Abstract
The first total synthesis of (+)-merobatzelladine B was accomplished using an iterative sequence of stereoselective Pd-catalyzed alkene carboamination reactions for formation of two of the three rings. This represents a new strategy for the generation of polycyclic guanidine natural products, and provides access to compounds with a syn-relationship between the C6 H-atom and the C8 alkyl group.
Keywords: Alkaloids, Natural Products, Asymmetric Synthesis, Palladium, Stereoselective
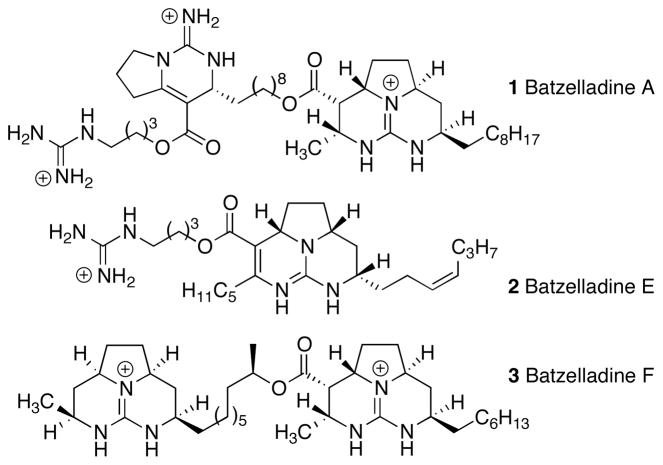
Polycyclic guanidine natural products, such as batzelladines A, E, and F, exhibit a rich and diverse array of interesting biological activities (Figure 1).[1,2] Some polycyclic guanidine alkaloids have been shown to inhibit protein-protein interactions, including binding of HIV gp120 to CD4 on human T-cells. In addition, many polycyclic guanidines display potent antiviral, antimalarial, and immunosuppressive properties.
Figure 1.
Polycyclic guanidine natural products
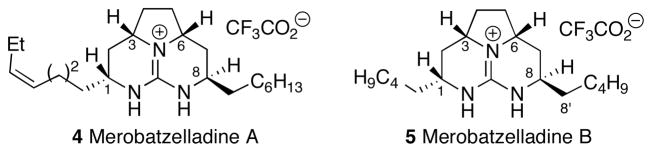
In 2009 Matsunaga et.al. reported the isolation of merobatzelladines A and B (4 and 5) from the marine sponge Monanchora sp. (Figure 2).[3] These compounds are members of a new sub-class of the batzelladine alkaloids that possess the signature tricyclic guanidine core common to all batzelladines, but display a unique stereochemical feature that differs from other members in this family. The C8 alkyl substituents in merobatzelladines A and B are positioned in a syn-relationship with the C6 hydrogen atoms, whereas other related natural products, such as batzelladines A, E, or F (1–3), contain an anti-relationship between these groups. Merobatzelladines A and B exhibit moderate antimicrobial activity against Vibrio anguillarum, and also show inhibitory activity against the K1 strain of Plasmodium falciparum (IC50 = 0.48 μg/mL and 0.97 μg/mL, respectively). Given the rich biological activity of the related batzelladine alkaloids, it is possible that merobatzelladines A and B may exhibit additional useful properties that have yet to be reported.
Figure 2.
Merobatzelladine alkaloids
Given the importance of polycyclic guanidine alkaloids, several different approaches have been employed for the synthesis of these compounds. The most widely utilized routes typically generate the fused ring system through condensation reactions,[4] cycloaddition reactions,[5] radical cyclizations,[6] and substitution reactions.[7] Although these routes have proven highly useful, none provide a means for generation of a C–C bond adjacent to the ring (such as the C8′–C4H9 bond in 5) during the ring-closing event. In addition, none of these routes have been employed for the generation of molecules with the syn-relationship between the C8 alkyl group and the C6 H-atom such as that displayed in merobatzelladines A and B. In this communication we describe the first total synthesis of merobatzelladine B (5), which provides the natural product as a single stereoisomer in high optical purity, and represents a new strategy for the construction of polycyclic guanidine alkaloids.
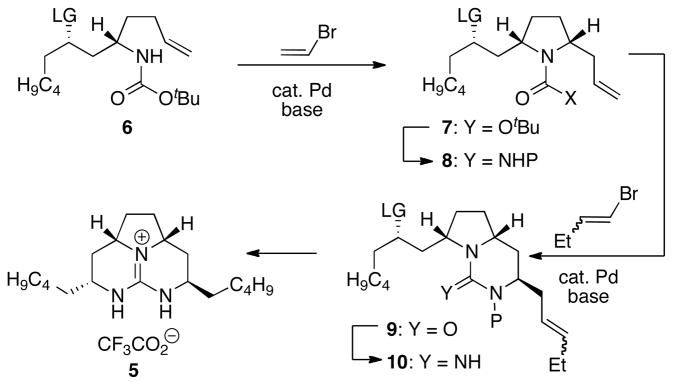
Our approach to the synthesis of merobatzelladine B was centered around the use of Pd-catalyzed alkene carboamination reactions for the formation of two of the three rings in the natural product.8 As shown in Scheme 1, we envisioned that a Pd-catalyzed carboamination between vinyl bromide and an appropriately functionalized γ-aminoalkene derivative 6 would generate cis-disubstituted pyrrolidine 7 with high stereocontrol. A second carboamination reaction between allylpyrrolidine derivative 8 and 1-bromo-1-butene would afford bicyclic product 9, which could then be transformed to the polycyclic guanidine natural product 5 through functional group interconversion and ring-closure via an intramolecular SN2 reaction.
Scheme 1.
Iterative carboamination strategy for polycyclic guanidine synthesis.
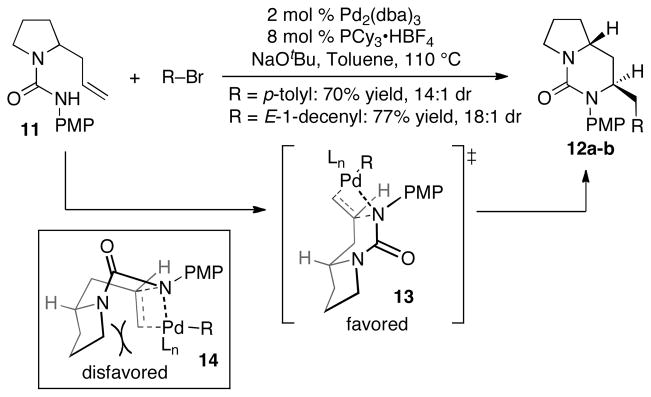
Our prior studies on Pd-catalyzed alkene carboamination reactions have illustrated that the conversion of N-boc-γ-aminoalkenes to 2,5-disubstituted pyrrolidines typically proceeds in good yield with > 20:1 diastereoselectivity favoring the cis-isomer.[8,9] As such, the transformation of 6 to 7 appeared quite feasible; however, the likelihood of success in the planned Pd-catalyzed carboamination between 8 and an alkenyl halide was less clear. The generation of six-membered rings via Pd-catalyzed carboamination is considerably more difficult than formation of five-membered rings,[10] and this has not previously been accomplished with an unsaturated urea substrate.[11] To test the feasibility of this key transformation, we examined the Pd-catalyzed carboamination of 2-allyl-pyrrolidine-derived urea 11 with simple aryl and alkenyl halides. After optimization of conditions, we found that a catalyst composed of Pd2(dba)3/PCy3 provided satisfactory results in these reactions (Scheme 2). The bicyclic urea products 12a–b were obtained in good yield and high diastereoselectivity, which may arise via cyclization through boat-like transition state 13.[10b,c,12] The alternative boat-like transition state 14, which leads to the minor diastereomer, appears to suffer from significant steric interactions between the alkene and the pyrrolidinyl ring. Moreover, cyclization through a chair-like transition state appears to be less accessible due to poor overlap between the alkene pi-system and the Pd–N bond.[10b,c]
Scheme 2.
Synthesis of bicyclic ureas via Pd-catalyzed carboamination.
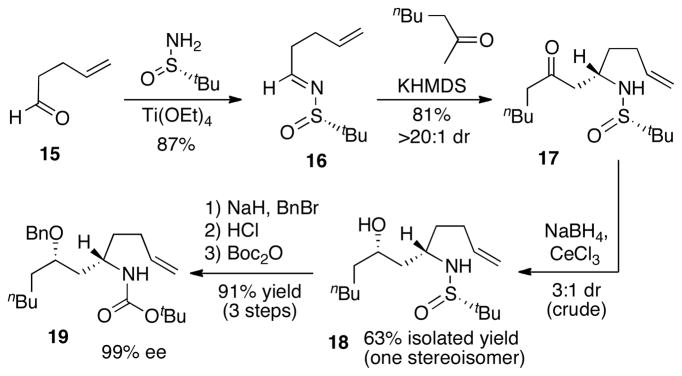
Having illustrated the feasibility of our approach to the generation of fused bicyclic ureas, we undertook the synthesis of merobatzelladine B by constructing an appropriately functionalized γ-aminoalkene derivative for the pyrrolidine-forming carboamination. As shown in Scheme 3, the amine-bearing stereocenter was generated via a highly efficient asymmetric Mannich reaction of sulfinyl imine 16.[13] The stereocontrolled reduction of ketone 17 proved quite challenging,[14] and after examining many different reducing agents we found that the combination of NaBH4 and CeCl3 led to formation of 18 with 3:1 diastereoselectivity. However, the two diastereomers were separable by column chromatography, and 18 was isolated as a single stereoisomer in 63% yield. Protection of the alcohol as a benzyl ether followed by exchange of the sulfinyl group for a boc-group provided 19 in 91% yield over three steps and 99% ee.
Scheme 3.
Synthesis of γ-aminoalkene 19.
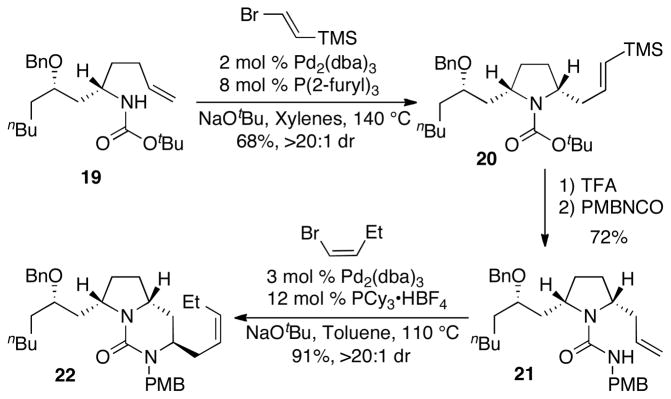
With intermediate 19 in hand, the key sequence of carboamination reactions was undertaken (Scheme 4). The Pd/P(2-furyl)3-catalyzed carboamination of 19 with E-2-bromovinyltrimethylsilane provided pyrrolidine 20 in 68% yield and with excellent stereocontrol (>20:1 dr).[15] Treatment of 20 with TFA led to cleavage of the boc group and protodesilylation of the alkene. The resulting pyrrolidine was coupled with p-methoxybenzylisocyanate to generate pyrrolidinyl urea 21 in 72% yield over the two-step sequence.[16] The Pd/PCy3-catalyzed carboamination of 21 with Z-1-bromo-1-butene proceeded smoothly to yield bicyclic urea 22 in 91% yield and >20:1 dr.
Scheme 4.
Carboamination reaction sequence for bicyclic urea construction.
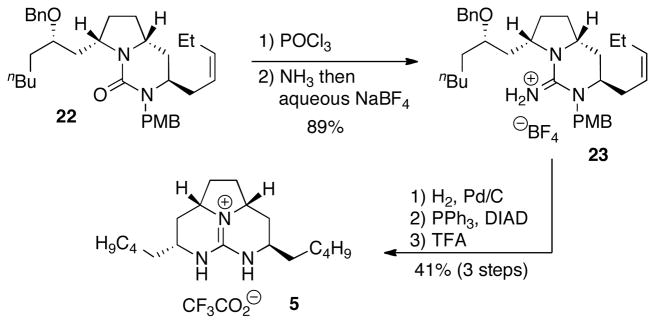
Bicyclic urea 22 was converted to guanidinium salt 23 in 89% yield by treatment with POCl3 followed by addition of ammonia (Scheme 5).[17] The tetrafluoroborate counterion was introduced during the workup procedure by washing a dichloromethane solution of the crude guanidine product with aqueous NaBF4. This anion exchange was essential to avoid complications during the subsequent ring-closing step.[18] Guanidinium salt 23 was then transformed to the natural product 5 in a three-step sequence involving initial hydrogenation with Pd/C to effect reduction of the alkene and cleavage of the benzyl ether protecting group. Ring-closure was achieved via an intramolecular Mitsunobu reaction,[7a] and deprotection of the N-PMB group provided merobatzelladine B (5) in 41% yield over the three step sequence from 23. The synthetic alkaloid was obtained in enantiopure form {[α]23D + 40.1 (c 0.7, MeOH) [lit.[3] [α]23D + 27 (c 0.15 MeOH)]}, and NMR spectra were identical to the data previously reported for the natural product.[3]
Scheme 5.
Completion of the synthesis.
In summary, we have developed the first asymmetric total synthesis of (+)-merobatzelladine B (5), which confirms the structural and stereochemical assignments of the natural product. Our route afforded the desired alkaloid in 15 steps and 6.7% overall yield from commercially available pent-4-enal (15). The results described above represent a fundamentally new strategy for the stereocontrolled synthesis of polycyclic guanidine natural products. This new approach allows for formation of a carbon-carbon bond during the ring-closing event, and is the first route shown to provide access to alkaloids with a syn-relationship between the C6 hydrogen atom and the C8 alkyl group. This strategy could potentially be employed to access other guanidine alkaloids that contain this stereochemical feature, and could also be used for the generation of novel analogs of the batzelladine alkaloids. In addition, this work also illustrates the feasibility of forming 5,6-fused bicyclic urea ring systems via Pd-catalyzed carboamination, which could be of value for preparation of other interesting biologically active heterocycles.
Supplementary Material
Acknowledgments
The authors acknowledge the NIH-NIGMS (GM 098314) and the University of Michigan Associate Professor Support Fund for financial support of this work. Additional funding was provided by GlaxoSmithKline and Amgen.
Footnotes
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
Contributor Information
Nicholas R. Babij, Department of Chemistry, University of Michigan, 930 N. University Ave, Ann Arbor, MI 48109-1055, USA
John P. Wolfe, Email: jpwolfe@umich.edu, Department of Chemistry, University of Michigan, 930 N. University Ave, Ann Arbor, MI 48109-1055, USA.
References
- 1.a) Berlinck RGS, Burtoloso ACB, Trindade-Silva AE, Romminger S, Morais RP, Bandeira K, Mizuno CM. Nat Prod Rep. 2010;27:1871–1907. doi: 10.1039/c0np00016g. [DOI] [PubMed] [Google Scholar]; b) Berlinck RGS, Burtoloso ACB, Kossuga MH. Nat Prod Rep. 2008;25:919–954. doi: 10.1039/b507874c. [DOI] [PubMed] [Google Scholar]; c) Berlinck RGS, Kossuga MH. Nat Prod Rep. 2005;22:516–550. doi: 10.1039/b209227c. [DOI] [PubMed] [Google Scholar]; d) Bewley CA, Ray S, Cohen F, Collins SK, Overman LE. J Nat Prod. 2004;67:1319–1324. doi: 10.1021/np049958o. [DOI] [PubMed] [Google Scholar]
- 2.a) Patil AD, Kumar NV, Kokke WC, Bean MF, Freyer AJ, De Brosse C, Mai S, Truneh A, Faulkner DJ, Carte B, Breen AL, Hertzberg RP, Johnson RK, Westley JW, Potts BCM. J Org Chem. 1995;60:1182–1188. [Google Scholar]; b) Snider BB, Chen J. Tetrahedron Lett. 1998;39:5697–5700. [Google Scholar]
- 3.a) Takishima S, Ishiyama A, Iwatsuki M, Otoguro K, Yamada H, Omura S, Kobayashi H, van Soest RWM, Matsunaga S. Org Lett. 2009;11:2655–2658. doi: 10.1021/ol9006794. [DOI] [PubMed] [Google Scholar]; b) Takishima S, Ishiyama A, Iwatsuki M, Otoguro K, Yamada H, Omura S, Kobayashi H, van Soest RWM, Matsunaga S. Org Lett. 2010;12:896. doi: 10.1021/ol9006794. [DOI] [PubMed] [Google Scholar]
- 4.a) Murphy PJ, Williams HL, Hursthouse MB, Abdul Malik KM. J Chem Soc, Chem Commun. 1994:119–120. [Google Scholar]; b) Snider BB, Chen J, Patil AD, Freyer AJ. Tetrahedron Lett. 1996;37:6977–6980. [Google Scholar]; c) Cohen F, Overman LE. J Am Chem Soc. 2001;123:10782–10783. doi: 10.1021/ja017067m. [DOI] [PubMed] [Google Scholar]; d) Overman LE, Wolfe JP. J Org Chem. 2001;66:3167–3175. doi: 10.1021/jo0100998. [DOI] [PubMed] [Google Scholar]; e) Aron ZD, Overman LE. Chem Comm. 2004:253–265. doi: 10.1039/b309910e. [DOI] [PubMed] [Google Scholar]
- 5.a) Arnold MA, Day KA, Duron SG, Gin DY. J Am Chem Soc. 2006;128:13255–13260. doi: 10.1021/ja063860+. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Butters M, Davies CD, Elliott MC, Hill-Cousins J, Kariuki BM, Ooi L-L, Wood JL, Wordingham SV. Org Biomol Chem. 2009;7:5001–5009. doi: 10.1039/b914744f. [DOI] [PubMed] [Google Scholar]
- 6.Evans PA, Qin J, Robinson JE, Bazin B. Angew Chem. 2007;119:7561–7563. doi: 10.1002/anie.200700840. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2007;46:7417–7419. doi: 10.1002/anie.200700840. [DOI] [PubMed] [Google Scholar]
- 7.Ishiwata T, Hino T, Koshino H, Hashimoto Y, Nakata T, Nagasawa K. Org Lett. 2002;4:2921–2924. doi: 10.1021/ol026303a. [DOI] [PubMed] [Google Scholar]
- 8.For reviews on Pd-catalyzed alkene carboamination reactions, see: Wolfe JP. Synlett. 2008:2913–2937.Schultz DM, Wolfe JP. Synthesis. 2012;44:351–361. doi: 10.1055/s-0031-1289668.
- 9.a) Bertrand MB, Neukom JD, Wolfe JP. J Org Chem. 2008;73:8851–8860. doi: 10.1021/jo801631v. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Bertrand MB, Wolfe JP. Tetrahedron. 2005;61:6447–6459. [Google Scholar]
- 10.a) Nakhla JS, Wolfe JP. Org Lett. 2007;9:3279–3282. doi: 10.1021/ol071241f. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Nakhla JS, Schultz DM, Wolfe JP. Tetrahedron. 2009;65:6549–6570. doi: 10.1016/j.tet.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Leathen ML, Rosen BR, Wolfe JP. J Org Chem. 2009;74:5107–5110. doi: 10.1021/jo9007223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.a) Fritz JA, Nakhla JS, Wolfe JP. Org Lett. 2006;8:2531–2534. doi: 10.1021/ol060707b. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Fritz JA, Wolfe JP. Tetrahedron. 2008;64:6838–6852. doi: 10.1016/j.tet.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.For further details on the mechanism of Pd-catalyzed alkene carboamination, see: Neukom JD, Perch NS, Wolfe JP. Organometallics. 2011;30:1269–1277.Neukom JD, Perch NS, Wolfe JP. J Am Chem Soc. 2010;132:6276–6277. doi: 10.1021/ja9102259.
- 13.a) Davis FA, Yang B. Org Lett. 2003;5:5011–5014. doi: 10.1021/ol035981+. [DOI] [PubMed] [Google Scholar]; b) Tang TP, Ellman JA. J Org Chem. 2002;67:7819–7832. doi: 10.1021/jo025957u. [DOI] [PubMed] [Google Scholar]
- 14.Davis FA, Gaspari PM, Nolt BM, Xu P. J Org Chem. 2008;73:9619–9626. doi: 10.1021/jo801653c. [DOI] [PubMed] [Google Scholar]
- 15.2-Bromovinyltrimethylsilane was used in place of vinyl bromide due to the volatility of the latter compound.
- 16.The PMB protecting group was employed due to the relative ease of deprotection as compared to the PMP group used in the model study.
- 17.This transformation must be conducted under rigorously anhydrous conditions to avoid HCl-mediated side reactions.
- 18.Use of the analogous guanidinium chloride salt in the ring-closing Mitsunobu reaction led to the formation of a chlorinated side product resulting from chloride for hydroxyl group substitution. In addition, a diastereomeric side product resulting from double inversion at C1 was also formed. Use of the BF4 salt prevented formation of these side products.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.