Abstract
Background/Purpose
Developmental defects of the enteric nervous system lead to a variety of disorders including Hirschprung’s disease. We have previously shown that heparin-binding EGF-like growth factor (HB-EGF) exerts neuroprotective effects on injured neurons. The goal of this study was to assess the role of HB-EGF in enteric nervous system (ENS) development and evaluate the effect of HB-EGF on enteric neural crest-derived cell (ENCC) migration in the developing gastrointestinal tract of mice.
Materials and Methods
HB-EGF immunohistochemistry was used to examine HB-EGF protein expression in the hindgut of embryonic mice. Gut specimens were stained for PGP9.5 (a neuronal cell marker) to examine the extent of ENCC migration in the intestine at different embryonic stages in HB-EGF knockout (KO) and wild type (WT) mice. Embryonic gut organ cultures were established to examine the effect of HB-EGF on ENCC migration.
Results
The expression of HB-EGF was limited to the endodermal epithelium of the hindgut in early gestation, but rapidly involved the hindgut mesenchyme after ENCC migrated into this region. ENCC migration was significantly delayed in HB-EGF KO compared to WT embryos, leading to defects in neural colonization of the distal gut in postnatal HB-EGF KO mice. Addition of HB-EGF to WT embryonic intestine significantly promoted ENCC migration, as demonstrated by a significant increase in the ratio of ENCC migration distance towards the distal hindgut/total colon length (78 ± 4% vs. 53 ± 2%, p=0.001).
Conclusions
Deletion of the HB-EGF gene leads to ENS developmental defects. HB-EGF stimulates ENCC migration in the gut, supporting a potential role for administration of HB-EGF in the future for the treatment of patients with intestinal neuronal disorders.
Keywords: heparin-binding EGF-like growth factor, enteric neural crest derived cells, migration, enteric nervous system
The enteric nervous system (ENS) is a collection of neurons and glia which are highly organized into a network of interconnected ganglia distributed in two major plexi including the myenteric plexus and the submucosal plexus in the wall of the intestine [1, 2]. It is the largest and most complex division of the peripheral nervous system. The ENS regulates intestinal motility, mucosal secretion, intestinal blood flow, and sensation in the gut [1]. To form the ENS, ENS precursor cells originating from the hindbrain neural crest enter the foregut and advance rostro-caudally in the intestine to populate the entire gastrointestinal tract through a complex process of migration, proliferation and differentiation [3, 4]. Failure of enteric neural crest-derived cells (ENCC) to colonize the distal bowel results in Hirschprung’s disease, characterized by the absence of enteric neurons in the distal bowel [5, 6].
Heparin-binding EGF-like growth factor (HB-EGF) was initially identified as a secreted product of cultured human macrophages [7], and then identified as a member of the epidermal growth factor (EGF) family [8]. In the central nervous system, HB-EGF acts as a neurotrophic molecule, serving to enhance neural stem cell survival, neural proliferation and synapse formation [9–12]. HB-EGF is highly enriched in cortical neurons during the development of the brain [13], and it specifically induces the migration of cortical neurons in a chemotactic fashion [14]. Interestingly, HB-EGF guides the emigration of cranial neural crest cells from the hindbrain to the periphery during the development of cranial nerves. Knockdown of HB-EGF using siRNA results in disruption of cranial neural crest cell migration and subsequent cranial nerve defects [15]. These results suggest that HB-EGF may be responsible for the proper migration of neural crest cells. The current study examines the role of HB-EGF in enteric neuronal crest cell migration and nerve network formation during ENS development.
1. Materials and methods
1.1. HB-EGF(−/−) knockout (KO) mice
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee of Nationwide Children’s Hospital (Protocol # AR06-00092). To investigate the effects of HB-EGF loss-of-function on ENS development, HB-EGF KO mice (n=48) and their HB-EGF(+/+) WT counterparts (n=45) were examined. HB-EGF KO mice on a C57BL/6J × 129 background and their HB-EGF WT C57BL/6J × 129 counterparts were kindly provided by Dr. David Lee (Chapel Hill, NC). In these HB-EGF KO mice, HB-EGF exons 1 and 2 were replaced with PGK-Neo, thus deleting the signal peptide and propeptide domains [16]. The desired targeting events were verified by Southern blots of genomic DNA and exon-specific polymerase chain reaction, with Northern blots confirming the absence of the respective transcripts. Adult ENS was analyzed at 4 weeks of age. For embryo studies, the morning of the vaginal plug was designated as Day E05..
1.2. Immunohistochemistry
Mouse colon was harvested at embryonic stages E11.5 and E14.5 and postnatal stages P4W. Transverse intestinal cryosections (10μm) were prepared and incubated with polyclonal HB-EGF antibody (10 μg/ml; R&D Systems, Inc., Minneapolis, MN) or polyclonal anti-protein gene product 9.5 antibody (PGP9.5, 1:500 dilution; Millipore, Jaffrey, NH) overnight at 4°C. The anti-HB-EGF antibody was selected for its ability to detect HB-EGF while not cross-reacting with other EGF family members such as EGF, TGF-α or Amphiregulin. Slides were then incubated with fluorophore-conjugated donkey anti-goat IgG (green, 1.5 μg/ml; Jackson ImmunoResearch Laboratories Inc., West Grove, PA) or donkey anti-rabbit IgG (red, 1μg/ml; Cy3, Jackson ImmunoResearch Laboratories Inc.) for 1 h at room temperature, washed in PBS, and mounted using anti-fade reagent (SlowFade, Molecular Probes, Carlsbad, CA). For whole mount immunostaining, the distal guts of E12.5 and E14.5 embryos were harvested. In addition, muscle strips including the myenteric plexuses were dissected from the distal colons of postnatal day 3 (P3) or week 4 (P4W) mice. Tissues were permeabilized in PBS containing 2% Triton X-100 and 0.5% DMSO at 4°C for 3 days, incubated in blocking solution (10% normal goat serum in 2% Triton X-100 and 0.5% DMSO) for 1 h at room temperature, incubated with anti-PGP9.5 antibody (1:200 dilution) or with anti-Neurofilament 160 Kd antibody (1:50 dilution; Invitrogen, Camarillo, CA) for 24h, washed with PBS several times, and then incubated with goat anti-rabbit IgG (green, 10μg/ml; Alexa 488, Molecular Probes, Eugene, OR) or goat anti-mouse IgG (red, 1μg/ml; Cy3, Jackson ImmunoResearch Laboratories Inc.) for 2h. Fluorescent staining was visualized using a fluorescence microscope (LSM510; Carl Zeiss Inc., Thornwood, NY).
1.3. Stool analysis and colonic transit
Four week old nonfasted male HB-EGF KO mice and their WT counterparts were euthanized by carbon dioxide asphyxiation, and the colon removed and photographed. Colonic stool was carefully collected and the wet weight determined. Stool retention was represented as the average stool weight. For stool frequency studies, animals were caged separately with a white paper sheet at the base of the cage to allow for easier detection of stool pellets. The number of stool pellets extruded per hour was used as an index of colonic emptying. Pellets were harvested immediately after expulsion and weighed to obtain wet weights. Stools were then dried in a 60°C incubator overnight to obtain dry weights, and the water content calculated using the formula: water content = (wet weight − dry weight)/wet weight × 100%. For colonic motility studies, 4 week old HB-EGF KO and WT mice were fasted for 12h. Mice were lightly anesthetized with isoflurane and a glass bead (3 mm diameter) was inserted through the anus and placed into the colon at a distance of 2 cm from the anal verge. After bead insertion, mice were placed individually in cages with white paper to aid in visualization of bead expulsion. Colonic motility was assessed by measuring the time between bead placement and expulsion of the bead. The time to bead expulsion was used as an estimate of the rate of colonic transit [17, 18].
1.4. Chemoattractant assay
Midguts (containing neural crest-derived cells) were dissected from E11.5 WT mice and 2 mm segments were planted in collagen-coated culture dishes in Neurobasal medium containing 1% fetal bovine serum (FBS). HB-EGF impregnated agarose beads were prepared as follows [19]. Affi-gel Blue Gel beads (BioRad, Hercules, CA) were incubated with human recombinant HB-EGF (1 μg/μl) for 1h at 37°C, agitating every 10 min. Control beads were incubated with bovine serum albumin (BSA). To confirm that HB-EGF was successfully bound to the agarose beads, HB-EGF soaked beads were washed in PBS and then subjected to SDS-PAGE and Western blotting using anti-HB-EGF antibodies. For the chemoattractant assay, HB-EGF soaked beads were placed on one side of cultured intestinal explants, with control beads placed at an equal distance on the opposite side of the tissue. Beads were located ~500–800 μm from the edge of the tissue. Cultures were maintained in an incubator with 5% CO2 at 37C° for 5 days and then fixed in 4% paraformaldehyde. The cultured explants were cut into 5 μm paraffin cross sections and processed for PGP9.5 immunohistochemistry.
1.5. Embryonic intestinal organ culture
WT embryonic intestines were dissected from E11.5 embryos, and cultured in Neurobasal medium supplemented with 1% FBS in the presence or absence of HB-EGF (20 ng/ml). After 2 days in culture, the gut was immunostained using the neuronal marker PGP9.5. ENCC migration distance was determined by measuring the distance between the ileocecal junction and most caudally migrated cells. ENCC migration was expressed as the distance of ENCC migration/total colonic length × 100%.
1.6. Statistical analysis
All data are presented as mean ± SEM. Statistical analyses were performed using Student’s t test or ANOVA (SigmaPlot 11.0). Absolute p values are shown, with p values < 0.05 considered statistically significant.
2. Results
2.1. Expression of HB-EGF in embryonic and postnatal intestine
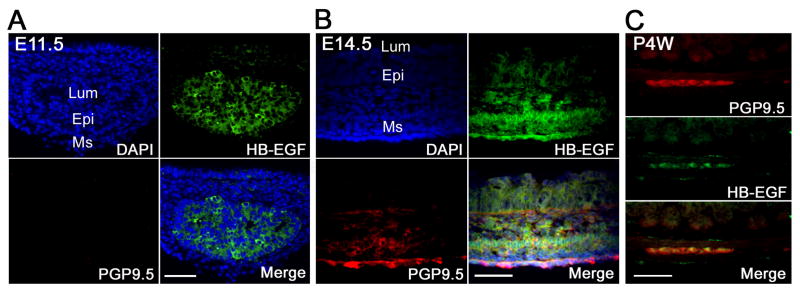
In mice, ENCC enter the foregut at approximately embryonic day 8.5 (E8.5), reach the cecum at E11.5, and complete their colonization by E14.5 [20]. To examine the potential role of HB-EGF in ENS development, the expression pattern of HB-EGF in mouse embryonic hindgut was analyzed by HB-EGF immunohistochemistry. At E11.5, a stage when ENCC have just reached the cecum but are not present in the remainder of the colon, HB-EGF expression was limited to the endodermal epithelium of the hindgut but absent in the surrounding mesenchyme (Figure 1A). At E14.5, prominent HB-EGF immunostaining was detected in the outer mesenchyme with weak HB-EGF expression noted in the inner epithelium, thus demonstrating a gradient of immunoreactivity across the gut wall (inner layer low, outer layer high expression) (Figure 1B). With the invasion of ENCC into the hindgut mesenchyme at this embryonic stage, robust HB-EGF immunostaining was noticed in the PGP9.5 positive cells (Figure 1B), which are migrating into the presumptive myenteric regions between the developing longitudinal and circular muscles located in the outer layer of the gut. This suggests that HB-EGF is initially expressed in epithelial cells at early embryonic stages, and then expressed in mesenchymal cells including migrating ENCC in the developing gut. To determine whether HB-EGF expression persisted in enteric neurons postnatally, we examined the ENS including myenteric plexus from 4 week old adult mouse colon. HB-EGF was expressed in myenteric plexus neurons (Figure 1C), suggesting that it may be involved in the neuroplasticity and remodeling of the ENS after birth.
Figure 1. Localization of HB-EGF in embryonic and postnatal WT mouse intestine.
Shown are representative photomicrographs of HB-EGF immunostaining of transverse sections of embryonic hindgut harvested at E11.5 (A) and E14.5 (B) and the colon of a WT mouse at 4 weeks postnatal age (C). HB-EGF protein was identified using anti-HB-EGF antibodies (green staining), neuronal cells were identified using anti-PGP9.5 antibodies (red staining), and DAPI was used to counter-stain nuclei (purple staining). E11.5, embryonic day 11.5; E14.5, embryonic day 14.5; P4W, postnatal week 4; Lum, lumen; Epi, epithelium; Ms, mesenchyme; scale bar = 100 μm.
2.2. HB-EGF knockout mice have increased stool retention and delayed colonic emptying
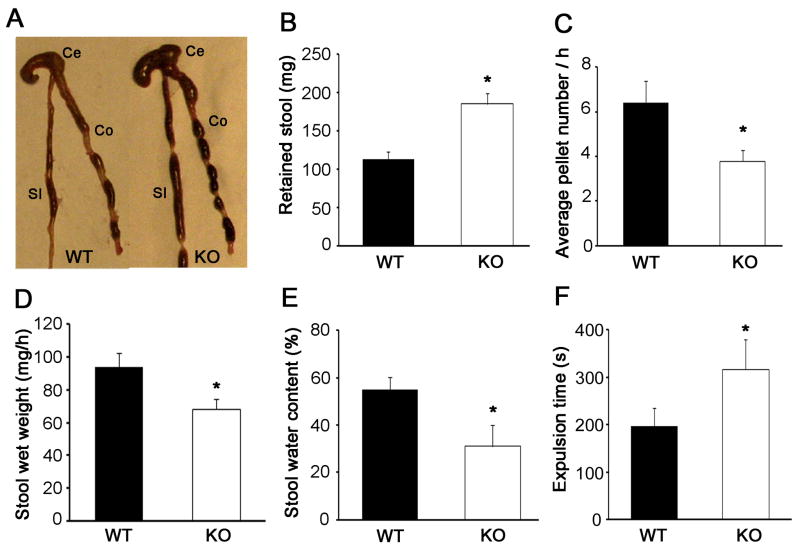
HB-EGF KO mice displayed significant colonic stool retention compared to WT mice (Figure 2A, B). To evaluate whether differences in stool retention were due to differences in the frequency of bowel movements, we assessed colonic emptying in WT and HB-EGF KO mice. Consistent with the stool retention data, we found that HB-EGF KO mice had delayed colonic emptying. The frequency of bowel movements, measured as the average number of pellets extruded per hour, was significantly lower in HB-EGF KO mice compared to WT mice (Figure 2C). In addition, the wet weight of the stool extruded per hour in HB-EGF KO mice was significantly lower compared to WT mice (Figure 2D). The percent water content in the stool was also significantly reduced in HB-EGF KO mice compared to WT mice (Figure 2E). Colonic motility, as assessed by the bead extrusion test, was significantly reduced in HB-EGF KO mice compared to WT mice (Figure 2F). The impaired colonic transit in HB-EGF KO mice suggests that deletion of the HB-EGF gene is associated with ENS defects.
Figure 2. Stool retention and colonic transit in HB-EGF KO and WT mice.
(A) Representative photograph showing increased stool retention in HB-EGF KO mouse colon; (B) weight of retained colonic contents in WT and HB-EGF KO mice. n=6 animals/group; (C) average number of stool pellets passed per hour in WT and HB-EGF KO mice. n=5 animals/group; (D) stool wet weight in WT and HB-EGF KO mice. n=5 animals/group; (E) stool water content in WT and HB-EGF KO mice. n=5 animals/group; and (F) glass bead expulsion time. n=8 animals/group. All data are represented as mean ± SEM. *p<0.05, **p<0.01. SI: small intestine; Ce: cecum; Co: colon.
2.3. HB-EGF knockout mice exhibit delayed ENCC migration in the developing intestine and ENS defects postnatally
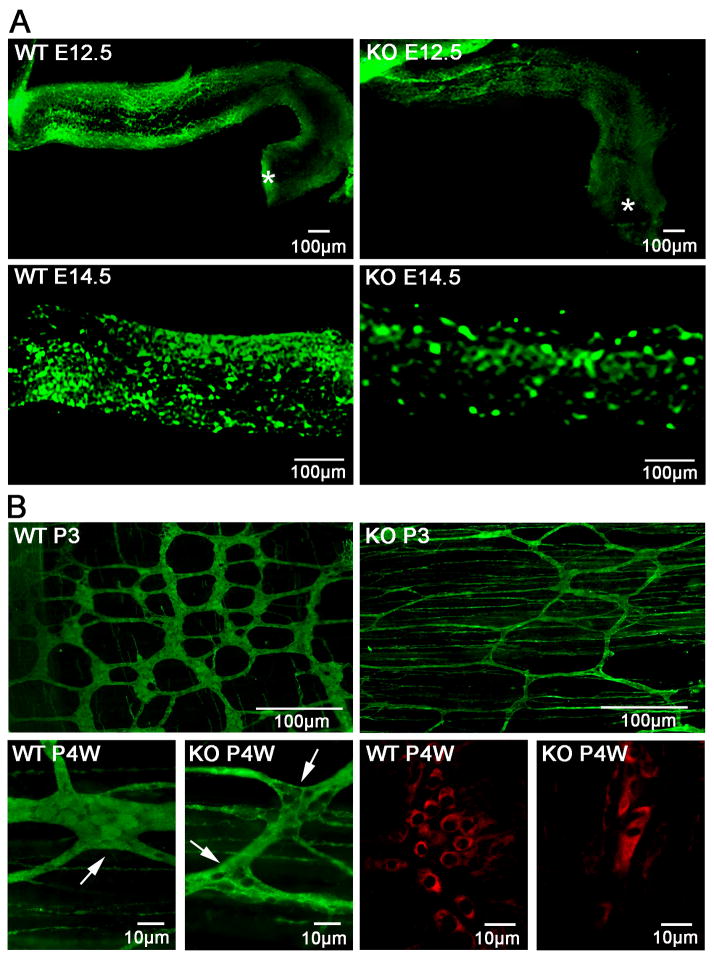
To determine whether HB-EGF is involved in the migration of ENCC during gut development, we first analyzed ENS development at embryonic stages E12.5 to E14.5, during which ENCC migrate within the gut wall towards the distal part of the hindgut. At E12.5, ENCC migrated more than of the length of the hindgut in WT mice, but just entered the proximal hindgut in HB-EGF KO mice (Figure 3A). At E14.5, WT hindguts were abundantly colonized with PGP9.5 positive neurons, while fewer neurons were seen in the hindgut of HB-EGF KO mice (Figure 3A). Normally, as ENCC migrate through the outer part of the mesenchyme towards the hindgut, differentiated neuronal cells cluster to form ganglia interconnected by neural fibers. Therefore, we next harvested the distal colons at postnatal day 3 (P3) and postnatal 4 weeks (P4W), and stained myenteric plexus neurons using whole mount PGP9.5 immunohistochemistry. Significant neuronal cell reduction in the myenteric plexus ganglia with abnormally sparse neural fibers were noted in the distal colons of HB-EGF KO mice, whereas WT mice had clustered neuronal cells in ganglia with well-developed neural strands (Figure 3B). The structure of myenteric ganglia was confirmed by immunohistochemistry using anti-Neurofilament 160 Kd antibody, showing strikingly decreased neuronal cells in the myenteric plexus ganglia of HB-EGF KO mice (Figure 3B). These results suggest that deletion of the HB-EGF gene delays the migration of ENCC during embryonic development, with subsequent hypoganglionosis and an abnormal ENS in the distal colon postnatally. To eliminate the possibility that HB-EGF KO mice had simultaneous defects in the structure of the intestinal smooth muscle layer and the interstitial cell of Cajal (ICC), we examined the morphology of smooth muscle cells and ICCs in HB-EGF KO and WT mice, and found no significant differences between the two (data not shown).
Figure 3. ENS development in WT and HB-EGF KO mice.
(A) Prenatal delay in migration of ENCC in HB-EGF KO mice. Whole-mount PGP9.5 immunohistochemistry of the distal intestine of WT and HB-EGF KO mice harvested at E12.5 and E14.5 was performed to examine the migration of ENCC. Asterisks mark the terminus of the hindgut. Note the significantly delayed migration and colonization of ENCC in the distal intestine of HB-EGF KO mice at E12.5 and E14.5. (B) Postnatal neural abnormalities in the distal colon of HB-EGF KO mice. Note the marked reduction of myenteric plexuses including fewer PGP9.5 positive enteric neurons in the distal colons of postnatal HB-EGF KO mice at P3D and P4W compared to WT control. White arrows indicate the myenteric plexuses ganglia in the distal colon. The alternative neuronal marker anti-Neurofilament 165 kD confirmed reduced neuronal cells in the ganglia of the distal colon in HB-EGF KO mice at P4W.
2.4. HB-EGF exerts chemoatttactant effects on ENCC
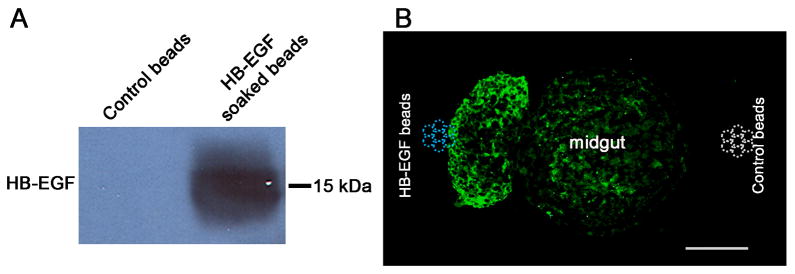
To further assess whether HB-EGF functions as a chemoattractant factor for ENCC, we cultured midgut segments from WT mice at E11.5, a time point when ENCC are abundant in the mouse midgut. We planted HB-EGF impregnated beads on one side of midgut segments and BSA soaked control beads on the opposite side of the midgut segments. After 5 days, ENCC were visualized by PGP9.5 immunostaining. ENCC migrated from the gut segments towards the HB-EGF impregnated beads (Figure 4). Extremely weak migration was noted on the opposite side of the gut segments towards the BSA control beads. This confirms that HB-EGF exerts potent chemoattractive effects on migrating ENCC.
Figure 4. Neuronal cell chemoattractant assay.
(A) Western blot of HB-EGF soaked beads. HB-EGF protein was abundant in HB-EGF soaked agarose beads compared to control BSA beads. (B) E11.5 midgut segments were grown in collagen-coated petri dishes with HB-EGF-impregnated agarose beads on the left and control BSA agarose beads on the right. The blue and white dashed circles indicate the approximate locations of HB-EGF soaked beads and control BSA beads, respectively. After 5 days, explant cross sections were cut and processed for PGP9.5 immunohistochemistry. Note the directional migration of intestinal neuronal cells and neurites towards the HB-EGF impregnated beads. Scale bar = 100 μm.
2.5. HB-EGF promotes ENCC migration
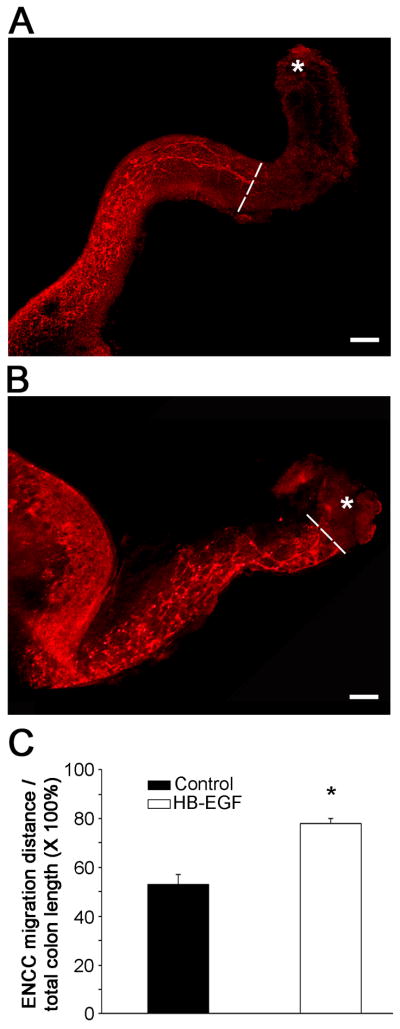
To further confirm that HB-EGF promotes ENCC migration rostro-caudally in the developing gut, we next examined intestine from WT mice harvested at 11.5 days of gestation. Enteric neurons in the embryonic gut were visualized with PGP9.5 immunohistochemistry after 2 days of in vitro culture in the presence or absence of HB-EGF. We found that addition of HB-EGF significantly promoted ENCC colonization in the distal gut from the cecum towards the anus (Figure 5A, B). Quantification of ENCC migration revealed 53 ± 2% migration in the absence of HB-EGF, which increased significantly to 78 ± 4% migration in the presence of HB-EGF (p<0.01) (Figure 5C).
Figure 5. ENCC colonization in gut organ explant cultures.
Embryonic guts were dissected from WT mice at E11.5 and cultured in (A) control medium or (B) medium containing HB-EGF (20 ng/ml). PGP9.5 immunohistochemistry was performed after 2 days. White dashed lines indicate the position of the most distally migrated ENCC; asterisks mark the terminus of the hindgut. Scale bar = 100 μm. (C) Quantitative analysis of ENCC migration. ENCC migration was expressed as the distance of ENCC migration from the cecocolic junction distally/total colonic length ×100%. Data are based on 4 independent experiments; *p<0.01.
3. DISCUSSION
It has become increasingly recognized that HB-EGF regulates neural progenitor cells during the development of the CNS and the peripheral nervous system. HB-EGF is involved in the path finding course of migrating neural progenitor cells [15]. During early brain development, HB-EGF expression is increased in the cerebral cortex, specifically at the marginal zone and in the subplate, to induce cortex neuron migration [13, 21]. HB-EGF regulates the migratory behavior of neural cells upon its activation of ErbB receptors, as ErbB expression is found in migrating neurons in all migratory routes [14]. Whereas much information is known about the role of HB-EGF in the development of the CNS, there are no previous reports of the role(s) played by HB-EGF in the developing ENS.
In the present study, we have confirmed HB-EGF protein expression in embryonic mouse intestine and ENCC, suggesting an important role for HB-EGF in the development of the ENS. Our results show that during the development of the ENS, HB-EGF is expressed in a precise temporal and spatial pattern. At early embryonic stages (E11.5), a low level of HB-EGF is detected only in the hindgut epithelium. However, at middle embryonic stages (E14.5), higher levels of HB-EGF are expressed by a heterogeneous population of cells including epithelial cells, mesenchymal cells and invading ENCC. HB-EGF is present in an inner layer-low, outer layer-high gradient of immunoreactivity across the gut wall at mid to late embryonic stages, a period when ENCC migrate through the mesenchyme and colonize the distal gut. The specific expression pattern of HB-EGF in embryonic gut suggests that it may be involved in regulating the normal migration of ENCC from the proximal intestine to the distal intestine. Moreover, the ENS undergoes continuous development after birth, with a population of neural crest-derived stem cells remaining in the gut into adulthood [22]. We have demonstrated continued HB-EGF expression in adult myenteric plexus ganglia, suggesting that HB-EGF may play a role in postnatal ENS maturation and plasticity.
Deletion of the HB-EGF gene impairs the development of several neural crest cell derived structures including craniofacial tissue and cardiac valves, with HB-EGF KO mice displaying heart valve malformation and cardiac dysfunction [15, 23, 24]. Knockout of the HB-EGF gene leads to aberrant migration of hindbrain-derived cranial neural crest cells within the paraxial mesenchyme, and abnormal axonal projection during the development of the cranial nerves [25]. We now demonstrate that HB-EGF KO mice exhibit delayed embryonic ENCC migration as well as abnormalities of the ENS after birth, including failure of neuronal cells to form normal numbers of ganglion cells, or hypoganglionosis. We have also shown that deletion of the HB-EGF gene in primary intestinal neurons retards neurite outgrowth and decreases the expression of synapse scaffold protein (unpublished data). These defects of interneuronal connectivity may be responsible, in part, for the ENS abnormalities seen in HB-EGF KO mice. HB-EGF KO mice display increased stool retention and delayed colonic emptying, consistent with impaired colon transit. The ENS abnormalities we have documented in these mice are likely to be responsible for their colonic dysmotility.
ENCC migration is a complex process. The rostro-caudal migration of ENCC is influenced by multiple factors including the autonomous tendency of ENCC to migrate, ENCC cell-cell interactions, and the presence of attractive or repulsive molecules in the gut mesenchyme [26, 27]. Enteric neurons project neurites caudally, which is the same direction in which the ENCC are migrating, indicating that the same mechanism drives both neurite polarity and cell migration. We have previously shown that HB-EGF promotes neurite outgrowth by constitutive activation of the MAPK signaling pathway [28]. The current study demonstrating chemoattraction of neurons and growing neurites towards HB-EGF soaked beads strongly supports the chemoattractant effects of HB-EGF on ENCC. Our gut organ culture studies confirm that HB-EGF promotes ENCC colonization towards the distal gut.
Transgenic technology, especially in the mouse, has provided us with a unique tool to study the genes associated with human inherited diseases including Hirschsprung’s disease. However, one must be very careful in interpreting the findings observed in genetic mouse models since not all of the genetic abnormalities that have been correlated with human diseases are exactly comparable to the analogous mutation in mouse models. Hirschsprung’s disease in humans is genetically heterogeneous and multigenic [29–32]. Dominant mutations of the receptor tyrosine kinase gene Ret at human chromosome 10q11.1 account for ~50% of familial cases of Hirschsprung’s disease and ~35% of sporadic cases, with a penetrance of 50–70% [33–35]. In contrast, mice carrying the Ret mutation differ from humans since aganglionosis occurs only in the homozygous null genotype for Ret with complete penetrance. The ENS is normal in heterozygous mice that carry only one single mutated Ret allele [36]. We do not yet have clinical data showing the HB-EGF expression pattern in Hirschsprung’s disease patients. In the future, genetic screening for HB-EGF gene expression in Hirschsprung’s disease patients compared with healthy individuals, or testing the expression levels of HB-EGF in the aganglionic colon of Hirschsprung’s disease patients or other intestinal dysganglionosis, might contribute to a better understanding of the roles of HB-EGF in the pathogenesis of the ENS disorders.
In conclusion, the current study demonstrates that HB-EGF KO mice have abnormalities of the ENS, manifested by delayed migration of ENCC and defects in the ENS in the distal colon. HB-EGF is a potent chemoattractant for neural precursor cells, and addition of HB-EGF promotes ENCC colonization of the gut. Future experiments will be designed to determine whether clinical administration of HB-EGF may be beneficial in disease states manifested by primary ENS abnormalities such as Hirschsprung’s disease, or after damage to the ENS secondary to intestinal injury including disease processes such as necrotizing enterocolitis or ischemia/reperfusion injury.
Acknowledgments
Authors thank Amanda Darbyshire for breeding of all timed pregnant mice. This work was supported by grants R01 DK65306, R01 GM61193 and R01 DK074611 from the National Institutes of Health (GEB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goyal RK, Hirano I. The enteric nervous system. N Engl J Med. 1996;334:1106–1115. doi: 10.1056/NEJM199604253341707. [DOI] [PubMed] [Google Scholar]
- 2.Young HM, Hearn CJ, Newgreen DF. Embryology and development of the enteric nervous system. Gut. 2000;47(Suppl 4):iv12–14. doi: 10.1136/gut.47.suppl_4.iv12. discussion iv26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burns AJ, Pasricha PJ, Young HM. Enteric neural crest-derived cells and neural stem cells: biology and therapeutic potential. Neurogastroenterol Motil. 2004;16 (Suppl 1):3–7. doi: 10.1111/j.1743-3150.2004.00466.x. [DOI] [PubMed] [Google Scholar]
- 4.Le Douarin NM, Dupin E, Ziller C. Genetic and epigenetic control in neural crest development. Curr Opin Genet Dev. 1994;4:685–695. doi: 10.1016/0959-437x(94)90135-p. [DOI] [PubMed] [Google Scholar]
- 5.Holschneider AM, Meier-Ruge W, Ure BM. Hirschsprung’s disease and allied disorders--a review. Eur J Pediatr Surg. 1994;4:260–266. doi: 10.1055/s-2008-1066115. [DOI] [PubMed] [Google Scholar]
- 6.Martucciello G. Hirschsprung’s disease, one of the most difficult diagnoses in pediatric surgery: a review of the problems from clinical practice to the bench. Eur J Pediatr Surg. 2008;18:140–149. doi: 10.1055/s-2008-1038625. [DOI] [PubMed] [Google Scholar]
- 7.Besner G, Higashiyama S, Klagsbrun M. Isolation and characterization of a macrophage-derived heparin-binding growth factor. Cell Regul. 1990;1:811–819. doi: 10.1091/mbc.1.11.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reynolds LF, Eng C. RET mutations in multiple endocrine neoplasia type 2 and Hirschsprung disease. Curr Opin Pediatr. 1995;7:702–709. doi: 10.1097/00008480-199512000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Jin K, Mao XO, Del Rio Guerra G, et al. Heparin-binding epidermal growth factor-like growth factor stimulates cell proliferation in cerebral cortical cultures through phosphatidylinositol 3′-kinase and mitogen-activated protein kinase. J Neurosci Res. 2005;81:497–505. doi: 10.1002/jnr.20510. [DOI] [PubMed] [Google Scholar]
- 10.Jin K, Mao XO, Sun Y, et al. Heparin-binding epidermal growth factor-like growth factor: hypoxia-inducible expression in vitro and stimulation of neurogenesis in vitro and in vivo. J Neurosci. 2002;22:5365–5373. doi: 10.1523/JNEUROSCI.22-13-05365.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viti J, Feathers A, Phillips J, et al. Epidermal growth factor receptors control competence to interpret leukemia inhibitory factor as an astrocyte inducer in developing cortex. J Neurosci. 2003;23:3385–3393. doi: 10.1523/JNEUROSCI.23-08-03385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagner B, Natarajan A, Grunaug S, et al. Neuronal survival depends on EGFR signaling in cortical but not midbrain astrocytes. EMBO J. 2006;25:752–762. doi: 10.1038/sj.emboj.7600988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakagawa T, Sasahara M, Hayase Y, et al. Neuronal and glial expression of heparin-binding EGF-like growth factor in central nervous system of prenatal and early-postnatal rat. Brain Res Dev Brain Res. 1998;108:263–272. doi: 10.1016/s0165-3806(98)00057-1. [DOI] [PubMed] [Google Scholar]
- 14.Caric D, Raphael H, Viti J, et al. EGFRs mediate chemotactic migration in the developing telencephalon. Development. 2001;128:4203–4216. doi: 10.1242/dev.128.21.4203. [DOI] [PubMed] [Google Scholar]
- 15.Hoshino H, Uchida T, Otsuki T, et al. Cornichon-like protein facilitates secretion of HB-EGF and regulates proper development of cranial nerves. Mol Biol Cell. 2007;18:1143–1152. doi: 10.1091/mbc.E06-08-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson LF, Qiu TH, Sunnarborg SW, et al. Defective valvulogenesis in HB-EGF and TACE-null mice is associated with aberrant BMP signaling. EMBO J. 2003;22:2704–2716. doi: 10.1093/emboj/cdg264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen JJ, Li Z, Pan H, et al. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: Abnormal intestinal motility and the expression of cation transporters. J Neurosci. 2001;21:6348–6361. doi: 10.1523/JNEUROSCI.21-16-06348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sibaev A, Yuce B, Kemmer M, et al. Cannabinoid-1 (CB1) receptors regulate colonic propulsion by acting at motor neurons within the ascending motor pathways in mouse colon. Am J Physiol Gastrointest Liver Physiol. 2009;296:G119–128. doi: 10.1152/ajpgi.90274.2008. [DOI] [PubMed] [Google Scholar]
- 19.Olwin BB, Hauschka SD. Fibroblast growth factor receptor levels decrease during chick embryogenesis. J Cell Biol. 1990;110:503–509. doi: 10.1083/jcb.110.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sasselli V, Pachnis V, Burns AJ. The enteric nervous system. Dev Biol. 2012 Jan 24; doi: 10.1016/j.ydbio.2012.01.012. [Epub ahead of print]; http://dx.doi.org/10.1016/j.ydbio.2012.01.012. [DOI] [PubMed]
- 21.Mishima K, Higashiyama S, Nagashima Y, et al. Regional distribution of heparin-binding epidermal growth factor-like growth factor mRNA and protein in adult rat forebrain. Neurosci Lett. 1996;213:153–156. doi: 10.1016/0304-3940(96)12850-0. [DOI] [PubMed] [Google Scholar]
- 22.Kruger GM, Mosher JT, Bixby S, et al. Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential, and factor responsiveness. Neuron. 2002;35:657–669. doi: 10.1016/s0896-6273(02)00827-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwamoto R, Yamazaki S, Asakura M, et al. Heparin-binding EGF-like growth factor and ErbB signaling is essential for heart function. Proc Natl Acad Sci U S A. 2003;100:3221–3226. doi: 10.1073/pnas.0537588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwamoto R, Mine N, Kawaguchi T, et al. HB-EGF function in cardiac valve development requires interaction with heparan sulfate proteoglycans. Development. 2010;137:2205–2214. doi: 10.1242/dev.048926. [DOI] [PubMed] [Google Scholar]
- 25.Golding JP, Trainor P, Krumlauf R, et al. Defects in pathfinding by cranial neural crest cells in mice lacking the neuregulin receptor ErbB4. Nat Cell Biol. 2000;2:103–109. doi: 10.1038/35000058. [DOI] [PubMed] [Google Scholar]
- 26.Druckenbrod NR, Epstein ML. Behavior of enteric neural crest-derived cells varies with respect to the migratory wavefront. Dev Dyn. 2007;236:84–92. doi: 10.1002/dvdy.20974. [DOI] [PubMed] [Google Scholar]
- 27.Rothman TP, Goldowitz D, Gershon MD. Inhibition of migration of neural crest-derived cells by the abnormal mesenchyme of the presumptive aganglionic bowel of ls/ls mice: analysis with aggregation and interspecies chimeras. Dev Biol. 1993;159:559–573. doi: 10.1006/dbio.1993.1264. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Y, Besner GE. Heparin-binding epidermal growth factor-like growth factor is a potent neurotrophic factor for PC12 cells. Neurosignals. 2010;18:141–151. doi: 10.1159/000319823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edery P, Pelet A, Mulligan LM, et al. Long segment and short segment familial Hirschsprung’s disease: variable clinical expression at the RET locus. J Med Genet. 1994;31:602–606. doi: 10.1136/jmg.31.8.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puffenberger EG, Hosoda K, Washington SS, et al. A missense mutation of the endothelin-B receptor gene in multigenic Hirschsprung’s disease. Cell. 1994;79:1257–1266. doi: 10.1016/0092-8674(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 31.Puffenberger EG, Kauffman ER, Bolk S, et al. Identity-by-descent and association mapping of a recessive gene for Hirschsprung disease on human chromosome 13q22. Hum Mol Genet. 1994;3:1217–1225. doi: 10.1093/hmg/3.8.1217. [DOI] [PubMed] [Google Scholar]
- 32.Martucciello G, Ceccherini I, Lerone M, et al. Pathogenesis of Hirschsprung’s disease. J Pediatr Surg. 2000;35:1017–1025. doi: 10.1053/jpsu.2000.7763. [DOI] [PubMed] [Google Scholar]
- 33.Romeo G, Ronchetto P, Luo Y, et al. Point mutations affecting the tyrosine kinase domain of the RET proto-oncogene in Hirschsprung’s disease. Nature. 1994;367:377–378. doi: 10.1038/367377a0. [DOI] [PubMed] [Google Scholar]
- 34.Edery P, Lyonnet S, Mulligan LM, et al. Mutations of the RET proto-oncogene in Hirschsprung’s disease. Nature. 1994;367:378–380. doi: 10.1038/367378a0. [DOI] [PubMed] [Google Scholar]
- 35.Lantieri F, Griseri P, Puppo F, et al. Haplotypes of the human RET proto-oncogene associated with Hirschsprung disease in the Italian population derive from a single ancestral combination of alleles. Ann Hum Genet. 2006;70:12–26. doi: 10.1111/j.1529-8817.2005.00196.x. [DOI] [PubMed] [Google Scholar]
- 36.Robertson K, Mason I, Hall S. Hirschsprung’s disease: genetic mutations in mice and men. Gut. 1997;41:436–441. doi: 10.1136/gut.41.4.436. [DOI] [PMC free article] [PubMed] [Google Scholar]