Abstract
Some mutations in the connection subdomain of the polymerase domain and in the RNase H domain of HIV-1 reverse transcriptase (RT) have been shown to contribute to resistance to RT inhibitors. However, the clinical relevance of such mutations is not well understood. To address this point we determined the prevalence of such mutations in a cohort of antiretroviral treatment-naïve patients (n=123) and assessed whether these substitutions are associated with drug resistance in vitro and in vivo. We report here significant differences in the prevalence of substitutions among subtype B, and non-subtype B HIV isolates. Specifically, the E312Q, G333E, G335D, V365I, A371V and A376S substitutions were present in 2–6% of subtype B, whereas the G335D and A371V substitutions were commonly observed in 69 and 75% of non-B HIV-1 isolates. We observed a significant decline in the viral loads of patients that were infected with HIV-1 carrying these substitutions and were subsequently treated with triple drug regimens, even in the case where zidovudine (AZT) was included in such regimens. We show here that generally, such single substitutions at the connection subdomain or RNase H domain have no influence on drug susceptibility in vitro by themselves. Instead, they generally enhance AZT resistance in the presence of excision-enhancing mutations (EEMs, also known as thymidine analogue-associated mutations, TAMs). However, N348I, A376S and Q509L did confer varying amounts of nevirapine resistance by themselves, even in the absence of EEMs. Therefore, our cohort establishes that several connection subdomain and RNase H domain substitutions typically act as pre-therapy polymorphisms.
Keywords: HIV-1, antiretroviral treatment-naïve patient, resistant mutation, connection subdomain, RNase H domain
1. INTRODUCTION
The zidovudine (AZT)-resistance mutations reside at the DNA polymerase domain of HIV-1 reverse transcriptase (RT). They are associated either with (a) the exclusion mechanism that enhances discrimination at the point of AZT-monophosphate (MP) incorporation through a set of mutations at codons A62, V75, F77, F116, and Q151, of the polymerase domain (Deval et al., 2002; Ueno and Mitsuya, 1997), or with (b) the excision mechanism that involves selective removal of AZT-MP after it has been incorporated by RT into the viral DNA (Boyer et al., 2001; Meyer et al., 1999). The excision mechanism is associated with mutations at the polymerase domain, including M41L, D67N, K70R, L210W, T215F/Y and K219E/Q (excision-containing mutations, EEMs, also known as thymidine analogue-associated mutations [TAMs]).
Certain mutations in the connection subdomain (codons 322 to 440) of the polymerase domain or in the RNase H domain (codons 441 to 560)of HIV-1 RT have been recently shown to be associated with resistance to AZT (Brehm et al., 2007; Hachiya et al., 2008; Kemp et al., 1998; Nikolenko et al., 2007; Ntemgwa et al., 2007; Yap et al., 2007). In some cases it appears that mutations that affect AZT resistance have different phenotypes, depending on the presence or absence of other resistance mutations. For example, the polymorphism G333D/E does not confer drug resistance by itself, but has been reported to contribute significantly to dual AZT-lamivudine (3TC) resistance when combined with EEMs and M184V (Caride et al., 2000; Gallego et al., 2002; Kemp et al., 1998; Zelina et al., 2008). Similarly, A371V and Q509L, which were selected in the background of D67N and K70R by high concentrations of AZT in vitro, show strong resistance to AZT and weak cross-resistance to 3TC, abacavir (ABC) and tenofovir (TNF/PMPA) in the presence of EEMs (Brehm et al., 2007). Santos et al. also recently reported that the A360V and A371V mutations are frequently observed in AZT-treated patients (Santos et al., 2008). In contrast, one of the connection subdomain mutations, N348I, is associated with resistance to both nucleoside RT inhibitors (NRTIs) and non-nucleoside RT inhibitors (NNRTIs) and appears to be induced by regimens containing AZT, didanosine (ddI) and/or nevirapine (NVP) (Hachiya et al., 2008; Yap et al., 2007). Recently, it has been shown that the N348I mutation decreases the efficiency of RNase H cleavage and increases excision of AZT from AZT-terminated primer/templates, in the presence of the pyrophosphate donor ATP (Delviks-Frankenberry et al., 2008; Ehteshami et al., 2008; Yap et al., 2007). The decreased degradation of the RNA template by the diminished RNase H cleavage has been proposed to provide additional time for RT to excise AZT-MP and hence result in the observed increased AZT resistance(Delviks-Frankenberry et al., 2008; Ehteshami et al., 2008)..
With the exception of N348I, the clinical relevance of these mutations remains to be clarified. A major obstacle to understanding the contribution of connection subdomain mutations to NRTI or NNRTI resistance has been the shortage of relevant sequencing data. This is because, until recently, the majority of commercially available genotypic and phenotypic assays have not been targeting this region of the enzyme. This is now changing, as more attention is being focused on such substitutions, following recent publications from us (Hachiya et al., 2008) and others (Yap et al., 2007) showing that at least one connection subdomain mutation, N348I, contributes to multi-class drug resistance. However, it has not yet been determined if the genotypic substitutions encountered in the connection subdomain of polymerase or in the RNase H domain of RT have any phenotypic impact or any effect on virologic response to subsequent therapies. Another important question is whether resistance testing now performed should include these mutations.
To determine whether some mutations at the connection subdomain or at the RNase H domain of RT that appear in the absence of known drug-resistance mutations of the polymerase domain are induced by RTI treatment or are simply pre-existing polymorphisms, we determined the frequency of amino acid substitutions in antiretroviral treatment-naïve patients and assessed whether these substitutions at the reported sites (Brehm et al., 2007; Hachiya et al., 2008; Kemp et al., 1998; Nikolenko et al., 2007; Yap et al., 2007) can cause drug resistance by themselves. We also determined if they have any effect on the virologic response to subsequent therapies.
2. MATERIALS AND METHODS
2.1 Patients
A total of 123 clinical isolates were obtained from fresh plasma of treatment-naïve HIV-infected patients using MAGIC-5 cells as described previously (Hachiya et al., 2001). Written informed consent was obtained from each patient under approval by Institutional Review Board of the International Medical Center of Japan (IMCJ-H13-80). The clinical course and antiretroviral therapies used were reviewed retrospectively.
2.2 Recombinant molecular clones
Recombinant molecular clones were generated as described previously (Hachiya et al., 2008). Briefly, the pBS-RTWT contains almost entire RT coding sequence (amino acid position 14 to 560) which is introduced silent mutations for cloning (restriction enzyme sites, Xma I and Xba I at 5’- and 3’-end of DNA fragment, respectively). After site directed mutagenesis, the mutated RT was ligated into the corresponding restriction enzyme site introduced HIV infectious clone, pNL101 (Hachiya et al., 2008; Shimura et al., 2008).
2.3 Genotypic and phenotypic assays
For the genotypic assay, viral RNA was extracted from culture supernatant of clinical isolates, amplified by nested RT-PCR, and then directly sequenced as described previously (Hachiya et al., 2008). For subtype classification, the RT sequences were analyzed using the ‘Genotyping’ software (http://www.ncbi.nlm.nih.gov/projects/genotyping/formpage.cgi) which uses the BLAST algorithm. HIV-1 sequences in worldwide, treatment-naïve patients were obtained from the Stanford HIV Drug Resistance Database (http://hivdb.stanford.edu/index.html, accessed as late as 26 Feb. 2008) and compared with our cohort. Prevalence of mutations at each codon were compared by the χ2-test, or Fisher’s exact test when the number of patients was smaller than 5.
For phenotypic assay, each clinical isolate was directly tested for drug susceptibility in triplicates, using the MAGIC-5 cell-based assay as described previously (Hachiya et al., 2001). Infectious viruses were obtained by transfection of 293T cells with individual HIV molecular clones containing desired mutations that were introduced by site directed mutagenesis. Cells were subsequently harvested and examined with the MAGIC-5 cell based assay (Hachiya et al., 2001; Hachiya et al., 2008).
2.4 Measurements of HIV-1 viral load
To assess virologic outcome, HIV-1 viral loads in plasma were measured using the commercially available Amplicor HIV-1 Monitor Test (Version 1.5, Roche Diagnostics K.K., Basel, Switzerland). Mean change from 0 at weeks 4, 8, 12, 16, 20 and 24 were evaluated. The statistical significance of the longitudinal changes of HIV-1 viral load in plasma was assessed by the Mann-Whitney U test.
2.5 Molecular modeling studies
The SYBYL and O programs were used to prepare molecular model of the complexes of HIV-1 RT in complex with RNA/DNA (Protein Data Bank code number 1HYS), and containing mutations A376S, N348I and Q509L that were introduced manually into the original 1HYS structure. After introduction of the mutations, the structure coordinates were optimized through 100 cycles of Coleman energy minimization protocol.
3. RESULTS
3.1 Sequence analysis of RT region
We sequenced nearly the entire RT coding region (amino acid position 9 to 560) of 123 clinical isolates obtained from treatment-naïve patients. Among these isolates, 6 contained the known RTI-associated resistant mutations, D67N (n=2), K238S (n=2) (http://www.hiv.lanl.gov/content/index), V108I/K238S (n=1) and V106A/V108I/K238S (n=1), and thus were excluded from further analysis. The clinical isolates were obtained from 6 patients within 1 year of the diagnoses for HIV-1 infection. Prevalence of HIV-1 with drug-associated mutations in Japanese treatment-naïve patients is estimated at approximately 4% (Gatanaga et al., 2007) and in American and European patients at 8 to 27% (Descamps et al., 2005; Little et al., 2002; UK_Collaborative_Group_on_Monitoring_the_Transmission_of_HIV_Drug_Resistance, 2001; Weinstock et al., 2004). Therefore, the prevalence in our cohort (4.8%) seems to be comparable or lower than in previous reports, suggesting that the 6 patients are treatment-naïve and newly infected from treating or treated patients. The strong majority of the remaining samples in our cohort were of subtype B (n=101 of a total of 117 isolates), while other subtypes were also identified (CRF01_AE, A, C and CRF12_BF, with12, 2, 1 and 1 isolates, respectively).
Substitutions at the connection subdomain and RNase H domain observed in this cohort and in previous reports (Brehm et al., 2007; Hachiya et al., 2008; Kemp et al., 1998; Nikolenko et al., 2007; Yap et al., 2007) are shown in Table 1. In the treatment-naïve patients of our cohort that were infected with subtype B (n=101), the frequencies of all mutations associated with AZT-resistance (Brehm et al., 2007; Hachiya et al., 2008; Kemp et al., 1998; Nikolenko et al., 2007; Yap et al., 2007) were comparable to those (treatment-naïve) deposited in the Stanford HIV Drug Resistance Database, except for that of A360T. The G335D and A371V substitutions were more prevalent in the non-B, rather than in the B isolates of our cohort. Moreover, the G335D/A371V combination was observed in 9 (56.3%) of the non-B isolates. Other polymorphisms, including E312A/D/N/T, G335E/N/S, A360S/T, A371T, A376T/V and Q509H, were widely observed in all subtypes in our cohort as well as in the Stanford HIV Drug Resistance Database. None of the clinical isolates of our cohort had the G333D, G335C, N348I, A360I/V, and Q509L mutations.
Table 1.
Drug susceptibilities of 117 clinical isolates obtained from treatment-naïve patients
| Amino acid substitutions |
Frequencya % (n) |
Median fold change in resistanceb |
||||
|---|---|---|---|---|---|---|
| Subtype B (n=101) |
Non-B (n=16) |
AZT | 3TC | NVP | EFV | |
| E312 | 84.1 (85) | 18.8 (3) | 1.2 | 1.3 | 1 | 1.1 |
| Qc | 3 (3) | 0 | 1.3 | 1.4 | 1.2 | 1.1 |
| A | 6.9 (7) | 0 | 1.1 | 1.1 | 1.3 | 1 |
| D | 1 (1) | 6.3 (1) | 1.7 | 1.2 | 1.7 | 1.1 |
| N | 0 | 6.3 (1) | 0.1 | 1.3 | 0.2 | 1.2 |
| T | 5 (5) | 68.8 (11)d | 0.8 | 1 | 1.1 | 1.1 |
| G333 | 94.1 (95) | 100 (16) | 1.1 | 1.3 | 1.1 | 1.1 |
| Dc | 0 | 0 | - | - | - | - |
| Ec | 5.9 (6) | 0 | 1.4 | 1.5 | 1 | 1.4 |
| G335 | 95 (96) | 25 (4) | 1.2 | 1.4 | 1 | 1.1 |
| Cc | 0 | 0 | - | - | - | - |
| Dc | 2 (2) | 68.8 (11)d | 0.7 | 0.9 | 1.1 | 1.1 |
| E | 0 | 6.3 (1) | 0.3 | 0.06 | 0.2 | 0.5 |
| N | 1 (1) | 0 | 0.2 | 0.2 | 0.8 | 1.5 |
| S | 2 (2) | 0 | 0.6 | 0.9 | 1.3 | 1.4 |
| N348 | 100 (101) | 100 (16) | 1.1 | 1.3 | 1 | 1.1 |
| Ic,e | 0 | 0 | - | - | - | - |
| A360 | 79.2 (80) | 87.5 (14) | 1.1 | 1.3 | 1.1 | 1.1 |
| Ic | 0 | 0 | - | - | - | - |
| Vc | 0 | 0 | - | - | - | - |
| S | 0 | 6.3 (1) | 0.7 | 1.2 | 1.5 | 0.8 |
| T | 20.8 (21)f | 6.3 (1) | 0.9 | 1.4 | 1 | 1.2 |
| V365 | 96 (97) | 100 (16) | 1.1 | 1.2 | 1 | 1.1 |
| Ic | 4 (4) | 0 | 1 | 2.3 | 1.7 | 1.3 |
| A371 | 96 (97) | 25 (4) | 1.2 | 1.3 | 1.1 | 1.1 |
| Vc | 3 (3) | 75 (12)d | 0.7 | 0.9 | 0.9 | 1.1 |
| T | 1 (1) | 0 | 0.5 | 1.3 | 0.7 | 0.7 |
| A376 | 92.1 (93) | 75 (12) | 1.1 | 1.3 | 1 | 1.1 |
| Sc | 3 (3) | 6.3 (1) | 1.3 | 0.9 | 1 | 0.6 |
| T | 5 (5) | 12.5 (2) | 1.2 | 1 | 1.4 | 1.2 |
| V | 0 | 6.3 (1) | 0.1 | 1.3 | 0.2 | 1.2 |
| Q509 | 98 (99) | 100 (16) | 1.1 | 1.3 | 1.1 | 1.1 |
| Lc | 0 | 0 | - | - | - | - |
| H | 2 (2) | 0 | 0.6 | 0.7 | 0.7 | 0.8 |
Of 123 clinical isolates, 6 carried the known RTI-associated mutations and were excluded from this analysis.
The drug susceptibility assay (Hachiya et al., 2001) was clinically approved in Japan.
Resistant mutations reported previously [2–6] are indicated in bold. Fold increase of EC50 which was compared to that of NL4–3 was greater than 3-fold defines as resistance.
The prevalence of these substitutions (E312T, G335D and 371V) is significantly difference among treatment-naïve patients between subtype B and non-B isolates (p<0.0001).
N348I confers cross-resistance to NRTIs and NNRTIs (Hachiya et al., 2008; Yap et al., 2007).
The prevalence of A360T is significantly high in our cohort compared to the Stanford HIV Drug Resistance Database (8.7%, p=0.0021).
3.2 Phenotypic assay for clinical isolates
Phenotypically, all clinical isolates showed little resistance to tested drugs (Table 1). Isolates with the V365I substitution (n=4 in subtype B) showed slightly reduced susceptibility to 3TC (2.3-fold). However, V365I may not be clinically relevant, since generally at least 3-fold resistance is required for assigning 3TC resistance in vivo (Parkin et al., 2004; Rhee et al., 2006). Furthermore, the prevalence of V365I in treated and untreated patients in the Stanford HIV Drug Resistance Database is comparable (3.7 and 3.6 %, respectively). Notably, clinical isolates from treatment-naïve patients from our cohort with HIV carrying the E312N, G335E/N or A376V substitutions displayed rather enhanced susceptibility (over 5-fold) to AZT and NVP, AZT, 3TC and NVP, and AZT and NVP, respectively (Table 1). In our cohort, in the absence of EEM mutations, A371V had no significant effect on drug resistance (Table 1). However, other studies have shown that combined with EEMs, A371V can confer strong resistance to AZT and has also been recently reported to be associated with weak cross-resistance to 3TC, TNF/PMPA and ABC (Brehm et al., 2007). In our cohort, ABC inhibits efficiently the clinical isolates that contain the A371V substitution in the absence of EEMs (n=13) either in a subtype B, or non-B background (median fold increase was 0.9-fold, data not shown). Further, the combination of A371V and G335D commonly observed in non-B isolates also showed no resistance to AZT, 3TC or ABC (0.7-, 1.0- and 1.1-fold increase in susceptibility as compared to wild-type HIV, respectively). These results demonstrate that none of the above substitutions that were observed in clinical isolates confer any significant resistance to NRTIs or NNRTIs in the absence of EEMs.
3.3 Phenotypic assay for molecular clones
To further expand our understanding of the role of substitutions in these RT regions on drug resistance we also prepared HIV-1 recombinant viruses with related mutations that have been reported previously in similar drug resistance studies (Brehm et al., 2007; Hachiya et al., 2008; Kemp et al., 1998; Nikolenko et al., 2007; Yap et al., 2007). The results shown in Table 2 confirm that in the absence of RTI resistance mutations, most substitutions in the connection subdomain and RNase H domain (with the exception of N348I, A376S and Q509L) show no significant resistance to AZT, 3TC, TNF/PMPA, NVP or efavirenz (EFV) (less than 3-fold), suggesting that these mutations act as secondary mutations and may enhance resistance that is caused by primary mutations and/or may somehow improve replication kinetics impaired by the primary mutations. Q509L, which has been reported to enhance cross resistance to NRTIs in the presence of EEMs (Brehm et al., 2007), conferred little resistance to at least AZT, 3TC and TNF/PMPA in this study. Unlike N348I that confers dual resistance to NRTIs and NNRTIs, A376S and Q509L provided only NVP resistance.
Table 2.
Drug susceptibilities of molecular HIV-1 clones
| EC50, µM (fold increase)a |
||||||
|---|---|---|---|---|---|---|
| Mutation | NRTI |
NNRTI |
||||
| AZT | 3TC | TNFb | NVP | EFV | ||
| WT | 0.026 ± 0.009 | 0.42 ± 0.04 | 6.2 ± 1.5 | 0.023 ± 0.01 | 0.0012 ± 0.0001 | |
| E312Q | 0.037 ± 0.006 | 0.36 ± 0.05 | 4.1 ± 1.4 | 0.056 ± 0.007 | 0.0009 ± 0.0002 | |
| (1.4) | (0.9) | (0.7) | (2.4) | (0.8) | ||
| G333D | 0.04 ± 0.01 | 0.28 ± 0.1 | 4.5 ± 1.8 | 0.055 ± 0.01 | 0.0017 ± 0.0003 | |
| (1.5) | (0.7) | (0.7) | (2.4) | (1.4) | ||
| G335C | 0.04 ± 0.02 | 0.45 ± 0.1 | 7.7 ± 1.1 | 0.065 ± 0.02 | 0.0007 ± 0.00009 | |
| (1.5) | (1.1) | (1.2) | (2.8) | (0.6) | ||
| N348I | 0.14 ± 0.01 | 0.56 ± 0.07 | 8.8 ± 1.9 | 0.24 ± 0.04 | 0.0032 ± 0.0005 | |
| (5.4) | (1.3) | (1.4) | (10) | (2.7) | ||
| A360I | 0.037 ± 0.01 | 0.35 ± 0.1 | 7.1 ± 2.1 | 0.038 ± 0.01 | 0.0009 ± 0.00008 | |
| (1.4) | (0.8) | (1.1) | (1.7) | (0.8) | ||
| A360V | 0.03 ± 0.002 | 0.28 ± 0.09 | 5.7 ± 2.3 | 0.051 ± 0.01 | 0.0016 ± 0.0006 | |
| (1.2) | (0.7) | (0.9) | (2.2) | (1.3) | ||
| V365I | 0.045 ± 0.008 | 0.27 ± 0.06 | 6.1 ± 2.0 | 0.066 ± 0.02 | 0.0013 ± 0.0002 | |
| (1.7) | (0.6) | (1) | (2.9) | (1.1) | ||
| A376S | 0.053 ± 0.02 | 0.3 ± 0.03 | 5.9 ± 1.6 | 0.084 ± 0.02 | 0.0022 ± 0.0004 | |
| (2) | (0.7) | (1) | (3.7) | (1.8) | ||
| Q509L | 0.072 ± 0.02 | 0.45 ± 0.1 | 8.1 ± 2.7 | 0.21 ± 0.06 | 0.0032 ± 0.0009 | |
| (2.8) | (1.1) | (1.3) | (9.1) | (2.7) | ||
Data means ± standard deviations from at least three independent experiments. The relative increase in the EC50 value compared with that in HIV-1WT is given in parenthesis. Bold indicates an increase in EC50 value greater than three fold.
TNF (PMPA), 9-R-(2-phosphonomethoxypropyl) adenine or tenofovir, which is an active nucleotide of clinical prodrug tenofovir disoproxil fumarate.
3.4 Virological response after initiation of combination therapy
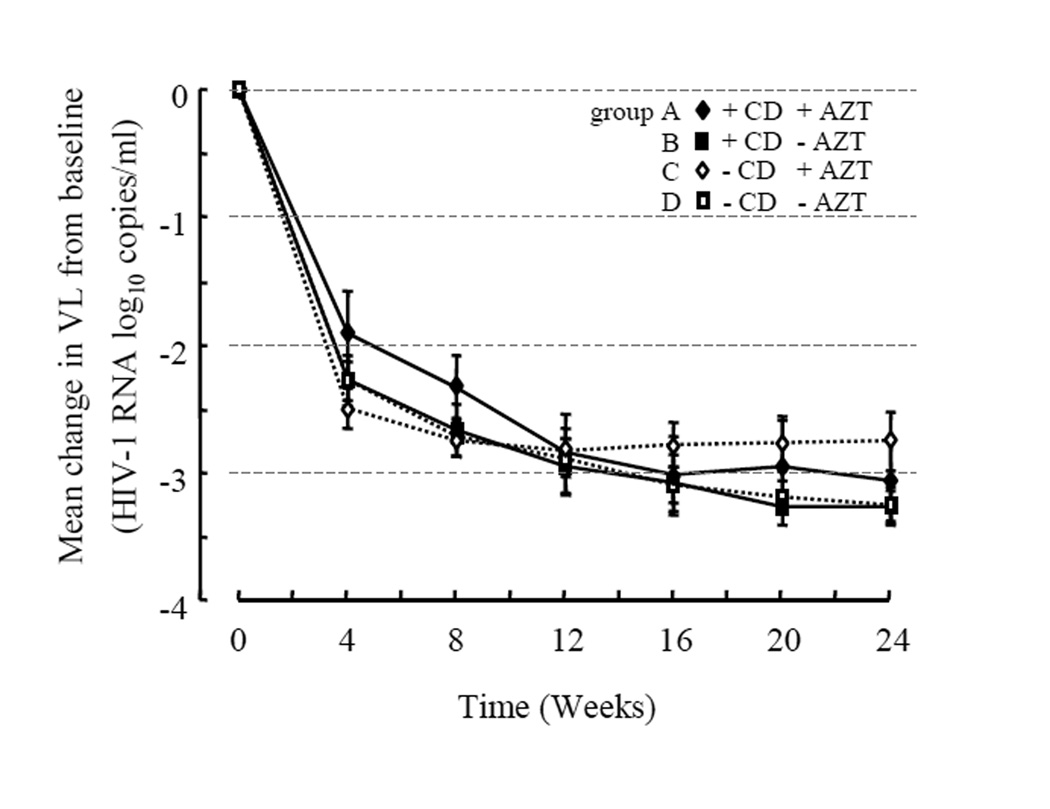
To further assess whether connection subdomain (CD) substitutions at baseline are one of predictive factors of virologic outcome, we examined clinical samples from the treatment-naïve patients who subsequently received combination therapy through measuring virus load in plasma from 0 to 24 weeks (Table 3 and Fig. 1). The treatment-naïve patients were classified in four groups; (A) patients who were infected by HIV-1 that carried one or two of the CD substitutions E312Q, G333E, G335D, V365I, A371V or A376S and who subsequently received combination therapy that contained AZT (n=8); (B) patients who were infected by HIV-1 that carried the above CD substitutions and who subsequently received combination therapy that did not contain AZT (n=13); (C) patients who were infected by HIV-1 that did not carry any of the above CD substitutions and who subsequently received combination therapy containing AZT (n=16); and (D) patients who were infected by HIV-1 that did not carry the above CD substitutions and who subsequently received combination therapy that did not contain AZT (n=24). The mean change in viral load from baseline (week 0) to week 24 was from −2.76 to −3.28 log10 copies/ml among four groups. There were no significant differences in viral load changes up to 24 weeks among these groups (Figure 1). Marginal viral suppression was observed in one patient who was infected by HIV-1 carrying A376S and who received combination therapy containing AZT. Any of the drug-associated resistant mutations were detected during the first 5 months of receiving combination therapy. However, HIV-1 protease mutations D30N and M36I that are responsible for resistance to NFV and HIV-1 RT D67N mutation that is responsible for AZT resistance eventually emerged. After switching to a new combination regimen (d4T/3TC/LPV), the viral load was successfully declined. These results indicate that at least combination of two substitutions in the connection subdomain that are observed in treatment-naïve patients do not affect the virologic response of the ensuing combination therapy. Instead, they merely act as polymorphisms among the treatment-naïve patients.
Table 3.
Profiles of patients infected with HIV carrying connection subdomain substitutions, and initial therapies used in patient treatments
| Combination for treatment-naïve patients infected HIV-1 |
||||
|---|---|---|---|---|
| Parameter | With substitutions |
Without substitutions |
||
| With AZT n=8 |
Without AZT n=13 |
With AZT n=16 |
Without AZT n=24 |
|
| Male, n (%) | 5 (63) | 10 (77) | 15 (94) | 23 (96) |
| Median age (range) | 37 (27–60) | 41 (27–54) | 36 (24–55) | 38 (26–59) |
| Median baseline viral load, log10 copies/ml (range) | 5.0 (3.0–6.0) | 5.0 (4.2–5.8) | 5.0 (4.1–6.4) | 5.2 (4.2–6.3) |
| Median baseline CD4 cell count, cell/µl (range) | 217 (3–549) | 110 (3–332) | 225 (9–613) | 170 (4–760) |
| Substitutions in the connection subdomain, n (%)a | ||||
| E312Q | - | 3 (23) | - | - |
| G333E | 2 (25) | 2 (15) | - | - |
| G335D | 3 (38) | 6 (46) | - | - |
| V365I | 2 (25) | - | - | - |
| A371V | 2 (25) | 5 (38) | - | - |
| A376S | 1 (13)b | 2 (15) | - | - |
| Initial therapy, n (%) | ||||
| Zidovudine | 8 (100) | - | 16 (100) | - |
| Lamivudine | 4 (50) | 11 (85) | 12 (75) | 24 (100) |
| Stavudine | - | 11 (85) | - | 20 (83) |
| Didanosine | 4 (50) | - | 4 (25) | - |
| Abacavir | 1 (13) | 1 (8) | 1 (6) | 3 (13) |
| Tenofovir | - | 1 (8) | - | 1 (4) |
| Emtricitabine | - | 1 (8) | - | - |
| Nevirapine | - | - | - | 3 (13) |
| Efavirenz | 2 (25) | 3 (23) | 9 (56) | 9 (38) |
| One protease inhibitor (PI) | 3 (38) | 7 (54) | 5 (31) | 7 (29) |
| Dual-boosted PI | 1(13) | 2 (15) | 1 (6) | 5 (21) |
E312Q, G333E, G335D, V365I, A371V and V376S were reported to be AZT-resistant mutations (Brehm et al., 2007; Kemp et al., 1998; Nikolenko et al., 2007)
This case observed not to achieve a viral load below the limits of detection by the initial combination therapy containing AZT.
Figure 1. Virological response up to 24 weeks after initiation of combination therapy.
Mean (± standard error of the mean; SEM) changes in plasma viral load (VL) were measured by Amplicor HIV-1 Monitor Test (Version 1.5, Roche Diagnostics K.K., Basel, Switzerland) from 0 to 24 weeks. Treatment-naive patients that were subsequently treated with combination therapy regimens are classified into 4 groups: patients that were infected with HIV-1 containing connection subdomain (CD) mutations and that subsequently received either combination therapy with AZT (n=8, closed diamonds, group A) or without AZT (n=13, closed squares, group B) and patients who were infected with HIV-1 with none of connection subdomain substitutions, and who subsequently received combination therapy either with AZT (n=16, open diamonds with broken line, group C) or without AZT (n=24, open squares with broken line, group D).
4. DISCUSSION
According to the crystal structure of HIV-1 RT in complex with RNA/DNA, some amino acids in the connection subdomain may affect binding to the RNA/DNA substrate (Sarafianos et al., 2001). It has been proposed that mutations at the connection subdomain may alter the binding affinity for nucleic acid at the connection subdomain and lead to enhanced resistance to AZT when combined with EEMs. This is thought to happen through a decrease in template RNA degradation which in turn provides additional time for RT to excise AZT-MP from the AZT-terminated template-primerAZT-MP,, thus causing resistance to AZT (Delviks-Frankenberry et al., 2007; Nikolenko et al., 2007; Nikolenko et al., 2005). In our cohort, as well as in the Stanford HIV Drug Resistance Database, we observed a considerable number of treatment-naïve clinical samples containing substitutions (E312Q, G333E, G335D, V365I, A371V and A376S) that have been previously associated with AZT resistance. Our phenotypic studies with clinical isolates carrying mutations located in the connection subdomain of the polymerase or in the RNase H domain of RT revealed that in the absence of other known RTI resistance mutations they do not cause by themselves significant resistance to the tested RTIs. Additionally, results from our cohort establish that the presence of G333E, G335D, V365I or A371V among treatment-naïve patients does not play any significant role in the virologic response after initiation of therapies that may, or may not include AZT. We identified 25 isolates that have been deposited before 1986, prior to clinical trails for AZT in the Los Alamos HIV Sequence Database (http://www.hiv.lanl.gov/content/index). Some of these isolates also contain E312V, V365I, A376S/T/P, indicating that at least these substitutions are polymorphisms that preceded any antiviral therapy.
None of the isolates in our cohort had the H539N or H549N substitutions which have been proposed to be associated with resistance to NRTIs due to decreasing the frequency of RT template-switching and the level of RNase H activity (Nikolenko et al., 2004; Roquebert and Marcelin, 2008). Furthermore, the G333D, G335C, N348I, A360I/V and Q509L substitutions were not observed in our cohort, and were also rarely observed among treatment-naïve patients (less than 1%) in the Stanford HIV Drug Resistance Database. Their increased incidence among NRTI-treated patients as compared to untreated patients (>3-fold, >40-fold and >12-fold increases for G333C, N348I, and A360V respectively [http://hivdb.stanford.edu/cgi-bin/RTPosMutSummary.cgi]) and in the case of Q509L reported by others (Brehm et al., 2007; Roquebert et al., 2007) suggest that they are associated with AZT-resistance. However, site directed mutagenesis studies showed that G333D (Kemp et al., 1998), G335C (Nikolenko et al., 2007), A360I/V (Nikolenko et al., 2007) and Q509L (Brehm et al., 2007) did not confer significant AZT resistance in the absence of other AZT resistance mutations. At present, only N348I has been shown to be involved in resistance to multiple RTIs (Hachiya et al., 2008; Yap et al., 2007). HIV with a serine at codon 376 also exhibits some NVP resistance in the absence of other mutations (Table 2). However, clinical isolates harboring different residues at position 376 exhibited no significant changes in their drug susceptibilities (Table 1). This discrepancy may arise from strain-specific polymorphisms that are present in the clinical isolates or the reference virus used in this study that may influence NVP susceptibility positively or negatively, respectively. In fact, we observe several polymorphisms in the majority of these isolates and it is possible that they somehow affect drug resistance. For instance, V118I has been identified in 2% of treatment-naïve patients as one of strain-specific polymorphisms, but more frequently observed in RTI-treated patients (Delaugerre et al., 2001). Although this mutation by itself confers no resistance, it has been reported to contribute to hypersusceptibility to NNRTI (Clark et al., 2006) as well as resistance to NRTI in the presence of E44A/D and/or EEMs (Romano et al., 2002). Therefore, it is possible that polymorphisms present in our clinical isolates may also affect drug-susceptibility leading to minor discrepancies with the results with recombinant virus.
In this study, the reference clone has an A376T polymorphism that is observed in a wide range of subtypes. Therefore, it is unlikely that A376T affects NVP susceptibility. Q509L confers moderate (~9fold) resistance to NVP (Table 2). Although Q509L was not observed in our cohort, this mutation was found in the pretreated patients of another survey (n=118) (Roquebert et al., 2007). These results indicate that introduction of Q509L may alter virologic responses, especially for NVP, although so far the clinical relevance and virological response of Q509L among the antiretroviral-experienced patients remains to be elucidated by further experiments.
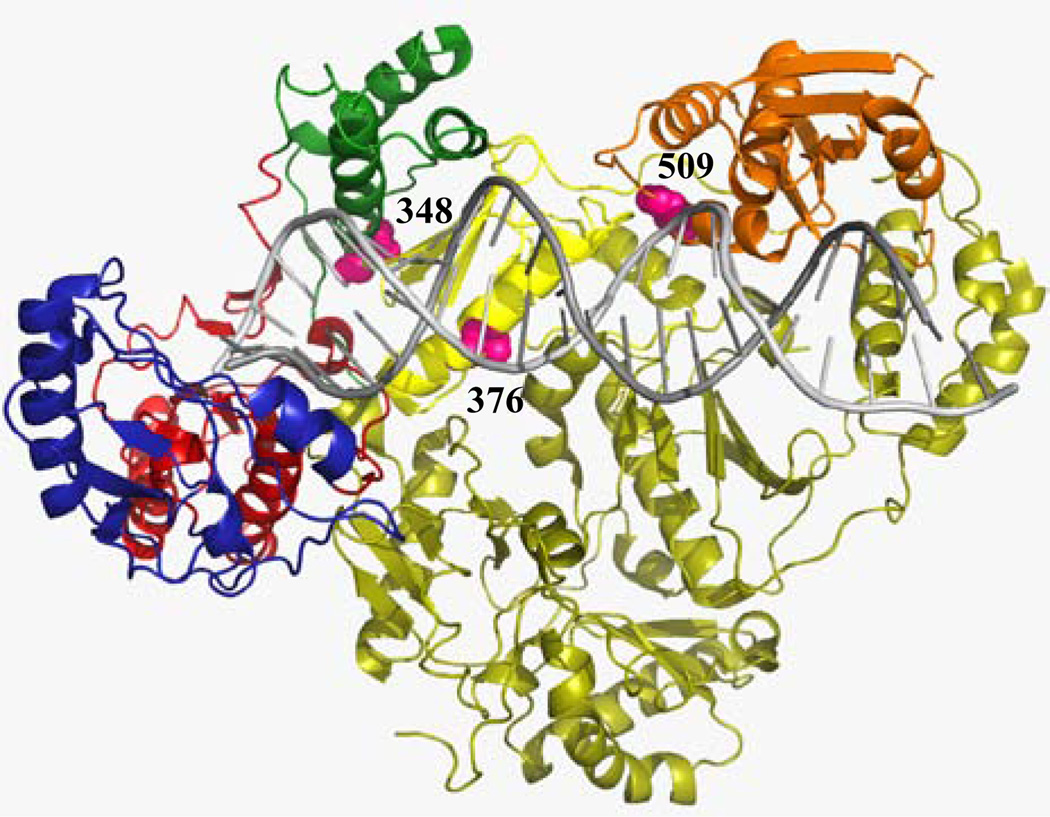
Analysis of the crystal structure of RT bound to RNA/DNA showed that residues 376 (of the p66 subunit) and 509 are located relatively close to the nucleic-acid binding cleft of RT, and residue 348 of the p66 subunit is located close to the hinge region of the thumb subdomain and to the NNRTI binding pocket (Fig. 2). Recently, Abbondanzieri et al. demonstrated that binding of nevirapine to RT causes conformational changes to the enzyme, allowing it to somehow relax the grip on nucleic-acid substrate (Abbondanzieri et al., 2008; Arnold and Sarafianos, 2008) . NVP acts as a rapid-equilibrium inhibitor, not a tight-binding inhibitor as EFV (Maga et al., 2000; Motakis and Parniak, 2002), and it might be more sensitive to changes in the interaction between RT and the nucleic acid substrate. Thus, changes in the interactions of RT with nucleic-acid substrate could also influence the interaction balance between polymerase and RNase H activity and consequently might lead to RTI resistance. Nevertheless, additional biochemical and structural studies are warranted to define the exact mechanisms by which these mutations in the connection subdomain and RNase H domains confer NVP resistance.
Figure 2. Structure of HIV-1 RT in complex with RNA/DNA.
The fingers, palm, thumb, connection subdomains, and RNase H domain of the p66 subunit colored in blue, red, green, yellow and orange, respectively. The p51 subunit is shown in dark yellow. Residue 348 of the p66 subunit is shown as pink Van der Waals spheres, and located proximal to the hinge region of the thumb subdomain and to the NNRTI binding pocket. Residues 376 and 509 of the p66 subunit are also shown, and are located proximal to the nucleic acid binding cleft.
Several studies have reported a correlation between two distinct types of EEMs in various HIV subtypes (Kantor et al., 2005; Montes et al., 2004; Novitsky et al., 2007). The Type I EEMs (M41L, L210W, T215Y and occasionally the D67N mutation) appear twice as frequently as Type II EEMs (D67N, K70R, T215F and K219Q mutation) in subtype B (Marcelin et al., 2004), whereas Type II EEMs are mostly observed in non-B isolates (Montes et al., 2004; Novitsky et al., 2007). Type II EEMs confer lower level of AZT and TNF/PMPA resistance, as compared to Type I EEMs (Cozzi-Lepri et al., 2005; Miller et al., 2004). Addition of A371V to Type II EEM background conferred cross-resistance to AZT and tenofovir (Brehm et al., 2007). A371V was observed in the majority of non-B isolates in our cohort (75%) and the Stanford HIV Drug Resistance Database (96% in CRF01_AE). Therefore, it is possible that in the background of non-B isolates, the majority of which contains drug resistance associated connection subdomain mutations, smaller number of EEMs, especially Type II EEMs, might be preferentially selected for AZT and TNF/PMPA resistance. In the absence of EEMs, mutations at the connection subdomain of non-subtype B HIV, such as E312N, G335E or A376V, appear to act as simple polymorphisms, because they either maintain or enhance drug susceptibility in non-subtypes B HIV (Table 1). However, the A376S polymorphism in samples of treatment-naive patients or in a recombinant virus used in this study conferred mild NVP resistance (Table 2). These mutations were stable even in the absence of any drug treatment, suggesting that viral fitness of these variants is likely to be comparable to wild type non-subtype B HIV.
In this study we report the prevalence of amino acid substitutions in the connection subdomain of the polymerase domain and in the RNase H domain of RT in a cohort of treatment-naïve patients. We also determined the phenotypic susceptibility of these mutants to various RTIs. Our results support the hypothesis that the substitutions observed among treatment-naïve patients have little impact on therapeutic outcome by themselves in the absence of AZT-associated mutations, although certain substitutions, such as N348I, A376S, and Q509L, are involved in drug resistance even by themselves. These results may help improve existing interpretation algorithms and analysis of drug resistance mutations.
ACKNOWLEDGEMENTS
This work was supported by a grant for the promotion of AIDS Research from the Ministry of Health, Labor and Welfare (AH, EK, MM, MT, and SO), a grant for Research for Health Sciences Focusing on Drug Innovation from The Japan Health Sciences Foundation (EK and MM), and by National Institute of Health (NIH) research grants AI076119, AI079801, and AI074389 to SGS. The authors thank Yukiko Takahashi for sample preparation.
REFERENCES
- Abbondanzieri EA, Bokinsky G, Rausch JW, Zhang JX, Le Grice SF, Zhuang X. Dynamic binding orientations direct activity of HIV reverse transcriptase. Nature. 2008;453(7192):184–189. doi: 10.1038/nature06941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold E, Sarafianos SG. Molecular biology: an HIV secret uncovered. Nature. 2008;453(7192):169–170. doi: 10.1038/453169b. [DOI] [PubMed] [Google Scholar]
- Boyer PL, Sarafianos SG, Arnold E, Hughes SH. Selective excision of AZTMP by drug-resistant human immunodeficiency virus reverse transcriptase. J Virol. 2001;75(10):4832–4842. doi: 10.1128/JVI.75.10.4832-4842.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm JH, Koontz D, Meteer JD, Pathak V, Sluis-Cremer N, Mellors JW. Selection of mutations in the connection and RNase H domains of human immunodeficiency virus type 1 reverse transcriptase that increase resistance to 3'-azido-3'-dideoxythymidine. J Virol. 2007;81(15):7852–7859. doi: 10.1128/JVI.02203-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caride E, Brindeiro R, Hertogs K, Larder B, Dehertogh P, Machado E, de Sa CA, Eyer-Silva WA, Sion FS, Passioni LF, Menezes JA, Calazans AR, Tanuri A. Drug-resistant reverse transcriptase genotyping and phenotyping of B and non-B subtypes (F and A) of human immunodeficiency virus type I found in Brazilian patients failing HAART. Virology. 2000;275(1):107–115. doi: 10.1006/viro.2000.0487. [DOI] [PubMed] [Google Scholar]
- Clark SA, Shulman NS, Bosch RJ, Mellors JW. Reverse transcriptase mutations 118I, 208Y, and 215Y cause HIV-1 hypersusceptibility to non-nucleoside reverse transcriptase inhibitors. Aids. 2006;20(7):981–984. doi: 10.1097/01.aids.0000222069.14878.44. [DOI] [PubMed] [Google Scholar]
- Cozzi-Lepri A, Ruiz L, Loveday C, Phillips AN, Clotet B, Reiss P, Ledergerber B, Holkmann C, Staszewski S, Lundgren JD. Thymidine analogue mutation profiles: factors associated with acquiring specific profiles and their impact on the virological response to therapy. Antivir Ther. 2005;10(7):791–802. [PubMed] [Google Scholar]
- Delaugerre C, Mouroux M, Yvon-Groussin A, Simon A, Angleraud F, Huraux JM, Agut H, Katlama C, Calvez V. Prevalence and conditions of selection of E44D/A and V118I human immunodeficiency virus type 1 reverse transcriptase mutations in clinical practice. Antimicrob Agents Chemother. 2001;45(3):946–948. doi: 10.1128/AAC.45.3.946-948.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delviks-Frankenberry KA, Nikolenko GN, Barr R, Pathak VK. Mutations in human immunodeficiency virus type 1 RNase H primer grip enhance 3'-azido-3'-deoxythymidine resistance. J Virol. 2007;81(13):6837–6845. doi: 10.1128/JVI.02820-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delviks-Frankenberry KA, Nikolenko GN, Boyer PL, Hughes SH, Coffin JM, Jere A, Pathak VK. HIV-1 reverse transcriptase connection subdomain mutations reduce template RNA degradation and enhance AZT excision. Proc Natl Acad Sci U S A. 2008;105(31):10943–10948. doi: 10.1073/pnas.0804660105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descamps D, Chaix ML, Andre P, Brodard V, Cottalorda J, Deveau C, Harzic M, Ingrand D, Izopet J, Kohli E, Masquelier B, Mouajjah S, Palmer P, Pellegrin I, Plantier JC, Poggi C, Rogez S, Ruffault A, Schneider V, Signori-Schmuck A, Tamalet C, Wirden M, Rouzioux C, Brun-Vezinet F, Meyer L, Costagliola D. French national sentinel survey of antiretroviral drug resistance in patients with HIV-1 primary infection and in antiretroviral-naive chronically infected patients in 2001–2002. J Acquir Immune Defic Syndr. 2005;38(5):545–552. doi: 10.1097/01.qai.0000155201.51232.2e. [DOI] [PubMed] [Google Scholar]
- Deval J, Selmi B, Boretto J, Egloff MP, Guerreiro C, Sarfati S, Canard B. The molecular mechanism of multidrug resistance by the Q151M human immunodeficiency virus type 1 reverse transcriptase and its suppression using alpha-boranophosphate nucleotide analogues. J Biol Chem. 2002;277(44):42097–42104. doi: 10.1074/jbc.M206725200. [DOI] [PubMed] [Google Scholar]
- Ehteshami M, Beilhartz GL, Scarth BJ, Tchesnokov EP, McCormick S, Wynhoven B, Harrigan PR, Gotte M. Connection domain mutations N348I and A360V in HIV-1 reverse transcriptase enhance resistance to 3'-azido-3'-deoxythymidine through both RNase H-dependent and -independent mechanisms. J Biol Chem. 2008;283(32):22222–22232. doi: 10.1074/jbc.M803521200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego O, Corral A, de Mendoza C, Rodes B, Soriano V. Prevalence of G333D/E in naive and pretreated HIV-infected patients. AIDS Res Hum Retroviruses. 2002;18(12):857–860. doi: 10.1089/08892220260190335. [DOI] [PubMed] [Google Scholar]
- Gatanaga H, Ibe S, Matsuda M, Yoshida S, Asagi T, Kondo M, Sadamasu K, Tsukada H, Masakane A, Mori H, Takata N, Minami R, Tateyama M, Koike T, Itoh T, Imai M, Nagashima M, Gejyo F, Ueda M, Hamaguchi M, Kojima Y, Shirasaka T, Kimura A, Yamamoto M, Fujita J, Oka S, Sugiura W. Drug-resistant HIV-1 prevalence in patients newly diagnosed with HIV/AIDS in Japan. Antiviral Res. 2007;75(1):75–82. doi: 10.1016/j.antiviral.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Hachiya A, Aizawa-Matsuoka S, Tanaka M, Takahashi Y, Ida S, Gatanaga H, Hirabayashi Y, Kojima A, Tatsumi M, Oka S. Rapid and simple phenotypic assay for drug susceptibility of human immunodeficiency virus type 1 using CCR5-expressing HeLa/CD4(+) cell clone 1–10 (MAGIC-5) Antimicrob Agents Chemother. 2001;45(2):495–501. doi: 10.1128/AAC.45.2.495-501.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachiya A, Kodama EN, Sarafianos SG, Schuckmann MM, Sakagami Y, Matsuoka M, Takiguchi M, Gatanaga H, Oka S. Amino acid mutation N348I in the connection subdomain of human immunodeficiency virus type 1 reverse transcriptase confers multiclass resistance to nucleoside and nonnucleoside reverse transcriptase inhibitors. J Virol. 2008;82(7):3261–3270. doi: 10.1128/JVI.01154-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor R, Katzenstein DA, Efron B, Carvalho AP, Wynhoven B, Cane P, Clarke J, Sirivichayakul S, Soares MA, Snoeck J, Pillay C, Rudich H, Rodrigues R, Holguin A, Ariyoshi K, Bouzas MB, Cahn P, Sugiura W, Soriano V, Brigido LF, Grossman Z, Morris L, Vandamme AM, Tanuri A, Phanuphak P, Weber JN, Pillay D, Harrigan PR, Camacho R, Schapiro JM, Shafer RW. Impact of HIV-1 subtype and antiretroviral therapy on protease and reverse transcriptase genotype: results of a global collaboration. PLoS Med. 2005;2(4):e112. doi: 10.1371/journal.pmed.0020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp SD, Shi C, Bloor S, Harrigan PR, Mellors JW, Larder BA. A novel polymorphism at codon of human immunodeficiency virus type 1 reverse transcriptase can facilitate dual resistance to zidovudine and L-2',3'-dideoxy-3'-thiacytidine. J Virol. 1998;72(6):5093–5098. doi: 10.1128/jvi.72.6.5093-5098.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little SJ, Holte S, Routy JP, Daar ES, Markowitz M, Collier AC, Koup RA, Mellors JW, Connick E, Conway B, Kilby M, Wang L, Whitcomb JM, Hellmann NS, Richman DD. Antiretroviral-drug resistance among patients recently infected with HIV. N Engl J Med. 2002;347(6):385–394. doi: 10.1056/NEJMoa013552. [DOI] [PubMed] [Google Scholar]
- Maga G, Ubiali D, Salvetti R, Pregnolato M, Spadari S. Selective interaction of the human immunodeficiency virus type 1 reverse transcriptase nonnucleoside inhibitor efavirenz and its thio-substituted analog with different enzyme-substrate complexes. Antimicrob Agents Chemother. 2000;44(5):1186–1194. doi: 10.1128/aac.44.5.1186-1194.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelin AG, Delaugerre C, Wirden M, Viegas P, Simon A, Katlama C, Calvez V. Thymidine analogue reverse transcriptase inhibitors resistance mutations profiles and association to other nucleoside reverse transcriptase inhibitors resistance mutations observed in the context of virological failure. J Med Virol. 2004;72(1):162–165. doi: 10.1002/jmv.10550. [DOI] [PubMed] [Google Scholar]
- Meyer PR, Matsuura SE, Mian AM, So AG, Scott WA. A mechanism of AZT resistance: an increase in nucleotide-dependent primer unblocking by mutant HIV-1 reverse transcriptase. Mol Cell. 1999;4(1):35–43. doi: 10.1016/s1097-2765(00)80185-9. [DOI] [PubMed] [Google Scholar]
- Miller MD, Margot N, Lu B, Zhong L, Chen SS, Cheng A, Wulfsohn M. Genotypic and phenotypic predictors of the magnitude of response to tenofovir disoproxil fumarate treatment in antiretroviral-experienced patients. J Infect Dis. 2004;189(5):837–846. doi: 10.1086/381784. [DOI] [PubMed] [Google Scholar]
- Montes B, Vergne L, Peeters M, Reynes J, Delaporte E, Segondy M. Comparison of drug resistance mutations and their interpretation in patients infected with non-B HIV-1 variants and matched patients infected with HIV-1 subtype B. J Acquir Immune Defic Syndr. 2004;35(4):329–336. doi: 10.1097/00126334-200404010-00001. [DOI] [PubMed] [Google Scholar]
- Motakis D, Parniak MA. A tight-binding mode of inhibition is essential for anti-human immunodeficiency virus type 1 virucidal activity of nonnucleoside reverse transcriptase inhibitors. Antimicrob Agents Chemother. 2002;46(6):1851–1856. doi: 10.1128/AAC.46.6.1851-1856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolenko GN, Delviks-Frankenberry KA, Palmer S, Maldarelli F, Fivash MJ, Jr, Coffin JM, Pathak VK. Mutations in the connection domain of HIV-1 reverse transcriptase increase 3'-azido-3'-deoxythymidine resistance. Proc Natl Acad Sci U S A. 2007;104(1):317–322. doi: 10.1073/pnas.0609642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolenko GN, Palmer S, Maldarelli F, Mellors JW, Coffin JM, Pathak VK. Mechanism for nucleoside analog-mediated abrogation of HIV-1 replication: balance between RNase H activity and nucleotide excision. Proc Natl Acad Sci U S A. 2005;102(6):2093–2098. doi: 10.1073/pnas.0409823102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolenko GN, Svarovskaia ES, Delviks KA, Pathak VK. Antiretroviral drug resistance mutations in human immunodeficiency virus type 1 reverse transcriptase increase template-switching frequency. J Virol. 2004;78(16):8761–8770. doi: 10.1128/JVI.78.16.8761-8770.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novitsky V, Wester CW, DeGruttola V, Bussmann H, Gaseitsiwe S, Thomas A, Moyo S, Musonda R, Van Widenfelt E, Marlink RG, Essex M. The reverse transcriptase 67N 70R 215Y genotype is the predominant TAM pathway associated with virologic failure among HIV type 1C-infected adults treated with ZDV/ddI-containing HAART in southern Africa. AIDS Res Hum Retroviruses. 2007;23(7):868–878. doi: 10.1089/aid.2006.0298. [DOI] [PubMed] [Google Scholar]
- Ntemgwa M, Wainberg MA, Oliveira M, Moisi D, Lalonde R, Micheli V, Brenner BG. Variations in reverse transcriptase and RNase H domain mutations in human immunodeficiency virus type 1 clinical isolates are associated with divergent phenotypic resistance to zidovudine. Antimicrob Agents Chemother. 2007;51(11):3861–3869. doi: 10.1128/AAC.00646-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin NT, Hellmann NS, Whitcomb JM, Kiss L, Chappey C, Petropoulos CJ. Natural variation of drug susceptibility in wild-type human immunodeficiency virus type 1. Antimicrob Agents Chemother. 2004;48(2):437–443. doi: 10.1128/AAC.48.2.437-443.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SY, Taylor J, Wadhera G, Ben-Hur A, Brutlag DL, Shafer RW. Genotypic predictors of human immunodeficiency virus type 1 drug resistance. Proc Natl Acad Sci U S A. 2006;103(46):17355–17360. doi: 10.1073/pnas.0607274103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano L, Venturi G, Bloor S, Harrigan R, Larder BA, Major JC, Zazzi M. Broad nucleoside-analogue resistance implications for human immunodeficiency virus type 1 reverse-transcriptase mutations at codons 44 and 118. J Infect Dis. 2002;185(7):898–904. doi: 10.1086/339706. [DOI] [PubMed] [Google Scholar]
- Roquebert B, Marcelin AG. The involvement of HIV-1 RNAse H in resistance to nucleoside analogues. J Antimicrob Chemother. 2008;61(5):973–975. doi: 10.1093/jac/dkn060. [DOI] [PubMed] [Google Scholar]
- Roquebert B, Wirden M, Simon A, Deval J, Katlama C, Calvez V, Marcelin AG. Relationship between mutations in HIV-1 RNase H domain and nucleoside reverse transcriptase inhibitors resistance mutations in naive and pre-treated HIV infected patients. J Med Virol. 2007;79(3):207–211. doi: 10.1002/jmv.20788. [DOI] [PubMed] [Google Scholar]
- Santos AF, Lengruber RB, Soares EA, Jere A, Sprinz E, Martinez AM, Silveira J, Sion FS, Pathak VK, Soares MA. Conservation Patterns of HIV-1 RT Connection and RNase H Domains: Identification of New Mutations in NRTI-Treated Patients. PLoS ONE. 2008;3(3):e1781. doi: 10.1371/journal.pone.0001781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarafianos SG, Das K, Tantillo C, Clark AD, Jr, Ding J, Whitcomb JM, Boyer PL, Hughes SH, Arnold E. Crystal structure of HIV-1 reverse transcriptase in complex with a polypurine tract RNA:DNA. Embo J. 2001;20(6):1449–1461. doi: 10.1093/emboj/20.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimura K, Kodama E, Sakagami Y, Matsuzaki Y, Watanabe W, Yamataka K, Watanabe Y, Ohata Y, Doi S, Sato M, Kano M, Ikeda S, Matsuoka M. Broad antiretroviral activity and resistance profile of the novel human immunodeficiency virus integrase inhibitor elvitegravir (JTK-303/GS-9137) J Virol. 2008;82(2):764–774. doi: 10.1128/JVI.01534-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno T, Mitsuya H. Comparative enzymatic study of HIV-1 reverse transcriptase resistant to 2',3'-dideoxynucleotide analogs using the single-nucleotide incorporation assay. Biochemistry. 1997;36(5):1092–1099. doi: 10.1021/bi962393d. [DOI] [PubMed] [Google Scholar]
- UK_Collaborative_Group_on_Monitoring_the_Transmission_of_HIV_Drug_Resistance. Analysis of prevalence of HIV-1 drug resistance in primary infections in the United Kingdom. Bmj. 2001;322(7294):1087–1088. doi: 10.1136/bmj.322.7294.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock HS, Zaidi I, Heneine W, Bennett D, Garcia-Lerma JG, Douglas JM, Jr, LaLota M, Dickinson G, Schwarcz S, Torian L, Wendell D, Paul S, Goza GA, Ruiz J, Boyett B, Kaplan JE. The epidemiology of antiretroviral drug resistance among drug-naive HIV-1-infected persons in 10 US cities. J Infect Dis. 2004;189(12):2174–2180. doi: 10.1086/420789. [DOI] [PubMed] [Google Scholar]
- Yap SH, Sheen CW, Fahey J, Zanin M, Tyssen D, Lima VD, Wynhoven B, Kuiper M, Sluis-Cremer N, Harrigan PR, Tachedjian G. N348I in the connection domain of HIV-1 reverse transcriptase confers zidovudine and nevirapine resistance. PLoS Med. 2007;4(12):e335. doi: 10.1371/journal.pmed.0040335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelina S, Sheen CW, Radzio J, Mellors JW, Sluis-Cremer N. Mechanisms by Which the G333D Mutation in Human Immunodeficiency Virus Type 1 Reverse Transcriptase Facilitates Dual Resistance to Zidovudine and Lamivudine. Antimicrob Agents Chemother. 2008;52(1):157–163. doi: 10.1128/AAC.00904-07. [DOI] [PMC free article] [PubMed] [Google Scholar]