Abstract
Establishment and maintenance of functional stem cells is critical for organ development and tissue homeostasis. Little is known about the mechanisms underlying stem establishment during organogenesis. Drosophila testes are among the most thoroughly characterized systems for studying stem cell behavior, with germline stem cells (GSCs) and somatic cyst stem cells (CySCs) cohabiting a discrete stem cell niche at the testis apex. GSCs and CySCs are arrayed around hub cells that also comprise the niche and communication between hub cells, GSCs, and CySCs regulates the balance between stem cell maintenance and differentiation. Recent data has shown that functional, asymmetrically dividing GSCs are first established at ~23 hrs after egg laying during Drosophila testis morphogenesis (Sheng et al., 2009). This process correlates with coalescence of the hub, but development of CySCs from somatic gonadal precursors (SGPs) was not examined. Here, we show that functional CySCs are present at the time of GSC establishment, and that Jak-STAT signaling is necessary and sufficient for CySC maintenance shortly thereafter. Furthermore, hyper-activation of Jak in CySCs promotes expansion of the GSC population, while ectopic Jak activation in the germline induces GSC gene expression in GSC daughter cells but does not prevent spermatogenic differentiation. Together, these observations indicate that, similar to adult testes, Jak-STAT signaling from the hub acts on both GSCs and CySC to regulate their development and differentiation, and that additional signaling from CySCs to the GSCs play a dominant role in controlling GSC maintenance during niche formation.
Keywords: Stem cell niche, testis, organogenesis, gametogenesis, cyst stem cell, Jak-STAT
Introduction
Stem cells are vital for maintenance and generation of healthy tissues. Their ability to undergo asymmetric, self-renewing divisions that produce healthy, differentiated daughter cells, as well as cells that retain undifferentiated character, allows for replacement and amplification of specific cell types within a tissue (Spradling et al., 2001). While stem cells vary in type and location, they typically rely on a specialized microenvironment, or niche, to control their behavior (Lander, 2012; Spradling et al., 2001). Dysregulation of stem cells within their niche has been linked to developmental disorders, cancer and senescence (Kuhn, 2011; Martin-Belmonte and Perez-Moreno, 2012; Warren and Rossi, 2009). Furthermore, functional engraftment into the proper stem cell niche is critical to the long-term success of most stem cell therapies (Kaufman, 2009; Mohsin et al., 2011). Despite this, understanding of how stem cells and their niches form under dynamic conditions such as tissue regeneration and organogenesis is limited.
Drosophila testes are among the most accessible and thoroughly characterized systems for studying the regulation of stem cell behavior (see (de Cuevas, 2011; Fuller, 1993) for comprehensive reviews). In adult flies, testes form a coiled tube with one blind end where the germline stem cell (GSC) niche is localized, and the other end connected to the genital tract. Two populations of stem cells reside within the male GSC niche: sperm producing GSCs and somatic cyst stem cells (CySCs) that help regulate GSC maintenance and whose progeny nurture spermatogenic differentiation (Fabrizio et al., 2003; Kawase et al., 2004; Kiger et al., 2000; Leatherman and Dinardo, 2008, 2010; Matunis et al., 1997; Sarkar et al., 2007; Schulz et al., 2002; Shivdasani and Ingham, 2003; Tazuke et al., 2002; Tran et al., 2000; Wang et al., 2008; Zheng et al., 2011). 5–9 GSCs are anchored around a tight cluster of non-mitotic somatic cells called the hub (Aboim, 1945; Boyle et al., 2007; Hardy et al., 1979; Leatherman and Dinardo, 2010; Wang et al., 2006; Yamashita et al., 2003); integrin-mediated adhesion anchors the hub to the testis apex (Lee et al., 2008; Tanentzapf et al., 2007). CySCs are similarly arrayed around the hub, although hub-CySC contact is made through membrane projections that inter-digitate between GSCs (Cheng, 2011; Gonczy and DiNardo, 1996; Hardy et al., 1979; Issigonis et al., 2009; Schulz et al., 2002; Voog et al., 2008). Initiation of spermatogenesis proceeds through the asymmetric division of GSCs away from the hub (Inaba M., 2010; Yamashita et al., 2003). GSC divisions typically produce one GSC that retains hub-GSC contact, and one daughter cell called a gonialblast. Each gonialblast divides 4 times with incomplete cytokinesis, yielding 16 interconnected spermatogonia that undergo meiosis and terminally differentiate into functional sperm. Similarly, dividing CySCs align their mitotic spindle perpendicular to the hub during anaphase (Cheng, 2011) so that the daughter CySC remains at the hub, while the other daughter cell, termed a cyst cell, ceases mitosis and acts in tandem with a second cyst cell to ensheath each gonialblast. Cyst cell pairs then enlarge so that they encase dividing spermatogonia throughout spermatid differentiation (Fuller, 1993; Hardy et al., 1979).
The bone morphogenetic protein (BMP) and Janus kinase-signal transducer and activator of transcription (Jak-STAT) signaling pathways play key roles in regulation of GSC and CySC maintenance in adult testes (see (de Cuevas, 2011) for comprehensive review). The Jak-STAT activating ligand, unpaired (Upd), is expressed in the hub; activating Jak-STAT signaling in adjacent CySCs and GSCs (Flaherty et al., 2010; Kiger et al., 2001; Leatherman and Dinardo, 2008, 2010; Tulina and Matunis, 2001). In the soma, localized Jak-STAT activation promotes expression of the transcriptional regulators, Zinc finger homeodomain-1 (ZFH-1) and Chinmo, which are required for CySC maintenance (Flaherty et al., 2010; Leatherman and Dinardo, 2008). In GSCs, Jak-STAT signaling promotes formation of polarized GSC-hub adhesions that orient the mitotic spindle, while Jak-STAT activation in CySCs is required for stem cell maintenance (Leatherman and Dinardo, 2010). Additionally, secretion of the BMP ligands, Decapentaplegic (DPP) and Glass-bottom-boat (GBB) from the hub and CySCs is required for GSC maintenance (Kawase et al., 2004; Leatherman and Dinardo, 2008; Shivdasani and Ingham, 2003; Wang et al., 2008; Zheng et al., 2011).
Testis formation begins mid-embryogenesis and early stages of spermatogonial differentiation are detectable ~24 hours later; near the middle of the 1st larval instar stage [see (Jemc, 2011) for comprehensive review]. At the onset of testis formation, migrating primordial germ cells (PGCs) contact somatic gonadal precursor (SGP) cells specified with bilateral symmetry in embryonic parasegments (PS) 10–12 of the gonadal mesoderm at ~7 hrs AEL (Boyle et al., 1997; Boyle and DiNardo, 1995; Brookman et al., 1992; Sonnenblick, 1941). PGCs and SGPs then compact into a spherical gonad by ~10.5 hrs AEL, with SGPs fully ensheathing the PGCs (Boyle and DiNardo, 1995; Jenkins et al., 2003; Van Doren et al., 2003; Warrior, 1994; Weyers et al., 2011). Subsequently, hub precursor cells specified from SGPs in the anterior two-thirds of the testis (Dinardo et al., 2011; Kitadate and Kobayashi, 2010; Kitadate et al., 2007; Le Bras and Van Doren, 2006; Okegbe and DiNardo, 2011) initiate morphogenesis so that formation of a tightly coalesced hub is complete by ~23 hrs AEL, which coincides with the embryo-larval transition (Gönczy et al., 1992; Le Bras and Van Doren, 2006; Sheng et al., 2009). During hub formation, PGCs form polarized adhesions with hub precursor cells localized to the gonad anterior, and gradually orient their mitotic spindles away from the coalescing hub, so that functional, asymmetrically diving male GSCs are established from PGCs at the time of hub formation (Jenkins et al., 2003; Le Bras and Van Doren, 2006; Sheng et al., 2009).
The observation that spermatogenic differentiation occurs shortly after GSC establishment and hub formation implies that functional CySCs are also present in 1st instar larval testes. Indeed, recent data indicate that a subset of CySCs found in the adult testis is specified from SGPs within PS11 of the embryonic gonad (Dinardo et al., 2011). However, no study to date has specifically examined CySC development in newly formed testes, or whether CySCs regulate GSC establishment and maintenance within the developing niche. By examining reporters of adult CySC and cyst cell identity during late embryonic and early larval gonad formation, we show here that the establishment of functional, asymmetrically dividing CySCs is a coordinated process that occurs simultaneously with hub formation and GSC establishment. Furthermore, as in adult testes, we find that developing CySCs play a critical role in regulating GSC maintenance within the newly formed GSC niche, and that the Jak-STAT signaling pathway acts on both GSCs and CySCs to regulate their development and differentiation.
Methods
Fly Stocks
y, w1118 flies were used as controls. nanos-Gal4∷VP16 on III (M. Van Doren) was used to drive UAS- transgene expression in the germline, while c587-Gal4 (A. Spradling;(Kai and Spradling, 2003)) was used to drive expression in the somatic gonad. UAS- lines used include: UAS-hopTumL (D. Harrison; (Hanratty and Dearolf, 1993)), UAS-β-Gal.nls, and UAS-mCD8∷GFP (Lee and Luo, 1999), Stat92E06346 (Hou et al.) as well as mwh red e Stat92EFrankenstein lines (C. Dearolf; (Baksa et al., 2002)), which we refer to as Stat92ETS, were balanced over Tm3, Sb, Kr-GFP for genotype selection of late stage embryos and larvae. The 10XSTAT92E-GFP reporter (line#1 on the 2nd chromosome; E. Bach; (Bach et al., 2007)) as well as Socs36E-PZ1647 (A. Spradling; (Issigonis et al., 2009)) and Mgm1 (M. Steinmann-Zwicky; (Staab et al., 1996)) enhancer trap lines were also used. Fly stocks were obtained from the Bloomington Stock Center (http://flystocks.bio.indiana.edu/), unless otherwise specified.
Collection of embryos, larvae & adult testes
Wild type and Stat92E06346 homozygous mutant embryos and larvae were collected at times ranging from 0–24 (embryos/L1e), 24–48 (L1) or 48–72 (L2) hours after egg laying (AEL) at 24°C. Embryos were staged according to morphology (Campos-Ortega and Hartenstein, 1985) with late stage 17/early L1 testes confirmed by presence of a tightly coalesced hub and/or polarized GSCs arrayed around the hub (Le Bras and Van Doren, 2006; Sheng et al., 2009). Wild type and Stat92E06346 larvae were sorted by age according to larval size compared with age standards at 24–30 (early-L1), 30–42 (mid-L1), 42–48 (late-L1) and 48–55 (early-L2) hours AEL. To obtain Stat92ETS mutant larvae, Stat92EFrankenstein/Stat92E06346 virgin females reared at 18°C were mated to Stat92E06346/TM3,Sb,KrGFP males and embryos collected for 6–12 h AEL at 18°C followed by incubation for 24–30 hours at 29°C. To obtain larvae with ectopic hopTumL expression, c587-Gal4 or nanos-Gal4∷VP16 virgins were crossed with UAS- hopTumL males, embryos collected 0–7 h AEL at 24°C, then incubated for either 41 (late L1), 48 (early L2) or 55 (mid L2) hours at 29°C. All adult testes were isolated 5–7 days after eclosion.
Antibodies and Immunostaining
Immunostaining of embryos, larvae, and adult testes was performed as described (Matunis et al., 1997; Sheng et al., 2009). The following primary antibodies were used: chick anti-Vasa at 1:3000 (K. Howard); rabbit anti-Vasa at 1:5000 (R. Lehmann); rabbit anti-ZFH1 at 1:5000 (R. Lehmann); rabbit anti-GFP at 1:2500 (Torrey Pines Labs); rabbit anti-β-galactosidase at 1:5000 (Cappel); rabbit anti-phosphorylated-Histone H3 at 1:1000 (Upstate Cell Signaling Solutions); guinea pig anti-Traffic Jam at 1:2500 (D. Godt); mouse anti-βeta-galactosidase at 1:5000 (Promega); mouse anti-Fasciclin 3 at 1:10 (C. Goodman; Developmental Studies Hybridoma Bank [DSHB]); mouse anti-EYA at 1:25 (S. Benzer/N. Bonini; DSHB); mouse anti-1B1 at 1:4 (H. Lipshitz; DSHB); mouse anti-Sex lethal M18 at 1:25 (P. Schedl; DSHB); mouse anti-GFP at 1:50 (Santa Cruz Biotechnology); rat anti-N-Cadherin at 1:20 (T. Uemura; DSHB). Secondary antibodies (Molecular Probes) used were: goat anti-chick 546, goat anti-chick 633, goat anti-mouse 488, goat anti-mouse 546, goat anti-mouse 633, goat anti-rabbit 488, goat anti-rabbit 633, goat anti-rat 546, goat anti-rat 633, goat anti-guinea pig 633. All secondary antibodies were used at 1:500. Nuclei were stained using DAPI at 1μg/mL (Roche) for 3 minutes.
Genotyping and quantitative analyses
Male embryos and larvae were distinguished from females by immunostaining for either the presence of Sex-lethal, EYA-positive msSGPs, or a coalesced hub. The genotype of Stat92E06346 and Stat92ETS embryos or larvae was determined by presence or absence of Kr-GFP on a balancer chromosome. For analysis of ZFH-1 expression after somatic Jak hyper-activation, c587-Gal4/c587-Gal4; +/+ females were mated to UAS-hopTumL/CyO males and > 40 larvae were examined to ensure a ~1:1 ratio of c587-Gal4/+; UAS-hopTumL/+ testes and c587-Gal4/+; CyO/+ sibling controls. For analysis of fusome morphology or EYA expression in these same larvae, co-immunostaining with ZFH-1 was performed to distinguish c587-Gal4/+; UAS-hopTumL/+ testes with expanded ZFH-1 expression from c587-Gal4/+; CyO/+ sibling controls which display ZFH-1 in a manner indistinguishable from that in wild type larvae. To determine the ratio of CySCs to GSCs, the number of germ cells immediately adjacent the hub, and the number of somatic cells expressing high-levels of ZFH-1 arrayed within 1–2 cell diameters from the hub, was used to identify GSC and CySC, respectively. To determine the rate of somatic cell division in developing testes, the total number of testes with one or more pHH3/TJ double-positive somatic cell was determined at a given stage of development, and the number and location of these cells within the gonad was noted. pHH3 positive cells in embryonic testes prior to hub formation were scored as adjacent to hub precursor cells when localized to the anterior half of the testes where hub precursor cells are known to be specified (Dinardo et al., 2011; Le Bras and Van Doren, 2006; Okegbe and DiNardo, 2011). A Chi-squared test was used to determine if changes in division rate within different regions of the gonad are significant over time. Significance for calculations of the average number of pHH3/TJ double positive cells in a given gonad region and the average number of CySCs and GSCs present in developing testes, were determined using a 2-tailed Student's T-test with equal variance. For both Chi-squared and Student's T-tests, we assumed significance at P<0.05. For analysis of Mgm1 expression after germline or somatic Jak hyper-activation, c587-Gal4/c587-Gal4; Mgm1, CyO/Sp or Mgm1, CyO/Sp; nanos-Gal4∷VP16/nanos-Gal4∷VP16 females were mated to either UAS-hopTumL/CyO or wild-type males and larval testes with Mgm1 expression analyzed. To quantify changes in Mgm1 expression after somatic Jak hyper-activation, Mgm1 expressing testes were categorized on a scale of 0–5 based on the following criteria: 0 (no posterior Mgm1 positive germ cells), 1 (1 posterior Mgm1 germ cell), 2 (2–5 posterior Mgm1 positive germ cells), 3 (5–10 posterior Mgm1 positive germ cells), 4 (>10 posterior Mgm1 positive germ cells).
Confocal microscopy
Embryos, larvae and testes were mounted in 70% glycerol containing 2.5% DABCO (Sigma) and p-phenylenediamine anti-fade agent (Sigma) at a final concentration of 0.2 mg/mL. Slides were viewed with an Olympus BX51 microscope equipped with a DSU spinning disc confocal system and Q-imaging RETIGA-SRV CCD camera. Images were captured and analyzed with Slidebook 5.0 software by 3I.
Results
CySC establishment correlates with GSC niche formation
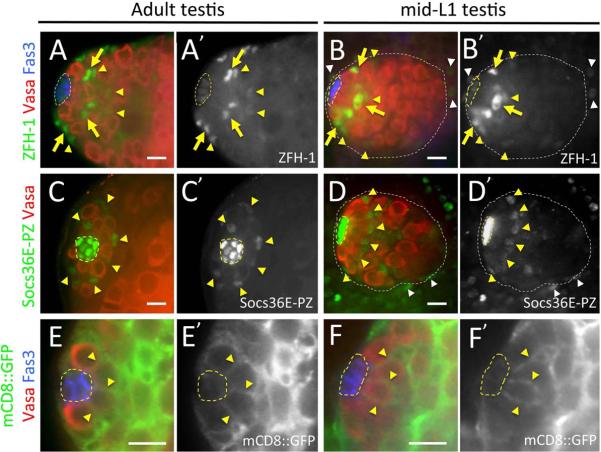
Functional, asymmetrically dividing GSCs are established in the Drosophila testis at the embryo-larval transition which occurs ~23 hrs AEL at embryonic stage 17-late/early L1 (st17-late/L1e)(Sheng et al., 2009). This process correlates temporally with coalescence of the hub, which is an essential component of the male GSC niche (Gönczy et al., 1992; Le Bras and Van Doren, 2006; Sheng et al., 2009). As maintenance and differentiation of GSCs in adult Drosophila testes also depends on the presence of CySCs and their progeny (Fabrizio et al., 2003; Issigonis M., 2012; Kawase et al., 2004; Kiger et al., 2000; Leatherman and Dinardo, 2008, 2010; Matunis et al., 1997; Sarkar et al., 2007; Schulz et al., 2002; Shivdasani and Ingham, 2003; Tazuke et al., 2002; Tran et al., 2000; Wang et al., 2008; Zheng et al., 2011), we sought to examine development of this cell type during testis morphogenesis. We first examined expression of the adult CySC marker, ZFH-1(Broihier et al., 1998; Leatherman and Dinardo, 2008) in mid-1st instar larval testes (mid-L1) shortly after GSC niche formation (~36 hrs AEL). In adult testes, ZFH-1 is detected at high-levels in nuclei of CySCs located adjacent to the hub, and weakly in early cyst cells as well as the hub (Figure 1A; (Leatherman and Dinardo, 2008)). At mid-L1, a similar pattern of expression is observed, with ZFH-1 enriched in nuclei of somatic cells localized immediately adjacent to the hub, weakly detected in hub cells and somatic cells 2–3 cell diameters from the hub, and also observed at low levels in somatic nuclei around the testis periphery (Figure 1B). All ZFH-1 enriched somatic cells immediately adjacent to the newly formed hub, as well as the weakly stained somatic cells away from the hub, co-express Traffic Jam (TJ) (Figure 2D), a reporter for CySCs and early cyst cells in adult testes that labels SGPs during embryonic development (Leatherman and Dinardo, 2010; Li et al., 2003). Furthermore, we find an average of 11.5 ZFH-1 enriched cells adjacent to the hub in mid-L1 testes (Supplemental Figure 1; n=18); yielding an ~1.5:1 ratio of putative CySCs to GSCs at this stage. This is similar to the 1.3:1 ratio of CySC to GSCs previously observed in L3 testes (Hardy et al., 1979). Together, these observations suggest that a mature GSC niche with functional CySCs has formed by mid-L1.
Figure 1. Cyst stem cells are present at mid- larval first instar.
Adult and mid-first instar larval testes immunostained with anti-Vasa (red) to detect germ cells, anti-Fasciclin 3 (Fas 3; A, B, E, F; blue) to detect the hub, and either anti-ZFH-1 (A, B, green; A', B' alone), anti-β-galactosidase (C, D, green; C', D' alone) to reveal Socs36E-PZ expression, or anti-GFP (G, H, green; E', F' alone). Testis apex/anterior oriented to the left in all images. Hub (yellow dashed lines) and testes (white dotted lines) outlined. [A, B] Adult (A) and mid-L1 (B) testes with high-level ZFH-1 expression (yellow arrows) restricted to nuclei of somatic cells immediately adjacent to the hub in adult, and lower-level ZFH-1 (yellow arrowheads) observed in somatic cells 2–3 cell layers away from the hub. ZFH-1 is also detected in somatic cells surrounding the testis (white arrowheads) at mid-L1. [C, D] Adult (C) and mid-L1 (D) testes with Socs36E-PZ expression detected strongly in the hub, at lower levels in CySCs (yellow arrows), and in somatic cells surrounding the testes (white arrowheads). Location of the hub was confirmed by Fas 3 expression (not shown). [E, F] Adult (E) and mid-L1 (F) testes with expression of a plasma membrane-tethered GFP transgene (UAS-mCD8∷GFP) driven by a somatic driver (c587-Gal4) observed in cells that interdigitate GSCs (yellow arrows). Scale bars at 10 μm.
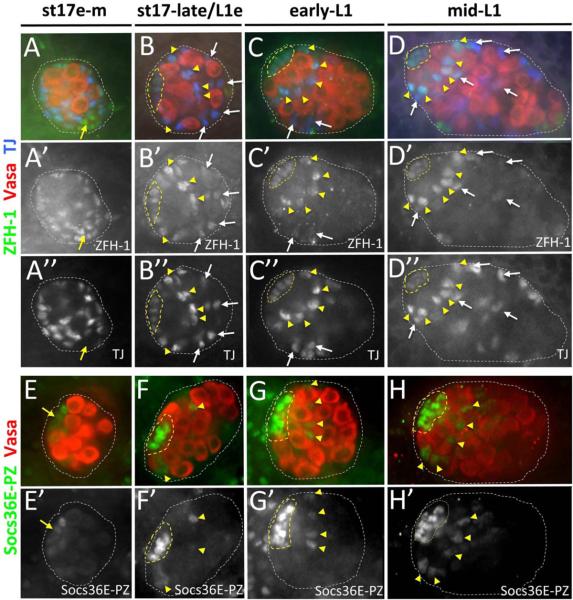
Figure 2. CySC reporters are detected at the embryo-larval transition.
Late-stage embryonic and first instar larval testes immunostained with anti-Vasa (A–H; red), either anti-ZFH-1 (A–D, green; A'–D' alone) and anti-Traffic Jam (TJ; A–D, blue; A”–D” alone) to detect somatic gonadal cells, or anti-β-galactosidase (E–H, green; E'–H' alone) to reveal Socs36E-PZ expression. Testes are from early-mid stage 17 embryos (~19 hrs AEL; A, E), stage 17-late/early L1 (~23 hrs AEL; B, F), early- 1st instar larvae (~36 hrs AEL; D, H). All images oriented with testis anterior to the left. Hub (yellow dashed lines) and testes (while dotted line) are outlined. [A–D] Early-mid stage 17 testis (A) with uniform ZFH-1 expression in SGPs that co-express TJ, and ZFH-1 expressed in likely msSGPs that are TJ negative (yellow arrow). Testis at the embryo-larval transition (B) with ZFH-1 expression enriched in somatic cells located adjacent to the hub (yellow arrowheads). Early- L1 (C) and mid-L1 (D) testes with high-level ZFH-1 restricted to hub-adjacent somatic cells (yellow arrowheads), and lower-level ZFH-1 detected in hub cells and also in somatic cells 2–3 cell diameters away from the hub (white arrows). [E–H] Early-mid stage 17 testis (E) with low-level Socs36E-PZ detected in somatic cells clustered at the testes anterior (yellow arrow). Testes at the embryo-larval transition (F), early-L1 (G) and mid-L1 (H) with low-level Socs36E-PZ (yellow arrowheads) in somatic cells immediately adjacent to hub cells that show high-level Socs36E-PZ expression.
To obtain further evidence for presence of CySCs in larval testes, we assessed expression of another potential adult CySC reporter, Suppressor of cytokine signaling 36E (Socs36E)(Issigonis et al., 2009). We examined the expression pattern of Socs36E using the LacZ enhancer trap, Socs36E-PZ1647 (Socs36E-PZ)(Issigonis et al., 2009; Singh et al., 2010), whose expression has not previously been characterized in the testis. In the adult, we find that Socs36E-PZ is strongly expressed in hub cells and differentiating cyst cells, while lower-level expression is observed in CySCs (Figure 1C; Supplemental Figure 2). Expression was also detected in the testes sheath (not shown) and in somatic cells at the base of the testis (Supplemental Figure 2). While Socs36E-PZ was consistently detected in the adult hub, CySC and sheath cell expression was heterogeneous, with CySC expression observed in 41% of testes examined and sheath cell expression observed in only 9% of testes (Supplemental Figure 2; n=51). Furthermore, among adult testis with CySC staining, co-labeling with TJ revealed that 57% of testes express Socs36E-PZ in all CySC arrayed around the hub, while Socs36E-PZ is detected in a subset of CySCs (from 1–3 CySCs; n=21) in the remainder of testes (Supplemental Figure 2). Heterogeneity in Socs36E-PZ expression is not due to transgene dose, as testes from the same adult male showed different expression patterns in the CySCs. (n=5). Similar to the adult, our analysis of mid-L1 testes revealed low-level Socs36E-PZ expression in putative CySCs localized immediately adjacent to the hub in 48% of testis examined, while high-level Socs36E-PZ expression was always observed in the hub (Figure 1D, n=31). Socs36E-PZ in putative CySCs at this stage also co-stained with ZFH-1 (Supplemental Figure 1); indicating that ZFH-1 and Socs36E-PZ are expressed in the same somatic cell population. Also similar to adults, among mid-L1 testes with Socs36E-PZ observed in putative CySCs, 53% of testes co-express Socs36E-PZ in all putative CySCs enriched with ZFH-1, while Socs36E-PZ is only observed in a subset of ZFH-1 enriched cells in the remainder of testes (n=19). Thus, while Socs36E-PZ expression is heterogeneous, the Socs36E-PZ enhancer trap is a marker for adult CySCs in a subset of testes and it's expression pattern in mid-L1 testes is consistent with presence of CySCs at this early stage of development.
As CySCs in adult testes contact hub cells through membrane projections that inter-digitate between GSCs (Cheng, 2011; Hardy et al., 1979; Issigonis et al., 2009), we also examined somatic cell morphology in developing testes. Consistent with adult CySC morphology nuclei of hub-adjacent cells strongly expressing either ZFH-1 or Socs36E-PZ are arrayed around the hub in late-stage embryonic and L1 testes (Figure 1A–D; see also (Leatherman and Dinardo, 2008)). To directly examine cell morphology, however, we expressed a cell surface GFP fusion protein (UAS-mCD8∷GFP) in somatic gonadal cells using the c587-Gal4 driver (Kai and Spradling, 2003). This driver marks CySCs and early cyst cells in the adult testis apex (Kawase et al., 2004). In both mid-L1 and adult testes, we find that c587-Gal4 marks somatic cells in the testis anterior that make hub-contacting membrane projections (Figure 1E, F). As expression of this driver is typically absent or detected only at low-levels in the developing hub compared to other somatic cells in the testis (Supplemental Figure 3), membrane projections are likely to extend inward from putative CySCs arrayed around the newly formed hub, rather than outward from the hub itself. Together, these data suggest the presence of CySCs in developing testes by the mid 1st instar larval stage of development.
To further examine timing of CySC development, we assessed the expression pattern of ZFH-1 and Socs36E-PZ at earlier stages of testis morphogenesis. Prior to formation of a fully coalesced hub at the embryo-larval transition, ZFH-1 is detected in somatic cells dispersed throughout the testes (Broihier et al., 1998; Jenkins et al., 2003; Mathews et al., 2006). Indeed, ZFH-1 is co-expressed with the SGP marker, TJ (Dinardo et al., 2011; Li et al., 2003), in all SGPs except a sub-population of likely male-specific SGPs at the testis posterior (DeFalco et al., 2003) which express ZFH-1 but not TJ (Figure 2A). At the time of hub coalescence (~23 hrs AEL), ZFH-1 expression remains detectable throughout the testes (Figure 2B). However, somatic nuclei adjacent to the hub are enriched for ZFH-1 expression and these same cells are arrayed around the hub in a manner characteristic of CySCs in the adult (compare Figure 2B with Figure 1A). Shortly thereafter, in early-L1 testes (~28 hrs AEL), high-level ZFH-1 expression is restricted to somatic cells adjacent to the hub (Figure 2C), and this expression pattern persists into later stages of development (Figure 2D). Interestingly, as these changes in ZFH-1 expression occur, the number of ZFH-1 enriched somatic cells immediately adjacent to the hub remains constant (Supplemental Figure 1). In comparison to ZFH-1 expression, Socs36E-PZ is initially detected only in hub precursor cells at the testis anterior in early-mid stage 17 embryos (~18 hrs AEL; Figure 2E). As development progresses, however, Socs36E-PZ expression in the newly formed hub becomes more prominent, and a sub-set of testes (60%, n=20) express Socs36E-PZ in somatic cells adjacent to the hub at the time of hub coalescence (Figure 2F). Co-staining with ZFH-1 reveals that Socs36E-PZ is co-expressed in the same somatic population adjacent to the hub at this time, although Socs36E-PZ expression is not always present in the ZFH-1 positive population (Supplemental Figure 1). This expression pattern persists into larval development, with diminishing levels of Socs36E-PZ observed in the somatic gonad 2–3 cell layers away from the hub by mid-L1 (Figure 2G, H; Supplemental Figure 1). Interestingly, at the embryo-larval transition when changes in ZFH-1 and Socs36E-PZ expression are first observed adjacent to the hub, somatic membrane projections extend between newly established GSCs in a manner consistent with adult CySCs (Supplemental Figure 3). Taken together, these data indicate that CySCs are present at the embryo-larval transition in a process that is coordinated with hub formation and GSC establishment.
Functional CySCs develop by mid-L1
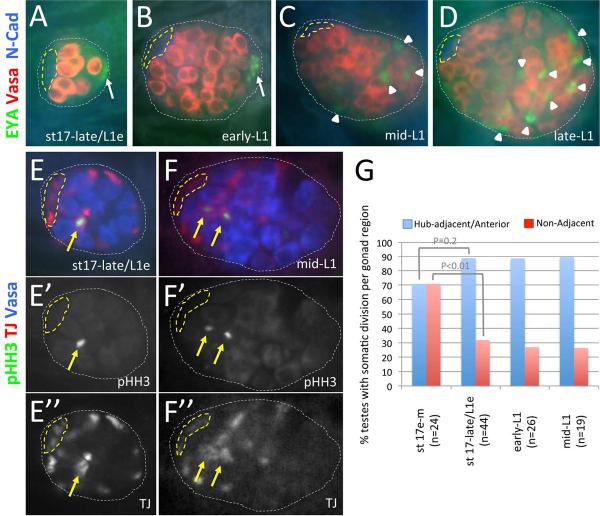
In the adult testis, functional CySCs produce cyst cell daughters through asymmetric cell division (Cheng, 2011; Gonczy and DiNardo, 1996). To determine when CySCs become functional during development, we first examined the timing and pattern of cyst cell differentiation in embryonic and larval testes. In the adult, Eyes Absent (EYA) is enriched in nuclei of cyst cells that surround differentiating spermatogonial clusters (Fabrizio et al., 2003). EYA is also enriched in nuclei of male-specific SGPs (msSGPs) that arise within PS13 of the embryonic mesoderm and associate with SGPs in PS10–12 mid-embryogenesis (Boyle et al., 1997; Boyle and DiNardo, 1995; DeFalco et al., 2003). As testis development progresses, we find that EYA expression persists in the testis posterior (Figure 3A, B; msSGP expression in early- and mid-L1 testes not shown). By mid-L1, however, EYA is also detected in somatic nuclei in the posterior half of the gonad (Figure 3C, D). Induction of EYA occurs after the enrichment of ZFH-1 to somatic cells immediately adjacent the hub, and correlates both temporally and spatially with onset of spermatogonial differentiation in the testes posterior (as previously characterized by Bag-of-marbles expression, (Sheng et al., 2009)). Furthermore, somatic cells in the posterior half of the gonad ensheath spermatogonia formed at this time (Supplemental Figure 3F). Thus, cyst cells that support spermatogonial differentiation distal to the hub have arisen by mid-L1.
Figure 3. CySC division and cyst differentiation are observed by mid-L1.
Late-stage embryonic and 1st instar larval testes immunostained with anti-Vasa (A–D, red; E, F, blue), either anti-Eyes Absent (EYA; A–D, green) to detect cyst cells and msSGPs or anti-phosphorylated-Histone-H3 (pHH3; E, F, green; E', F' alone) to detect mitotic chromatin, and either anti-N-Cadherin (N-Cad; A–D, blue) to detect the hub or anti-TJ (TJ; E, F, red; E”, F”, alone) to detect somatic gonadal cells. Images oriented with anterior to the left. Hub (yellow dashed lines) and testes (white dotted lines) outlined. [A–D] Testes at the embryo-larval transition (A) and early-L1 (B) with EYA expressed in male-specific somatic gonadal precursors (msSGPs; white arrows). Mid-L1 (C) and late-L1 (D) testes with EYA detected in somatic nuclei in the posterior half of the gonad (white arrowheads) where spermatogonia are known to form. [E, F] Testes at the embryo-larval transition (E) and mid-L1 (F) with pHH3 detected in somatic cells adjacent to the hub (yellow arrows). [G] Graph showing the percentage of pHH3/TJ double positive testes over time with pHH3 detected in somatic cells adjacent to the developing hub (in the anterior half of the testes in early-mid stage 17 embryos) or in cells elsewhere in the testes (Non-Adjacent). P-values from Chi-squared analyses are shown.
We next assessed whether posterior cyst cells arise from functional CySCs located adjacent to the hub at earlier stages of development. As lineage analysis is not possible for individual somatic cells in embryonic and 1st instar larval testes, we examined the pattern of somatic cell division in developing testes. If cyst cells in 1st instar larval testes arise from functional CySCs established adjacent to the hub at the embryo-larval transition, one would expect hub-associated somatic cells to be mitotically active, and cells localized away from the hub to be quiescent. If division is observed in somatic cells elsewhere in the testis, however, these cells may or may not give rise to cyst cells. To examine division in developing testes, we assessed for presence of phosphorylated Histone-H3 (pHH3) in somatic cells marked by TJ expression (Li et al., 2003) during late embryonic and early larval development (Figure 3E, F). Specifically, we determined the cell division rate in different regions of the gonad, which we define as the percentage of pHH3/TJ double-positive testes with one or more somatic cell undergoing mitosis in anterior/hub-adjacent cells vs. pHH3/TJ double-positive cells located elsewhere in the testes (see methods). As the average number of pHH3/TJ double-positive cells is equivalent in different gonad regions throughout L1 (1.26±0.5, n=66 and 1.25±0.4, n=25 in anterior/hub-adjacent cells and cells elsewhere in the gonad respectively; P=0.94), this definition provides an unbiased description of cell division rate. In early to mid stage 17 testes prior to hub formation, we find that somatic division is observed at equal rates in the anterior (70.8%) and posterior (70.8%) halves of the testis (Figure 3G, n=24). At the time of hub formation, there may be a slight increase in the cell division rate of somatic cells localized immediately adjacent to the hub (88.6%, n=44; P 0.2), after which the division rate of hub-adjacent cells remains constant through mid-L1 (88.5%, n=26 and 89.7%, n=19 at early- and mid-L1, respectively; P>0.95). Elsewhere in the testis, however, the rate of somatic cell division decreases significantly at the time of hub formation (31%, n=44; P<0.01), after which it remains at a consistently low rate (27%, n=26 and 26%, n=19 at early- and mid-L1, respectively; P>0.95). Thus, while it is possible that a subset of somatic cells localized away from the hub give rise to cyst cells during early stages of spermatogenic differentiation, the relatively constant rate of mitotic division in somatic cells adjacent to the hub, combined with a significant decrease in the rate of somatic division elsewhere in the testes at the time of hub formation, suggest that hub-adjacent cells are, indeed, functional CySCs that divide asymmetrically to produce differentiating cyst cells present in the testis posterior by mid-L1. A constant number of ZFH-1 enriched somatic cells localized immediately adjacent to the hub during and after hub formation (Supplemental Figure 1), further suggests that these cells initiate steady-state asymmetric divisions by the embryo to larval transition.
Jak-STAT regulates CySC maintenance in larval testes
Jak-STAT signaling is required for CySC maintenance in adult Drosophila testes (Issigonis et al., 2009; Leatherman and Dinardo, 2010; Singh et al., 2010). We, therefore, sought to examine the pattern of Jak-STAT activation in developing testes as well as the impact of altered Jak-STAT signaling on CySC behavior. This pathway proceeds through binding of the Upd ligand to its receptor on the surface of a target cell (Hombria and Brown, 2002). This results in activation of a receptor-associated Jak, Hopscotch (Hop), which, in turn, phosphorylates the transcription factor STAT92E.
To test for Jak-STAT activation in developing testis, we examined the pattern of GFP expression in late stage embryos and larvae containing the 10XSTAT92E-GFP reporter (Bach et al., 2007). This reporter contains 10 copies of the STAT92E DNA binding sites isolated from the Socs36E gene inserted upstream of the GFP coding sequence. Prior to hub formation, GFP expression is detected at low levels within SGPs and also in cells on the testis periphery where pigment cells derived from the surrounding fat body are known to reside (DeFalco et al., 2008; Hempel and Oliver, 2007; Nanda et al., 2009)(Supplemental Figure 4A). At the time of hub formation, 10XSTAT92E-GFP expression in the main body of the gonad becomes restricted to hub cells and newly established CySCs located immediately adjacent to the hub (Supplemental Figure 4B). By L1, this reporter is no longer detected in the testis periphery, and expression in the hub and CySCs becomes stronger, with a gradient of decreasing expression observed in cyst cells away from the hub (Supplemental Figure 4C, D). Interestingly, expression of the 10XSTAT92E-GFP reporter is never detected in germ cells that have previously been shown to activate Jak-STAT signaling in all PGCs and later in GSCs using high-level STAT92E expression as an assay (Casper et al., 2011; Dinardo et al., 2011; Leatherman and Dinardo, 2010; Sheng et al., 2009; Wawersik et al., 2005). Despite this, the expression pattern of the 10XSTAT92E-GFP reporter is similar to that of the Socs36E-PZ enhancer trap in late embryonic and early larval testes (Figure 2E–H). Furthermore, increased STAT92E expression has previously been observed in SGPs surrounding the germline prior to hub formation (Wawersik et al., 2005). Thus, while 10XSTAT92E-GFP and Socs36E-PZ do not accurately report Jak-STAT activity in the developing germline, Jak-STAT signaling appears to be activated in functional, asymmetrically dividing CySCs that are established by the embryo-larval transition.
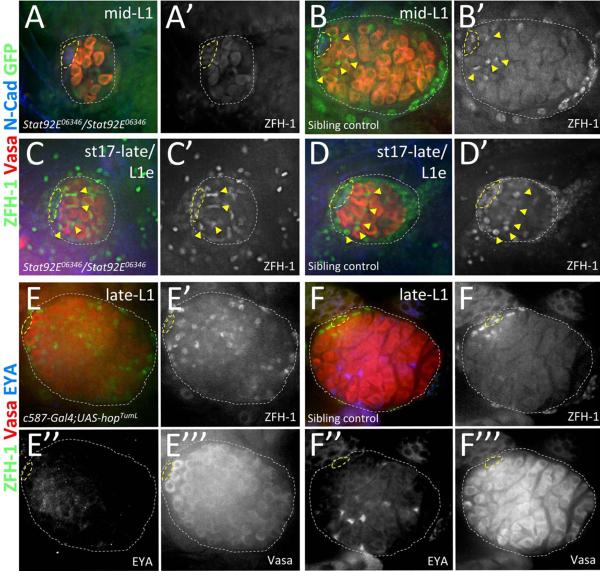
We next assessed whether Jak-STAT signaling is required for CySC maintenance in the newly formed GSC niche. We first examined whether Jak-STAT is required for CySC maintenance in late-stage embryonic and 1st instar larval testes that lack zygotic Stat92E gene function (Stat92E06346/Stat92E06346)(Hou et al., 1996; Luo et al., 1997). We find that expression of the CySC marker, ZFH-1, is absent or reduced in Stat92E06346 homozygous mutant testes by mid- larval 1st instar (Figure 4A), with ~86% of testes (n=22) lacking high-level ZFH-1 expression in somatic cells adjacent the hub, and all other testes showing a single ZFH-1 expressing nucleus in this location. Similar observations are also made in mid-Ll testes carrying a temperature-sensitive heteroallelic combination of Stat92E (Stat92ETS; see methods) that inhibits both maternal and zygotic Stat92E gene function at increased temperatures (Baksa et al., 2002; Hou et al., 1996; Sheng et al., 2009))(Supplemental Figure 5). In contrast, ZFH-1 expression in sibling control testes (Figure 4B, Supplemental Figure 5; n=18 and 20 for Stat92E06346 and Stat92ETS controls respectively) is indistinguishable from wild type (compare with Figure 1A). Thus, Jak-STAT signaling is required for CySC maintenance shortly after GSC niche formation.
Figure 4. Jak-STAT signaling is necessary and sufficient for CySC maintenance in larval testes.
Late-stage embryos and 1st instar larval testes immunostained with anti-Vasa (A–F, red; E'”& F'” alone), anti-ZFH-1 (A–F, green; A'–D', in black and white with GFP; E' & F' alone), and either anti-GFP (A–D, green; A' & B' in black and white with ZFH-1) to determine genotype of Stat92E06346 mutants (see methods) or anti-EYA (E & F, blue; E” & F” alone) or. All images with testis anterior oriented left. Hub (yellow dashed lines) and testes (white dotted lines) outlined. [A, B] mid-L1 testes from (A) a Stat92E06346 homozygous mutant lacking high-level ZFH-1 expression in somatic cells around the hub and from (B) a sibling controls with ZFH-1 enriched in hub-adjacent cells (yellow arrowheads). [C, D] stage 17-late/L1 early testes from (A) a Stat92E06346 homozygous mutant and (B) a sibling control both showing high-level ZFH-1 in somatic cells adjacent to the hub (yellow arrowheads). [E, F] Late-L1 testes with Jak hyper-activated in the somatic gonad (E; c587-Gal4/+;UAS-hopTumL/+) show expanded expression of high-level ZFH-1 and no EYA expression in cyst cells, while age-matched sibling controls (F; c587-Gal4/+;CyO/+) show high-level ZFH-1 restricted to hub-adjacent CySCs and EYA expression in somatic nuclei interspersed throughout the testis posterior.
Interestingly, at the embryo to larval transition, Stat92E06346 homozygous mutant testes show normal ZFH-1 expression and morphology compared with age-matched sibling controls (Figure 4 C, D). Furthermore, while GFP expression in the testis periphery used for genotyping made it impossible to accurately count the number of ZFH-1 enriched CySCs adjacent the hub in sibling controls, Stat92E06346 homozygous mutant testes that lack GFP showed a CySC to GSC ratio of ~1.5 (12.5±2.6 CySCs and 8.3±1.9 GSCs, n=15) consistent with that observed in wild-type testes at the embryo to larval transition (see Supplemental Figure 1). Analyses for Stat92ETS mutants that lack both maternal and zygotic Stat92E were, however, not performed at this early time-point since growth at non-permissive temperature did not reliably block STAT92E function (not shown). Furthermore, mutations completely ablating maternal Stat92E function cause severe segmentation defects and embryonic lethality (Hou et al., 1996). Therefore, while zygotic Stat92E is not required for CySC establishment, it is possible that the maternal contribution of Stat92E is sufficient to promote this process.
We next sought to assess the impact of Jak-STAT hyperactivation on CySC development. Over-expression of a temperature-activated Jak allele, hopTumL (Hanratty and Dearolf, 1993), specifically in somatic cells using the c587-Gal4 driver (see methods) results in expression of high-level ZFH-1 throughout the somatic gonad in late 1st instar larval testes (Figure 4E; n=21), while expression in sibling controls processed in parallel is unchanged (Figure 4F; n=20). Furthermore, the induction of ZFH-1 throughout the testis directly correlates with loss of the cyst cell marker, EYA (Figure 4E; n=16). While the hopTumL allele has been described to cause precocious differentiation of immune cells at increased temperatures (Silvers and Hanratty, 1984), this is not likely the case in developing testes upon UAS-hopTumL expression, as we observe over-proliferation of CyCSs at the expense of cyst cell differentiation. Thus, Jak-STAT signaling in somatic cells of the newly formed GSC niche is both necessary and sufficient to promote the maintenance of newly established CySCs in 1st instar larval testes shortly after GSC niche formation.
Jak-STAT acts both directly and indirectly on newly formed GSCs
Jak-STAT signaling from the hub has previously been implicated in the regulation of GSC maintenance in both adult and developing testes (Kiger et al., 2001; Sheng et al., 2009; Tulina and Matunis, 2001). Recent work in adult males indicates that this is an indirect consequence of defects in the maintenance of CySCs, which act upon GSCs to promote their maintenance by repressing spermatogonial differentiation (Leatherman and Dinardo, 2008, 2010; Wang et al., 2008). Our analysis of Jak-STAT's impact on CySC development is consistent with this observation (Figure 4), but the impact on GSC behavior at this stage has not been tested. We, therefore, examined the effect of either somatic or germline Jak-STAT hyper-activation on GSC maintenance and differentiation shortly after GSC establishment. To do so, changes in fusome morphology were examined in larval testes after hopTumL over-expression in either germ cells (nanos-Gal4∷VP16) or the soma (c587-Gal4). Fusomes are germline-specific organelles that show spherical morphology in the cytoplasm of GSCs and GB cells, but form elongated structures that interconnect 2- cell spermatogonia and then mature into branched structures that span the cytoplasm of 4-, 8- and 16- cell spermatogonia (Hime et al., 1996; Lin et al., 1994). Presence of spherical vs. elongated or branched fusomes are, therefore, a functional read-out for GSC maintenance and differentiation (Hime et al., 1996; Lin et al., 1994; Tran et al., 2000; Tulina and Matunis, 2001).
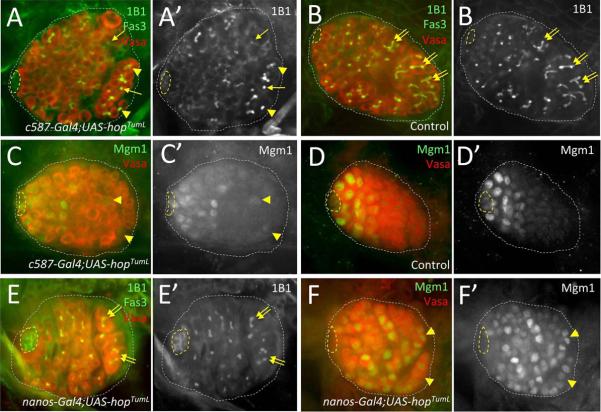
Upon hopTumL over-expression in the soma (Figure 5A), we observe altered germ cell differentiation, with germ cells bearing spherical fusomes found throughout the gonad and partial disruption of fusome branching in posterior germ cells where spermatogonial differentiation normally occurs (n=24). In contrast, age-matched sibling controls show normal fusome maturation and spermatogonial differentiation (Figure 5B; n=20). Similar to controls, testes where hopTumL is over-expressed specifically in the germline show no obvious defects in gonad morphology or fusome maturation (Figure 5E; n=34). These data, along with our observations that Jak-STAT signaling regulates CySC maintenance in L1 testes, indicates that Jak-STAT signaling from the hub acts on the newly formed niche in a manner similar to the adult: By promoting CySC maintenance which, in turn, acts through a Jak-STAT independent mechanism to repress GSC differentiation.
Figure 5. Impact of germline vs. somatic Jak-STAT activation on GSC maintenance and gene expression.
Late- 1st instar and early 2nd instar larval testes immunostained with anti-Vasa (A–F, red; A”–F” alone), and either anti-1B1 E' alone) to reveal fusomes, or anti-(β-galactosidase (C, D, & F, green; C', D' & F' alone) to reveal expression of the enhancer trap Mgml. All images with testis anterior oriented left. Hub (yellow dashed lines) and testes (white dotted lines) outlined. [A, B] Early-L2 testis with Jak hyper-activated in somatic cells (A; c587-Gal4/+;UAS-hopTumL/+) showing germ cells with rounded fusomes (yellow arrows) and aberrant branching (yellow arrowheads) in the testis posterior, whereas elongation and branching of fusomes (yellow double-arrows) is observed in posterior germ cells of an age-matched sibling control (B; c587-Gal4/+; CyO/+). [C, D] Late-Ll testis after somatic Jak hyper-activation (C; c587-Gal4/+; Mgm1/UAS-hopTumL) showing Mgm1 expression in a small subset of germ cells at the testes posterior (yellow arrowheads), while Mgml expression is restricted to GSCs and early gonialblasts in an age-matched sibling control (D; c587-Gal4/+; Mgm1/+). [E, F] Early-L1 testis with Jak hyper-activated specifically in the germline (E, F; UAS-hopTumL/Mgml; nanos-Gal4/+) showing (E) normal germ cell differentiation with spherical fusomes in GSCs and gonialblasts adjacent to the hub, and branched fusomes (yellow double-arrows) in distally-localized spermatogonia, as well as (F) expression of Mgml in germ cells throughout the testis, including differentiating spermatogonia at the testis posterior (yellow arrowheads).
To further examine germline vs. somatic Jak-STAT regulation of GSC behavior, we also assessed the impact of Jak hyper-activation on the expression of an additional GSC reporter, Male germline marker 1 (Mgm1). Mgm1 is an enhancer trap line with LacZ inserted near the escargot gene (Streit et al., 2002) that is detected in male GSCs and gonialblast cells near the testis anterior in larval and adult testes (Figure 5D; (Sheng et al., 2009; Staab et al., 1996). Upon Jak-STAT hyper-activation in the somatic gonad, we find that Mgm1 expression remains largely restricted to germ cells in the testis anterior, though a subset of testes (~20%; n=29) show Mgm1 expression in a small number of posterior germ cells (2–5 on average). In contrast, Jak-STAT hyper-activation specifically in the germline induces Mgm1 expression in germ cells throughout the gonad, including differentiating spermatogonia (Figure 5F; Supplemental Figure 4; n=25). As germline Jak hyper-activation does not alter spermatogenic differentiation (Figure 5E), these preliminary observations suggest that Mgm1 induction may be a transcriptional read-out for additional aspects of male GSC behavior that are directly regulated by the Jak-STAT pathway. Thus, as in adult testes (Leatherman and Dinardo, 2008, 2010) Jak-STAT signaling acts both indirectly and directly on GSCs to regulate their development, while additional mechanisms of signaling from CySCs to GSCs play the primary role in controlling the balance between GSC maintenance and differentiation.
Discussion
Our data indicate that functional CySCs are established in the Drosophila testis stem cell niche by the embryo-larval transition (~23 hrs AEL; Figure 6). Dividing CySCs that express adult CySC markers are organized around the newly formed hub at this time, and onset of cyst cell differentiation that supports steady state production of differentiating spermatogonia is observed shortly thereafter. Jak-STAT signaling is necessary and sufficient for CySC maintenance in the newly formed niche, and expansion of the CySC population due to somatic Jak hyper-activation represses GSC differentiation. Conversely, while our data suggest that germline Jak activation alters aspects of gene expression in germ cells, this has no impact on GSC maintenance or spermatogonial differentiation. Thus, less than 14 hours after onset of testis formation, a functional stem niche that regulates stem cell self-renewal in a manner similar to the adult Drosophila testis, has formed through the coordinated development of multiple stem cell types that are established from stem cell progenitors in the embryonic gonad.
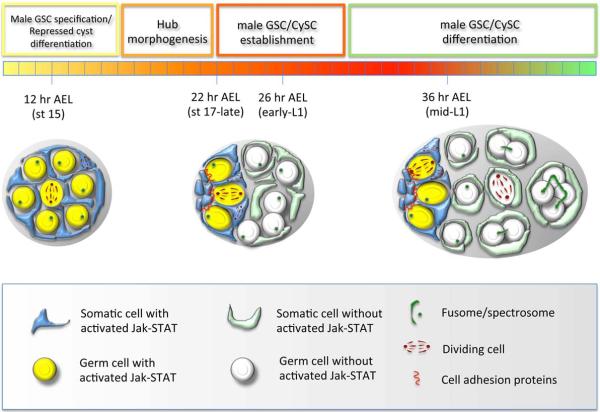
Figure 6. Model of testis stem cell development.
(left gonad) Jak-STAT activation represses SGP differentiation and specifies male GSC fate after gonad formation. (middle gonad) Hub morphogenesis results in restricted expression of UPD to the testes anterior and Jak-STAT activation only in germ cells and somatic cells adjacent the hub. Restriction of Jak-STAT activation in germ cells promotes formation of adherens junctions between GSCs and hub cells that are required for orienting germ cell divisions away from the hub, while restricted Jak-STAT activation in somatic cells promotes CySC behavior and represses cyst differentiation. (right gonad) Somatic cells away from the hub that lack Jak-STAT activation differentiate into cyst cells that act in pairs to promote spermatogonial differentiation, while CySCs adjacent to the hub repress differentiation of neighboring GSCs and divide asymmetrically to produce more cyst cells. Fusomes and spectrosomes are shown in dark green, cell adhesions shown in red, and the mitotic spindle of diving cells shown in maroon.
A model for testis stem cell development
Jak-STAT signaling plays a critical role in controlling both GSC and CySC behavior in developing testes. The Jak-STAT activating ligand, upd, is initially expressed in somatic cells at the anterior half of the testis just after gonad formation, and STAT is activated in all PGCs, as well as in SGPs ((Wawersik et al., 2005); Supplemental Figure 4). During hub morphogenesis, however, upd gradually becomes restricted to hub cells that coalesce at the testis apex (Le Bras and Van Doren, 2006; Sheng et al., 2009). This correlates both temporally and spatially with restriction of STAT activity to newly established GSCs and CySCs docked at the hub, and the concomitant loss of STAT activity in posterior germ cells and somatic cells ((Sheng et al., 2009); Supplemental Figure 4). Interestingly, germline STAT activation characterized by high-level STAT92E expression (Casper et al., 2011; Dinardo et al., 2011; Leatherman and Dinardo, 2010; Sheng et al., 2009; Wawersik et al., 2005) is not detected with the 10XSTAT92E-GFP reporter we used to reveal STAT activity in SGPs and CySCs. While the rationale for these differences is not clear, restriction of STAT activity to GSCs and CySC adjacent to the hub correlates both temporally and spatially with the re-distribution of E-Cadherin to the GSC-hub interface (Le Bras and Van Doren, 2006; Sheng et al., 2009). Furthermore, STAT activation is required for the maintenance of adherens junctions at the GSC-hub interface in adult testes (Leatherman and Dinardo, 2010), and also appears to modulate formation of cell adhesions between CySCs and the hub (Issigonis et al., 2009; Voog et al., 2008).
Our new data, along with data discussed above, leads us to propose a model for regulation of GSC and CySC development (Figure 6) whereby, differentiation of SGPs into cyst cells is initially inhibited during embryonic gonad formation, potentially through expression of the transcriptional repressor ZFH-1 and/or function of maternal Stat92E (see below), while male GSC fate is specified via Jak-STAT activation. By the embryo-larval transition, however, restriction of Jak-STAT activity to the testis apex during hub morphogenesis dramatically changes the behavior of both PGC and SGPs to promote GSC establishment and the maintenance of both GSCs and CySCs within the niche. In the germline, restricted STAT activation promotes the re-distribution of adherens junctions to the germline-hub interface, which serves to orient germ cell divisions away from the developing hub. In the soma, STAT activation in cells located immediately adjacent to the hub promotes the continued repression of cyst differentiation, while hub-distal cells that lack STAT activity, are permitted to differentiate. Additionally, STAT activity in newly established CySC results in activation of signaling from the soma to the germline that promotes GSC behavior and represses spermatogenic differentiation. Thus, when germ cells initiate oriented divisions at the end of embryogenesis, cells adhering to the hub remain undifferentiated, while germ cells displaced away from the hub differentiate into gonialblasts. Similarly, onset of asymmetric CySC division at the embryo-larval transition results in production of cyst cells that are displaced from the hub and promote spermatogenic differentiation in neighboring germ cells. We hypothesize that integrin-based and/or cadherin-based cell adhesions between newly established CySCs and the hub promote the continued association of asymmetrically dividing CySCs with the niche.
GSC renewal and CySC establishment
While existing data in adult or developing testes support the above model, a number of questions remain: What signals emanating from the newly established CySCs promote GSC maintenance? Additionally, how is CySC fate first specified, and what regulates CySC establishment? As BMP signaling from CySCs and hub cells is required for GSC maintenance in the adult testis (Kawase et al., 2004; Leatherman and Dinardo, 2008; Shivdasani and Ingham, 2003; Wang et al., 2008; Zheng et al., 2011), this pathway may play a similar role during testis development. Furthermore, Zfh-1, chinmo, as well as ken & barbie (ken) have all been implicated in regulation of signaling, either directly or indirectly, from adult CySCs to the GSCs (Flaherty et al., 2010; Issigonis M., 2012; Leatherman and Dinardo, 2008). These three genes are also required for adult CySC maintenance, and are thought to do so by repressing CySC differentiation (Flaherty et al., 2010; Issigonis M., 2012; Leatherman and Dinardo, 2008). Given the dynamic pattern of ZFH-1 expression in developing testis, it is possible that ZFH-1 plays a similar role during CySC establishment. While Zfh-1 promotes SGP specification prior to gonad coalescence, we hypothesize that ZFH-1 expression in the embryonic gonad also serves to repress SGP differentiation along the cyst cell fate after gonad formation has occurred. Based on this hypothesis, as high-level ZFH-1 becomes restricted to newly established CySCs adjacent to the hub, somatic cells located away from the hub that now lack ZFH-1 would be permitted to differentiate into cyst cells. Furthermore, since Zfh-1 is a STAT92E target (Leatherman and Dinardo, 2008), and Jak-STAT signaling is required for the maintenance of newly established CySCs, we propose that the restriction of Jak-STAT signaling to the testis anterior directly regulates the dynamics of ZFH-1 expression. While loss of zygotic Stat92E does not obviously impact ZFH-1 expression or gonad morphology during CySC establishment, it is possible that maternal Stat92E promotes expression of ZFH-1 prior to stem cell niche formation. In the future, it will be very interesting to assess the role of Zfh-1 and other known STAT targets in the regulation of CySC establishment. This, and the elucidation of additional mechanisms regulating CySC specification, morphogenesis, as well as oriented division and the displacement of CySC progeny from the developing hub, will provide a more complete understanding of stem cell development during testis niche formation and a better understanding of general paradigms controlling stem cell formation during organogenesis in other systems.
Supplementary Material
Supplemental Figure 1 – Number of ZFH-1 enriched cells in developing testes and coexpression with Socs36E-PZ: [A] Graph showing average number of ZFH-1 enriched somatic cells (blue) and GSCs (red) located adjacent to the hub at the time of hub formation (st17-late/L1e; ∼23 hrs AEL), at early-L1 (∼28 hrs AEL), and at mid-L1 (∼36 hrs AEL). Error bar shows standard deviation. The average number of CySCs and GSCs shows no significant change over time (P>0.5 in all instances). Based on these data, and the number of pHH3/TJ double positive CySCs in L1 testes, the mitotic index of CySCs in L1 is ∼0.11. [B, C] Developing testes immunostained with anti-ZFH-1 (green; B’, C’ alone), anti-β-galactosidase to reveal Socs36E-PZ (blue; B’’, C” alone) and anti-Vasa (red). Socs36E-PZ is co-expressed in cells with high-level ZFH-1 (yellow arrows) located immediately adjacent to the hub at mid-L1 (B) and at the embryo-larval transition (C). Not all hub-adjacent cells expressing high-level ZFH-1 express Socs36E-PZ (yellow arrowheads).
Supplemental Figure 2 – Socs36E-PZ expression in the adult testis: Adult Socs36E-PZ testes from 3–5 day old males immunostained with anti-Vasa (red), anti-β-galactosidase (green; B’–D’ alone) to reveal Socs36E-PZ expression, and anti-TJ (blue; B”–D” alone) to reveal CySCs and cyst cells. [A] Image of entire testis with Socs36E-PZ expression detected in hub cells at the testis apex (yellow arrow), cyst cells along the length of the testes (white arrowheads) and somatic cells at the base of the testis (red arrow). [B–D] Images of the testis apex showing high-level Socs36E-PZ expression in hub cells (B–D; yellow dashed line), and variable levels of Socs36E-PZ expression in CySC ranging from expression in all CySCs (B), a subset of CySCs (C), to expression in no CySCs (D). CySCs with and without Socs36E-PZ expression are indicated (yellow arrows or arrowheads, respectively).
Supplemental Figure 3 – c587-Gal4 expression in the developing testis: Late-stage embryonic and 1st instar larval testes with c587-Gal4 driving expression of nuclear β-Gal (UAS-β-Gal.nls; A–D) or membrane localized GFP (UAS-mCD8∷GFP; E, F). Testes immunostained with anti-Vasa (red), and either anti-N-Cad (A–D, blue) and anti-β-Gal (A–D, green; A’–D’ alone), or anti-Fas3 (E,F, blue) and anti-GFP (E,F, green; E’ & F’ alone). Hub (yellow dashed lines) and testes (while dotted line) are outlined. [A–D] Lowlevel nuclear β-Gal (yellow arrow head) is occasionally detected in somatic cells (14.3% with β-gal nuclei detected, n=21) randomly dispersed throughout early-mid stage 17 testes (A). At the embryo-larval transition (B) nuclear β-Gal is observed throughout the testis, with expression occasionally seen in newly coalesced hub cells (7.4% of testes with low-level hub expression as in panel B, and 7.4% of testes with nuclear β-gal expressed at levels similar to the rest of the gonad, n=27). In early- and mid- L1 larvae (C, D), nuclear β-Gal remains throughout the testes except in hub cells, where it is undetectable (n=45 and 15 for early- and mid- L1, respectively). Strong nuclear β-gal expression is, however, occasionally detected in cells on the testis periphery adjacent to the hub (not shown; 16.7%, n=36). [E, F] GFP is observed in somatic cells that interdigitate the GSCs (yellow arrowheads) at the embryo-larval transition (E) and at mid-L1 (F). At mid-L1, somatic cells ensheath 2–4 cell spermatogonia (yellow arrows) located in the testes posterior.
Supplemental Figure 4 – STAT is activated in newly established CySCs: Testes from 10xSTAT92E-GFP embryos or larvae immunostained with anti-GFP (green; alone in black and white), anti-Vasa (red), and anti-Fas3 (blue). Images oriented with testis anterior to the left and hub indicated (arrow). (A) Early stage 17 testis, just prior to hub formation, showing GFP expression in cells on the testis periphery and low-level expression in SGPs within the main body of the gonad. (B) Testis at the embryo-larval transition, at the time of hub formation, showing GFP expression in the testis periphery as well as lower-level expression in hub cells and somatic cells immediately adjacent to the hub. (C) Early- and (D) mid- 1st instar larval testes showing strong GFP expression in hub cells and CySC that diminishes in somatic cells localized away from the hub.
Supplemental Figure 5 – Jak-STAT signaling is required for CySC maintenance: mid-L1 testis immunostained with anti-ZFH-1 (green; A’, B’ in black & white with GFP), anti-GFP (green; A’, B’ in black & white with ZFH-1), anti-Fas3 (blue; A”, B” alone), and anti-Vasa (red; A”’, B”’ alone). All images with testis anterior oriented left. Hub(yellow dashed lines) and testes (white dotted lines) outlined. (A) Stat92ETS testis mutant for both maternal and zygotic stat92E at high temperatures (29°C) lacking ZFH-1 expression in somatic nuclei adjacent to the hub. (B) Sibling control testis with high-level ZFH-1 expression detected in somatic cells adjacent to the hub.
Highlights
We examine the process of cyst stem cell (CySC) development in Drosophila testes
CySC establishment correlates with formation of the testis stem cell niche
Jak-STAT signaling is necessary and sufficient for CySC maintenance in larval testes
Zygotic Stat92E is not required for the initial establishment of CySCs at the embryo-larval transition
Signaling from CySCs promotes the maintenance of germline stem cells (GSCs) in larval testes
Acknowledgements
We are grateful to all our colleagues who have supplied us with suggestions, antibodies, stocks and technical assistance. We would also like to acknowledge the Bloomington Stock Center at the Indiana University for maintaining and providing fly stocks, and the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology. We specifically thank Andrea Lin and Rebecca Obniski for assistance with analysis of adult testes, as well as Stephen DiNardo, Mark Van Doren, Mathias Leu and members of the Wawersik and Matunis labs for helpful discussions. This work was supported by NSF grant IOS-082315 (MW), NIH grant RO1HD040307 (EM), William & Mary Summer Research Grants (DS and MB), and the HHMI Undergraduate Science Education Program (summer research grants to JF & TJ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aboim AN. Developpement embryonnaire et post-embryonnaire des gonades normales et agametiques de Drosophila melanogaster. Revue Suisse de Zoologie. 1945;52:53–154. [Google Scholar]
- Bach EA, Ekas LA, Ayala-Camargo A, Flaherty MS, Lee H, Perrimon N, Baeg GH. GFP reporters detect the activation of the Drosophila JAK/STAT pathway in vivo. Gene Expr Patterns. 2007;7:323–331. doi: 10.1016/j.modgep.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Baksa K, Parke T, Dobens LL, Dearolf CR. The Drosophila STAT protein, stat92E, regulates follicle cell differentiation during oogenesis. Developmental Biology. 2002;243:166–175. doi: 10.1006/dbio.2001.0539. [DOI] [PubMed] [Google Scholar]
- Boyle M, Bonini N, DiNardo S. Expression and function of clift in the development of somatic gonadal precursors within the Drosophila mesoderm. Development. 1997;124:971–982. doi: 10.1242/dev.124.5.971. [DOI] [PubMed] [Google Scholar]
- Boyle M, DiNardo S. Specification, migration, and assembly of the somatic cells of the Drosophila gonad. Development. 1995;121:1815–1825. doi: 10.1242/dev.121.6.1815. [DOI] [PubMed] [Google Scholar]
- Boyle M, Wong C, Rocha M, Jones DL. Decline in self-renewal factors contributes to aging of the stem cell niche in the Drosophila testis. Cell Stem Cell. 2007;1:470–478. doi: 10.1016/j.stem.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Broihier HT, Moore LA, Van Doren M, Newman S, Lehmann R. zfh-1 is required for germ cell migration and gonadal mesoderm development in Drosophila. Development. 1998;125:655–666. doi: 10.1242/dev.125.4.655. [DOI] [PubMed] [Google Scholar]
- Brookman J, Toosy A, Shashidhara L, White R. The 412 retrotransposon and the development of gonadal mesoderm in Drosophila. Development. 1992;116:1185–1192. doi: 10.1242/dev.116.4.1185. [DOI] [PubMed] [Google Scholar]
- Campos-Ortega J, Hartenstein V. The embryonic development of Drosophila melanogaster. Springer-Verlag; New York: 1985. [Google Scholar]
- Casper AL, Baxter K, Van Doren M. no child left behind encodes a novel chromatin factor required for germline stem cell maintenance in males but not females. Development. 2011;138:3357–3366. doi: 10.1242/dev.067942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Tiyaboonchai A, Yamashita YM, Hunt AJ. Asymmetric division of cyst stem cells in Drosophila testis is ensured by anaphase spindle repositioning. Development. 2011;138:831–837. doi: 10.1242/dev.057901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cuevas M, Matunis EL. The stem cell niche: Lessons from Drosophila testis. Development. 2011;138:2861–2869. doi: 10.1242/dev.056242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFalco T, Camara N, Le Bras S, Van Doren M. Nonautonomous sex determination controls sexually dimorphic development of the Drosophila gonad. Dev Cell. 2008;14:275–286. doi: 10.1016/j.devcel.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFalco TJ, Verney G, Jenkins AB, McCaffery JM, Russell S, Van Doren M. Sex-specific apoptosis regulates sexual dimorphism in the Drosophila embryonic gonad. Dev Cell. 2003;5:205–216. doi: 10.1016/s1534-5807(03)00204-1. [DOI] [PubMed] [Google Scholar]
- Dinardo S, Okegbe T, Wingert L, Freilich S, Terry N. lines and bowl affect the specification of cyst stem cells and niche cells in the Drosophila testis. Development. 2011;138:1687–1696. doi: 10.1242/dev.057364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizio JJ, Boyle M, DiNardo S. A somatic role for eyes absent (eya) and sine oculis (so) in Drosophila spermatocyte development. Dev Biol. 2003;258:117–128. doi: 10.1016/s0012-1606(03)00127-1. [DOI] [PubMed] [Google Scholar]
- Flaherty MS, Salis P, Evans CJ, Ekas LA, Marouf A, Zavadil J, Banerjee U, Bach EA. chinmo is a functional effector of the JAK/STAT pathway that regulates eye development, tumor formation, and stem cell self-renewal in Drosophila. Dev Cell. 2010;18:556–568. doi: 10.1016/j.devcel.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller M. Spermatogenesis. In: Bate M, Martinez Arias A, editors. The Development of Drosophila melanogaster. Cold Spring Harbor Press; Cold Spring Harbor: 1993. pp. 71–147. [Google Scholar]
- Gonczy P, DiNardo S. The germ line regulates somatic cyst cell proliferation and fate during Drosophila spermatogenesis. Development. 1996;122:2437–2447. doi: 10.1242/dev.122.8.2437. [DOI] [PubMed] [Google Scholar]
- Gönczy P, Viswanathan S, DiNardo S. Probing spermatogenesis in Drosophila with P-element enhancer detectors. Development. 1992;114:89–98. doi: 10.1242/dev.114.1.89. [DOI] [PubMed] [Google Scholar]
- Hanratty WP, Dearolf CR. The Drosophila Tumorous-lethal hematopoietic oncogene is a dominant mutation in the hopscotch locus. Mol Gen Genet. 1993;238:33–37. doi: 10.1007/BF00279527. [DOI] [PubMed] [Google Scholar]
- Hardy RW, Tokuyasu KT, Lindsley DL, Garavito M. The germinal prolieration center in the testis of Drosophila melanogaster. J.Ultrastruct.Res. 1979;69:180–190. doi: 10.1016/s0022-5320(79)90108-4. [DOI] [PubMed] [Google Scholar]
- Hempel LU, Oliver B. Sex-specific DoublesexM expression in subsets of Drosophila somatic gonad cells. BMC Dev Biol. 2007;7:113. doi: 10.1186/1471-213X-7-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hime GR, Brill JA, Fuller MT. Assembly of ring canals in the male germ line from structural components of the contractile ring. J Cell Sci. 1996;109(Pt 12):2779–2788. doi: 10.1242/jcs.109.12.2779. [DOI] [PubMed] [Google Scholar]
- Hombria JC, Brown S. The fertile field of Drosophila Jak/STAT signalling. CurrBiol. 2002;12:R569–575. doi: 10.1016/s0960-9822(02)01057-6. [DOI] [PubMed] [Google Scholar]
- Hou XS, Melnick MB, Perrimon N. marelle acts downstream of the Drosophila HOP/JAK kinase and encodes a protein similar to the mammalian STATs. Cell. 1996;84:411–419. doi: 10.1016/s0092-8674(00)81286-6. [DOI] [PubMed] [Google Scholar]
- Inaba M, Y.H., Salzmann V, Fuller MT, Yamashita YM. E-cadherin is required for centrosome and spindle orientation in Drosophila male germ line stem cells. PLoS One. 2010;5:e12473. doi: 10.1371/journal.pone.0012473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issigonis M, Tulina N, de Cuevas M, Brawley C, Sandler L, Matunis E. JAK-STAT signal inhibition regulates competition in the Drosophila testis stem cell niche. Science. 2009;326:153–156. doi: 10.1126/science.1176817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issigonis M, M.E. The Drosophila BCL6 homolog ken and barbie promotes somatic stem cell self-renewal in the testis niche. Dev Biol. 2012;368:181–192. doi: 10.1016/j.ydbio.2012.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemc JC. Somatic gonadal cells: the supporting cast for the germline. Genesis. 2011;49:753–775. doi: 10.1002/dvg.20784. [DOI] [PubMed] [Google Scholar]
- Jenkins AB, McCaffery JM, Van Doren M. Drosophila E-cadherin is essential for proper germ cell-soma interaction during gonad morphogenesis. Development. 2003;130:4417–4426. doi: 10.1242/dev.00639. [DOI] [PubMed] [Google Scholar]
- Kai T, Spradling A. An empty Drosophila stem cell niche reactivates the proliferation of ectopic cells. Proc Natl Acad Sci U S A. 2003;100:4633–4638. doi: 10.1073/pnas.0830856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman DS. Toward clinical therapies using hematopoietic cells derived from human pluripotent stem cells. Blood. 2009;114:3513–3523. doi: 10.1182/blood-2009-03-191304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawase E, Wong MD, Ding BC, Xie T. Gbb/Bmp signaling is essential for maintaining germline stem cells and for repressing bam transcription in the Drosophila testis. Development. 2004;131:1365–1375. doi: 10.1242/dev.01025. [DOI] [PubMed] [Google Scholar]
- Kiger AA, Jones DL, Schulz C, Rogers MB, Fuller MT. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science. 2001;294:2542–2545. doi: 10.1126/science.1066707. [DOI] [PubMed] [Google Scholar]
- Kiger AA, White-Cooper H, Fuller MT. Somatic support cells restrict germline stem cell self-renewal and promote differentiation. Nature. 2000;407:750–754. doi: 10.1038/35037606. [DOI] [PubMed] [Google Scholar]
- Kitadate Y, Kobayashi S. Notch and Egfr signaling act antagonistically to regulate germ-line stem cell niche formation in Drosophila male embryonic gonads. Proc Natl Acad Sci U S A. 2010;107:14241–14246. doi: 10.1073/pnas.1003462107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitadate Y, Shigenobu S, Arita K, Kobayashi S. Boss/Sev signaling from germline to soma restricts germline-stem-cell-niche formation in the anterior region of Drosophila male gonads. Dev Cell. 2007;13:151–159. doi: 10.1016/j.devcel.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Kuhn G, Blomgren K. Developmental dysregulation of adult neurogenesis. Eur J Neurosci. 2011;33:1115–1122. doi: 10.1111/j.1460-9568.2011.07610.x. [DOI] [PubMed] [Google Scholar]
- Lander AD, Kimble J, Clevers H, Fuchs E, Montarras D, Buckingham M, Calof AL, Trumpp A, Oskarsson T. What does the concept of the stem cell niche really mean today? BMC Biology. 2012;10 doi: 10.1186/1741-7007-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bras S, Van Doren M. Development of the male germline stem cell niche in Drosophila. Dev Biol. 2006;294:92–103. doi: 10.1016/j.ydbio.2006.02.030. [DOI] [PubMed] [Google Scholar]
- Leatherman JL, Dinardo S. Zfh-1 controls somatic stem cell self-renewal in the Drosophila testis and nonautonomously influences germline stem cell self-renewal. Cell Stem Cell. 2008;3:44–54. doi: 10.1016/j.stem.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leatherman JL, Dinardo S. Germline self-renewal requires cyst stem cells and stat regulates niche adhesion in Drosophila testes. Nat Cell Biol. 2010;12:806–811. doi: 10.1038/ncb2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Zhou L, Kim J, Kalbfleisch S, Schock F. Lasp anchors the Drosophila male stem cell niche and mediates spermatid individualization. Mech Dev. 2008;125:768–776. doi: 10.1016/j.mod.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- Li MA, Alls JD, Avancini RM, Koo K, Godt D. The large Maf factor Traffic Jam controls gonad morphogenesis in Drosophila. Nat Cell Biol. 2003;5:994–1000. doi: 10.1038/ncb1058. [DOI] [PubMed] [Google Scholar]
- Lin H, Yue L, Spradling AC. The Drosophila fusome, a germline-specific organelle, contains membrane skeletal proteins and functions in cyst formation. Development. 1994;120:947–956. doi: 10.1242/dev.120.4.947. [DOI] [PubMed] [Google Scholar]
- Luo H, Rose P, Barber D, Hanratty WP, Lee S, Roberts TM, D'Andrea AD, Dearolf CR. Mutation in the Jak kinase JH2 domain hyperactivates Drosophila and mammalian Jak-Stat pathways. Mol Cell Biol. 1997;17:1562–1571. doi: 10.1128/mcb.17.3.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Belmonte F, Perez-Moreno M. Epithelial cell polarity, stem cells and cancer. Nat Rev Cancer. 2012;12:23–38. doi: 10.1038/nrc3169. [DOI] [PubMed] [Google Scholar]
- Mathews WR, Ong D, Milutinovich AB, Van Doren M. Zinc transport activity of Fear of Intimacy is essential for proper gonad morphogenesis and DE-cadherin expression. Development. 2006;133:1143–1153. doi: 10.1242/dev.02256. [DOI] [PubMed] [Google Scholar]
- Matunis E, Tran J, Gonczy P, Caldwell K, DiNardo S. punt and schnurri regulate a somatically derived signal that restricts proliferation of committed progenitors in the germline. Development. 1997;124:4383–4391. doi: 10.1242/dev.124.21.4383. [DOI] [PubMed] [Google Scholar]
- Mohsin S, Siddiqi S, Collins B, Sussman MA. Empowering adult stem cells for myocardial regeneration. Circ Res. 2011;109:1415–1428. doi: 10.1161/CIRCRESAHA.111.243071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda S, DeFalco TJ, Loh SH, Phochanukul N, Camara N, Van Doren M, Russell S. Sox100B, a Drosophila group E Sox-domain gene, is required for somatic testis differentiation. Sex Dev. 2009;3:26–37. doi: 10.1159/000200079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okegbe TC, DiNardo S. The endoderm specifies the mesodermal niche for the germline in Drosophila via Delta-Notch signaling. Development. 2011;138:1259–1267. doi: 10.1242/dev.056994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A, Parikh N, Hearn SA, Fuller MT, Tazuke SI, Schulz C. Antagonistic roles of Rac and Rho in organizing the germ cell microenvironment. Curr Biol. 2007;17:1253–1258. doi: 10.1016/j.cub.2007.06.048. [DOI] [PubMed] [Google Scholar]
- Schulz C, Wood CG, Jones DL, Tazuke SI, Fuller MT. Signaling from germ cells mediated by the rhomboid homolog stet organizes encapsulation by somatic support cells. Development. 2002;129:4523–4534. doi: 10.1242/dev.129.19.4523. [DOI] [PubMed] [Google Scholar]
- Sheng XR, Posenau T, Gumulak-Smith JJ, Matunis E, Van Doren M, Wawersik M. Jak-STAT regulation of male germline stem cell establishment during Drosophila embryogenesis. Dev Biol. 2009;334:335–344. doi: 10.1016/j.ydbio.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivdasani AA, Ingham PW. Regulation of stem cell maintenance and transit amplifying cell proliferation by tgf-beta signaling in Drosophila spermatogenesis. Curr Biol. 2003;13:2065–2072. doi: 10.1016/j.cub.2003.10.063. [DOI] [PubMed] [Google Scholar]
- Silvers M, Hanratty WP. Alterations in the production of hemocytes due to a neoplastic mutation of Drosophila melanogaster. J Invertebr Pathol. 1984;44:324–328. doi: 10.1016/0022-2011(84)90030-2. [DOI] [PubMed] [Google Scholar]
- Singh SR, Zheng Z, Wang H, Oh SW, Chen X, Hou SX. Competitiveness for the niche and mutual dependence of the germline and somatic stem cells in the Drosophila testis are regulated by the JAK/STAT signaling. J Cell Physiol. 2010;223:500–510. doi: 10.1002/jcp.22073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenblick BP. Germ cell movements and sex differentiation of the gonads in the Drosophila embryo. Proc. Natl. Acad. Sci. USA. 1941;26:373–381. doi: 10.1073/pnas.27.10.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;414:98–104. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- Staab S, Heller A, Steinmann-Zwicky M. Somatic sex-determining signals act on XX germ cells in Drosophila embryos. Development. 1996;122:4065–4071. doi: 10.1242/dev.122.12.4065. [DOI] [PubMed] [Google Scholar]
- Streit A, Bernasconi L, Sergeev P, Cruz A, Steinmann-Zwicky M. mgm 1, the earliest sex-specific germline marker in Drosophila, reflects expression of the gene esg in male stem cells. Int J Dev Biol. 2002;46:159–166. [PubMed] [Google Scholar]
- Tanentzapf G, Devenport D, Godt D, Brown NH. Integrin-dependent anchoring of a stem-cell niche. Nat Cell Biol. 2007;9:1413–1418. doi: 10.1038/ncb1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazuke SI, Schulz C, Gilboa L, Fogarty M, Mahowald AP, Guichet A, Ephrussi A, Wood CG, Lehmann R, Fuller MT. A germline-specific gap junction protein required for survival of differentiating early germ cells. Development. 2002;129:2529–2539. doi: 10.1242/dev.129.10.2529. [DOI] [PubMed] [Google Scholar]
- Tran J, Brenner TJ, DiNardo S. Somatic control over the germline stem cell lineage during Drosophila spermatogenesis. Nature. 2000;407:754–757. doi: 10.1038/35037613. [DOI] [PubMed] [Google Scholar]
- Tulina N, Matunis E. Control of stem cell self-renewal in Drosophila spermatogenesis by JAK-STAT signaling. Science. 2001;294:2546–2549. doi: 10.1126/science.1066700. [DOI] [PubMed] [Google Scholar]
- Van Doren M, Mathews WR, Samuels M, Moore LA, Broihier HT, Lehmann R. fear of intimacy encodes a novel transmembrane protein required for gonad morphogenesis in Drosophila. Development. 2003;130:2355–2364. doi: 10.1242/dev.00454. [DOI] [PubMed] [Google Scholar]
- Voog J, D'Alterio C, Jones DL. Multipotent somatic stem cells contribute to the stem cell niche in the Drosophila testis. Nature. 2008;454:1132–1136. doi: 10.1038/nature07173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Singh SR, Zheng Z, Oh SW, Chen X, Edwards K, Hou SX. Rap-GEF signaling controls stem cell anchoring to their niche through regulating DE-cadherin-mediated cell adhesion in the Drosophila testis. Dev Cell. 2006;10:117–126. doi: 10.1016/j.devcel.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Wang L, Li Z, Cai Y. The JAK/STAT pathway positively regulates DPP signaling in the Drosophila germline stem cell niche. J Cell Biol. 2008;180:721–728. doi: 10.1083/jcb.200711022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren LA, Rossi DJ. Stem cells and aging in the hematopoietic system. Mech Ageing Dev. 2009;130:46–53. doi: 10.1016/j.mad.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrior R. Primordial germ cell migration and the assembly of the Drosophila embryonic gonad. Developmental Biology. 1994;166:180–194. doi: 10.1006/dbio.1994.1306. [DOI] [PubMed] [Google Scholar]
- Wawersik M, Milutinovich A, Casper AL, Matunis E, Williams B, Van Doren M. Somatic control of germline sexual development is mediated by the JAK/STAT pathway. Nature. 2005;436:563–567. doi: 10.1038/nature03849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyers JJ, Milutinovich AB, Takeda Y, Jemc JC, Van Doren M. A genetic screen for mutations affecting gonad formation in Drosophila reveals a role for the slit/robo pathway. Dev Biol. 2011;353:217–228. doi: 10.1016/j.ydbio.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita YM, Jones DL, Fuller MT. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science. 2003;301:1547–1550. doi: 10.1126/science.1087795. [DOI] [PubMed] [Google Scholar]
- Zheng Q, Wang Y, Vargas E, DiNardo S. magu is required for germline stem cell self-renewal through BMP signaling in the Drosophila testis. Dev Biol. 2011;357:202–210. doi: 10.1016/j.ydbio.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 – Number of ZFH-1 enriched cells in developing testes and coexpression with Socs36E-PZ: [A] Graph showing average number of ZFH-1 enriched somatic cells (blue) and GSCs (red) located adjacent to the hub at the time of hub formation (st17-late/L1e; ∼23 hrs AEL), at early-L1 (∼28 hrs AEL), and at mid-L1 (∼36 hrs AEL). Error bar shows standard deviation. The average number of CySCs and GSCs shows no significant change over time (P>0.5 in all instances). Based on these data, and the number of pHH3/TJ double positive CySCs in L1 testes, the mitotic index of CySCs in L1 is ∼0.11. [B, C] Developing testes immunostained with anti-ZFH-1 (green; B’, C’ alone), anti-β-galactosidase to reveal Socs36E-PZ (blue; B’’, C” alone) and anti-Vasa (red). Socs36E-PZ is co-expressed in cells with high-level ZFH-1 (yellow arrows) located immediately adjacent to the hub at mid-L1 (B) and at the embryo-larval transition (C). Not all hub-adjacent cells expressing high-level ZFH-1 express Socs36E-PZ (yellow arrowheads).
Supplemental Figure 2 – Socs36E-PZ expression in the adult testis: Adult Socs36E-PZ testes from 3–5 day old males immunostained with anti-Vasa (red), anti-β-galactosidase (green; B’–D’ alone) to reveal Socs36E-PZ expression, and anti-TJ (blue; B”–D” alone) to reveal CySCs and cyst cells. [A] Image of entire testis with Socs36E-PZ expression detected in hub cells at the testis apex (yellow arrow), cyst cells along the length of the testes (white arrowheads) and somatic cells at the base of the testis (red arrow). [B–D] Images of the testis apex showing high-level Socs36E-PZ expression in hub cells (B–D; yellow dashed line), and variable levels of Socs36E-PZ expression in CySC ranging from expression in all CySCs (B), a subset of CySCs (C), to expression in no CySCs (D). CySCs with and without Socs36E-PZ expression are indicated (yellow arrows or arrowheads, respectively).
Supplemental Figure 3 – c587-Gal4 expression in the developing testis: Late-stage embryonic and 1st instar larval testes with c587-Gal4 driving expression of nuclear β-Gal (UAS-β-Gal.nls; A–D) or membrane localized GFP (UAS-mCD8∷GFP; E, F). Testes immunostained with anti-Vasa (red), and either anti-N-Cad (A–D, blue) and anti-β-Gal (A–D, green; A’–D’ alone), or anti-Fas3 (E,F, blue) and anti-GFP (E,F, green; E’ & F’ alone). Hub (yellow dashed lines) and testes (while dotted line) are outlined. [A–D] Lowlevel nuclear β-Gal (yellow arrow head) is occasionally detected in somatic cells (14.3% with β-gal nuclei detected, n=21) randomly dispersed throughout early-mid stage 17 testes (A). At the embryo-larval transition (B) nuclear β-Gal is observed throughout the testis, with expression occasionally seen in newly coalesced hub cells (7.4% of testes with low-level hub expression as in panel B, and 7.4% of testes with nuclear β-gal expressed at levels similar to the rest of the gonad, n=27). In early- and mid- L1 larvae (C, D), nuclear β-Gal remains throughout the testes except in hub cells, where it is undetectable (n=45 and 15 for early- and mid- L1, respectively). Strong nuclear β-gal expression is, however, occasionally detected in cells on the testis periphery adjacent to the hub (not shown; 16.7%, n=36). [E, F] GFP is observed in somatic cells that interdigitate the GSCs (yellow arrowheads) at the embryo-larval transition (E) and at mid-L1 (F). At mid-L1, somatic cells ensheath 2–4 cell spermatogonia (yellow arrows) located in the testes posterior.
Supplemental Figure 4 – STAT is activated in newly established CySCs: Testes from 10xSTAT92E-GFP embryos or larvae immunostained with anti-GFP (green; alone in black and white), anti-Vasa (red), and anti-Fas3 (blue). Images oriented with testis anterior to the left and hub indicated (arrow). (A) Early stage 17 testis, just prior to hub formation, showing GFP expression in cells on the testis periphery and low-level expression in SGPs within the main body of the gonad. (B) Testis at the embryo-larval transition, at the time of hub formation, showing GFP expression in the testis periphery as well as lower-level expression in hub cells and somatic cells immediately adjacent to the hub. (C) Early- and (D) mid- 1st instar larval testes showing strong GFP expression in hub cells and CySC that diminishes in somatic cells localized away from the hub.
Supplemental Figure 5 – Jak-STAT signaling is required for CySC maintenance: mid-L1 testis immunostained with anti-ZFH-1 (green; A’, B’ in black & white with GFP), anti-GFP (green; A’, B’ in black & white with ZFH-1), anti-Fas3 (blue; A”, B” alone), and anti-Vasa (red; A”’, B”’ alone). All images with testis anterior oriented left. Hub(yellow dashed lines) and testes (white dotted lines) outlined. (A) Stat92ETS testis mutant for both maternal and zygotic stat92E at high temperatures (29°C) lacking ZFH-1 expression in somatic nuclei adjacent to the hub. (B) Sibling control testis with high-level ZFH-1 expression detected in somatic cells adjacent to the hub.