Abstract
The epidermis increases pigmentation and epidermal thickness in response to ultraviolet exposure to protect against UV-associated carcinogenesis; however, the contribution of epidermal thickness has been debated. In a humanized skin mouse model that maintains interfollicular epidermal melanocytes, we found that forskolin, a small molecule that directly activates adenylyl cyclase and promotes cAMP generation, up-regulated epidermal eumelanin accumulation in fair-skinned melanocortin-1-receptor (Mc1r)-defective animals. Forskolin-induced pigmentation was associated with a reproducible expansion of epidermal thickness irrespective of melanization or the presence of epidermal melanocytes. Rather, forskolin-enhanced epidermal thickening was mediated through increased keratinocyte proliferation, indirectly through secreted factor(s) from cutaneous fibroblasts. We identified keratinocyte growth factor (Kgf) as a forskolin-induced fibroblast-derived cytokine that promoted keratinocyte proliferation, as forskolin induced Kgf expression both in the skin and in primary fibroblasts. Lastly, we found that even in the absence of pigmentation, forskolin-induced epidermal thickening significantly diminished the amount of UVA and UVB that passed through whole skin and reduced the amount of UVB-associated epidermal sunburn cells. These findings suggest the possibility of pharmacologic-induced epidermal thickening as a novel UV-protective therapeutic intervention, particularly for individuals with defects in pigmentation and adaptive melanization.
Keywords: Photoprotection, epidermal thickness, topical cAMP, melanin
Introduction
Cutaneous UV responses represent a complex interaction between cell-autonomous events as well as intercellular signaling involving keratinocytes, melanocytes and a variety of supporting cells (1–2). The physiologic and molecular events that occur after exposure of the skin to UV radiation are complex, well-studied and involve components of the neuroendocrine (3–4), immunologic (5–6) and pigmentary systems (1, 7–8). Bioactive molecules such as α-melanocyte stimulating hormones (9–11) and molecules known to increase melanocyte cAMP levels (12) result in activation of pro-differentiation pathways mediated in part through microphthalmia (Mitf) and CREB and ultimately in induction of melanin synthesis. We previously reported a pharmacologic strategy to induce melanization in Mc1r-defective mice using the small molecule forskolin (13). This strategy, based on inducing epidermal synthesis and accumulation of melanin, protected mice against the formation of UV-induced photodimers in the skin as well as development of UV-induced carcinomas (13). However, besides inducing melanization of the epidermis, topical forskolin also caused the epidermis to thicken (14). In this report, we studied the mechanisms of forskolin-induced epidermal keratosis and examined the capacity of epidermal thickening to protect against UV damage in the skin independent of melanization. We found that proliferation of basal keratinocytes contributed to forskolin-induced epidermal thickening and that this proliferation was mediated by fibroblast-derived soluble factors. Further, we found that forskolin-induced epidermal thickening protected against UV penetration and UV-induced epidermal apoptosis even in the absence of melanization, raising the possibility of pharmacologic UV protection based on epidermal thickening in individuals with defective melanin synthesis.
Methods
Animals and primary cells
K14-Scf transgenic C57BL/6J mice were generated as described (13, 15–16). Epidermal primary keratinocytes isolated from C57BL/6J wild type mice were grown in keratinocyte-selective media as previously published (17–19) until no pigmented, dendritic cells (i.e. melanocytes) or spindle-shaped cells (fibroblasts) were observed among the cuboidal-appearing keratinocytes (typically after 4–6 weeks in culture). As an additional control, neither fibroblast (vimentin) or melanocyte (mitf) mRNA were distinguishable above background after selection (data not shown). Melanins were quantitatively analyzed by HPLC as described (20).
Topical treatments
Mice were used between 4 and 8 weeks of age and were depilated by trimming with electric shears followed by topical depilatory cream (Nair®). Preparations of topical agents were spread evenly over the entire dorsal skin. Forskolin was prepared as described (13). Solvent (vehicle) control consisted of 70% ethanol/30% propylene glycol. Animals were treated once daily for 5 days a week unless otherwise stated; for forskolin-treated animals, this equated to roughly 80 micromoles of drug applied daily to the skin.
Skin color measurement
Skin reflective colorimetry was assessed with a CR-400 Colorimeter (Minolta Corporation, Japan) calibrated against a white background. Degree of melanization (darkness) was quantified as the colorimetric measurement on the *L axis (white-black axis) of the CIE standard color axis (21).
UV exposure
For most experiments, mice were exposed to a double bank of UVB lamps (UV Products, Upland, CA). Unless stated otherwise, animals were exposed to radiation once daily, five days a week for three weeks (fifteen total doses), with a daily dose of 2 kJ/m2 UVB and 0.5 kJ/m2 UVA (therefore 2.5 kJ/m2 total UV per day and 37.5 kJ/m2 cumulative total UV dose). 2 kJ/m2 per day was chosen because it represents a sub-erythemal dose of UV light previously found to induce tanning in our animal model (in an Mc1r-intact background) (13). For the UV penetration experiments, a 1.6 kW Xenon short arc solar simulator UV source (Sciencetech, London, ON Canada) was used with custom filters to eliminated all but UVB or UVA resulting in >90% pure UVB or UVA radiation respectively. UV emittance was measured with a Model IL1400A handheld flash measurement photometer (International Light, Newburyport, MA) equipped with separate UVB (measuring wavelengths from 265–332 nm; peak response at 290 nm) and UVA (measuring wavelengths from 315–390 nm; peak response at 355 nm) corresponding to International Light product numbers TD# 26532 and TD# 27108 respectively. Spectral output of the lamps was determined to be roughly 75% UV-B and 25% UV-A.
Histology and immunohistochemistry
Skin biopsies were fixed (10% buffered formalin), paraffin-embedded, sectioned (5 μm) and Fontana-Masson stained (American Master*Tech Scientific, Inc., Lodi, CA) (22). Epidermal thickness was determined by multiple measurements in fields without sectioning artifacts. To quantify nucleated cells, sections were stained with 4′,6-diamidino-2-phenylindole (DAPI; Sigma, St. Louis, MO) (23), and numbers of nuclei in the epidermis were quantified. Nuclei were quantified along the entire biopsy, with roughly 15–20 high power fields per biospy, excluding sections that displayed major artifactual disruptions (e.g. torn or folded cutting artifacts) or evidently non-perpendicular lengths. Non-follicular epidermal nuclei were counted along the entire image. For keratin 14 and Ki67 staining, antigen retrieval was performed by heating (45 minutes, 10 mM citrate buffer pH 6.0) in a rice cooker. Immunohistochemistry was performed using rabbit anti-keratin 14 antibody (1:500 dilution of 1 mg/ml stock; Covance, Princton, NJ), rabbit anti-Ki67 antibody (1:1000 dilution of 2 mg/ml stock; Abcam, Cambridge, MA) and FITC-conjugated goat anti-rabbit secondary antibody (1:1000, Abcam, Cambridge, MA). Images were captured and measurements were made using the QCapture Pro program (QImaging Software).
Proliferation assays
Primary keratinocytes were serum starved overnight and treated for 24h as described (24–25). 3H-thymidine (Perkin-Elmer, Waltham, MA) was added during the last 3h of treatment. DNA was precipitated with 10% trichloroacetic acid (Sigma-Aldrich, St. Louis, MO) and collected with 0.2N NaOH (Sigma-Aldrich, St. Louis, MO); incorporated radiolabeled thymidine was measured in a Beckman LS 6500 (Beckman Coulter, USA).
mRNA quantification (qPCR)
Total RNA was harvested from cells using the RNeasy extraction kit (Qiagen RNA) and from skin using Trizol (Invitrogen). cDNA was generated (Fermentas first strand synthesis kit; Thermo Scientific, Glen Burnie, MD) and dual color quantitative real-time PCR analysis was performed using a Roche Lightcycler 480 (20 ng cDNA/reaction using mouse β-actin as the reference gene. Primer sets for Kgf/Fgf7 were 5′-TCTCATCAATCTCCAGTTCACAA (left) and 5′-CTTGCGTTGATTGCTACTCCT (right), for Fgf10 were 5′-CGGGACCAAGAATGAAGACT (left) and 5′-GCAACAACTCCGATTTCCAC (right) and for pro-EGF 5′-CATGCCCCACAGGATTTG (left) and 5′-GGGCAGGAAACAAGTTCG (right).
Measurement of UVR transmission
Dorsal skins of animals treated as described were removed, spread flat and placed over a UVR sensor. UV transmission was measured and percent transmission was calculated for UVA and UVB.
Sunburn cells
Five days after the last topical treatment (21d, 5d/wk), animals were irradiated. Skin biopsies were harvested (24h), processed and stained by hematoxylin and eosin. Sunburn cells, defined as epidermal cells with pyknotic condensed nuclei (26), were quantified.
Statistical analysis
Statistical comparisons between cohorts of control- versus forskolin-treated animals were evaluated by a 1-way ANOVA with Tukey’s correction unless otherwise stated. Differences were considered statistically significant if the p value was < 0.05.
Results
K14-Scf transgenic C57BL/6 animals (27) were crossed with established C57BL/6J coat color variants to generate genetically-matched animals with “humanized skin”. Thus, unlike non-transgenic mice, K14-Scf animals maintain interfollicular epidermal melanocytes in the stratum basale and retain the capacity for epidermal pigmentation (13). In this model, “wild type” K14-Scf C57BL/6 mice with functional melanocortin 1 receptor (Mc1r) and tyrosinase (Tyr) exhibited an intensely eumelanotic phenotype in both the fur and the epidermal skin (Fig. S1a), whereas incorporation of the Mc1r extension (Mc1re/e) mutation essentially switched the pigment phenotype to a red/blonde pheomelanotic complexion. Loss of tyrosinase, the rate limiting enzyme critical to production of all types of melanin, resulted in near complete loss of pigmentation, irrespective of Mc1r function (Fig. S1a). HPLC-based quantification of melanin species of whole depilated skin from these transgenic animals confirmed that the skin of wild type (Mc1rE/E Tyr+/+) animals contained abundant eumelanin, skin of extension (Mc1re/e Tyr+/+) animals contained little eumelanin but preferentially contained pheomelanin, and the dorsal skin of tyrosinase-null (Mc1re/e Tyrc2j/c2j or Mc1rE/E Tyr−/−) adult animals contained insignificant amounts of either melanin species irrespective of Mc1r status (Fig. S1b).
Using pheomelanotic Mc1r-defective animals that model fair-skinned humans, we found that daily topical applications of the adenylyl cyclase activator forskolin, but neither vehicle nor daily UV exposure, robustly induced skin darkening (Fig. S2a, b) (13). Epidermal darkening was due to accumulation of melanin throughout the epidermis, as determined by Fontana-Masson melanin staining (Fig. 1a). Previously, we determined that topically-applied forskolin may also lead to increased numbers of epidermal melanocytes in our animal model, which may also contribute to epidermal darkening (14). When skin sections of control- vs. forskolin-treated extension animals were carefully compared, we noted that in addition to the increased melanization, the epidermis of forskolin-treated animals was thicker than that of vehicle-treated counterparts (Fig 1a, b). Interestingly, UV-exposed Mc1r-null animals demonstrated robust epidermal thickening but failed to exhibit melanization (Fig. 1a, b). Thus, pharmacologic cAMP induction lead to both melanization and epidermal thickening, whereas UV exposure of animals with defective Mc1r promoted epidermal thickening without melanization, suggesting that the physiologic mechanisms regulating melanization must be distinct from those regulating epidermal thickening. Forskolin-induced thickening was noted as early as day 7 of treatment, reached maximal levels between 14–21 days of therapy and remained fairly constant through three months of daily treatment (data not shown) (14). To control for the confounding effects on epidermal thickness related to different stages of hair cycling (28);(29), we repeated the three week experiment in 8 week-old C57BL/6 animals, all of which remained in non-anagen phases of the hair cycle throughout the three week experiment. Again, topically-applied forskolin promoted a statistically significant (p<0.05) increase in epidermal thickness, suggesting that this effect was unrelated to hair cycling (Fig. S3). Pharmacologically-mediated epidermal thickening by forskolin appeared to be independent of an inflammatory component, as determined by lack of erythema and by the lack of epidermal or dermal inflammatory cell infiltrate (Fig. S4). Furthermore, we noted no global reproducible effects on keratinocyte differentiation as judged by expression of keratin-1, keratin-14 or involucrin within seven days of treatment (Fig. S5).
Figure 1.
Forskolin treatment increases epidermal thickness. (a, b) Representative images and epidermal thickness measurements of Fontana-Masson stained dorsal skin biopsies taken from K14-Scf Mc1re/e Tyr+/+ (extension) animals treated (3wk, 5d/wk) with vehicle (n=4), forskolin (n=4), or UVB (2kJ/m2) (n=3). (c, d) Representative images (c) and epidermal thickness measurements (d) of Fontana-Masson stained skin sections from K14-Scf Mc1re/e Tyr+/+ (extension) (n=4) or amelanotic K14-Scf Mc1re/e Tyrc2j/c2j (albino extension) (n=3) animals treated as indicated above. (e) Epidermal thickness measurements of amelanotic K14-Scf transgenic (n=3) vs. non-transgenic (n=2) Mc1re/e Tyrc2j/c2j albino extension animals treated (3wk, 5d/wk) with vehicle or forskolin. Scale bar=303m, * p ≤ 0.05. Note that forskolin-induced epidermal thickening is independent of either melanin (c, d) or stem cell factor (e).
Since forskolin promoted robust melanization along with epidermal thickening, we reasoned that forskolin-induced epidermal thickening may be due to melanin accumulation. To test this hypothesis, we determined the effect of topical forskolin on Mc1r-defective albino K14-Scf animals incapable of making melanin due to a defective tyrosinase gene (Tyrc2j/c2j). Unlike their fair-skinned Mc1r-defective tyrosinase-intact extension counterparts, albino animals showed no forskolin-induced skin darkening (Fig. S2c, d). Fontana-Masson-stained skin biopsies confirmed robust epidermal expansion in the absence of melanization in forskolin-treated K14-Scf albino extension animals (Fig. 1c). In fact, the degree of forskolin-induced epidermal thickening was similar between tyrosinase-intact (melanin-containing) and albino animals (Fig. 1d), indicating that forskolin-induced epidermal thickening was independent of tyrosinase function or melanin deposition. We next tested the contribution of the K14-Scf transgene and interfollicular melanocytes on forskolin-induced epidermal thickening by comparing non-transgenic albino animals with their K14-Scf counterparts. We found similar levels of forskolin-dependent epidermal thickening in either group of animals (Fig. 1d), suggesting that forskolin-induced epidermal thickening is independent of c-kit signaling or the presence of interfollicular melanocytes.
We reasoned that forskolin-induced epidermal thickening could result either from an increase in the number of epidermal keratinocytes or from hypertrophy of existing cells. To help distinguish between these possibilities, we stained skin sections from control-versus forskolin-treated K14-Scf extension animals with 4′,6-diamidino-2-phenylindole (DAPI), a fluorescent stain that avidly binds DNA and facilitates enumeration of nucleated cells. We found that topically-applied forskolin, like daily low-dose UV (positive control), promoted an accumulation of nucleated cells in the epidermis (Fig. 2a, b). An increase in epidermal keratinocyte number could result from either increased proliferation of basal keratinocytes or from interference with terminal differentiation wherein maturing keratinocytes lose their nuclei as they migrate beyond the stratum spinosum to form the cornified layer. To distinguish between these possibilities, we determined the effect of topical forskolin on keratin 14 expression, a cytokeratin expressed by proliferating basal keratinocytes (30–31). We found that both UV (positive control) or topical forskolin promoted expansion of keratin 14-expressing cells in the epidermis (Fig. 2c, d). Furthermore, forskolin treatment increased the number of Ki67-stained cells in the stratum basale (Fig. 2e, f), consistent with cAMP-induced enhanced proliferation of basal keratinocytes (32). Curiously, there was no difference in the number of epidermal nuclei between control-treated and forskolin-treated animals at day 3 (Fig. 2g), suggesting that the accumulation of epidermal cells caused by enhanced proliferation took longer than 3 days to occur.
Figure 2.
Forskolin promotes epidermal cell accumulation and basal keratinocyte proliferation in vivo. (a, b) Representative skin sections (a) and quantification (b) of DAPI-stained epidermal nuclei in K14-Scf Mc1re/e Tyr+/+ (extension) animals treated (3wk, 5d/wk) as described in Fig. 1. Scale bar=30μm. (c, d) Representative skin sections stained for keratin 14 (c) and keratin 14 thickness measurements (d) from animals treated as described in “a”. Scale bar=30μm. (e, f, g) Representative Ki67-stained skin sections (e) Ki67 quantification (f) and DAPI-stained nuclei (g) from K14-Scf Mc1re/e Tyr+/+ (extension) animals treated (0 or 3 days) with vehicle (n=3) or forskolin (n=4). Scale bar=603m, * p ≤ 0.05.
We reasoned that forskolin could increase keratinocyte proliferation either directly via cAMP stimulation or indirectly through activation of other skin cells. The literature is mixed with respect to the effect of cAMP stimulation on keratinocytes, with some manuscripts reporting enhanced proliferation (33–34) and others describing cAMP-mediated effects on migration and differentiation instead (35–36). In order to clarify between these possibilities, we purified primary keratinocytes from C57BL/6 animals (19) and tested the ability of purified forskolin to affect their proliferative capacity in vitro. Instead of enhancing the proliferative rate of primary keratinocytes, purified forskolin consistently inhibited keratinocyte thymidine incorporation (Fig. 3a), unlike the positive control epidermal growth factor (EGF) (37). Thus, we concluded that cAMP stimulation does not directly increase proliferation of purified primary murine keratinocytes.
Figure 3.
Forskolin promotes primary keratinocyte proliferation in the presence of fibroblasts. (a) 3H-thymidine incorporation (expressed as fold change over vehicle control) of purified primary C57BL/6 keratinocytes treated with vehicle (1% DMSO; n=3), 100 μM forskolin (n=3) or 20 ng/ml EGF (n=3). (b) 3H-thymidine incorporation of purified primary C57BL/6 keratinocytes co-cultured in the presence of either primary C57BL/6 keratinocytes, primary C57BL/6 fibroblasts, or transformed B16 melanocytes in Transwell® inserts and treated with either vehicle or forskolin as described above. (c) Expression of Kgf, Fgf10, or Egf mRNA in primary C57BL/6 murine fibroblasts treated (6h) with vehicle or forskolin as described above. (d) Kgf mRNA expression at 6 hours in whole skin of C57BL/6 animals treated topically with either vehicle or forskolin as previously described. Kgf mRNA data are expressed as fold induction over vehicle control and normalized to β-actin expression. (e) 3H-thymidine incorporation of primary C57BL/6 keratinocytes pretreated with forskolin (100 μM; 6h) and then incubated with vehicle (1% DMSO), forskolin (100 μM), or Kgf (20 ng/ml) for an additional 24h. * p ≤ 0.05.
Since topically-administered forskolin enhanced keratinocyte proliferation the context of whole skin but not in purified keratinocytes, we hypothesized that forskolin promoted keratinocyte proliferation indirectly via the participation of other skin cell type(s). We tested the role of soluble factors in forskolin-mediated keratinocyte proliferation with the use of Transwell© chambers. Into the upper chamber were placed either B16 murine transformed melanocytes, primary C57BL/6 keratinocytes (negative control) or primary C57BL/6 fibroblasts. The proliferative rate of purified primary keratinocytes (in the lower chamber) was measured in the presence of either vehicle or forskolin. Whereas forskolin inhibited keratinocyte proliferation directly, it potently enhanced keratinocyte proliferation if primary fibroblasts were included in the Transwell© chambers (Fig. 3b). Transformed B16 melanocytes had a lesser stimulatory effect (Fig. 3b). We concluded that forskolin stimulated keratinocyte proliferation indirectly through one or more soluble factors produced by other skin cells, particularly fibroblasts. We then sought to determine the identity of the cAMP-induced fibroblast growth factors responsible for forskolin-induced keratinocyte proliferation. We noted marked up-regulation in Kgf (Fgf7) mRNA in forskolin-treated primary fibroblasts with a modest increase in Fgf10 mRNA and no change in Egf expression (Fig. 3c). We found a similar increase in Kgf mRNA in forskolin-exposed skin (Fig. 3d). To determine if Kgf was able to overcome the anti-proliferative effect of forskolin treatment seen in vitro, we repeated the 3H-thymidine incorporation assay with primary keratinocytes pretreated with forskolin for 6 hours. We found that recombinant Kgf (20 ng/ml) partially rescued forskolin-mediated keratinocyte proliferation (Fig. 3e). Together, these data suggested that forskolin-induced keratinocyte proliferation may be mediated indirectly through induction of fibroblast-derived Kgf.
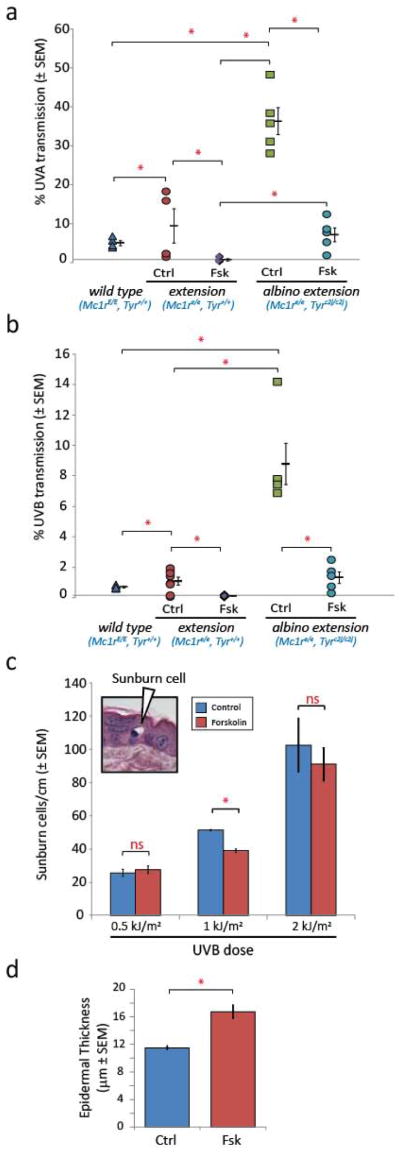
Finally, we sought to determine whether forskolin-induced epidermal thickening could protect skin against UV penetration. We compared cutaneous responses between tyrosinase-intact, melanin-inducible animals (K14-Scf Mc1re/e, Tyr+/+) with those of their tyrosinase-defective genetically-matched counterparts (K14-Scf Mc1re/e, Tyrc2j/c2j). We assessed UV protection by measuring the amount of UV radiation that would pass through dorsal skin treated topically (3 wk) with either forskolin or vehicle. Consistent with previous observations, daily application of forskolin to K14-Scf Mc1r-defective animals promoted skin thickening irrespective of tyrosinase function and melanin deposition (Fig. S6a), however, only in the tyrosinase-intact background did significant melanization occur (Fig. S6b). Control-treated albino extension skin allowed 36.3% and 8.8% of UVA or UVB photons to pass through respectively (Fig. 4a, b). In contrast, albino extension skin that had been pretreated with forskolin allowed only 7.1% (UVA) and 1.3% (UVB) to pass through (Fig. 4a, b). Therefore, melanin-independent forskolin-induced epidermal thickening was associated with 80.4% (UVA) and 85.2% (UVB) reductions in the amount of UV penetration through the skin as measured in this manner. If melanization was added to the system by using tyrosinase-intact K14-Scf extension (Mc1re/e, Tyr+/+) animals, then forskolin pretreatment was associated with transmission of 0.7% UVA and 0.1% UVB, representing reductions of 92.5% and 90.0% respectively over control-treated counterparts (Fig. 4a, b). The importance of eumelanin to UV photoprotection was also demonstrated by noting the UV-blocking capacity of untreated K14-Scf Mc1r-intact (fully eumelanotic) wild type animals. In these mice, thought to be maximally pigmented yet with a thin, pharmacologically-unstimulated epidermis, only 5.1% UVA and 0.73% UVB passed through the skin (Fig. 4a, b). Overall, these data showed that epidermal thickening blocked penetration of UVA and UVB into the skin even in the complete absence of pigmentation.
Figure 4.
Forskolin-mediated epidermal thickening is photoprotective. (a, b) Transmission of (a) UVA (315–390 nm) or (b) UVB (265–332 nm) through whole depilated dorsal skins of age-matched K14-Scf animals treated (3wk, 5d/wk) as indicated (n=5 animals per group). Untreated K14-Scf Mc1rE/E Tyr+/+ (wild type) animals animals are included as “maximally pigmented” eumelanotic controls. (c) Quantification of sunburn cells 24h after UV exposure in albino extension animals pre-treated (3wk, 5d/wk) with either vehicle (n=4) or forskolin (n=5) and exposed to the indicated dose of UVB. The inset shows an example of a sunburn cell dense and (d) describes mean epidermal thickness of cohorts of animals used in the sunburn cell assay. * p ≤ 0.05.
In order to determine whether forskolin-induced epidermal thickening was associated with functional UV protection, we quantified “sunburn cells” in the epidermis at varying doses of UVB radiation. Sunburn cells are thought to represent keratinocytes dying apoptotically after UV exposure (26). By this measure, we found that animals pre-treated with forskolin had fewer sunburn cells in the epidermis 24h after exposure to 1 kJ/m2 UVB (Fig. 4c). This protection was due to increased epidermal thickness alone since these experiments were carried out in albino extension animals incapable of melanizing their epidermis (Fig. 4d). This photoprotection could be overcome by increasing the dose of UV administered (2 kJ/m2; Fig. 4c). These findings are novel because of the ability of this animal model to disconnect epidermal thickening from pigmentation in a genetically defined system. Together, these data show that pharmacologic-induced epidermal thickening is protective against UV even in the absence of epidermal melanization.
Discussion
Using a genetically-defined animal model with “humanized skin”, we found that topical cAMP up-regulation promoted epidermal thickening through enhanced keratinocyte proliferation. This pharmacologically-induced epidermal thickening was UV-protective independently of pigmentation. Since most reports of UV-induced epidermal thickening involved pigmented human or porcine skin (38–39) or non-transgenic murine models wherein the epidermis lacks interfollicular melanocytes, the role of melanocytes and their melanin products in UV-induced epidermal thickening has been unclear. We found that epidermal thickening occurred even in tyrosinase-null albino animals implying that UV-induced epidermal thickening was not simply due to an accumulation of melanin in epidermal keratinocytes. Rather, forskolin-induced epidermal thickening was associated with an increase in the proliferative rate of basal keratinocytes and a subsequent accumulation of nucleated cells in the epidermis, findings which might have implications for wound healing and other cutaneous processes reliant on keratinocyte proliferation.
Our studies suggest that direct cAMP stimulation inhibits proliferation of primary keratinocytes, in agreement with previous findings by others (40). Instead, our data suggest that one or more soluble cAMP-induced factors derived from fibroblasts, likely Kgf or Fgf10, mediates keratinocyte proliferation. Both of these cytokines are known to be produced by fibroblasts (41–42) and to stimulate keratinocyte proliferation (43–44). In our system, Kgf was the growth factor whose levels increased the most upon cAMP stimulation. Though Kgf is known to promote keratinocyte proliferation in the context of wound healing (45–47) and in certain pathologic conditions (e.g. psoriasis) (48), its contribution to UV-induced epidermal keratosis is unclear. Nonetheless, Kgf seems to exert UV-protective effects on epidermal skin cells, specifically mediating resistance to UV-mediated oxidative injury (49), epidermal apoptosis (50) and increased uptake of melanosomes (51). Though forskolin clearly induced Kgf mRNA directly in purified fibroblasts, our data do not rule out the contribution of other mediators or cytokines (e.g. keratinocyte-derived IL-1 (52)) in affecting fibroblast Kgf production or epidermal thickening. It is important to point out that Kgf was identified by mRNA expression alone. We were technically unable to detect Kgf protein by conventional means (Western analysis, ELISA), presumably because anti-Kgfcommercial reagents that were used were designed to measure human Kgf rather than murine Kgf. Therefore, it will be important in future studies to verify the involvement of Kgf at the protein level in forskolin-induced epidermal thickening.
Pharmacologically-enhanced epidermal thickening significantly contributed to UV photoprotection even in amelanotic skin. Indeed, the degree of photoprotection afforded by forskolin-induced epidermal thickening in an amelanotic (albino) background approached that observed in untreated pheomelanotic (fair-skinned) animals (Fig. 4). This observation may be clinically significant, as amelanotic persons who suffer from albinism tend to be much more UV sensitive than otherwise fair-skinned persons whose epidermis contains pheomelanin and little eumelanin. Though it might offer the potential benefit of enhanced UV-protection, pharmacologically-induced epidermal thickening might also be associated with potential negative effects as well, including potentiating conditions of hyperkeratosis including psoriasis and atopic dermatitis. Clearly, preclinical studies will have to be done before this approach can safely be considered for clinical practice.
We realize that forskolin, a potent and promiscuous activator of adenylyl cyclases, may not be an optimal translational product for inducing UV photoprotection in humans. Rather, we view these data as proof-of-concept studies to show the potential benefit of topical cAMP-mediated epidermal thickening in UV resistance. The use of topical agents to increase epidermal cAMP may also protect the skin in other ways, most notably by enhancing removal of UV-induced DNA damage as was shown by Hearing and coworkers in a reconstructed skin model (53). Nonetheless, a more targeted approach may have to be identified before translating these findings into clinical application. Topical application of clinically available phosphodiesterase inhibitors, for example, also increase cellular levels of cAMP and exhibited activities similar to forskolin in the murine model that we used in our studies (54). Recent work by Hwang and colleagues, for example, showed that 5,7-dimethoxyflavone up-regulated pigment synthesis in melanocytes through cAMP-dependent signaling (12), suggesting other potential agents for topical cAMP manipulation. Overall, these data suggest that pharmacologic manipulation of epidermal thickness may represent a novel means to enhance the photoprotective properties of the skin.
Supplementary Material
Figure S1. Humanized skin mouse model system. (a) Phenotypes of the C57BL/6 K14-Scf transgenic mice used in this study. Epidermal pigmentation, like that of the fur, is dependent on function of Mc1r and Tyr loci. (b) Eumelanin and pheomelanin contents of depilated dorsal skins of K14-Scf transgenic animals (n=3 per group). * p ≤ 0.05.
Figure S2. Forskolin promotes epidermal melaninization. (a) K14-Scf Mc1re/e Tyr+/+ (extension) animals were treated (3wk, 5d/wk) with vehicle, forskolin, or UVB (2 kJ/m2). Note the darkening of the dorsal skin in the forskolin-treated but not UV-exposed animals. (b) Skin color as measured by reflective colorimetry of animals in “a”. We previously showed that forskolin-induced skin darkening was due to accumulation of epidermal eumelanin (13) (c, d) photographs (c) and reflective colorimetric skin color quantification (d) of K14-Scf Mc1re/e Tyrc2j/c2j (albino extension) animals treated with vehicle (n=3), forskolin, (n=3), or UVB (n=2) as described. Note the lack of dorsal skin darkening in forskolin-treated animals. * p ≤ 0.05.
Figure S3. Epidermal thickening experiment in age- and gender-matched C57BL/6 animals. 8 week-old C57BL/6 Mc1rE/E Tyrc2j/c2j (non-transgenic wild type) animals were treated (3wk, 5d/wk) with vehicle (n=8), forskolin, (n=8) or 2 kJ/m2UVB (n=4). (a) Representative photos of animals at the end of the 3 wk course. (b) representative Hematoxylin and Eosin stained cross-section skin images and (c) epidermal thickness measurements from animals treated in “a”. * p ≤ 0.05.
Figure S4. Inflammatory cell infiltrate in the skin of 21 day-treated animals. Quantification of number of inflammatory cells per high power field in the epidermis and dermis of hematoxylin and eosin-stained skin from K14-Scf Mc1re/e Tyrc2j/c2j (albino extension) animals treated (3wk, 5d/wk) with vehicle or forskolin as described (n=4 for vehicle; n=6 for forskolin). * p ≤ 0.05.
Figure S5. Keratin expression in the skin of C57BL/6 Mc1rE/E Tyrc2j/c2j (non-transgenic wild type) animals treated with vehicle or forskolin daily for 7 days. Western blot analysis to indicate keratinocyte differentiation was performed for the intermediate-filaments keratin 14 (basal), keratin 1 (suprabasal), and involucrin (suprabasal). We noted much variability in keratin protein expression between animals. Each experimental condition (vehicle or forskolin) involved the same animal biopsied at day 0, day 3 and day 7. We show two representative animals for each treatment group.
Figure S6. Forskolin increases whole skin thickness (a) and darkens skin color (b) in tyrosinase intact animals. Thicknesses (a) or skin color (b) of whole dorsal depilated and defatted skins of animals described in Fig. 4 were determined by micrometer quantification and reflectometry respectively. * p ≤ 0.05.
Acknowledgments
The authors wish to thank Malinda Spry, Jess Christian, Danielle Ronis, Osama Abona-Ama, Cynthia Long and Patrick Michael for technical assistance. We also thank current and past funding sources: the National Cancer Institute (R01 CA131075-02), the Wendy Will Case Cancer Research Fund, the Markey Cancer Foundation, the Children’s Miracle Network and the Jennifer and David Dickens Melanoma Research Foundation. T. Scott, P. Christian and K. Donohue performed the experiments, B. Shelton assisted with statistical interpretation of the data, M. Kesler quantified inflammatory cell infiltrate in skin biopsies, K, Wakamatsu and S. Ito quantified melanins and J. D’Orazio and T. Scott designed the research study and wrote the paper.
References
- 1.Abdel-Malek ZA, Kadekaro AL, Swope VB. Stepping up melanocytes to the challenge of UV exposure. Pigment Cell Melanoma Res. 2010;23:171–186. doi: 10.1111/j.1755-148X.2010.00679.x. [DOI] [PubMed] [Google Scholar]
- 2.Seiberg M. Keratinocyte-melanocyte interactions during melanosome transfer. Pigment Cell Res. 2001;14:236–242. doi: 10.1034/j.1600-0749.2001.140402.x. [DOI] [PubMed] [Google Scholar]
- 3.Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocrine reviews. 2000;21:457–487. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- 4.Slominski A, Wortsman J, Paus R, Elias PM, Tobin DJ, Feingold KR. Skin as an endocrine organ: implications for its function. Drug discovery today. Disease mechanisms. 2008;5:137–144. doi: 10.1016/j.ddmec.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper KD, Baron ED, Matsui MS. Implications of UV-induced inflammation and immunomodulation. Cutis. 2003;72:11–15. discussion 16. [PubMed] [Google Scholar]
- 6.Clydesdale GJ, Dandie GW, Muller HK. Ultraviolet light induced injury: immunological and inflammatory effects. Immunol Cell Biol. 2001;79:547–568. doi: 10.1046/j.1440-1711.2001.01047.x. [DOI] [PubMed] [Google Scholar]
- 7.Abdel-Malek ZA, Knittel J, Kadekaro AL, Swope VB, Starner R. The melanocortin 1 receptor and the UV response of human melanocytes--a shift in paradigm. Photochem Photobiol. 2008;84:501–508. doi: 10.1111/j.1751-1097.2008.00294.x. [DOI] [PubMed] [Google Scholar]
- 8.Chakraborty AK, Funasaka Y, Slominski A, et al. UV light and MSH receptors. Ann N Y Acad Sci. 1999;885:100–116. doi: 10.1111/j.1749-6632.1999.tb08668.x. [DOI] [PubMed] [Google Scholar]
- 9.Abdel-Malek Z, Swope VB, Suzuki I, et al. Mitogenic and melanogenic stimulation of normal human melanocytes by melanotropic peptides. Proc Natl Acad Sci U S A. 1995;92:1789–1793. doi: 10.1073/pnas.92.5.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki I, Cone RD, Im S, Nordlund J, Abdel-Malek ZA. Binding of melanotropic hormones to the melanocortin receptor MC1R on human melanocytes stimulates proliferation and melanogenesis. Endocrinology. 1996;137:1627–1633. doi: 10.1210/endo.137.5.8612494. [DOI] [PubMed] [Google Scholar]
- 11.Im S, Moro O, Peng F, et al. Activation of the cyclic AMP pathway by alpha-melanotropin mediates the response of human melanocytes to ultraviolet B radiation. Cancer Res. 1998;58:47–54. [PubMed] [Google Scholar]
- 12.Kang YG, Choi EJ, Choi Y, Hwang JK. 5,7-dimethoxyflavone induces melanogenesis in B16F10 melanoma cells through cAMP-dependent signalling. Exp Dermatol. 2011;20:445–447. doi: 10.1111/j.1600-0625.2010.01236.x. [DOI] [PubMed] [Google Scholar]
- 13.D’Orazio JA, Nobuhisa T, Cui R, et al. Topical drug rescue strategy and skin protection based on the role of Mc1r in UV-induced tanning. Nature. 2006;443:340–344. doi: 10.1038/nature05098. [DOI] [PubMed] [Google Scholar]
- 14.Spry ML, Vanover JC, Scott T, et al. Prolonged treatment of fair-skinned mice with topical forskolin causes persistent tanning and UV protection. Pigment Cell Melanoma Res. 2009;22:219–229. doi: 10.1111/j.1755-148X.2008.00536.x. [DOI] [PubMed] [Google Scholar]
- 15.Kunisada T, Lu SZ, Yoshida H, et al. Murine cutaneous mastocytosis and epidermal melanocytosis induced by keratinocyte expression of transgenic stem cell factor. J Exp Med. 1998;187:1565–1573. doi: 10.1084/jem.187.10.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanover JC, Spry ML, Hamilton L, Wakamatsu K, Ito S, D’Orazio JA. Stem cell factor rescues tyrosinase expression and pigmentation in discreet anatomic locations in albino mice. Pigment Cell Melanoma Res. 2009;22:827–838. doi: 10.1111/j.1755-148X.2009.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halaban R, Alfano FD. Selective elimination of fibroblasts from cultures of normal human melanocytes. In Vitro. 1984;20:447–450. doi: 10.1007/BF02619590. [DOI] [PubMed] [Google Scholar]
- 18.Tamura A, Halaban R, Moellmann G, Cowan JM, Lerner MR, Lerner AB. Normal murine melanocytes in culture. In Vitro Cell Dev Biol. 1987;23:519–522. doi: 10.1007/BF02628423. [DOI] [PubMed] [Google Scholar]
- 19.Pirrone A, Hager B, Fleckman P. Primary mouse keratinocyte culture. Methods Mol Biol. 2005;289:3–14. doi: 10.1385/1-59259-830-7:003. [DOI] [PubMed] [Google Scholar]
- 20.Ito S, Nakanishi Y, Valenzuela RK, Brilliant MH, Kolbe L, Wakamatsu K. Usefulness of alkaline hydrogen peroxide oxidation to analyze eumelanin and pheomelanin in various tissue samples: application to chemical analysis of human hair melanins. Pigment Cell Melanoma Res. 2011;24:605–613. doi: 10.1111/j.1755-148X.2011.00864.x. [DOI] [PubMed] [Google Scholar]
- 21.Wagner JK, Jovel C, Norton HL, Parra EJ, Shriver MD. Comparing quantitative measures of erythema, pigmentation and skin response using reflectometry. Pigment Cell Res. 2002;15:379–384. doi: 10.1034/j.1600-0749.2002.02042.x. [DOI] [PubMed] [Google Scholar]
- 22.Zappi E, Lombardo W. Combined Fontana-Masson/Perls’ staining. Am J Dermatopathol. 1984;6 (Suppl):143–145. [PubMed] [Google Scholar]
- 23.Chazotte B. Labeling nuclear DNA using DAPI. Cold Spring Harb Protoc. 2011;2011 doi: 10.1101/pdb.prot5556. pdb prot5556. [DOI] [PubMed] [Google Scholar]
- 24.Jackman J, O’Connor PM. Current Protocols in Cell Biology. Wiley; 2001. Methods for Synchronizing Cells at Specific Stages of the Cell Cycle. [DOI] [PubMed] [Google Scholar]
- 25.Zetterberg A, Skold O. The effect of serum starvation on DNA, RNA and protein synthesis during interphase in L-cells. Exp Cell Res. 1969;57:114–118. doi: 10.1016/0014-4827(69)90374-7. [DOI] [PubMed] [Google Scholar]
- 26.Bayerl C, Taake S, Moll I, Jung EG. Characterization of sunburn cells after exposure to ultraviolet light. Photodermatol Photoimmunol Photomed. 1995;11:149–154. doi: 10.1111/j.1600-0781.1995.tb00157.x. [DOI] [PubMed] [Google Scholar]
- 27.Kunisada T, Yoshida H, Yamazaki H, et al. Transgene expression of steel factor in the basal layer of epidermis promotes survival, proliferation, differentiation and migration of melanocyte precursors. Development. 1998;125:2915–2923. doi: 10.1242/dev.125.15.2915. [DOI] [PubMed] [Google Scholar]
- 28.Slominski A, Paus R. Melanogenesis is coupled to murine anagen: toward new concepts for the role of melanocytes and the regulation of melanogenesis in hair growth. J Invest Dermatol. 1993;101:90S–97S. doi: 10.1111/1523-1747.ep12362991. [DOI] [PubMed] [Google Scholar]
- 29.Alonso L, Fuchs E. The hair cycle. J Cell Sci. 2006;119:391–393. doi: 10.1242/jcs.02793. [DOI] [PubMed] [Google Scholar]
- 30.Galvin S, Loomis C, Manabe M, Dhouailly D, Sun TT. The major pathways of keratinocyte differentiation as defined by keratin expression: an overview. Adv Dermatol. 1989;4:277–299. discussion 300. [PubMed] [Google Scholar]
- 31.Del Bino S, Vioux C, Rossio-Pasquier P, et al. Ultraviolet B induces hyperproliferation and modification of epidermal differentiation in normal human skin grafted on to nude mice. Br J Dermatol. 2004;150:658–667. doi: 10.1111/j.0007-0963.2004.05886.x. [DOI] [PubMed] [Google Scholar]
- 32.Van Erp PE, De Mare S, Rijzewijk JJ, Van de Kerkhof PC, Bauer FW. A sequential double immunoenzymic staining procedure to obtain cell kinetic information in normal and hyperproliferative epidermis. Histochem J. 1989;21:343–347. doi: 10.1007/BF01798497. [DOI] [PubMed] [Google Scholar]
- 33.Green H. Cyclic AMP in relation to proliferation of the epidermal cell: a new view. Cell. 1978;15:801–811. doi: 10.1016/0092-8674(78)90265-9. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi H, Honma M, Miyauchi Y, Nakamura S, Ishida-Yamamoto A, Iizuka H. Cyclic AMP differentially regulates cell proliferation of normal human keratinocytes through ERK activation depending on the expression pattern of B-Raf. Arch Dermatol Res. 2004;296:74–82. doi: 10.1007/s00403-004-0478-z. [DOI] [PubMed] [Google Scholar]
- 35.Tong PS, Marcelo CL. Augmentation of keratinocyte differentiation by the epidermal mitogen, 8-bromo-cAMP. Exp Cell Res. 1983;149:215–226. doi: 10.1016/0014-4827(83)90393-2. [DOI] [PubMed] [Google Scholar]
- 36.Iwasaki T, Chen JD, Kim JP, Wynn KC, Woodley DT. Dibutyryl cyclic AMP modulates keratinocyte migration without alteration of integrin expression. J Invest Dermatol. 1994;102:891–897. doi: 10.1111/1523-1747.ep12383031. [DOI] [PubMed] [Google Scholar]
- 37.Hashimoto K. Regulation of keratinocyte function by growth factors. J Dermatol Sci. 2000;24 (Suppl 1):S46–50. doi: 10.1016/s0923-1811(00)00141-9. [DOI] [PubMed] [Google Scholar]
- 38.Ortonne JP. The effects of ultraviolet exposure on skin melanin pigmentation. J Int Med Res. 1990;18 (Suppl 3):8C–17C. [PubMed] [Google Scholar]
- 39.Yamaguchi Y, Hearing VJ. Physiological factors that regulate skin pigmentation. Biofactors. 2009;35:193–199. doi: 10.1002/biof.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goto M, Kadoshima-Yamaoka K, Murakawa M, et al. Phosphodiesterase 7A inhibitor ASB16165 impairs proliferation of keratinocytes in vitro and in vivo. Eur J Pharmacol. 2010;633:93–97. doi: 10.1016/j.ejphar.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 41.Brauchle M, Angermeyer K, Hubner G, Werner S. Large induction of keratinocyte growth factor expression by serum growth factors and pro-inflammatory cytokines in cultured fibroblasts. Oncogene. 1994;9:3199–3204. [PubMed] [Google Scholar]
- 42.Beer HD, Florence C, Dammeier J, McGuire L, Werner S, Duan DR. Mouse fibroblast growth factor 10: cDNA cloning, protein characterization, and regulation of mRNA expression. Oncogene. 1997;15:2211–2218. doi: 10.1038/sj.onc.1201383. [DOI] [PubMed] [Google Scholar]
- 43.O’Keefe EJ, Chiu ML, Payne RE., Jr Stimulation of growth of keratinocytes by basic fibroblast growth factor. J Invest Dermatol. 1988;90:767–769. doi: 10.1111/1523-1747.ep12560956. [DOI] [PubMed] [Google Scholar]
- 44.Schultz G, Rotatori DS, Clark W. EGF and TGF-alpha in wound healing and repair. J Cell Biochem. 1991;45:346–352. doi: 10.1002/jcb.240450407. [DOI] [PubMed] [Google Scholar]
- 45.Marchese C, Chedid M, Dirsch OR, et al. Modulation of keratinocyte growth factor and its receptor in reepithelializing human skin. J Exp Med. 1995;182:1369–1376. doi: 10.1084/jem.182.5.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Werner S. Keratinocyte growth factor: a unique player in epithelial repair processes. Cytokine Growth Factor Rev. 1998;9:153–165. doi: 10.1016/s1359-6101(98)00010-0. [DOI] [PubMed] [Google Scholar]
- 47.Werner S, Krieg T, Smola H. Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol. 2007;127:998–1008. doi: 10.1038/sj.jid.5700786. [DOI] [PubMed] [Google Scholar]
- 48.Finch PW, Murphy F, Cardinale I, Krueger JG. Altered expression of keratinocyte growth factor and its receptor in psoriasis. Am J Pathol. 1997;151:1619–1628. [PMC free article] [PubMed] [Google Scholar]
- 49.Kovacs D, Raffa S, Flori E, et al. Keratinocyte growth factor down-regulates intracellular ROS production induced by UVB. J Dermatol Sci. 2009;54:106–113. doi: 10.1016/j.jdermsci.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 50.Braun S, Krampert M, Bodo E, et al. Keratinocyte growth factor protects epidermis and hair follicles from cell death induced by UV irradiation, chemotherapeutic or cytotoxic agents. J Cell Sci. 2006;119:4841–4849. doi: 10.1242/jcs.03259. [DOI] [PubMed] [Google Scholar]
- 51.Cardinali G, Bolasco G, Aspite N, et al. Melanosome transfer promoted by keratinocyte growth factor in light and dark skin-derived keratinocytes. J Invest Dermatol. 2008;128:558–567. doi: 10.1038/sj.jid.5701063. [DOI] [PubMed] [Google Scholar]
- 52.Wang X, Bi Z, Chu W, Wan Y. IL-1 receptor antagonist attenuates MAP kinase/AP-1 activation and MMP1 expression in UVA-irradiated human fibroblasts induced by culture medium from UVB-irradiated human skin keratinocytes. Int J Mol Med. 2005;16:1117–1124. [PubMed] [Google Scholar]
- 53.Passeron T, Namiki T, Passeron HJ, Le Pape E, Hearing VJ. Forskolin protects keratinocytes from UVB-induced apoptosis and increases DNA repair independent of its effects on melanogenesis. J Invest Dermatol. 2009;129:162–166. doi: 10.1038/jid.2008.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khaled M, Levy C, Fisher DE. Control of melanocyte differentiation by a MITF-PDE4D3 homeostatic circuit. Genes Dev. 2010;24:2276–2281. doi: 10.1101/gad.1937710. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Humanized skin mouse model system. (a) Phenotypes of the C57BL/6 K14-Scf transgenic mice used in this study. Epidermal pigmentation, like that of the fur, is dependent on function of Mc1r and Tyr loci. (b) Eumelanin and pheomelanin contents of depilated dorsal skins of K14-Scf transgenic animals (n=3 per group). * p ≤ 0.05.
Figure S2. Forskolin promotes epidermal melaninization. (a) K14-Scf Mc1re/e Tyr+/+ (extension) animals were treated (3wk, 5d/wk) with vehicle, forskolin, or UVB (2 kJ/m2). Note the darkening of the dorsal skin in the forskolin-treated but not UV-exposed animals. (b) Skin color as measured by reflective colorimetry of animals in “a”. We previously showed that forskolin-induced skin darkening was due to accumulation of epidermal eumelanin (13) (c, d) photographs (c) and reflective colorimetric skin color quantification (d) of K14-Scf Mc1re/e Tyrc2j/c2j (albino extension) animals treated with vehicle (n=3), forskolin, (n=3), or UVB (n=2) as described. Note the lack of dorsal skin darkening in forskolin-treated animals. * p ≤ 0.05.
Figure S3. Epidermal thickening experiment in age- and gender-matched C57BL/6 animals. 8 week-old C57BL/6 Mc1rE/E Tyrc2j/c2j (non-transgenic wild type) animals were treated (3wk, 5d/wk) with vehicle (n=8), forskolin, (n=8) or 2 kJ/m2UVB (n=4). (a) Representative photos of animals at the end of the 3 wk course. (b) representative Hematoxylin and Eosin stained cross-section skin images and (c) epidermal thickness measurements from animals treated in “a”. * p ≤ 0.05.
Figure S4. Inflammatory cell infiltrate in the skin of 21 day-treated animals. Quantification of number of inflammatory cells per high power field in the epidermis and dermis of hematoxylin and eosin-stained skin from K14-Scf Mc1re/e Tyrc2j/c2j (albino extension) animals treated (3wk, 5d/wk) with vehicle or forskolin as described (n=4 for vehicle; n=6 for forskolin). * p ≤ 0.05.
Figure S5. Keratin expression in the skin of C57BL/6 Mc1rE/E Tyrc2j/c2j (non-transgenic wild type) animals treated with vehicle or forskolin daily for 7 days. Western blot analysis to indicate keratinocyte differentiation was performed for the intermediate-filaments keratin 14 (basal), keratin 1 (suprabasal), and involucrin (suprabasal). We noted much variability in keratin protein expression between animals. Each experimental condition (vehicle or forskolin) involved the same animal biopsied at day 0, day 3 and day 7. We show two representative animals for each treatment group.
Figure S6. Forskolin increases whole skin thickness (a) and darkens skin color (b) in tyrosinase intact animals. Thicknesses (a) or skin color (b) of whole dorsal depilated and defatted skins of animals described in Fig. 4 were determined by micrometer quantification and reflectometry respectively. * p ≤ 0.05.