Abstract
Purpose
We have observed a higher rate of Lhermitte's syndrome (LS) after chemo-IMRT of head and neck cancer than the published rates after conventional radiotherapy. We hypothesized that the inhomogeneous spinal cord dose distributions produced by IMRT caused a “bath and shower” effect, characterized by low doses in the vicinity of high doses, reducing spinal cord tolerance.
Methods and Materials
73 patients with squamous cell carcinoma of the oropharynx participated in a prospective study of IMRT concurrent with weekly carboplatin and taxol. 15 (21%) reported LS in at least 2 consecutive follow-up visits. Mean dose, maximum dose, partial (Vd) as well as the absolute volume (cc) of spinal cord receiving specified doses (≥10 Gy, 20 Gy, 30 Gy, 40 Gy), and the pattern of dose distributions at the “anatomical” (from the base of the skull to the aortic arch) and “plan-related” (from the top through the bottom of the PTV's) spinal cords were compared between LS and 34 non-LS patients.
Results
LS patients had significantly higher spinal cord mean doses, V30, V40, and volumes receiving ≥30 and ≥ 40 Gy compared to the non-LS patients (p < 0.05). Strongest predictors of LS were higher V40 and higher cord volumes receiving ≥40 Gy (p ≤ 0.007). There was no evidence of larger spinal cord volumes receiving low doses in the vicinity of higher doses (“bath and shower”) in LS compared to non-LS patients.
Conclusions
Greater mean dose, V30, V40, and cord volumes receiving ≥30 and ≥40 Gy characterized LS compared to non-LS patients. “Bath and shower” effects could not be validated in this study as a potential contributor to LS. The higher than expected rates of LS may be due to the specific concurrent chemotherapy agents, or to more accurate identification of LS in the setting of a prospective study.
Keywords: Lhermitte's syndrome, Predictive factors, Head and neck cancer, Bath and shower effect, IMRT
Introduction
Lhermitte's syndrome (LS) is an electric shock-like sensation in the spine and extremities exacerbated by neck flexion. It is caused by reversible demyelination of ascending sensory neurons due to inhibition of oligodendrocyte proliferation following radiotherapy (RT) of the cervical or thoracic spine (1-3). The denuded axons become sensitive to irritation from neck flexion, causing the characteristic shock sensations. Once oligodendrocytes recover and myelin synthesis is resumed, symptoms subside. Although LS is not usually associated with a progression to chronic progressive irreversible myelitis, delayed radiation myelopathy causing paralysis may be preceded by LS (4).
The incidence of LS in series of patients receiving RT for head and neck (HN) or thoracic malignancies has been reported to be between 3.6 and 13% (4-7). All these series used conventional RT, which typically delivers homogeneous dose distributions across the spinal cord. In a prospective study of chemo-IMRT for HN cancer, in which treatment toxicities including LS were recorded prospectively and longitudinally, we have observed a substantially higher incidence of LS than that reported in the literature. We hypothesized that the inhomogeneous dose distributions produced by IMRT across the spinal cord may have played a role in increasing the rate of LS.
Investigations of the tolerances of rat spinal cords to RT revealed a “bath and shower” dose-volume effect, where radiation tolerance is markedly reduced when a high dose volume segment (shower) is surrounded by low-dose volumes (bath) (8, 9). This effect was attributed to the interference of neighboring oligodendrocyte migration into high-dose regions by low-dose radiation, as well as to the inhibition of the release of cytokines, growth factors, and vasoactive mediators by astrocytes and microglia receiving low doses that regulate oligodendrocyte proliferation, differentiation, and migration. These investigations assessed dose-effect relationships for spinal cord tolerances in the rat, using an irreversible neurological deficit as an endpoint. We hypothesized that a similar phenomenon may have existed in the spinal cords of the patients receiving IMRT in our series, facilitating transient demyelination that manifests as LS.
In order to investigate potential “bath and shower” effects causing LS in patients who participated in this chemo-IMRT study, we have compared the pattern of inhomogeneous dose distributions produced by IMRT across the spinal cord in LS and non-LS patients. This study represents the first clinical assessment of potential “bath and shower” effects for the spinal cord.
Methods and Materials
Patients
73 patients with stage III-IV squamous cell carcinoma of the oropharynx participated in an Institutional Review Board-approved prospective longitudinal study of chemo-IMRT-associated toxicities and signed a study-specific informed consent. Details of the patients and therapy have been previously published (10). In brief, all patients were prescribed 70 Gy to the primary clinical target volume (CTV1) and 56-63 Gy to the CTV2-3, all in 35 fractions over 7 weeks. Dose constraints included maximum doses of 46 Gy to the spinal cord and 50 Gy to an expansion of the spinal cord by 5 mm (planning organ-at-risk volume). The whole neck was treated by IMRT in all patients. No patients received prior therapy. Concurrent chemotherapy consisted of carboplatin, (Area-under-the-curve 1) and paclitaxel 30 mg/m2, delivered once weekly during the 7 treatment weeks. All patients were treated between 2004 and 2008 at the University of Michigan.
As a part of a longitudinal assessment in the study using the Common Toxicities Criteria (CTCAE v2.0), patients were asked about symptoms suggesting LS prior to therapy and during each of their clinical follow-up visits, at 2-month intervals, through 2 years after treatment. Since LS has been reported to last on average 4-6 months (5, 7), and in order to exclude cases of very mild LS, patients were determined to have developed definitive LS for the purposes of our analysis if they answered “yes” to the question related to LS in at least two consecutive follow-up visits. Only patients with at least 12 months of follow-up who did not report LS were included in the “non-LS” group. Clinical and radiation treatment factors were compared between LS patients and those who did not report LS at any time point.
Spinal cord dosimetry
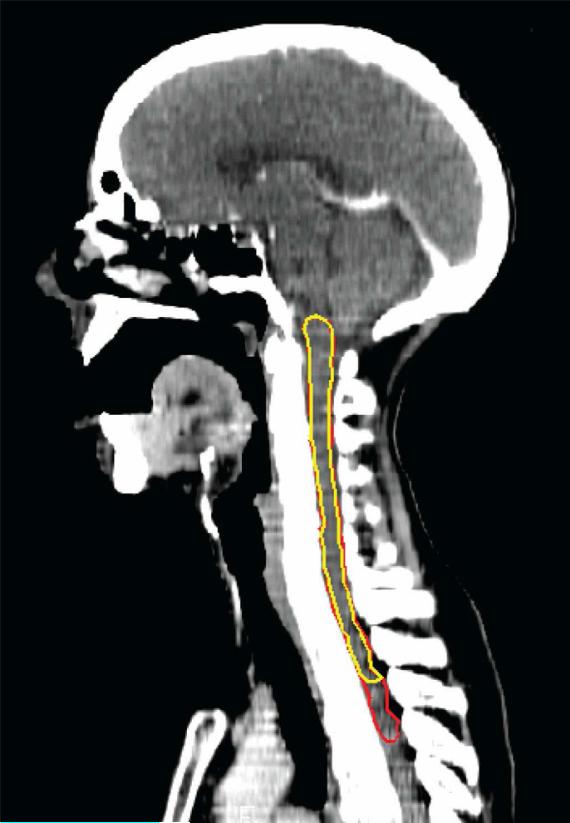
The dosimetry for the clinical treatment planning was based on an automatic delineation of the spinal cord based on the bony spinal canal, rather than the spinal cord itself. The spinal cord was therefore re-contoured manually for the purposes of this study on sequential axial CT slices of each patient. Also, for the purposes of this study, two different lengths of spinal cord were contoured for each patient: (a) from the base of the skull superiorly to the superior border of the aortic arch inferiorly (“anatomical spinal cord”), and (b) from the base of the skull superiorly, near the superior border of the PTVs, to the inferior border of the neck PTVs, representing the superior and inferior-most extents of the irradiated spinal cord (“plan-related spinal cord”) (Fig. 1).
Figure 1.
Sagittal CT slice displaying structure definitions of the plan-related (yellow) and anatomical (red) spinal cords.
Cumulative and direct dose-volume histograms (DVHs) for the spinal cords of each patient were computed, and dose distributions were assessed qualitatively. Additionally, dose metrics examined for association with LS included the following: mean dose, maximum dose, and the partial (Vd) as well as the absolute volume (cc) of spinal cord receiving specified doses (≥10 Gy, 20 Gy, 30 Gy, 40 Gy).
Statistical analysis
Fisher's exact tests and two-sample t-tests were used to test associations between patient characteristics and LS. Relationships between the development of LS and the dose metrics were modeled using logistic regression analysis on the binary outcome (LS vs. non-LS). All analyses were performed using SPSS v 18.0 statistical software. All reported p values were two-sided, and values ≤0.05 were considered to be significant.
Results
Of 73 protocol patients, 15 (21%) patients reported LS in at least 2 consecutive follow-up visits and were considered LS patients for analysis. The mean onset of LS after therapy completion and duration of symptoms for the LS population were 5 and 5.5 months, respectively. Symptoms resolved spontaneously in all cases and no patient developed radiation myelitis. The 15 LS patients were compared to 34 patients who did not develop any symptoms suggestive of LS with at least 12 months post-therapy follow-up (“non-LS group”). Of the remaining 24 patients, 19 did not have 12- months LS data and were therefor excluded (none reported LS through 10 months), and 5 reported LS only once during follow-up and were excluded due to uncertainty (see Methods).
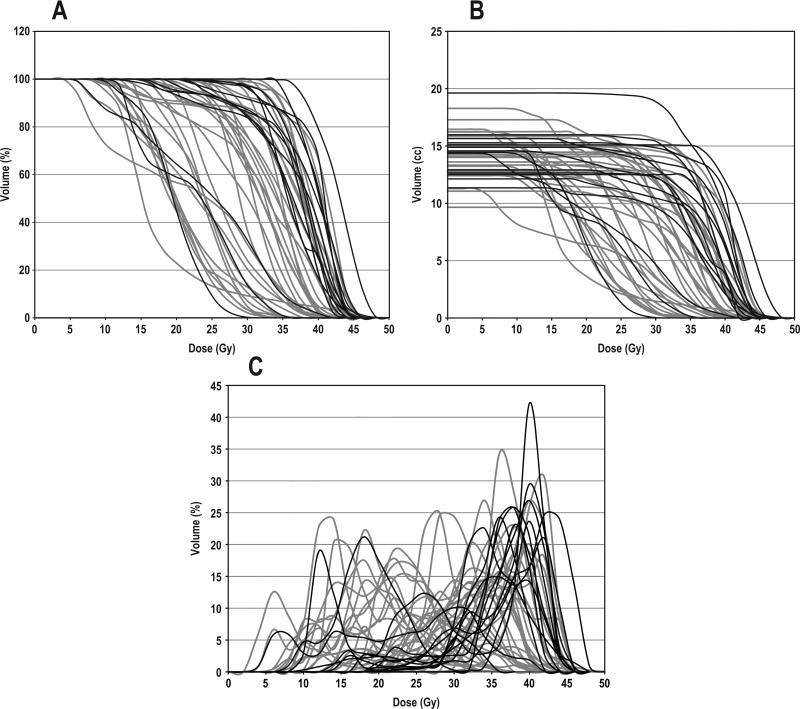
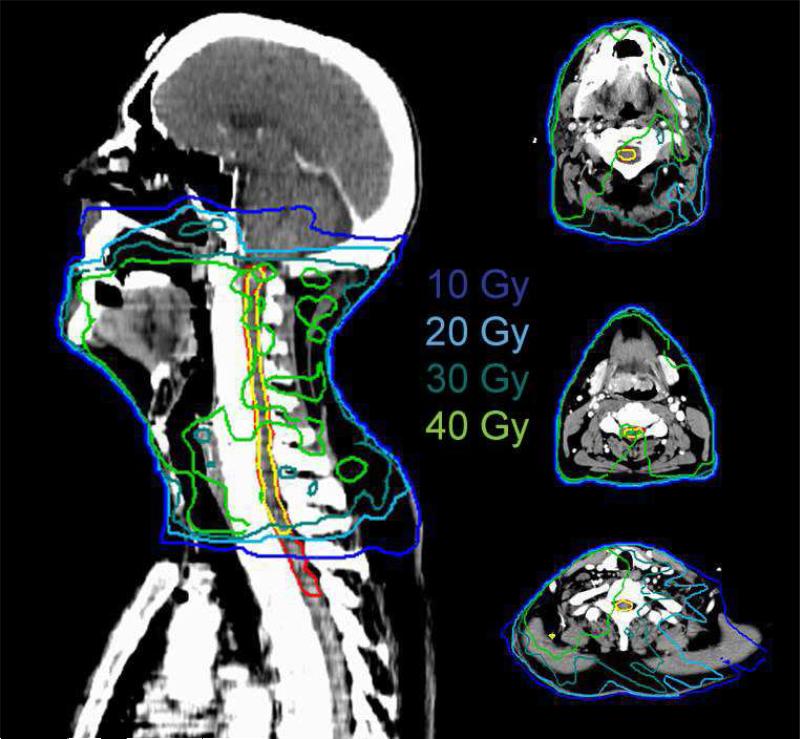
Patient and tumor characteristics such as gender, age, tumor location, AJCC stage, and clinical risk factors for neuropathy (diabetes, hypertension) are summarized in Table 1. None of these factors were significantly associated with LS (p ≥ 0.1). Cumulative DVHs for the “plan-related spinal cords” are shown in Fig. 2, and corresponding dosimetric comparisons between the LS and non-LS populations are detailed in Table 2. LS patients had significantly higher spinal cord mean doses, V30, V40, and volumes receiving ≥30, 40 Gy compared to non-LS patients (p < 0.05). The highest correlations with LS were associated with higher V40 and cord volumes receiving ≥40 Gy (p ≤ 0.006). V10, V20, maximum dose, and cord volumes receiving ≥10 or ≥20 Gy were not found to be significantly different between the two groups (p ≥ 0.15). The dose distribution for a LS patient in our series is shown in Fig. 3.
Table 1.
Patient and Tumor Characteristics by LS Status
| Non-LS (n = 34) | LS (n = 15) | |
|---|---|---|
| Gender | ||
| Male | 32 (94.1%) | 12 (80.0%) |
| Female | 2 (5.9%) | 3 (20.0%) |
| Age | ||
| Mean | 59 | 55 |
| Range | 42-78 | 45-74 |
| Tumor location | ||
| Tonsil | 13 (38.2%) | 10 (66.7%) |
| Base of tongue | 18 (52.9%) | 5 (33.3%) |
| Tonsil + Base of tongue | 2 (5.9%) | 0 |
| Pharyngeal wall | 1 (3.0%) | 0 |
| AJCC stage | ||
| II | 1 (3.0%) | 0 |
| III | 3 (8.8%) | 2 (13.3%) |
| IVA | 27 (79.4%) | 13 (86.7%) |
| IVB | 3 (8.8%) | 0 |
| Clinical risk factors | ||
| Hypertension | 6 (17.6%) | 3 (20.0%) |
| Diabetes | 1 (3.0%) | 1 (6.7%) |
| Hypertension + diabetes | 2 (5.9%) | 1 (6.7%) |
Figure 2.
DVHs for the plan-related spinal cord: non-LS (gray) and LS (black) patients. Cumulative DVHs with (A) percent cord volume and (B) absolute cord volume. (C) Direct DVH with percent cord volume.
Table 2.
Comparison of Dose Metrics by LS Status: Plan-Related Spinal Cord*
| Dose metric | Non-LS (n = 34) | LS (n = 15) | p-value |
|---|---|---|---|
| Mean dose (Gy) | 29.0 ± 6.9 | 33.6 ± 7.0 | 0.032 |
| Maximum dose (Gy) | 42.2 ± 4.2 | 43.1 ± 4.5 | 0.572 |
| V10 (%) | 99.1 ± 2.6 | 99.1 ± 3.2 | 0.451 |
| V20 (%) | 83.4 ± 22.1 | 89.9 ± 18.1 | 0.148 |
| V30 (%) | 54.9 ± 35.6 | 76.7 ± 32.8 | 0.044 |
| V40 (%) | 12.3 ± 17.5 | 32.6 ± 24.2 | 0.005 |
| Vol (cc) ≥10 Gy | 14.0 ± 2.0 | 14.3 ± 2.1 | 0.421 |
| Vol (cc) ≥20 Gy | 11.8 ± 3.5 | 13.0 ± 3.3 | 0.157 |
| Vol (cc) ≥30 Gy | 7.8 ± 5.2 | 11.1 ± 5.1 | 0.048 |
| Vol (cc) ≥40 Gy | 1.7 ± 2.5 | 5.0 ± 3.9 | 0.006 |
Defined from the skull base to the inferior border of the PTV.
Figure 3.
The dose distribution for a patient with Lhermitte's syndrome.
Table 3 summarizes the dosimetric comparisons between LS and non-LS patients for the “anatomical spinal cord.” Similar to the “plan-related spinal cord,” LS patients had significantly higher spinal cord mean doses, V30, V40, and absolute cord volumes receiving ≥30 or ≥40 Gy, compared to the non-LS patients (p < 0.04) while the other dosimetric parameters were not found to be significantly different.
Table 3.
Comparison of Dose Metrics by LS Status: Anatomical Spinal Cord*
| Dose metric | Non-LS (n = 34) | LS (n = 15) | p-value |
|---|---|---|---|
| Mean dose (Gy) | 26.3 ± 6.4 | 30.2 ± 6.3 | 0.039 |
| Maximum dose (Gy) | 42.9 ± 3.9 | 44.0 ± 4.6 | 0.327 |
| V10 (%) | 88.1 ± 7.8 | 89.1 ± 7.3 | 0.259 |
| V20 (%) | 71.9 ± 20.4 | 76.8 ± 17.0 | 0.204 |
| V30 (%) | 45.0 ± 29.7 | 64.6 ± 27.6 | 0.037 |
| V40 (%) | 10.6 ± 15.3 | 27.2 ± 20.0 | 0.007 |
| Vol (cc) ≥10 Gy | 16.8 ± 2.5 | 17.2 ± 2.4 | 0.293 |
| Vol (cc) ≥20 Gy | 13.6 ± 4.1 | 14.8 ± 3.7 | 0.160 |
| Vol (cc) ≥30 Gy | 8.4 ± 5.6 | 12.4 ± 5.7 | 0.031 |
| Vol (cc) ≥40 Gy | 1.8 ± 2.6 | 5.4 ± 4.3 | 0.005 |
Defined from the skull base to the superior border of the aortic arch.
For the purposes of investigating potential “bath and shower” dose-volume effects for LS, we used the direct DVHs for the plan-related spinal cords (Fig. 2C). LS patients generally did not receive low, “sub-threshold” doses to the spinal cord, with only 3 of 15 patients receiving doses ≤20 Gy to ≥10% of the spinal cord, and 4 of 15 receiving doses ≤30 Gy to ≥20% of the spinal cord. In comparison, non-LS patients received a substantially wider range of low doses, with 16 of 34 patients receiving ≤20 Gy to ≥10% of the spinal cord, and 27 of 34 receiving ≤30 Gy to ≥20% of the cord. A qualitative examination of dose distributions in the spinal cords revealed that volumes receiving >40 Gy in both the LS and non-LS patients were small, segmented and scattered, rather than in large contiguous volumes, and were superimposed on volumes of lower doses, with no qualitative differences in this distribution pattern between the LS and non-LS patients.
Discussion
The incidence of post-radiation LS (21%) in our chemo-IMRT study was higher than the rates previously published in retrospective series of patients receiving conventional 2D RT without concomitant chemotherapy. Our dosimetric study showed significantly higher doses, particularly V40, in the LS compared with the non-LS patients, but could not validate our hypothesis that IMRT caused a “bath and shower” effect that might have explained the high rate of LS.
The literature on dose-volume toxicity relationships for LS is limited. Word et al. (11) found that 4 (9%) of 44 patients with Hodgkin's disease who received mantle irradiation of 4000 rad developed LS, but a correlation between incidence and increasing dose could not be drawn. Fein et al. (5) reported that 8% of patients who received ≥50 Gy to the cervical spinal cord developed LS compared to 3.3% of those who received <50 Gy. They reported also that daily fractionations of ≥2 Gy were associated with increased incidence of LS. Similarly, Leung et al. (7) noted a correlation between incidence of LS and dose when the total dose to the cervical spinal cord exceeded 48.9 Gy. More detailed dose parameters derived from 3D treatment data were not provided in these past studies. Lewanski et al. (12) provided case analyses of four patients who developed LS following RT of the HN and noted that they all had received mean cord doses ≥40.5 Gy and had maximum doses of 40-50 Gy.
Very few studies have examined the effects of inhomogeneous dose distributions of IMRT on the spinal cord. Lim et al. (2) reported a patient who developed LS after being treated with IMRT and chemotherapy, finding a mean spinal cord dose of 2692 cGY and a maximum dose of 4478 cGy. In our series of patients treated with IMRT and concurrent chemotherapy for oropharyngeal cancer, LS was found to be significantly correlated with a higher mean dose, V30, V40, and spinal cord volume receiving ≥30 Gy and ≥40 Gy, with the highest correlations associated with a higher V40 and cord volume receiving ≥40 Gy. In comparison, the spinal cord doses typically delivered in conventional, 3-field irradiation of HN cancer are higher. In these plans the spinal cord typically receives 40-46 Gy homogeneously at 1.8-2.0 Gy/fraction, and then it is shielded for the “off cord” and final tumor boost, which deliver additional transmitted and scattered doses, such that the cumulative total doses exceed 50 Gy (13). In comparison, only small spinal cord volumes have received >40 Gy in the IMRT patients in our series, and their fraction doses were smaller (40-45 Gy maximal spinal cord dose was delivered at approximately 1.1-1.3 Gy/fraction, as the whole treatment course was delivered over 35 fractions).
As the total and fraction doses delivered by IMRT to the spinal cord were lower than those typically delivered by “conventional” RT while rates of LS were higher, we have investigated the importance of the dose distribution pattern. Bijl et al. (8) investigated “bath and shower” dose-volume effects for limb paralysis on rat spinal cords and found a decrease of 15-22 Gy in the iso-effective shower doses (ED50) when they were superimposed on bath doses as low as 4 Gy (compared to an ED50 of 53.7 Gy when irradiating a segment without any bath). This supported the hypothesis that surrounding sub-threshold doses inhibit neighboring oligodendrocytes or oligodendrocyte precursor cells (OPCs) from migrating to adjacent regions receiving higher doses, thus preventing remyelination of denuded axons and reducing the spinal cord's radiation tolerance (8, 14-16). There have been no clinical studies, however, of these effects on human spinal cords.
We did not observe a similar bath effect for LS in our series. LS patients generally did not receive low, sub-threshold doses to the spinal cord, with only 3 of 15 patients receiving doses ≤20 Gy to ≥10% of the spinal cord, and 4 of 15 receiving doses ≤30 Gy to ≥20% of the spinal cord; most of the irradiated spinal cord volumes received doses greater than 30 Gy. In comparison, non-LS patients received a substantially wider range of sub-threshold and threshold doses. A major weakness of our study is the obvious inability to reproduce the dose distributions in a “control” group mimicking the control groups in the animal studies of “bath and shower effect,” in which no dose at all was delivered in the vicinity of the high doses. Our clinical study cannot therefore exclude “bath and shower” effects contributing to LS. However, the most significant predictors of LS in our study were higher spinal cord doses, rather than any differences in the distributions of the low doses.
Other risk factors for neuropathy, such as diabetes mellitus and hypertension, were not found to be significantly associated with LS in our study. Yet, all patients in our series were treated with concurrent chemotherapy consisting of carboplatin and paclitaxel, each of which has been associated with peripheral neuropathies (17, 18). It is possible that the use of these specific concurrent chemotherapy agents increased substantially the rate of LS in our series compared to previous LS series, all of which used RT without concomitant chemotherapy. LS is very rare following chemotherapy alone, but previous treatment with a neurotoxic chemotherapeutic agent may predispose to the development of LS during the administration of another neurotoxic chemotherapy (19, 20). It is possible that the reason for the protection of the spinal cord from the effect of neurotoxic chemotherapy relates to the blood brain barrier (BBB) which protects the spinal cord but is not present in peripheral nerves. The administration of radiotherapy concurrent with neurotoxic chemotherapy may disrupt the BBB and increase the effect of chemotherapy on the spinal cord.
Lastly, to the best of our knowledge, our series was the only one in which LS was specifically addressed as a potential toxicity in a prospective, longitudinal manner. It is possible that the previous retrospective series under-estimated the real incidence of LS.
In conclusion, higher spinal cord V40 and other measures of spinal cord doses, such as mean doses, were found in patients with LS compared with patients without LS after chemo-IMRT of oropharyngeal cancer, but no evidence was found of larger low-dose volumes in the vicinity of high dose volumes in the LS patients. Other explanations for the relatively high rate of LS in our series, which delivered lower total as well as lower daily fraction doses to the spinal cord compared with conventional RT, include the concurrent chemotherapy used in the study, and the possibility that the actual rate of LS following RT of HN cancer is indeed higher than reported in previous retrospective studies.
We have found a 21% rate of Lhermitte's sign (LS) in a prospective study of chemo-IMRT for head and neck cancer, higher than reported previously after conventional RT. We explored the possibility that the inhomogeneous distribution of doses across the spinal cord, causing a “bath and shower” effect (which was found to increase spinal cord toxicity in pre-clinical studies) could explain this finding. While clear dose-effect relationships were found, there was no evidence of “bath and shower” effect. It is possible that the specific concurrent chemotherapy, or the prospective nature of the study, relate to the higher-than-usual rate of LS in our study.
Acknowledgments
Supported in part by NIH grant PO1 CA59827 and the Newman Family Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Notification: none
Financial Disclosure: none
Presented in part at the 52nd Annual Meeting of the American Society of Radiation Oncology (ASTRO), San Diego, CA, Oct 31-Nov 4, 2010.
References
- 1.Jones A. Transient Radiation Myelopathy (with Reference to Lhermitte's Sign of Electrical Paraesthesia). Br J Radiol. 1964;37:727–744. doi: 10.1259/0007-1285-37-442-727. [DOI] [PubMed] [Google Scholar]
- 2.Lim DC, Gagnon PJ, Meranvil S, et al. Lhermitte's Sign Developing after IMRT for Head and Neck Cancer. Int J Otolaryngol. 2010:907960. doi: 10.1155/2010/907960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.St Clair WH, Arnold SM, Sloan AE, et al. Spinal cord and peripheral nerve injury: current management and investigations. Semin Radiat Oncol. 2003;13:322–332. doi: 10.1016/s1053-4296(03)00025-0. [DOI] [PubMed] [Google Scholar]
- 4.Esik O, Csere T, Stefanits K, et al. A review on radiogenic Lhermitte's sign. Pathol Oncol Res. 2003;9:115–120. doi: 10.1007/BF03033755. [DOI] [PubMed] [Google Scholar]
- 5.Fein DA, Marcus RB, Jr., Parsons JT, et al. Lhermitte's sign: incidence and treatment variables influencing risk after irradiation of the cervical spinal cord. Int J Radiat Oncol Biol Phys. 1993;27:1029–1033. doi: 10.1016/0360-3016(93)90519-2. [DOI] [PubMed] [Google Scholar]
- 6.Gemici C. Lhermitte's sign: Review with special emphasis in oncology practice. Crit Rev Oncol Hematol. 74:79–86. doi: 10.1016/j.critrevonc.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Leung WM, Tsang NM, Chang FT, et al. Lhermitte's sign among nasopharyngeal cancer patients after radiotherapy. Head and Neck-Journal for the Sciences and Specialties of the Head and Neck. 2005;27:187–194. doi: 10.1002/hed.20140. [DOI] [PubMed] [Google Scholar]
- 8.Bijl HP, van Luijk P, Coppes RP, et al. Unexpected changes of rat cervical spinal cord tolerance caused by inhomogeneous dose distributions. Int J Radiat Oncol Biol Phys. 2003;57:274–281. doi: 10.1016/s0360-3016(03)00529-7. [DOI] [PubMed] [Google Scholar]
- 9.Joiner M, Van der Kogel A. Basic Clinical Radiobiology. 4th ed. Hodder Arnold; London: 2009. [Google Scholar]
- 10.Feng FY, Kim HM, Lyden TH, et al. Intensity-modulated radiotherapy of head and neck cancer aiming to reduce dysphagia: early dose-effect relationships for the swallowing structures. Int J Radiat Oncol Biol Phys. 2007;68:1289–1298. doi: 10.1016/j.ijrobp.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 11.Word JA, Kalokhe UP, Aron BS, et al. Transient radiation myelopathy (Lhermitte's sign) in patients with Hodgkin's disease treated by mantle irradiation. Int J Radiat Oncol Biol Phys. 1980;6:1731–1733. doi: 10.1016/0360-3016(80)90261-8. [DOI] [PubMed] [Google Scholar]
- 12.Lewanski CR, Sinclair JA, Stewart JS. Lhermitte's sign following head and neck radiotherapy. Clin Oncol (R Coll Radiol) 2000;12:98–103. [PubMed] [Google Scholar]
- 13.Martel MK, Eisbruch A, Lawrence TS, et al. Spinal cord dose from standard head and neck irradiation: implications for three-dimensional treatment planning. Radiother Oncol. 1998;47:185–189. doi: 10.1016/s0167-8140(97)00212-0. [DOI] [PubMed] [Google Scholar]
- 14.Bijl HP, van Luijk P, Coppes RP, et al. Dose-volume effects in the rat cervical spinal cord after proton irradiation. Int J Radiat Oncol Biol Phys. 2002;52:205–211. doi: 10.1016/s0360-3016(01)02687-6. [DOI] [PubMed] [Google Scholar]
- 15.Bijl HP, van Luijk P, Coppes RP, et al. Influence of adjacent low-dose fields on tolerance to high doses of protons in rat cervical spinal cord. Int J Radiat Oncol Biol Phys. 2006;64:1204–1210. doi: 10.1016/j.ijrobp.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 16.Withers R. Migration and myelination. Int J Radiat Oncol Biol Phys. 2003;57:9–10. doi: 10.1016/s0360-3016(03)00530-3. [DOI] [PubMed] [Google Scholar]
- 17.Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol. 2002;249:9–17. doi: 10.1007/pl00007853. [DOI] [PubMed] [Google Scholar]
- 18.Rose PG, Smrekar M. Improvement of paclitaxel-induced neuropathy by substitution of docetaxel for paclitaxel. Gynecol Oncol. 2003;91:423–425. doi: 10.1016/s0090-8258(03)00540-7. [DOI] [PubMed] [Google Scholar]
- 19.Ciucci G, De Giorgi U, Leoni M, et al. Lhermitte's sign following oxaliplatin-based chemotherapy in a cisplatin-pretreated ovarian cancer patient. Arch Neurol. 1982;39:719–20. doi: 10.1046/j.1468-1331.2003.00579.x. [DOI] [PubMed] [Google Scholar]
- 20.Heinzlef O, Lotz JP, Roullet E, et al. Severe neuropathy after high dose carboplatin in three patients receiving multidrug chemotherapy. J Neurol Neurosurg Psychiatry. 1998;64:667–9. doi: 10.1136/jnnp.64.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]