Abstract
Elastin-like polypeptide (ELP) fusions have been designed to allow large scale, non-chromatographic purification of many soluble proteins using the inverse transition cycling (ITC) method; however, the sensitivity of the aqueous lower critical solubility phase transition temperature (Tt) of ELPs to the addition of cosolutes, including detergents, may be a potential hindrance in purification of proteins with surface hydrophobicity in such a manner. To identify detergents that are known to solubilize such proteins (e.g., membrane proteins) and that have little effect on the Tt of the ELP, we screened a number of detergents with respect to their effects on the Tt and secondary structures of a model ELP (denoted here as ELP180). We found that mild detergents (e.g., DDM, Triton-X100, and CHAPS) do not alter the phase transition behavior or structure (as probed by circular dichroism) of ELP180. This result is in contrast to previous studies that showed a strong effect of other detergents (e.g., SDS) on the Ttof ELPs. Our results clearly indicate that mild detergents do not preclude ITC-based separation of ELPs, and thus that ELP fusions may prove to be useful in the purification of detergent-solubilized recombinant hydrophobic proteins, including membrane proteins, which are otherwise notoriously difficult to extract and purify by conventional separation methods (e.g., chromatography).
Keywords: elastin-like polypeptides, detergents, lower critical solution temperature, membrane protein purification
Introduction
Elastin-like polypeptides (ELPs) are polymers that are composed of repeats of the pentapeptide sequence Val-Pro-Gly-Xaa-Gly (where Xaa can be any amino acid except Pro) that undergo a sharp and reversible phase transition in water; they are soluble below their lower critical solution temperature (LCST or Tt) and insoluble in a coacervate phase as amorphous precipitates above their Tt.1–3 ELPs maintain their LCST phase transition behavior when they are recombinantly expressed as chimeric fusions with other proteins.3–5 This unusual characteristic of ELPs has been previously employed to separate a range of fusion proteins from cell lysate by repeated and alternating centrifugation below and above the LCST, a process known as inverse transition cycling (ITC).1 Fusion to ELPs can result in enhanced in vivo solubilization and thus higher yields of expressed proteins, while insertion of protease cleavage sites allows for facile recovery of the target protein from the purified chimera.4,5 Thus, fusion of ELPs with target recombinant proteins has provided several attractive features as an economical and straightforward method for their purification without the need for chromatography.
The predominant thermodynamic driving force for the LCST phase transition of ELPs is the release of bound water molecules from ELPs as the solution temperature is increased.6–8 The Tt of an ELP, which depends on the molecule’s molecular weight, concentration, the pH and ionic strength of the aqueous solution, and the presence of additives such as detergents, is an important design parameter in optimizing an effective purification strategy for a given recombinant protein.4,9 For example, if the addition of surfactants, or other cosolutes, results in a significant increase in Tt, ITC may result in poor or no purification.1 Furthermore, the activity of the recovered target protein may be compromised if the Tt is near or above the thermal denaturation temperature of that protein.3,5 It is therefore necessary to characterize the role of cosolutes on Tt if their addition is necessary for the separation of fusion proteins of interest.
Because integral membrane proteins play critical roles in cellular signaling, molecular trafficking, metabolism and adhesion, they are major targets in the development of pharmaceutical drugs.10 In such developmental research and in fundamental studies, significant quantities of membrane proteins are often required to elucidate their structure and function. Recombinant expression methods offer promise in addressing this issue, but the purification of integral membrane proteins following their solubilization in detergents remains challenging. Chromatographic purification of membrane proteins, the current standard of practice, is often problematic because they can interact strongly with the column matrix, thereby lowering column efficiency.11 While multiple chromatographic purification steps are often necessary to achieve purity, detergents, which are required to solubilize and stabilize integral membrane proteins, may also be harmful to the chromatographic column matrix, reducing purification efficiency as well as resolution.11,12
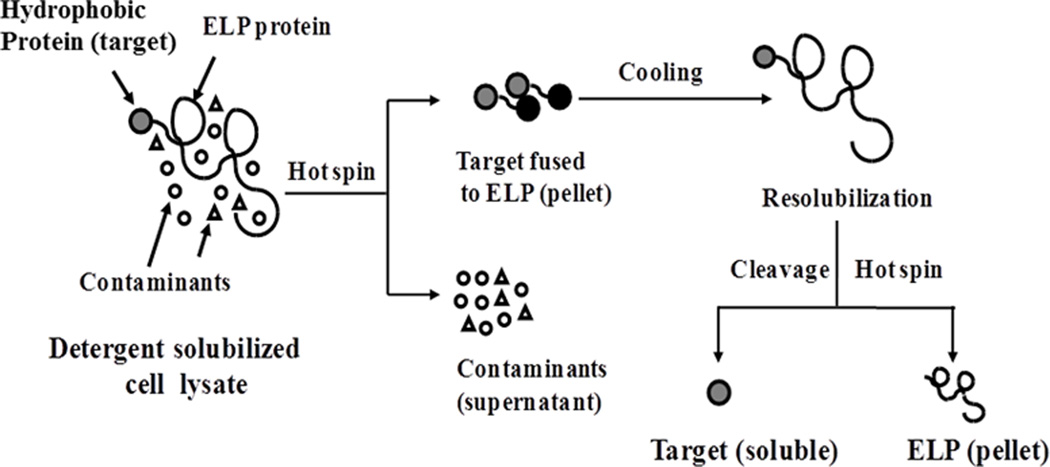
Motivated by the limitations of current methods, the goal of this study is to identify surfactants that are routinely used to solubilize hydrophobic proteins but have minimal perturbation of the LCST transitions of a model ELP. Achieving this goal is a necessary first step toward development of an alternative, single-step method to overexpress and purify integral membrane proteins (Scheme 1). Earlier studies have shown that sodium dodecylsulfate (SDS), a strong anionic detergent, can either increase or decrease the Tt of ELPs and poly(N-isopropylacrylamide) (PNIPAAm, a synthetic polymer that exhibits similar LCST transition characteristics to those of ELPs)8 depending on the concentration of SDS and the molecular weight of ELPs.9,13 SDS is known to modify the interactions between ELPs or PNIPAAm and water.8,9 Moreover, SDS is a strong denaturant and hence, mild detergents are often used for the isolation and stabilization of membrane proteins.
Scheme 1.
Schematic of the proposed ITC-based non-chromatographic purification method4 adapted for hydrophobic proteins solubilized in a detergent.
In this study, we examined the effects of three mild detergents – n-dodecyl-β-D-maltoside (DDM), Triton X-100, and the zwitterionic 3-[(3-cholamidopropyl) dimethylamino]-1-propanesulfonate (CHAPS) – that are commonly used in membrane protein solubilization and purification15 on the thermal transition behavior and secondary structures of a model ELP using temperature controlled turbidity measurements and circular dichroism spectroscopy (CD), respectively. We found that, in contrast to SDS, the mild detergents used herein do not affect the Tt or the secondary structures of ELP180 and, therefore, hold significant promise for purification of recombinantly expressed and detergent-solubilized membrane proteins utilizing the ITC method.
Materials and Methods
ELP[V5A2G3-180] expression and purification
For this study, we chose a model ELP construct, ELP[V5A2G3-180] (hereafter referred to as ELP180), that has been previously employed in ITC of recombinant soluble proteins (see reference 14 for complete sequence). ELP180 is an ELP containing 180 pentapeptide subunits (Val-Pro-Gly-Xaa-Gly), composed of 18 ten-pentapeptide repeats with the guest residues Val, Ala, and Gly in a 5:2:3 ratio.16 The expression vector pET25b(−) ELP[V5A2G3-180] was transformed into E. coli BLR (DE3) cells (Novagen). Cells were grown for 24 h at 37 °C without induction as described previously.4 Harvested cells were subsequently lysed by brief sonication in phosphate buffered saline (PBS, pH 7.3) [4.2 mM Na2HPO4, 1.4 mM KH2PO4, 137 mM NaCl, and 2.7 mM KCl] with or without 19.5 mM DDM. Lysed cells were then centrifuged at 13,000 rcf at 4 °C for 20 min. Soluble nucleic acids were precipitated by the addition of polyethylenimine (PEI) [0.5% (w/v) final concentration] and removed by centrifugation.3 ELP180 was then purified by ITC. Briefly, an aliquot of 0.5 M NaCl was added to the cell extract with or without DDM and the protein was selectively precipitated by centrifugation at 13,000 rcf at 35 to 40 °C for 20 min (termed hot spin in Scheme 1). The pellet fraction containing ELP180 was resuspended in ice-cold PBS with or without DDM. Typically, two to three rounds of ITC (hot centrifugation, pellet resuspension, and subsequent cold centrifugation) were performed to purify the protein. Purified ELP180 was characterized by SDS-PAGE analysis using copper stained gels.4 The concentration of the purified ELP180 was determined spectrophotometrically using a molar extinction coefficient of Trp at 280 nm (5690 M−1 cm−1).16 The yield of ELP180 was 35 mg per liter of bacterial culture.
Turbidity assays
To characterize the ELP’s LCST phase behavior, the optical density at 350 nm (OD350) of ELP solutions, ranging in concentration from 1 to 60 µM in PBS with or without detergents (DDM, Triton X-100, CHAPS, SDS), were measured as a function of temperature using a UV-visible spectrophotometer (UV-1601, Shimazdu, Japan) equipped with a thermoelectric temperature controller. The OD values of buffer or buffers containing detergents were subtracted from the measured values of ELP samples. The heating and cooling rates were 1 °C per minute and Tt values were determined as previously described.1
Critical micelle concentration (CMC) determination of DDM
Surface tension measurements were made using a Wilhelmy plate tensiometer and an electrobalance (KSV Instruments Ltd., Finland). The tensiometer was initially calibrated with ultrapure water (40 mL) to an air-water interfacial tension of 71 mN/m at room temperature.17 Aliquots of varying concentrations of DDM were added to the water, mixed and equilibrated, and the surface pressure of the mixture after the addition of each DDM aliquot was measured. A point at which no increase in surface pressure with the addition of DDM was taken as the CMC value.17
Dynamic light scattering
The size distributions and hydrodynamic radii (Rh) of the protein and protein-detergent mixtures were measured by dynamic light scattering (DAWN HELEOS II, Wyatt Technology, USA) at room temperature. A linearly polarized gallium arsenide laser was used as the light source (658 nm at 130 mW). Data were collected in batch mode using a microcuvette that contained 20 µL of 20 µM protein with or without detergents at a sampling time of 10 min. Size distribution plots were obtained from regularization fitting using ASTRA V software (Wyatt Technology, USA). Rh values were extrapolated using cumulant analysis, assuming scattering species are spherical and monodisperse.
Cirucular dichroism spectroscopy
The secondary structures of ELP (1 µM) in the presence or absence of detergents and either below or above LCST were determined by circular dichroism spectroscopy (CD) (Model 420 AVIV Biomedical Inc., USA). Far UV CD spectra were recorded using a quartz cuvette with 1 mm path length at 0.5 nm intervals between 190–260 nm at room temperature .21,22 Background spectra of the buffers were subtracted from the recorded values.
Results
Effect of DDM on ELP180 purification and phase transition temperature
Determination of detergent compatibility is an important prerequisite for the extraction of integral membrane proteins in their native structure.20 The ability of a detergent to solubilize and stabilize a membrane protein depends strongly on the particular characteristics of the protein and of the detergents.14 For the solubilization of a particular membrane protein, identification of a suitable detergent is generally empirical. The extraction and stabilization of membrane proteins from cell extracts typically require the addition of detergents at concentrations 3 to 5 times their critical micelle concentrations (CMC) or more.6,18 One such detergent, DDM, is commonly used to solubilize membrane proteins and has been shown to adequately preserve the activity of many membrane proteins.6,18 Under our experimental conditions, we found the CMC of DDM in water to be 0.17 mM (data not shown), which is close to a previously reported value.19
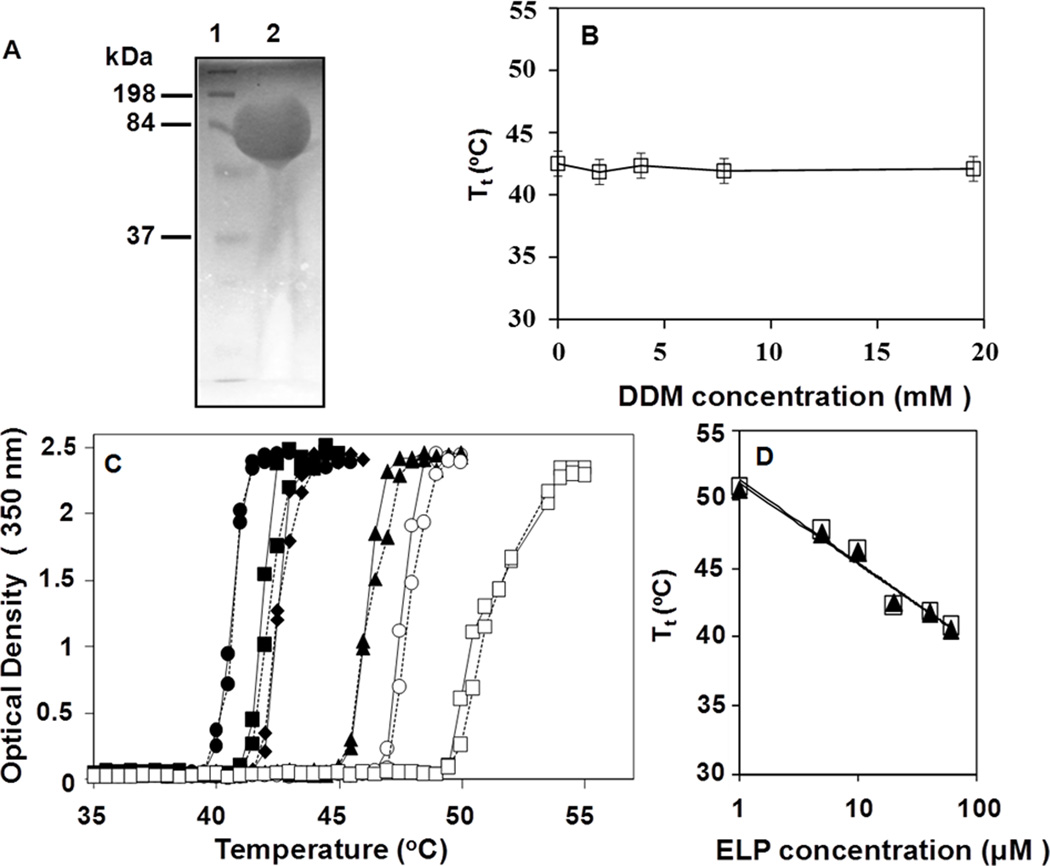
Earlier reports have shown that ELP180 is highly overexpressed in E. coli and can be purified by ITC (Scheme 1).1,4 This polypeptide (molecular weight ~72 kDa) has a relatively low Tt of approximately 35 to 40 °C, depending on the ELP and salt concentrations.3,16 To validate the ITC method for the purification of detergent solubilized proteins, ELP180 was expressed in E. coli and purified from the cell lysate using the ITC method (Scheme 1) either in the absence or presence of 19.5 mM DDM (~100 times the CMC). As shown in Figure 1A, highly pure ELP180 was isolated after 3 rounds of ITC carried out in the presence of DDM. Distinct impurities were not detected by SDS-PAGE when 60 µg of purified ELP180 was loaded. The yield of ITC purified ELP180 was not affected by the presence of DDM, suggesting that ELP-membrane fusions solubilized in DDM may be fully recovered.19
Figure 1.
ELP180 purification by ITC and turbidity assays. (A) Copper stained 10% SDS-PAGE gel of purified ELP180. Lane 1: protein standards and lane 2: purified ELP180. Lane 2 was overloaded with ~60 µg protein in order to detect impurities present. Molecular weights of protein standards are indicated on the left. (B) Effect of DDM on the Tt of 20 µM ELP180. (C) Turbidity profiles of varying concentrations of ELP180 with or without DDM as a function of temperature. ELP180 in the absence (broken lines) and presence (solid lines) of 19.5 mM DDM at 1 µM (open squares), 5 µM (open circles), 10 µM (filled triangles), 20 µM (filled diamonds), 40 µM (filled squares) and 60 µM (filled circles) (D) Effect of protein concentrations on the Tt of ELP180 in the absence (filled triangles) or presence (open squares) of 19.5 mM DDM.
The phase transition temperature (Tt) is an important characteristic of an ELP for ITC; a high Tt makes capturing the target protein difficult by ITC and often results in poor or no purification.3 To ascertain how the presence of DDM affects the Ttof ELP180, we measured the Tt of 20 µM ELP180 at various concentrations of DDM (Figure 1B). The turbidity curves show the characteristic transformation to a turbid solution upon heating of the ELP solution above its Tt. For each thermal transition curve, a Tt value was interpolated as the temperature at which 50% of maximum turbidity (OD350) was observed.1 DDM did not alter the Tt for all concentration studied, up to 19.5 mM, which is more than two orders of magnitude higher than its CMC.19 In fact, the thermal transition profile of ELP180 was more influenced by the protein concentration with higher polypeptide concentrations yielding turbid solutions at lower temperatures (Figure 1C), which is in agreement with published reports.3 Tt values for 1, 5, 10, 20, 40 and 60 µM ELP180 were experimentally determined to be 50.7, 47.5, 46.1, 42.5, 41.7, and 40.5 °C, respectively. With the addition of 19.5 mM DDM, the turbidity profiles of ELP180 at all concentrations overlapped with those without DDM (Figures 1C and 1D). Thus, DDM did not alter the thermal transition behavior of ELP180. Taken together, these data suggest that ELP fusions may be useful to purify DDM-solubilized integral membrane proteins by the ITC method.
Effect of other detergents on the Tt of ELP180
To determine if other commonly used mild detergents behave similarly to DDM, a nonionic detergent, we monitored the phase transition behavior of ELP180 in the presence of Triton X-100 (nonionic), CHAPS (zwitterionic) and compared them with results using SDS, a strong anionic detergent.
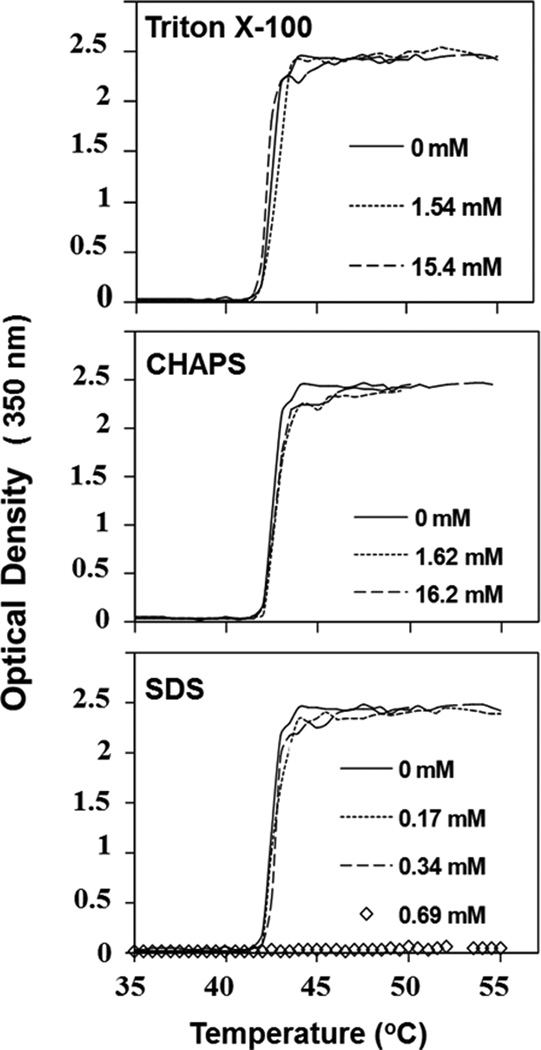
As shown in Figure 2 (upper and middle panels), no significant changes in the thermal transition curve of ELP180 (20 µM) were observed with the addition of Triton X-100 (1.54 mM and 15.4 mM) and CHAPS (1.62 mM and 16.2 mM). Typically 1 to 5 mM of Triton X-100 and 1.6 to 6.1 mM of CHAPS are used to solubilize membrane proteins, which are well within the ranges of both detergents used in this study. Therefore both Triton X-100 and CHAPS may be considered suitable detergents in the purification of membrane proteins by ITC. SDS also did not alter the phase transition of ELP180 for concentrations up to 0.34 mM (Figure 2, lower panel); however, dramatic alteration of the phase transition behavior occurred above 0.69 mM of SDS (Figure 2, lower panel). In contrast to published reports which show initial depression of Tt of ELP150 at low concentrations of SDS and gradual increase of Tt above 4 mM,13 our data show that 0.69 mM of SDS is enough to completely abolish the thermally induced phase transition of 20 µM ELP180 over the temperatures studied. A study of PNIPAAm, which behaves similarly to ELPs in response to temperature change, reported that 17 mM of SDS can completely inhibit its phase transition.8 This discrepancy observed in our work as compared to previous studies may result from the different ELP protein concentrations used as previous studies have used higher protein concentration ranges.8,13 However, both reports unambiguously demonstrate that the strong anionic SDS greatly affects Tt of ELPs at low concentrations and therefore may not be suitable for protein extraction and purification.
Figure 2.
Turbidity profiles of ELP180 (20 µM) in the absence or presence of varying concentrations of Triton X-100, CHAPS and SDS as a function of temperature. Detergents and their concentrations are indicated.
Effect of detergents on ELP180 secondary structures
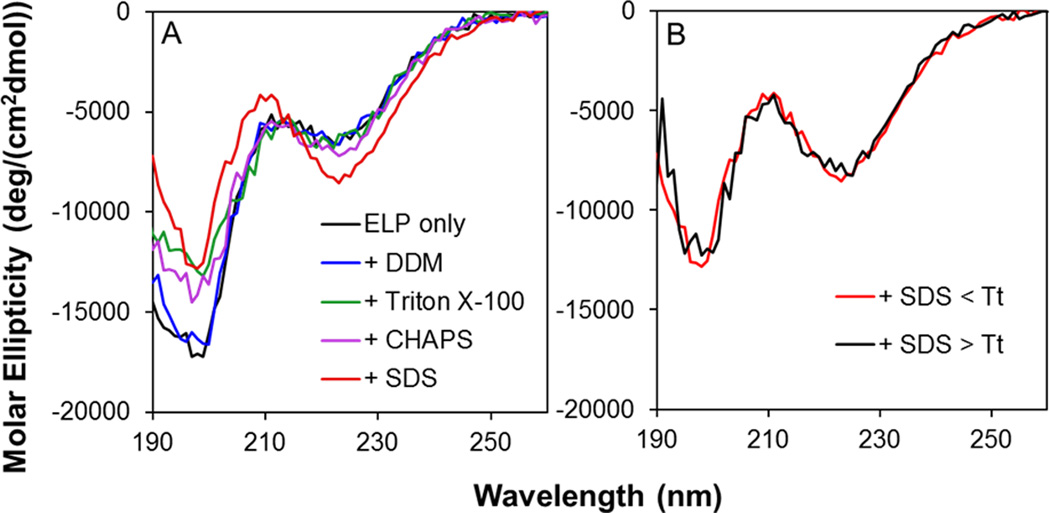
Our results demonstrate that the Tt, reflecting association and aggregation, of ELP180 is unaffected by mild detergents such as DDM, Triton X-100 and CHAPS within functionally relevant concentration ranges, while the phase transition was suppressed for temperatures up to 55 °C by the strong detergent, SDS (Figures 1 and 2). To examine the influence of detergents on the thermally-induced conformational change we assessed the secondary structures of ELP180 in the absence or presence of detergents using circular dichroism spectroscopy (CD) at the lowest ELP180 concentration used for measuring Tt, 1 µM. CD spectra of ELP180 samples with and without detergents below (25 °C) and above (51 °C) Tt were taken. As shown in Figure 3A, the CD spectrum of soluble ELP180 in PBS with no detergent shows a distinct minimum at 197 nm (ππ*-transition) and a less pronounced minimum at 218 nm (nπ*-transition) at 25 °C, indicative of the characteristic random coil and β-turn structures of ELPs, respectively.22 The CD spectrum of ELP180 in the presence of DDM overlapped with that of ELP alone (Figure 3A), indicating that this mild detergent does not perturb the secondary structures of ELP180. In the presence of Triton X-100 and CHAPS, the intensity of the peak at 197 nm is slightly decreased while the intensity of the peak at 218 nm remained roughly the same. This change indicates that the presence of these two detergents slightly reduced the random coil content in the soluble ELP180, but did not induce the formation of more ordered structures. In the presence of the strong detergent SDS, the intensity of the random coil peak decreased while the intensity of the β-turn peak increased (Figure 3A), indicating that SDS has induced ELP180 to adopt more ordered secondary structures in the soluble state. Thus our data show that the mild detergents had no or little effect on the structure of ELP180 at low temperature while the strong detergent SDS induced ELP180 to adopt a more ordered conformation.
Figure 3.
Background subtracted CD spectra of (A) ELP180 (1 µM) alone and with added DDM (19.5 mM), Triton X-100 (15.4 mM), CHAPS (16.2 mM), or SDS (0.69 mM) collected at 25 °C, and (B) ELP180 (1 µM) in the presence of SDS (0.69 mM) below and above LCST.
When the ELP180 samples were heated to 51 °C, a temperature above the Tt, all samples except ELP180 containing SDS, precipitated to form turbid solutions. Due to light scattering from these samples, direct comparison between CD spectra of samples below and above Tt were not made. It is expected, based on previous studies,8,9,21,22that the ELP transitions from a disordered to a more ordered structure when heated above Tt. Since the ELP sample containing SDS remained clear, with no phase transition, when heated to 51 °C, the CD spectra of the sample at 25 and 51 °C are directly compared (Figure 3B). The CD spectrum of ELP180 in the presence of SDS remained unchanged when heated from 25 to 51 °C, indicating a lack of structural changes, or phase transition, of the ELP in the same temperature range. Thus, although ELP180 at low temperature adopts a more ordered conformation in the presence of SDS, this soluble conformation is retained with heating.
Effect of detergents on ELP180 hydrodynamic radii
Aside from altering the phase transition behaviors of ELPs, the binding of detergents to ELPs are also known to solubilize and affect the aggregation state of the polypeptides. For example, even at temperatures below Tt where ELP solutions are clear, the ELPs tend to form soluble, thereby sub-visible, aggregates, and the addition of the strong detergent SDS has been shown to solubilize the aggregates and reduce the size distribution of ELPs.13 The mild detergents tested in this study do not affect either the phase transition behaviors or the secondary structures of soluble ELP180. To test if the interactions between these mild detergents with ELP180 exert any effect on the aggregation state of soluble ELP180, dynamic light scattering was used as a sensitive technique to measure the size distribution of the polypeptide at 25 °C in the absence and presence of the different detergents.
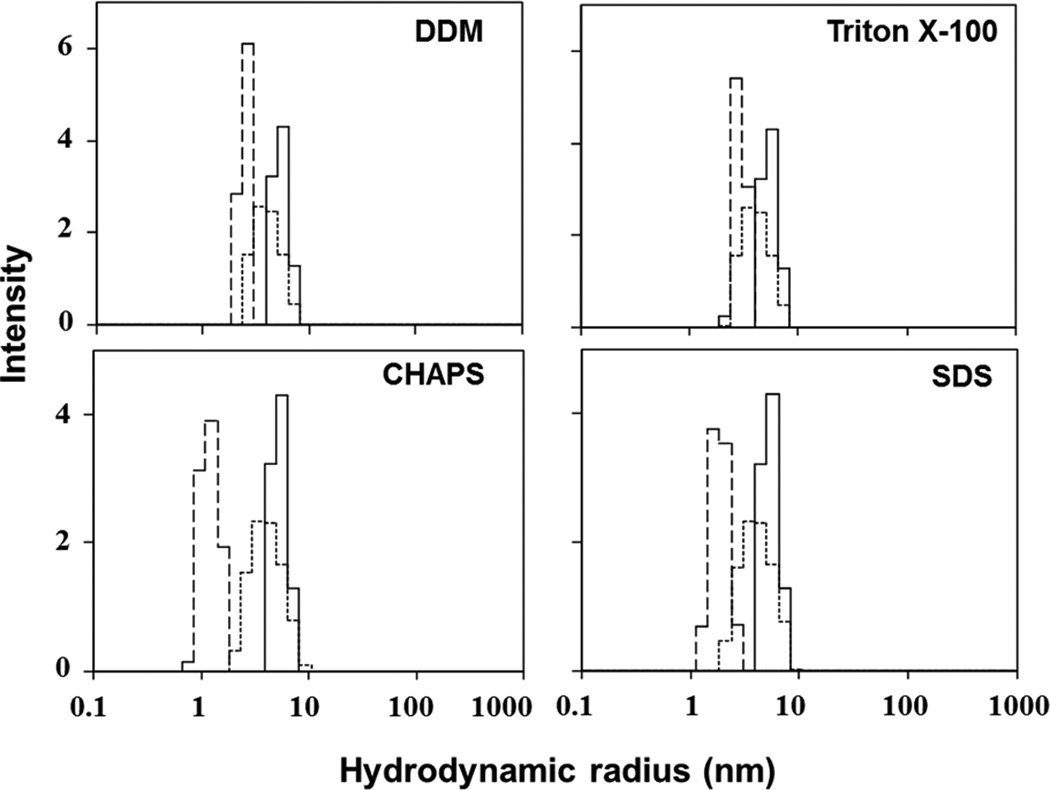
The hydrodynamic radii (Rh) value of soluble 20 µM ELP in PBS obtained from cumulant analysis was 7.16 ± 0.02 nm (Figure 4, distributions shown in solid lines). The Rh values of DDM, Triton X-100, CHAPS or SDS alone were 3.16 ± 0.10 nm, 1.57 ± 0.03 nm, 1.67 ± 0.04 nm and 2.43 ± 0.05 nm, respectively (Figure 4, distributions shown in dashed lines). Since detergent concentrations used in these measurements were five-fold higher than their respective CMC values, these Rh values correspond to those of detergent micelles. DLS measurements of detergents at the same concentrations added to 20 µM ELP80 yielded Rh values intermediate of that of ELP180 alone or detergents alone. The measured Rh values were 4.68 ± 0.11 nm, 5.08 ± 0.05 nm, 4.63 ± 0.06 nm and 4.73 ± 0.02 nm in the presence of DDM, Triton X-100, CHAPS or SDS, respectively (Figure 4, distributions shown in dotted lines). Size distribution of ELP180-detergent mixtures in most cases overlapped with those of ELP alone or detergent micelles alone, which suggests that the presence of mild detergent micelles did not significantly alter the size distribution of ELPs when considering the combined ELP and detergent micelle populations. The slight decrease in the maximum of the ELP size distribution with the addition of detergents may be due to the combined effect of two distinct distributions on the data acquisition, a weak solubilizing effect of small ELP aggregates, or weak interactions between the detergents and ELPs (as strong binding between ELPs and micelles that results in more extended conformations would result in increases in the apparent, or hydrodynamic, radii of the polypeptides).
Figure 4.
Effect of detergents on ELP180 (20 µM) particle size distribution in PBS as measured by dynamic light scattering at 25 °C. Solid lines, ELP alone; dotted lines, ELP in the presence of detergent (detergents are indicated); dashed line, detergents only.
Discussion
ELPs are increasingly being utilized as fusion tags because they are highly overexpressed in E. coli and have narrow ranges in LCST transition temperature, properties which facilitate purification of fused proteins in their native states by the simple and high throughput ITC method.1,2 Moreover ELP fusion proteins have been shown to improve yields, enhance solubility and maintain activity of the target protein irrespective of its size and complexity.4,5 On the other hand, ELPs are susceptible to increases in phase transition temperature upon the addition of cosolutes,4,9 which may lead to ELP phase transition temperatures above the thermal denaturation point of the target protein.
Previous reports have shown a dual effect of the strong anionic detergent SDS on ELPs: an initial depression of Tt of ELPs at low concentrations of SDS followed by a gradual increase of Tt up to the boiling point of water for SDS concentrations at, or higher than, the CMC of SDS.8,13 Similarly the cooperative binding between SDS and polymer segments and the repulsion between adjacent polymer-bound SDS micelles may be responsible for the increase in Tt of PNIPAAm.8 Additionally, it has been reported that concentrations of SDS above 4 mM can increase the Tt of ELP150 and can completely inhibit the thermally induced random coil-to-goluble transition of PNIPAAm at 17 mM or higher.8,13 The findings of these studies might discourage the possible use of ELP fusion proteins in the ITC method for the purification of insoluble integral membrane proteins due to the relatively high concentration of detergents typically required to enable complete solubilization.6,18 While the deleterious effects of strong detergents on the thermal responsive properties of ELPs have clearly been demonstrated, our results show that the use of mild detergents may provide the key to using ELPs as purification vehicles in the ITC methodology for hydrophobic proteins. We therefore hypothesize that ELPs, in presence of mild detergents that are commonly used to solubilize and stabilize membrane proteins,6,18 may be useful to purify such proteins (Scheme 1).
Our results have demonstrated differences in the effects of mild and strong detergents on the phase transition behavior of a model ELP that has been previously used as a fusion partner in ITC based purification of recombinant proteins. ELP180 was shown to be relatively unaffected by the addition of the mild detergents (DDM, Triton X-100 and CHAPS) above their CMC and concentrations typically used to solubilize membrane proteins. (Figures 1 and 2), whereas a strong detergent (SDS) inhibits the phase transition of ELP180 up to 55 °C at concentrations even below the CMC of SDS (8.1 mM)8 (Figure 2, lower panel). Below the Tt, CD spectra indicate that ELP180 retained the characteristic random coil and β-turn structures in the presence of mild detergents DDM (19.5 mM), Triton X-100 (15.4 mM), CHAPS (16.2 mM), and adopts a more ordered conformation in the presence of the strong anionic surfactant SDS (0.69 mM (Figure 3). ELP180 in the presence of the mild detergents likely undergoes a structural change throughout phase transition, while SDS greatly inhibited the thermally induced structural transition (Figure 3B) with the solution remaining clear after heating. Together with our data showing that mild detergents do not alter the Tt of ELP180 (Figure 1 and 2), these results indicate that mild detergents do not perturb the structure or phase transition behavior of ELP180. Our results also show that detergents, including SDS, did not significantly alter the size distribution of ELP180, indicating a lack of strong binding to the ELP (Figure 4).
The characteristic properties of ELPs –their reversible coil-to-globule thermal phase transition and secondary structures– are exclusively maintained in the presence of mild detergents, possibly by virtue of the nonionic or zwitterionic character of these surfactants. Altogether these data suggest that secondary structures of ELPs, which may play an important role in in vitro control of solubility of hydrophobic fusion proteins, are relatively unaffected by the presence of the mild detergents both below and above their LCST. Therefore, we propose the utilization of ELPs as potential fusion partners in the use of ITC of integral membrane proteins or other insoluble proteins in the presence of mild surfactants.
Conclusions
Unlike many cosolutes, several mild detergents employed in the extraction, solubilization and stabilization of membrane proteins do not significantly alter the transition temperature of ELP180. Furthermore, such detergents do not influence the ELP’s secondary structure. This study suggests that mild detergents, unlike strong detergents such as SDS, may facilitate the simple, fast and robust non-chromatographic purification of recombinant proteins that are hydrophobic and expressed as chimeric fusions to ELPs using the ITC method. Furthermore the results of this study may help to elucidate a possible design constraint in additional, applications that are based on both ELP and detergent functionality, including bio-assays,24 separations relying on ELP-based hybrid membranes or particles,25 and drug formulations.26 Indeed the effect of detergents on the thermal behavior of elastin-like polypeptides may play an important role in their success.
Acknowledgement
This work was supported by the NSF’s Triangle MRSEC (DMR-1121107), by the Army Research Office (Grant # W911NF-061-1-0333 awarded to G.P.L.) and by the NIH (grant # GM061232 awarded to A.C.). We thank Prof. Y. Yingling (NC State University) for help in assembling the TOC graphic.
Abbreviations
- CHAPS
3-[(3-cholamidopropyl) dimethylamino]-1-propanesulfonate
- CD
circular dichroism spectroscopy
- CMC
critical micelle concentration
- DDM
n-dodecyl-β-D-maltoside
- ELPs
elastin-like polypeptides
- ITC
inverse transition cycling
- PBS
phosphate buffer saline
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
References
- 1.Meyer DE, Chilkoti A. Nat Biotechnol. 1999;17:1112–1115. doi: 10.1038/15100. [DOI] [PubMed] [Google Scholar]
- 2.Meyer DE, Trabbic-Carlson K, Chilkoti A. Biotechnol Prog. 2001;17:720–728. doi: 10.1021/bp010049o. [DOI] [PubMed] [Google Scholar]
- 3.Meyer DE, Chilkoti A. Biomacromolecules. 2004;5:846–851. doi: 10.1021/bm034215n. [DOI] [PubMed] [Google Scholar]
- 4.Trabbic-Carlson K, Liu L, Kim B, Chilkoti A. Protein Sci. 2004;13:3274–3284. doi: 10.1110/ps.04931604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang HJ, Kim JH, Chang WJ, Kim ES, Koo YM. J Microbiol Biotechnol. 2007;17:1751–1757. [PubMed] [Google Scholar]
- 6.Seddon AM, Cumow P, Booth PJ. Biochim Biophys Acta. 2004;1666:105–117. doi: 10.1016/j.bbamem.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Trabbic-Carlson K, Meyer DE, Liu L, Piervincenzi R, Nath N, LaBean T, Chilkoti A. Protein Eng Des Sel. 2004;17:57–66. doi: 10.1093/protein/gzh006. [DOI] [PubMed] [Google Scholar]
- 8.Dharana D, Chatterji PR. J Macromol Sci Rev Macromol Chem Phys. 2000;C40:51–68. [Google Scholar]
- 9.Yamaoka T, Tamura T, Seto Y, Tada T, Kunugi S, Tirrell DA. Biomacromolecules. 2003;4:1680–1685. doi: 10.1021/bm034120l. [DOI] [PubMed] [Google Scholar]
- 10.Cho W, Stahelin R. Annu Rev Biophys Biomol Struct. 2005;34:119–151. doi: 10.1146/annurev.biophys.33.110502.133337. [DOI] [PubMed] [Google Scholar]
- 11.Bergfors TM. Protein crystallization: techniques, strategies, and tips: a laboratory manual. La Jolla, California: International University Line; 1999. [Google Scholar]
- 12.le Maire M, Champeil P, Moller JV. Biochim Biophys Acta. 2000;1508:86–111. doi: 10.1016/s0304-4157(00)00010-1. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Trabbic-Carlson K, Albertorio F, Chilkoti A, Cremer PS. Biomacromolecules. 2006;7:2192–2199. doi: 10.1021/bm060254y. [DOI] [PubMed] [Google Scholar]
- 14.Arnold T, Linke D. Curr Protoc Protein Sci. 2008;Chapter 4(Unit):4.8.1–4.8.30. doi: 10.1002/0471140864.ps0408s53. [DOI] [PubMed] [Google Scholar]
- 15.Witzmann F, Jamot B, Parker D. Electrophoresis. 1991;12:687–688. doi: 10.1002/elps.1150120919. [DOI] [PubMed] [Google Scholar]
- 16.Meyer DE, Chilkoti A. Biomacromolecules. 2002;3:357–367. doi: 10.1021/bm015630n. [DOI] [PubMed] [Google Scholar]
- 17.Chi EY, Weickmann J, Carpenter JF, Manning MC, Randolph TW. J Pharm Sci. 2005;94:256–274. doi: 10.1002/jps.20237. [DOI] [PubMed] [Google Scholar]
- 18.Ribosa II, Sanchez-Leal J, Comelles F, Garcia MT. J Colloid Interface Sci. 1997;187:443–446. doi: 10.1006/jcis.1996.4734. [DOI] [PubMed] [Google Scholar]
- 19.VanAken T, Foxall-VanAken S, Castleman S, Ferguson-Miller S. Methods Enzymol. 1986;125:27–35. doi: 10.1016/s0076-6879(86)25005-3. [DOI] [PubMed] [Google Scholar]
- 20.Drew D, Slotboom DJ, Friso G, Reda T, Genevaux P, Rapp M, Meindl-Beinker NM, Lambert W, Lerch M, Daley DO, Van Wijk KJ, Hirst J, Kunji E, De Gier JW. Protein Sci. 2005;14:2011–2017. doi: 10.1110/ps.051466205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nuhn H, Klok HA. Biomacromolecules. 2008;9:2755–2763. doi: 10.1021/bm800784y. [DOI] [PubMed] [Google Scholar]
- 22.Serrano V, Liu W, Franzen S. Biophys J. 2007;93:2429–2435. doi: 10.1529/biophysj.106.100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rika J, Meewes M, Nyffenegger R, Binkert TH. Phys Rev Lett. 1990;65:657–660. doi: 10.1103/PhysRevLett.65.657. [DOI] [PubMed] [Google Scholar]
- 24.Yang P, Marinakos SM, Chilkoti A. Langmuir. 2011;27:1463–1471. doi: 10.1021/la104186n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rama Rao GV, Balamurugan S, Meyer DE, Chilkoti A, Lopez GP. Langmuir. 2002;18:1819–1824. [Google Scholar]
- 26.McDaniel JR, Callahan DJ, Chilkoti A. Adv Drug Deliver Rev. 2010;62:1456–1467. doi: 10.1016/j.addr.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]