategies that bring together different modalities, such as immunomodulatory agents and small molecules (23–25). The approach outlined in this study remains worthy of pursuit, and should be revisited as our knowledge of melanoma biology and our access to high-potency inhibitors of the most important pathways both increase.
Figure 5.
Consort Diagram
STATEMENT OF TRANSLATIONAL RELEVANCE.
Sorafenib plus temsirolimus could potentially block both MAPK and AKT pathways, resulting in cytotoxicity against cells with activation of one or both pathways.
Acknowledgments
ACKNOWLEDGEMENT OF SUPPORT: This investigation was supported in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, DHHS: CA32102, CA38926, CA27057, CA14028, CA35178, CA35281, CA46368, CA35431, CA46282, CA45808, CA35128, CA46441, CA45377, CA67575, CA20319, CA35119, CA04919, CA12644, CA46113, CA58861 (SWOG); CA21115 and CA39229 (ECOG)
Footnotes
PREVIOUS PRESENTATION OF RESULTS: Results presented in part at the 46th Annual Meeting of the American Society of Clinical Oncology (ASCO), June 4-8, 2010, Chicago, IL.
References
- 1.Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–2147. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 2.Nazarian R, Shi H, Wang Q, Kog X, Koya RC, Lee H, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973–977. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villanueva J, Vultur A, Lee JT, Somasundaram R, Fukunaga-Kalabis M, Cipolla AK, et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 2010;18:683–95. doi: 10.1016/j.ccr.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patnaik A, Ricart A, Cooper J, Papadopoulos K, Beeram M, Mita C, et al. A phase I, pharmacokinetic and pharmacodynamic study of sorafenib (S), a multi-targeted kinase inhibitor in combination with temsirolimus (T), an mTOR inhibitor in patients with advanced solid malignancies. Proc Am Soc Clin Oncol. 2007;25S [Abstract 3512] [Google Scholar]
- 5.Kim KB, Davies MA, Papadopoulos NE, Bedikian AY, Hwu W, Woodard K, et al. Phase I/II study of the combination of sorafenib and temsirolimus in patients with metastatic melanoma. Proc Am Soc Clin Oncol. 2009;27S [Abstract 9026] [Google Scholar]
- 6.Hong DS, Sebti S, Newman R, Blaskovich MA, Ye L, Gagel RF, et al. Phase I trial of a combination of the multikinase inhibitor Sorafenib and the farnesyltransferase inhibitor Tipifarnib in advanced malignancies. Clin Cancer Res. 2009;15:7061–7068. doi: 10.1158/1078-0432.CCR-09-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 8.Madhunapantula SV, Robertson GP. The PTEN-AKT3 signaling cascade as a therapeutic target in melanoma. Pigment Cell Melanoma Res. 2009;22:400–419. doi: 10.1111/j.1755-148X.2009.00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorden A, Osman I, Gai W, He D, Huang W, Davidson A, et al. Analysis of BRAF and N-RAS mutations in metastatic melanoma tissues. Cancer Res. 2003;63:3955–3957. [PubMed] [Google Scholar]
- 10.Molhoek KR, Brautigan DL, Slingluff CL., Jr Synergistic inhibition of human melanoma proliferation by combination treatment with B-Raf inhibitor BAY43-9006 and mTOR inhibitor Rapamycin. J Transl Med. 2005;3:39. doi: 10.1186/1479-5876-3-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meier F, Busch S, Lasithiotakis K, Kulms D, Garbe C, Maczey E, et al. Combined targeting of MAPK and AKT signalling pathways is a promising strategy for melanoma treatment. Br J Dermatol. 2007;156:1204–1213. doi: 10.1111/j.1365-2133.2007.07821.x. [DOI] [PubMed] [Google Scholar]
- 12.Molhoek KR, Griesemann H, Shu J, Gershenwald JE, Brautigan DL, Slingluff CL., Jr Human melanoma cytolysis by combined inhibition of mammalian target of rapamycin and vascular endothelial growth factor/vascular endothelial growth factor receptor-2. Cancer Res. 2008;68:4392–4397. doi: 10.1158/0008-5472.CAN-07-5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green SJ, Dahlberg S. Planned versus attained design in phase II clinical trials. Stat Med. 1992;11:853–862. doi: 10.1002/sim.4780110703. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 15.Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics. 1982;38:29–41. [Google Scholar]
- 16.Eisen T, Ahmad T, Flaherty KT, Gore M, Kaye S, Marais R, et al. Sorafenib in advanced melanoma: a Phase II randomised discontinuation trial analysis. Br J Cancer. 2006;95:581–586. doi: 10.1038/sj.bjc.6603291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flaherty KT, Schiller J, Schuchter LM, Liu G, Tuveson DA, Redlinger M, et al. A phase I trial of the oral, multikinase inhibitor sorafenib in combination with carboplatin and paclitaxel. Clin Cancer Res. 2008;14:4836–4842. doi: 10.1158/1078-0432.CCR-07-4123. [DOI] [PubMed] [Google Scholar]
- 18.Margolin K, Longmate J, Baratta T, Synold T, Christensen S, Weber J, et al. CCI-779 in metastatic melanoma: a phase II trial of the California Cancer Consortium. Cancer. 2005;104:1045–1048. doi: 10.1002/cncr.21265. [DOI] [PubMed] [Google Scholar]
- 19.Gajewski TF, Niedzwiecki D, Johnson J, Linette G, Bucher C, Blaskovich M, et al. Phase II study of the farnesyltransferase inhibitor R115777 in advanced melanoma: CALGB 500104. Proc Am Soc Clin Oncol. 2006;24S doi: 10.1186/1479-5876-10-246. [Abstract 8014] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidinger M, Bellmunt J. Plethora of agents, plethora of targets, plethora of side effects in metastatic renal cell carcinoma. Cancer Treat Rev. 2010;36:416–424. doi: 10.1016/j.ctrv.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arkenau HT, Kefford R, Long GV. Targeting BRAF for patients with melanoma. Br J Cancer. 2011;104:392–398. doi: 10.1038/sj.bjc.6606030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Downward J. Targeting RAF: trials and tribulations. Nat Med. 2011;17:286–288. doi: 10.1038/nm0311-286. [DOI] [PubMed] [Google Scholar]
- 24.van der Heijden MS, Bernards R. Inhibition of the PI3K pathway: hope we can believe in? Clin Cancer Res. 2010;16:3094–3099. doi: 10.1158/1078-0432.CCR-09-3004. [DOI] [PubMed] [Google Scholar]
- 25.Abraham RT, Gibbons JJ. The mammalian target of rapamycin signaling pathway: twists and turns in the road to cancer therapy. Clin Cancer Res. 2007;13:3109–3114. doi: 10.1158/1078-0432.CCR-06-2798. [DOI] [PubMed] [Google Scholar]