Abstract
The expansive use of immunosuppressive medications in fields such as transplantational medicine and oncology, the higher frequency of invasive procedures in an aging population and the HIV/AIDS pandemic have increased the frequency of systemic fungal infections. At the same time, increased resistance of pathogenic fungi to classical antifungal agents has led to sustained research efforts targeting alternative antifungal strategies.
In this review, we focus on two promising approaches: cationic peptides and the targeting of fungal virulence factors. Cationic peptides are small, predominantly positively charged protein fragments which exert direct and indirect antifungal activities, one mechanism of action being the permeabilization of the fungal membrane. They include lysozyme, defensins, and cathelicidins, as well as novel synthetic peptides. Amongst fungal virulence factors, the targeting of candidal secreted aspartic proteinases seems to be a particularly promising approach.
Keywords: antifungal therapies, antimicrobial peptides, defensin, cathelicidin, Sap inhibitors
Facultative pathogenic fungal organisms such as Candida albicans colonize mucosal surfaces of the majority of humans, without provoking a clinical infection in immunocompetent individuals (1, 2). The role of antimicrobial peptides in the prevention of fungal infections has recently received increased attention. These small, predominantly cationic proteins are key elements of innate immunity and can directly kill multiple bacterial, viral and fungal pathogens. Far from being solely restricted to an effector role, they also exert their antimicrobial activity as immunomodulating agents (3) or inhibit virulence factors of the pathogen. They are found on the epidermis as well as the mucosa and form a soluble barrier against a pathogen invasion, acting jointly with an intact epidermis as a first line of defense. Another attractive approach is the use of inhibitors of fungal virulence factors such as candidal aspartic proteinases. We review alternative approaches to antifungal treatment with a focus on antifungal peptides and proteinase inhibitors.
Antifungal Peptides
RNase-7
Discovered in 2002 by J Harder and JM Schröder, this 14.5 kDa ribonuclease exerts broad antimicrobial activity against C. albicans, as well as numerous Gram positive and Gram negative bacteria, especially Enterococcus faecium. Crude extracts of callus stratum corneum of healthy individuals showed antimicrobial activity. Analysis with high performance liquid chromatography demonstrated a fraction with a high activity against S. aureus. This fraction was further purified and analyzed with mass spectrometry, showing a 14.5 kDa protein, which was sequenced with Edman degradation. The isolation of the corresponding cDNA from primary keratinocytes enabled the deduction of the complete amino-acid sequence which showed the greatest similarity to RNase A superfamily members. In vitro antimicrobial activity was quantified by counting colony forming units (CFUs) of microbes cultivated with increasing amounts of RNase-7. A mid-range dose of 4 µM achieved a CFU-reduction of C. albicans from 105 to a little more than 103 on a logarithmic scale. Analysis of tissue from various body regions revealed an RNase-7 expression in numerous epithelial tissues, such as skin, genitourinary tract and respiratory tract as well as at at low levels in the digestive tract. RNase-7 was also detected in the supernatant of the culture of unstimulated primary keratinocytes. Real-time PCR showed an upregulation of RNase-7 expression in primary cultured keratinocytes after treatment with the proinflammatory cytokines TNF-α (2.5 fold), Interferon-γ (7 fold) and Interferon-1β (8.5 fold), as well as after stimulation with heat-inactivated bacteria such as P. aeruginosa (9-fold) in vitro. The antifungal mechanisms of action so far remain unknown (4).
Lysozyme
Lysozyme is an enzyme classically known for its muramidase activity lysing bacterial peptidoglycan and killing bacteria. However, as early as 1970 it was shown that lysozyme was also active against C. albicans, its mechanism of antifungal action staying subject to speculation (5). Subsequently, broad antifungal activity was also shown against numerous clinical isolates of all Candida spp., with a significant variation of interstrain and intrastrain sensitivity, and against A. fumigatus and Penicillium spp. (6).
Lysozyme is found in virtually all human body fluids (e.g. saliva, respiratory secretions and liquor). Expression of lysozyme in the skin has been located in the cytoplasm of epidermal cells and throughout the pilosebaceous apparatus (6), in body secretions such as saliva and in neutrophils (7). Wu et al. investigated the effect of lysozyme on the viability and Sap activity of C. albicans. Saps are secreted aspartic proteases and their main functions are probably to provide nutrition, aid penetration and invasion, and evade immune responses (8). An incubation period of 24h with sub-lethal concentrations of lysozyme resulted in a dose-dependent reduction of Sap activity and secretion, measured by spectrophotometry and ELISA respectively. This finding was paralleled by decreasing Candida viability at higher, lethal concentrations (15 µg/ml and 20 µg/ml), with significant differences in various strains, quantified by colony-forming units yielded per ml cultivated medium. Electron microscopy showed ballooned cells; some appearing collapsed and deflated, after 24h-exposure to lysozyme at concentrations below 10 µg/ml which did not affect the viability of C. albicans. The fungicidal activity at high concentrations therefore was suggested to result of membrane or cell wall damage leading to osmotic imbalance (9). Also, the negatively charged domain of lysozyme has been assumed to target the Candida surface (9). Very recently it could be demonstrated that the digestion of human milk lysozyme by pepsin yields five different anti-microbial peptides that are also active against C. albicans (10).
Chitotriosidase
Besides lysozyme, other chitin cleaving enzymes have been found in human leukocytes (11). Activated human macrophages as well as neutrophils have been demonstrated to secrete chitotriosidase, an enzyme that is implied in the defense of fungal pathogens and is also a marker for Gaucher’s Disease, a lysosomal storage disorder (12). Recently, gene therapy with chitotriosidase transfected phagocytes has been suggested as a novel therapeutic approach to combat fungal infections (13). Although it seems an interesting target for the future development of antifungal therapies, it is not expressed either in skin or mucosa.
Lactoferrin
Lactoferrin is an iron bindin protease present in various body secretions, including saliva. Human lactoferrin has found to be effective against C. krusei and C. albicans (14). It has been originally thought that lactoferrin restricts microbial growth by iron depletion. However, additional mechanisms of antimicrobial action have been reported since that time. Most notably, like with lysozyme, peptic digestion yields a cationic antimicrobial peptide (lactoferricin) with a very broad microbicidal activity (15)
Antileukoprotease (ALP, SLPI)
Antileukoprotease, a serine protease inhibitor is found in secreted fluids from the genitourinary and respiratory tract, as well as in keratinocytes from the skin and the mucosa. Tomee et al. investigated the antifungal activity of recombinant ALP against Aspergillus fumigatus and C. albicans (16). The fungicidal activity was measured in the percentage reduction of CFUs on agar plates after incubation of A. fumigatus conidia and C. albicans yeast cells with rALP. Recombinant ALP had a dose-dependant fungicidal effect on metabolically active A. fumigatus conidia, while dormant A. fumigatus conidia were not affected. The N-terminal domain had a higher fungicidal activity in comparison to the C-terminal domain, in all concentrations tested. Lysozyme and human neutrophil defensins 1–3 showed similar fungicidal activity. Recombinant ALP had a dose-dependant effect on C. albicans yeast cells. It also had a dose-dependent, fungistatic effect on C. albicans, delaying the onset of growth of remaining colonies and increasing the lag phase of candidal growth in treated colonies. Lysozyme and human neutrophil defensins 1–3 showed similar maximum fungicidal activity, although only lysozyme showed effects at low doses. ALP also has an antiretroviral activity against HIV-1 and is bactericidal towards E. coli and S. aureus (16).
Calprotectin (Calgranulin)
Calprotectin is a heterodimer constituted by S100A8 and S100A9, two low-weight proteins with two calcium-binding helix-loop-helix structural domain each. It is distributed unequally over the human skin, with barely detectable levels in the epidermis of the abdomen and high levels in the epithelial tissue of the female genital area and of plantar skin (17). It is fungistatic towards C. albicans, possibly via sequestration of Zn2+ or Mn2+. Murthy et al. showed that Calprotectin inhibited candidal growth at concentrations equal to or greater than 18 µg/ml, by two orders of magnitude (18). Abtin et al. have shown that IL-1α and flagellin, a protein found on some Gram-negative bacteria, induced the expression of S100A8 and S100A9 as well as S100A8 and S100A9 transcription. Knock-down with TLR5-specific siRNA inhibited the increase of S100A8 and S100A9 expression, showing that the induction of S100A8 and S100A9 by flagellin requires the TLR5-mediated pathway. Culture supernatants of C. albicans could not increase S100A8 and S100A9 expression (17).
Histatins
Histatin-1 and -3 are salivary proteins secreted by the parotid and submandibular glands. Histatin-5 is a 24 amino acids long N-terminal fragment of Histatin 3 and is generated by proteolytic cleavage. Histatin-5 has the strongest fungicidal activity, killing yeast and filamentous forms of Candida species at physiological concentrations of 15 to 30 µM. It exerts its fungicidal activity by binding to a candidal 67 kDa Hst-5 binding protein and subsequent non-lytic ATP-efflux (19).
Defensins
Defensins are a family of small cationic peptides with six cysteine residues which can be divided into α-, β-, and θ-defensins according to the alignment of their disulfide bridges and molecular structure (3). Humans only express α- and β-defensins (20). Here, we would like to focus on β-defensins.
Human β-defensin-1 is constitutively expressed in all endothelial tissues and its mRNA can be up-regulated via TLR-mediated stimulation through pro-inflammatory interferon-γ, bacteria or LPS in monocytes (21). It exerts a fungicidal activity towards C. albicans. Reduction of the disulfide bonds of human β-defensin 1 increases its antimicrobial activity (22).
Human β-defensin-2 is only expressed in epithelial tissue and is highly upregulated upon stimulation with pro-inflammatoric cytokines such as TNF and upon contact with bacterial and fungal pathogens (23) via TLR-2 (24) as well as via IL-1 (25). Psoriatic skin loses its physical integrity, thus undermining its role as a mechanical barrier against infection. Nonetheless, it is rarely infected. The strong upregulation of Human β-Defensin-2 in inflamed psoriatic skin (26) can serve as one explanation.
Human β-Defensin-3 is expressed in keratinocytes and airway epithelial cells (27). It is induced via TGF-α – EGFR signaling (25) and exerts its fungicidal activity against C. albicans via the same mechanism as the human-β-defensins 1 and 2 (28).
Krishnakumari et al. analyzed the anticandidal activity by determining the minimal fungicidal concentration (MFC) of the human β-defensins 1–3, as well as that of their synthetic C-terminal analogs Phd1–3. Of the three defensins, human β-defensins 3 showed the strongest fungicidal activity against C. albicans with a MFC of 2.5 µM and HBD-2 the weakest with a MFC of 8 µM. The inactivity of HBD-1 and -2 in the presence of the metabolic inhibitors sodium azide and carbonyl cyanide m-chlorophenylhydrazone showed that the function of these two defensins was energy-dependent, quite contrary to HBD-3 whose fungicidal capabilities were not altered. The fungicidal activity of all defensins was reduced in an environment of high NaCl, CaCl2 and MgCl2. Confocal microscope imaging of C. albicans incubated with Phd1–3 showed membrane damage as well as location of Phd1–3 on the outer membrane. A membrane permeabilization assay with Sytox green showed a significant increase in fluorescence after the addition of defensins to C. albicans. These results show that at least one mechanism of action of HBD1–3 is increasing the membrane permeability of C. albicans (28).
Cathelicidins
Next to defensins, the cathelicidins constitute the other larger group of antimicrobial skin peptides. They are small cationic peptides and possess an N-terminal signal peptide, a highly conserved cathelin domain and a cationic antimicrobial peptide at the C-terminus (3). The cathelin domain has the double function of an antimicrobial peptide and of a protease inhibitor (29). The C-terminus is cleaved by proteases and shows broad, potent antimicrobial activity (3). Cathelicidins are produced by cutaneous and mucosal epithelial cells as well as mast cells (30). The secretion of LL- 37 by human eccrine sweat glands is subject to controversy, as Murakami et al. found LL-37 in human sweat of healthy volunteers but Rieg et al. could not (31, 32). Moreover, the gene encoding for precursor of human LL-37, CAMP, is expressed in keratinocytes during inflammation (33). Members of the cathelidicin family include human LL-37, murine CRAMP and porcine PR-39. They all show fungistatic and fungicidal activity against Malassezia furfur, Trichophyton mentagrophytes and Trichophyton rubrum (34). The minimal inhibitory concentration of LL-37 against two strains of T. mentagrophytes and two strains of T. rubrum were identical at 12.5 µM, the MFC were 12.5 µM for the two strains of Trichophyton mentagrophytes and 25 µM against the two strains of Trichophyton rubrum. LL-37 showed no relevant activity against a strain of Arthroderma otae. CRAMP showed a slightly higher antifungal activity at 10 µM against M. furfur in vitro. M. furfur and T. rubrum can also slightly increase cathelidicin mRNA expression in human keratinocytes in vitro. Cathelicidins CRAMP and LL-37 show fungicidal and fungistatic activity against C. albicans, with a similar MIC between 15 and 20 µM. At the skin surface, LL-37 is processed by a serine-protease into shorter peptides such as KS-30 and RK-31. They showed a higher fungicidal activity against C. albicans than LL-37 or CRAMP. A Sytox green permeabilization assay showed that LL-37 and RK-31 render the candidal membrane more permeable. Immunohistochemical analysis demonstrated a significant mCRAMP expression after C. albicans skin infection in vivo (Fig. 1). Furthermore, although mCRAMP-deficient mice showed no increased susceptibility to C. albicans in a blood-killing assay, human sweat as well as LL-37 and its products RK-31 and KS-30 showed anticandidal activity, suggesting that cathelicidins are more effective on the skin surface (35).
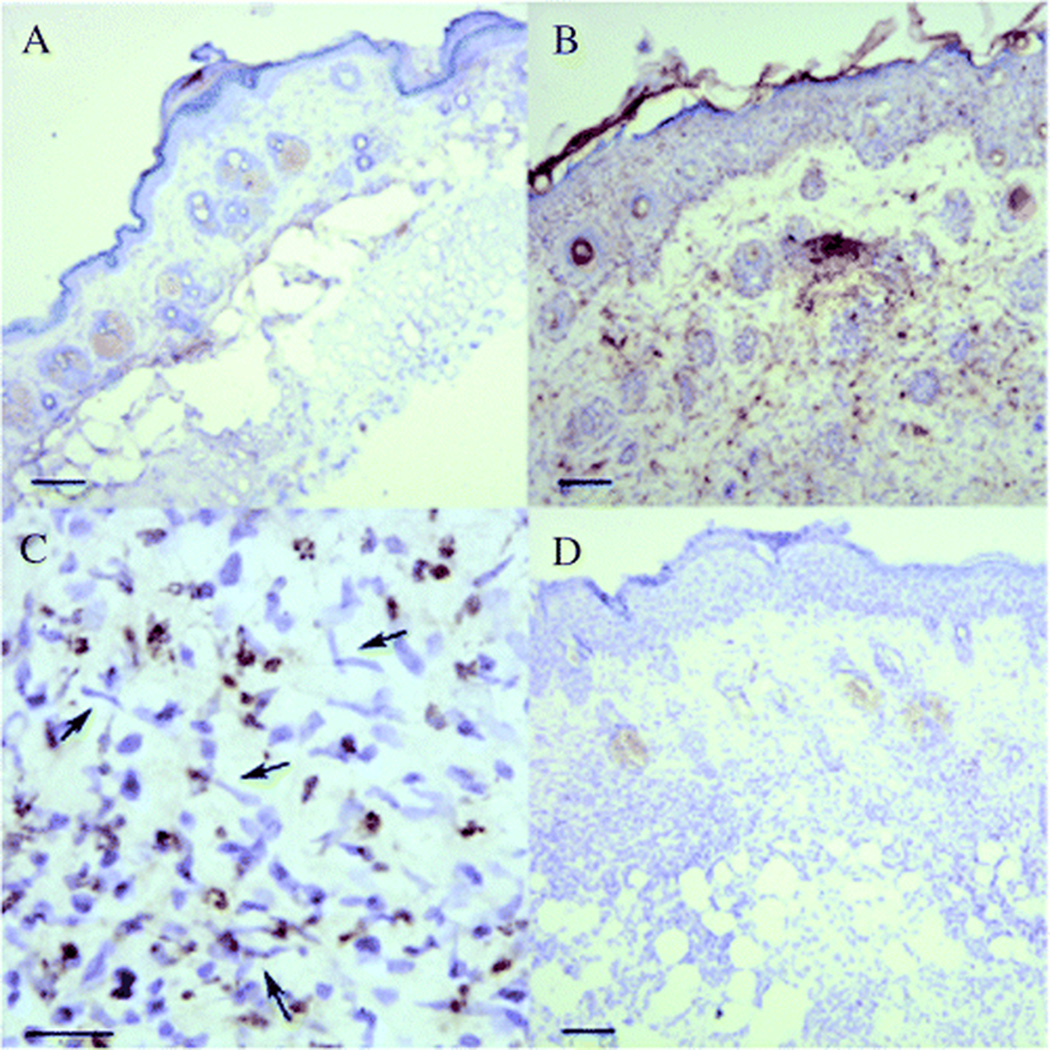
Fig. 1.
Immunohistochemical analysis of mCRAMP expression in Candida albicans-infected mouse skin. (A) Normal uninfected skin and (B–D) C. albicans-infected skin. (A–C) Stained with antibody to mCRAMP. (D) Stained with rabbit pre-immune serum used as a control. Brown staining in (B) shows increased expression of mCRAMP in C. albicans-infected skin compared with uninfected skin in (A). (C) High power (scale bar = 10 mm) of dermis from (B) shows evidence of multiple C. albicans organisms indicated by arrows and mCRAMP containing neutrophils (hematoxylin counterstain; scale bar = 40 mm in (A, B, D)) From Figure 4, (34) (With permission from the nature publishing group).
Synthetic peptides
Kamysz et al. published the antifungal activity of three chemically engineered antifungal peptides against different Candida spp. The peptides aurein 1.2, citropin 1.1 A and uperin 3.6 all had lower MICs than amphotericin B and nystatin in vitro (36).
Recently, the antifungal activity of two synthetic antifungal cationic peptides, VS2 and VS3 was shown. They irreversibly inhibited the growth of various Candida spp., multidrug resistance strains, Cryptococcus neoformans, Aspergillus niger, Fusarium oxysporium and Neurospora crassa at MIC80 values ranging from 15.62 µM to 250 µM. In combination with fluconazole, non-candicidal concentrations of both peptides showed high antifungal activity against a strain of fluconazole-resistant Candida spp. In addition to causing a direct permeabilization of the candidal cell membrane, they also induced necrosis by causing an intracellular accumulation of reactive oxygen species and showed fast killing kinetics (37).
A further synthetic peptide, KSL-W attenuated the virulence of C. albicans in vitro. Pre-treatment of C. albicans with KSL-W reduced CFUs as well as adhesion on engineered human oral mucosa. The reduction of virulence might also explain the reduction of TLR2, TLR4 and TLR9 expression in engineered human oral mucosa tissue cultivated with KSL-W pre-treated C. albicans. In contrast, TLR6 expression was increased. KSL-W also downregulated the gene expression of human β-defensins and modulated proinflammatory cytokine secretion. Indeed, KSL-W dose-dependently increased IL-1β and decreased IL-6 expression of gingival epithelial cells after culture with C. albicans in vitro (38).
Moreover, recently designed Sap inhibitors based on the known Sap2-substrate specificity data and X-ray analyses of Sap2/inhibitor complexes, taking the structure of the non-selective aspartic proteinase inhibitor pepstatin A as a blueprint. These designed antifungal agents showed high inhibitory activity for Sap1, Sap3, Sap5 and Sap6 (Cadicamo et al., unpublished data).
Dermcidin
Dermcidin is constitutively and specifically expressed in human eccrine sweat glands and is transported to the skin surface. The peptide is proteolyzed after secretion and its processed form, DCD-1L, is highly fungicidal against C. albicans under in vitro conditions similar to those of human sweat (39). In contrast to many cationic antimicrobial peptides, DCD-derived peptides do not permeabilize microbial membranes, suggesting a different mechanism of action (40).
hGAPDH(2–32)
Recently, a GAPDH-derived peptide from human placental tissue demonstrated antimicrobial activity in vitro (Fig. 2). hGAPDH (2–32) is 100 % identical to the N-terminus of human GAPDH. It was capable of inhibiting the growth of C. albicans in micromolar concentrations in vitro, exerting fungicidal activity confirmed by a flow cytometric killing assay. Moreover, it was capable of inhibiting two secreted aspartic proteinases that are important candidal virulence factors and of inducing epithelial IL-8 and GM-CSF secretion in addition to stimulating TLR-4 expression (Wagener et al., unpublished data).
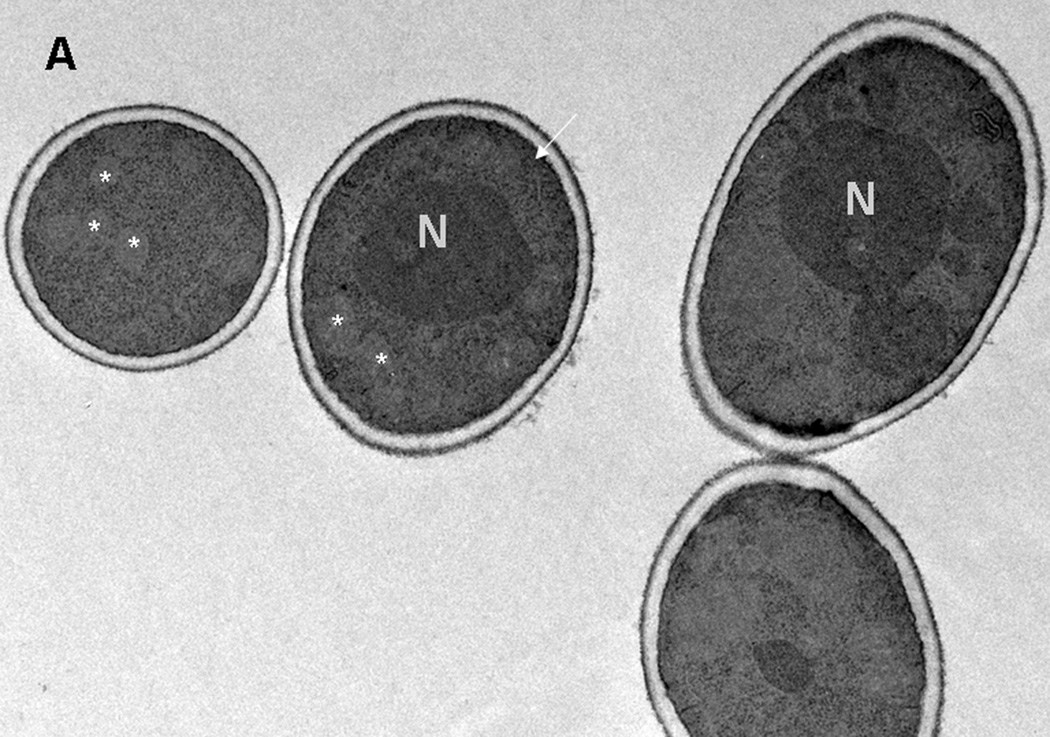
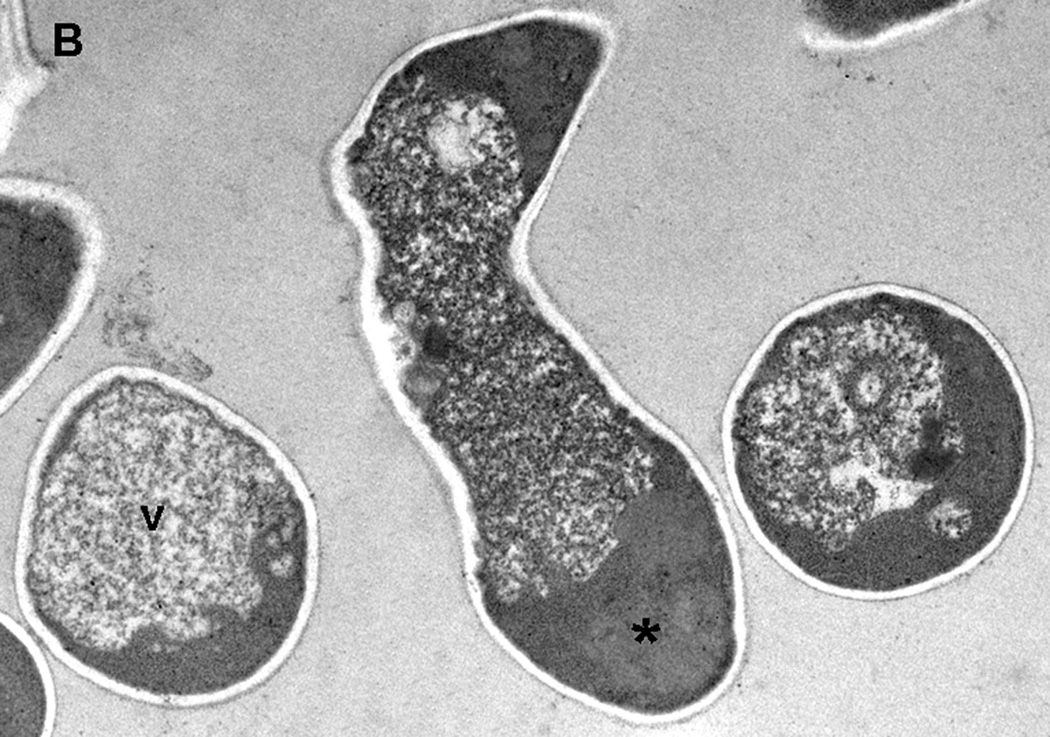
Fig. 2.
Electron microscopy of C. albicans SC5314 cells grown in 2% Sabouraud glucose medium without (A) and with (B) 125 mg/l hGAPDH (2–32) for 24 h. (A) Cells grown without hGAPDH (2–32) have a regular morphology. N: nuclei, arrow: endocytoplasmatic reticulum, *: mitochondria. (B) C. albicans grown under the influence of hGAPDH (2–32). v: enlargement of the fungal cytoplasmic vacuoles. *: disorganization of the internal organelles.
Targeting virulence factors
Significant improvements in other medical fields such as oncology and transplantational medicine have in turn increased the prevalence of immunocompromised patients. Moreover, increased longevity and a higher hospitalization rate in old age as well as the HIV pandemic have also led to an increase in individuals developing fungal infections. This has in turn led to an increment in antifungal treatments, giving rise to more and more frequent resistances to the drugs employed. We therefore now face an increased necessity to find and develop new antifungal therapies.
One approach has been the exploration of novel targets in fungal pathogens. Antifungal peptides, either synthetic or naturally occurring have the advantage of having different microbial targets in comparison to classic antifungal agents. Results are promising (37, 38). For example, azole resistance in Candida spp. can be due to mutations in genes encoding efflux pumps (41). Indeed, ATP binding cassette transporter gene mutations such as an over expression of CDR1 were found to correlate with increasing fluconazole MICs in a series of clinical isolates (42). Many antifungal peptides are small cationic molecules whose target is the fungal outer membrane. Not surprisingly, these peptides can overcome resistances to antifungal drugs currently in first-line use such as fluconazole (37). Moreover, these peptides occur naturally or are similar to natural products, so they can be designed with few side-effects on host cells (38). Nonetheless, the most promising application of these antifungal agents seems to be topical and not systemic use (35). The problem of increased drug-resistant, life-threatening systemic fungal infections remains. One possible use of antifungal peptides could therefore be the prophylaxis of systemic fungal infections in populations at risk by strengthening the epithelial barrier as a first line of defense by preventing invasion.
Another strategy is to target fungal virulence factors themselves. One promising target is the biofilm formation, especially of Candida spp.. Recently, Mandal et al. published an effective concentration dependant inhibition of Candida tropicalis growth and biofilm disruption with the novel antifungal plant peptide Tn-AFP1 extracted from Trapa natans fruits(43).
A further group of fungal pathogenicity factors of interest as therapeutic targets is the family of secreted aspartic proteinases (Saps) of C. albicans (44) (Fig. 4). It has been shown that the adhesion capabilities of C. albicans can be reduced either by mutations in SAP-coding genes or by using the SAP-inhibitor Pepstatin A (45).
Antiretrovirals of the proteinase inhibitor group such as ritonavir can themselves reduce C. albicans adhesion (46), a finding corroborated clinically by the finding that and HIV-patients under therapy with proteinase inhibitors had a lower oral Candida load (47). At first, the reduction of fungal infections, especially of oral candidiasis in HIV-patients treated with antiretrovirals was attributed to an improvement of the immune status. Since 1998, it has been known that antiretroviral proteinase inhibitors such as indinavir and saquinavir can dose-dependently inhibit secreted aspartic proteinases (Saps) (48). This was confirmed by Schaller et al. who investigated the inhibitory effect of saquinavir and amprenavir on Saps of three C. albicans strains from HIV-infected patients. The results showed significant reduction in their proteolytic activity, comparable to that of the classic Sap inhibitor pepstatin A (49). Borg-von-Zepelin et al. published an article in 1999 demonstrating the ability of four antiretrovirals (ritonavir, saquinavir, nelfinavir and indinavir) to inhibit Sap 1–3 in a dose-dependant manner. Nonetheless, only a slight inhibition of Sap 4–6 with the highest concentration of saquinavir could be achieved. Nelfinavir marginally reduced Sap-6-activity (50). All in all, the further development of protein inhibitors primarily targeting the virulence factors and not the pathogen itself as antifungal therapies seems to be a promising strategy (51).
Another promising development in the same direction has been the design and application of antibodies targeting fungal virulence factors. A targeted therapy against SAP-2 decreased candidal epithelial adhesion on the vaginal wall in a rat model and increased the clearance of the infection (52).
In summary, a better understanding of antifungal peptides will lead to the discovery of novel microbial targets, thus aiding in the design of new strategies to keep staying a step ahead in the fight against increasingly drug-resistant fungal infections.
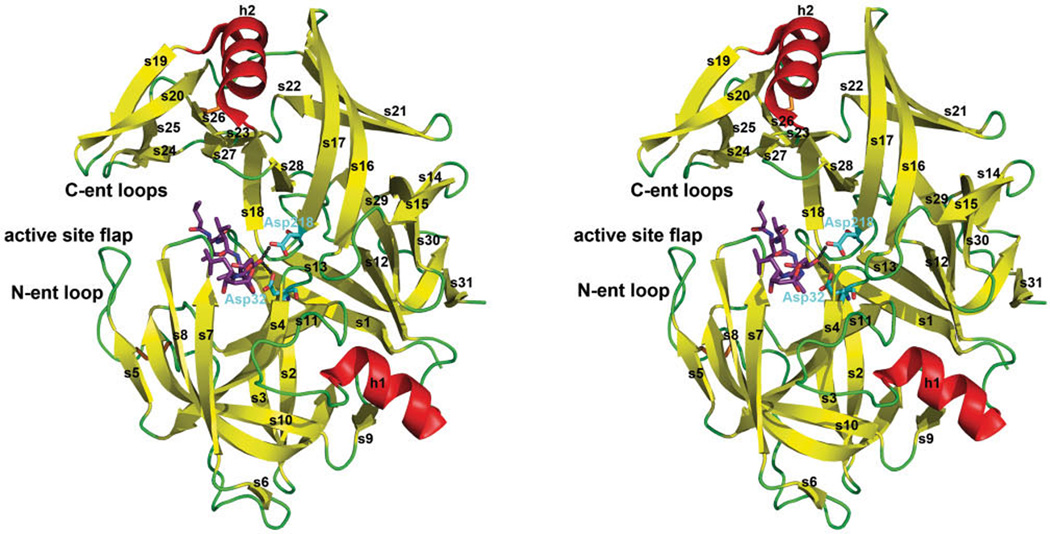
Fig. 3.
Structure of the Sap5 complexed with pepstatin A. Stereo overall view of Sap5 as example for the overall structure of the Sap isoenzymes. Sap isoenzymes mainly consist of β-strands arranging to a tightly packed, kidney-shaped globular protein. The side chains of the two catalytical aspartates are displayed in blue, the two disulphide bridges (Cys47–Cys59 and Cys256–Cys294) in orange. The loops tied together by the disulphide bridges are specified as N-terminal entrance loop (cysteine residues 47 and 59) (N-ent loop) and C-terminal entrance loops (cysteine residues 256 and 294) (C-ent loops). β-Sheet s7,8 (residues 81–91) represents the active site flap which plays an important role in inhibitor binding. Pepstatin A is shown as a stick model colored in magenta (54).
Table I.
The following table lists the species, against which an antifungal effect of the specified antimicrobial peptide has been confirmed
| Antifungal Peptide | Susceptible species | References |
|---|---|---|
| RNase-7 | C. albicans | (4) |
| Lysozyme |
C. albicans, P. parapsilosis, A. fumigatus, Penicillium spp |
(5) |
| Antileukoprotease | A. fumigatus, C. albicans | (16) |
| Calprotectin | C. albicans | (17) |
| Histatins | C. albicans | (19) |
| Defensins | ||
| Human β-defensin-1 | C. albicans | (20,21,28) |
| Human β-defensin-2 | C. albicans | (23,28) |
| Human β-Defensin-3 | C. albicans | (27,28) |
| Cathelicidins |
C. albicans, Malassezia furfur, T. mentagrophytes, T. rubrum |
(31) |
| Dermcidin | C. albicans | (39) |
| GAPDH | C. albicans | (Schneider et al., unpublished data). |
| Synthecic peptides aurein 1.2, citropin 1.1 A, uperin 3.6 |
C. albicans | (36) |
|
VS2 and VS3 |
Candida spp. incl. drug-resistant strains, Cryptococcus neoformans, A. niger, Fusarium oxysporium, Neurospora crassa |
(37) |
|
KSL-W |
C. albicans | (38) |
| Lactoferrin | C. albicans, C. glabrata | (53) |
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (Sch 897/1-3, graduate college 685) and by a NIDCR grant R01 DE017514-01. Walter Burgdorf is acknowledged for help in revising the manuscript.
Footnotes
All authors contributed to the literature review and writing of the manuscript.
Conflict of interests: None declared
References
- 1.Lalla RV, Latortue MC, Hong CH, et al. A systematic review of oral fungal infections in patients receiving cancer therapy. Support Care Cancer. 18:985–992. doi: 10.1007/s00520-010-0892-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chai LY, Netea MG, Vonk AG, Kullberg BJ. Fungal strategies for overcoming host innate immune response. Med Mycol. 2009;47:227–236. doi: 10.1080/13693780802209082. [DOI] [PubMed] [Google Scholar]
- 3.Braff MH, Bardan A, Nizet V, Gallo RL. Cutaneous defense mechanisms by antimicrobial peptides. J Invest Dermatol. 2005;125:9–13. doi: 10.1111/j.0022-202X.2004.23587.x. [DOI] [PubMed] [Google Scholar]
- 4.Harder J, Schroder JM. RNase 7, a novel innate immune defense antimicrobial protein of healthy human skin. J Biol Chem. 2002;277:46779–46784. doi: 10.1074/jbc.M207587200. [DOI] [PubMed] [Google Scholar]
- 5.Kamaya T. Lytic action of lysozyme on Candida albicans. Mycopathol Mycol Appl. 1970;42:197–207. doi: 10.1007/BF02051947. [DOI] [PubMed] [Google Scholar]
- 6.Papini M, Simonetti S, Franceschini S, Scaringi L, Binazzi M. Lysozyme distribution in healthy human skin. Arch Dermatol Res. 1982;272:167–170. doi: 10.1007/BF00510410. [DOI] [PubMed] [Google Scholar]
- 7.van de Merwe JP, Lindemans J, Mol GJ. Plasma lysozyme levels and decay of neutrophilic granulocytes in patients with Crohn's disease. Hepatogastroenterology. 1980;27:130–134. [PubMed] [Google Scholar]
- 8.Naglik JR, Challacombe SJ, Hube B. Candida albicans Secreted Aspartyl Proteinases in Virulence and Pathogenesis. 2003:400–428. doi: 10.1128/MMBR.67.3.400-428.2003. In. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu T, Samaranayake LP, Leung WK, Sullivan PA. Inhibition of growth and secreted aspartyl proteinase production in Candida albicans by lysozyme. J Med Microbiol. 1999;48:721–730. doi: 10.1099/00222615-48-8-721. [DOI] [PubMed] [Google Scholar]
- 10.Ibrahim HR, Imazato K, Ono H. Human lysozyme possesses novel antimicrobial peptides within its N-terminal domain that target bacterial respiration. J Agric Food Chem. 59:10336–10345. doi: 10.1021/jf2020396. [DOI] [PubMed] [Google Scholar]
- 11.Escott GM, Adams DJ. Chitinase activity in human serum and leukocytes. Infect Immun. 1995;63:4770–4773. doi: 10.1128/iai.63.12.4770-4773.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kzhyshkowska J, Gratchev A, Goerdt S. Human chitinases and chitinase-like proteins as indicators for inflammation and cancer. Biomark Insights. 2007;2:128–146. [PMC free article] [PubMed] [Google Scholar]
- 13.Gordon-Thomson C, Kumari A, Tomkins L, et al. Chitotriosidase and gene therapy for fungal infections. Cell Mol Life Sci. 2009;66:1116–1125. doi: 10.1007/s00018-009-8765-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samaranayake YH, Samaranayake LP, Wu PC, So M. The antifungal effect of lactoferrin and lysozyme on Candida krusei and Candida albicans. Apmis. 1997;105:875–883. [PubMed] [Google Scholar]
- 15.Orsi N. The antimicrobial activity of lactoferrin: current status and perspectives. Biometals. 2004;17:189–196. doi: 10.1023/b:biom.0000027691.86757.e2. [DOI] [PubMed] [Google Scholar]
- 16.Tomee JF, Hiemstra PS, Heinzel-Wieland R, Kauffman HF. Antileukoprotease: an endogenous protein in the innate mucosal defense against fungi. J Infect Dis. 1997;176:740–747. doi: 10.1086/514098. [DOI] [PubMed] [Google Scholar]
- 17.Abtin A, Eckhart L, Glaser R, Gmeiner R, Mildner M, Tschachler E. The antimicrobial heterodimer S100A8/S100A9 (calprotectin) is upregulated by bacterial flagellin in human epidermal keratinocytes. J Invest Dermatol. 130:2423–2430. doi: 10.1038/jid.2010.158. [DOI] [PubMed] [Google Scholar]
- 18.Murthy AR, Lehrer RI, Harwig SS, Miyasaki KT. In vitro candidastatic properties of the human neutrophil calprotectin complex. J Immunol. 1993;151:6291–6301. [PubMed] [Google Scholar]
- 19.Edgerton M, Koshlukova SE. Salivary histatin 5 and its similarities to the other antimicrobial proteins in human saliva. Adv Dent Res. 2000;14:16–21. doi: 10.1177/08959374000140010201. [DOI] [PubMed] [Google Scholar]
- 20.Selsted ME, Ouellette AJ. Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005;6:551–557. doi: 10.1038/ni1206. [DOI] [PubMed] [Google Scholar]
- 21.Duits LA, Ravensbergen B, Rademaker M, Hiemstra PS, Nibbering PH. Expression of beta-defensin 1 and 2 mRNA by human monocytes, macrophages and dendritic cells. Immunology. 2002;106:517–525. doi: 10.1046/j.1365-2567.2002.01430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schroeder BO, Wu Z, Nuding S, et al. Reduction of disulphide bonds unmasks potent antimicrobial activity of human beta-defensin 1. Nature. 2011;469:419–423. doi: 10.1038/nature09674. [DOI] [PubMed] [Google Scholar]
- 23.Harder J, Bartels J, Christophers E, Schroder JM. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 24.Hertz CJ, Wu Q, Porter EM, et al. Activation of Toll-like receptor 2 on human tracheobronchial epithelial cells induces the antimicrobial peptide human beta defensin-2. J Immunol. 2003;171:6820–6826. doi: 10.4049/jimmunol.171.12.6820. [DOI] [PubMed] [Google Scholar]
- 25.Sorensen OE, Thapa DR, Rosenthal A, Liu L, Roberts AA, Ganz T. Differential regulation of beta-defensin expression in human skin by microbial stimuli. J Immunol. 2005;174:4870–4879. doi: 10.4049/jimmunol.174.8.4870. [DOI] [PubMed] [Google Scholar]
- 26.Harder J, Schroder JM. Psoriatic scales: a promising source for the isolation of human skin-derived antimicrobial proteins. J Leukoc Biol. 2005;77:476–486. doi: 10.1189/jlb.0704409. [DOI] [PubMed] [Google Scholar]
- 27.Harder J, Bartels J, Christophers E, Schroder JM. Isolation and characterization of human beta –defensin-3, a novel human inducible peptide antibiotic. J Biol Chem. 2001;276:5707–5713. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- 28.Krishnakumari V, Rangaraj N, Nagaraj R. Antifungal activities of human beta-defensins HBD-1 to HBD-3 and their C-terminal analogs Phd1 to Phd3. Antimicrob Agents Chemother. 2009;53:256–260. doi: 10.1128/AAC.00470-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zaiou M, Nizet V, Gallo RL. Antimicrobial and protease inhibitory functions of the human cathelicidin (hCAP18/LL-37) prosequence. J Invest Dermatol. 2003;120:810–816. doi: 10.1046/j.1523-1747.2003.12132.x. [DOI] [PubMed] [Google Scholar]
- 30.McCormick TS, Weinberg A. Epithelial cell-derived antimicrobial peptides are multifunctional agents that bridge innate and adaptive immunity. Periodontol. 2000;54:195–206. doi: 10.1111/j.1600-0757.2010.00373.x. 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murakami M, Lopez-Garcia B, Braff M, Dorschner RA, Gallo RL. Postsecretory processing generates multiple cathelicidins for enhanced topical antimicrobial defense. J Immunol. 2004;172:3070–3077. doi: 10.4049/jimmunol.172.5.3070. [DOI] [PubMed] [Google Scholar]
- 32.Rieg S, Steffen H, Seeber S, et al. Deficiency of Dermcidin-Derived Antimicrobial Peptides in Sweat of Patients with Atopic Dermatitis Correlates with an Impaired Innate Defense of Human Skin In Vivo. The Journal of Immunology. 2005;174:8003–8010. doi: 10.4049/jimmunol.174.12.8003. [DOI] [PubMed] [Google Scholar]
- 33.Frohm M, Agerberth B, Ahangari G, et al. The Expression of the Gene Coding for the Antibacterial Peptide LL-37 Is Induced in Human Keratinocytes during Inflammatory Disorders. Journal of Biological Chemistry. 1997;272:15258–15263. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- 34.Lopez-Garcia B, Lee PH, Gallo RL. Expression and potential function of cathelicidin antimicrobial peptides in dermatophytosis and tinea versicolor. J Antimicrob Chemother. 2006;57:877–882. doi: 10.1093/jac/dkl078. [DOI] [PubMed] [Google Scholar]
- 35.Lopez-Garcia B, Lee PH, Yamasaki K, Gallo RL. Anti-fungal activity of cathelicidins and their potential role in Candida albicans skin infection. J Invest Dermatol. 2005;125:108–115. doi: 10.1111/j.0022-202X.2005.23713.x. [DOI] [PubMed] [Google Scholar]
- 36.Kamysz W, Nadolski P, Kedzia A, et al. In vitro activity of synthetic antimicrobial peptides against Candida. Pol J Microbiol. 2006;55:303–307. [PubMed] [Google Scholar]
- 37.Maurya IK, Pathak S, Sharma M, et al. Antifungal activity of novel synthetic peptides by accumulation of reactive oxygen species (ROS) and disruption of cell wall against Candida albicans. Peptides. 2011;32:1732–1740. doi: 10.1016/j.peptides.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 38.Semlali A, Leung KP, Curt S, Rouabhia M. Antimicrobial decapeptide KSL-W attenuates Candida albicans virulence by modulating its effects on Toll-like receptor, human beta-defensin, and cytokine expression by engineered human oral mucosa. Peptides. 2011;32:859–867. doi: 10.1016/j.peptides.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 39.Schittek B, Hipfel R, Sauer B, et al. Dermcidin: a novel human antibiotic peptide secreted by sweat glands. Nat Immunol. 2001;2:1133–1137. doi: 10.1038/ni732. [DOI] [PubMed] [Google Scholar]
- 40.Steffen H, Rieg S, Wiedemann I, et al. Naturally processed dermcidin-derived peptides do not permeabilize bacterial membranes and kill microorganisms irrespective of their charge. Antimicrob Agents Chemother. 2006;50:2608–2620. doi: 10.1128/AAC.00181-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White TC, Marr KA, Bowden RA. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev. 1998;11:382–402. doi: 10.1128/cmr.11.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White TC. Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob Agents Chemother. 1997;41:1482–1487. doi: 10.1128/aac.41.7.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mandal SM, Migliolo L, Franco OL, Ghosh AK. Identification of an antifungal peptide from Trapa natans fruits with inhibitory effects on Candida tropicalis biofilm formation. Peptides. 1741;32:1741–1747. doi: 10.1016/j.peptides.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 44.Bein M, Schaller M, Korting HC. The secreted aspartic proteinases as a new target in the therapy of candidiasis. Curr Drug Targets. 2002;3:351–357. doi: 10.2174/1389450023347542. [DOI] [PubMed] [Google Scholar]
- 45.Watts HJ, Cheah FS, Hube B, Sanglard D, Gow NA. Altered adherence in strains of Candida albicans harbouring null mutations in secreted aspartic proteinase genes. FEMS Microbiol Lett. 1998;159:129–135. doi: 10.1111/j.1574-6968.1998.tb12851.x. [DOI] [PubMed] [Google Scholar]
- 46.Bektić J, Lell CP, Fuchs A, et al. HIV protease inhibitors attenuate adherence of Candida albicans to epithelial cells in vitro. FEMS Immunology & Medical Microbiology. 2001;31:65–71. doi: 10.1111/j.1574-695X.2001.tb01588.x. [DOI] [PubMed] [Google Scholar]
- 47.Arribas JR, Hernandez-Albujar S, Gonzalez-Garcia JJ, et al. Impact of protease inhibitor therapy on HIV-related oropharyngeal candidiasis. Aids. 2000;14:979–985. doi: 10.1097/00002030-200005260-00009. [DOI] [PubMed] [Google Scholar]
- 48.Hoegl L, Thoma-Greber E, Rocken M, Korting HC. HIV protease inhibitors influence the prevalence of oral candidosis in HIV-infected patients: a 2-year study. Mycoses. 1998;41:321–325. doi: 10.1111/j.1439-0507.1998.tb00345.x. [DOI] [PubMed] [Google Scholar]
- 49.Schaller M, Krnjaic N, Niewerth M, Hamm G, Hube B, Korting HC. Effect of antimycotic agents on the activity of aspartyl proteinases secreted by Candida albicans. J Med Microbiol. 2003;52:247–249. doi: 10.1099/jmm.0.05048-0. [DOI] [PubMed] [Google Scholar]
- 50.Borg-von Zepelin M, Meyer I, Thomssen R, et al. HIV-Protease inhibitors reduce cell adherence of Candida albicans strains by inhibition of yeast secreted aspartic proteases. J Invest Dermatol. 1999;113:747–751. doi: 10.1046/j.1523-1747.1999.00747.x. [DOI] [PubMed] [Google Scholar]
- 51.Gauwerky K, Borelli C, Korting HC. Targeting virulence: a new paradigm for antifungals. Drug Discov Today. 2009;14:214–222. doi: 10.1016/j.drudis.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 52.De Bernardis F, Liu H, O'Mahony R, et al. Human domain antibodies against virulence traits of Candida albicans inhibit fungus adherence to vaginal epithelium and protect against experimental vaginal candidiasis. J Infect Dis. 2007;195:149–157. doi: 10.1086/509891. [DOI] [PubMed] [Google Scholar]
- 53.Kuipers ME, de Vries HG, Eikelboom MC, Meijer DKF, Swart PJ. Synergistic Fungistatic Effects of Lactoferrin in Combination with Antifungal Drugs against Clinical Candida Isolates. Antimicrobial Agents and Chemotherapy. 1999;43:2635–2641. doi: 10.1128/aac.43.11.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borelli C, Ruge E, Lee JH, et al. X-ray structures of Sap1 and Sap5: structural comparison of the secreted aspartic proteinases from Candida albicans. Proteins. 2008;72:1308–1319. doi: 10.1002/prot.22021. [DOI] [PubMed] [Google Scholar]