Abstract
Arabidopsis has been used as a model system to study many aspects of plant growth and development. However, fruit senescence in Arabidopsis has been less investigated and the underlying molecular and hormonal (especially ethylene) regulatory mechanisms are not well understood. It is reported here that the Arabidopsis silique has characteristics of a climacteric fruit, and that AtNAP, a NAC family transcription factor gene whose expression is increased with the progression of silique senescence, plays an important role in its senescence. Silique senescence was delayed for 4–5 d in the atnap knockout mutant plants. The ethylene climacteric was delayed for 2 d in the atnap silique and the associated respiratory climacteric was suppressed. Exogenous ethylene stimulated respiration in the wild type, but not in the atnap mutant. The decoupling of the ethylene and respiratory climacterics in the atnap mutant suggests that AtNAP is required for ethylene stimulation of respiration. qPCR analyses revealed that the expression patterns of genes involved in ethylene biosynthesis, perception, and signalling, ACS2, ETR1, CTR1, EIN2, EIN3, and ERF1, were also altered in the atnap mutant. The effects of exogenous ABA, SA, 6-BA, and NAA on ethylene production and respiration in siliques of the wild type and atnap mutant were also investigated. A model involving ABA-AtNAP-controlled stomatal opening in regulating ethylene-stimulated respiration in fruit senescence is presented.
Key words: Arabidopsis, AtNAP, climacteric, ethylene, fruit senescence, gene regulation
Introduction
Arabidopsis and tomato are model systems for the study of development, ripening, and senescence of dry and fleshy fruits, respectively (Alexander and Grierson, 2002; Giovannoni, 2004, 2007; Klee and Giovannoni, 2011). In Arabidopsis, significant progress on the regulation of fruit development by transcription factors has been made; MADS box genes play key roles in carpel identity (reviewed in Giovannoni, 2007; Klee and Giovannoni, 2011). A MADS box gene in tomato named LeMADS-RIN has been shown to control fruit ripening non-hormonally (Vrebalov et al., 2002). Although ectopic expression of AGL15 (an Arabidopsis MADS domain-containing gene) under the direction of the senescence-specific SAG12 promoter (Gan, 1995) delays flower senescence and silique maturation in Arabidopsis (Fang and Fernandez, 2002), this MADS domain gene is seed-specific and appears not to be expressed in carpels (silique walls). The role of AGL15 and other MADS genes in silique senescence in Arabidopsis remains elusive, and the transcriptional regulation of silique senescence is yet to be discovered. Guo and Gan (2006) found that AtNAP, a NAC family transcription factor gene, has an important role in leaf senescence in Arabidopsis. Leaf senescence was delayed for up to 10 d in the atnap knockout mutant plants whereas chemical induction of AtNAP in young leaves readily caused precocious leaf senescence. Recently, AtNAP is shown to be up-regulated early after anthesis, which corresponds with ovule senescence (Carbonell-Bejerano et al., 2010). Whether AtNAP also has a role in regulating the senescence of siliques, a derivative of leaves, is not known.
Although Arabidopsis has been a useful model for many aspects of plant growth and development, including leaf senescence, research into the molecular and hormonal regulation of its fruit senescence has been limited and has lagged behind that for tomato and other fruit. Recent studies on structural changes and senescence-associated gene expression during silique development and senescence revealed that it undergoes well-defined developmental and senescence processes in Arabidopsis (Wagstaff et al., 2009; Carbonell-Bejerano et al., 2010). Microarray analysis of gene expression profiles during silique senescence (Wagstaff et al, 2009) represents a significant step toward a molecular understanding of fruit senescence in Arabidopsis. However, such fundamental questions as whether the Arabidopsis silique is a climacteric fruit and how the silique responds to exogenous ethylene and other hormones have not yet been addressed.
It is reported here that the Arabidopsis silique exhibits characteristics of a climacteric fruit that is subjected to regulation by ethylene and other hormones. It is also reported that Arabidopsis silique senescence is highly controlled by AtNAP. Silique senescence is delayed and the respiration surge associated with endogenous or exogenous ethylene is suppressed in the atnap knockout mutant plants. The ethylene production of the atnap siliques in response to treatments with abscisic acid (ABA), salicylic acid (SA), 6-benzylaminopurine (6-BA), and 1-naphthaleneacetic acid (NAA) is reduced compared with that of the wild type. qPCR analyses reveal that the expression of AtACS2 (encoding a 1-amino-cyclopropane-1-carboxylic acid or ACC synthase), AtETR1 (an ethylene receptor gene), AtCTR1 (a gene for a negative regulator of ethylene signal transduction), AtEIN2 (a gene for a positive regulator of ethylene signal transduction), and AtERF1 (a target gene of AtEIN3) is down-regulated whereas the expression of AtEIN3 (also a positive regulator of ethylene signal transduction) is up-regulated in siliques of the atnap plants.
Materials and methods
Plant materials and growth conditions
Arabidopsis thaliana ecotype Columbia and the atnap knockout mutant plants were used in this research. Seeds were sterilized with three rinses in 70% EtOH containing 0.01% Triton X-100, and a final rinse in 95% EtOH. The seeds were air-dried in a laminar flow hood and then sown on Petri dishes that were then placed at 4 °C overnight. Ten-day-old seedlings were transplanted to Fafard soils (3 parts moss:2 parts vermiculite:1 part perlite) and grown in growth chambers at 23 °C with 64% relative humidity under constant light (150 µmol m–2 s–1 light from a mixture of fluorescent and incandescent bulbs (Guo and Gan, 2006).
Treatments
To evaluate ethylene production and respiration rates, siliques of WT or the atnap knockout mutant plants were harvested at 4, 6, 8, 10, 12, and 14 d post-anthesis (DPA), respectively, and were placed into 10ml vials; the vials were kept open for 30min to vent any ethylene possibly caused by wounding before being sealed with serum caps. The vials were kept at 23 °C for 4h (unless specified otherwise) for ethylene and CO2 measurements. The 30min delay between excision and sealing the vials was based on preliminary experiments in which WT siliques were placed into 10ml vials immediately after excision or at different time periods up to 120min. The ethylene production rates in WT siliques (4 DPA) were not affected by any time delay (P=0.353), means ±standard errors (n=4) being 30.5±0.9, 33.3±1.9, 29.9±2.0, and 33.1±1.5 nl g–1 h–1 after 0, 30, 60, and 120min, respectively. The 30min period was therefore used for convenience to allow the randomization of siliques to vials where necessary.
To evaluate the effects of ethylene treatment on the endogenous production of ethylene and CO2 production, siliques harvested at 4, 6, 8, 10, 12, and 14 DPA were placed in vials, which were injected with 5 µl of 2% (v/v) ethylene (air as a control; Airgas, NY) to obtain a final concentration of 10 µl l–1. The siliques were kept at 23 °C for 2h and then transferred into new vials and sealed. The vials with siliques were kept at 23 °C for 4h for ethylene and CO2 measurements. Four biological replications were performed for each sampling point.
For hormone treatments, siliques at 4 DPA were harvested and placed in 10ml vials containing 200 µl of 100 µM ABA, 6-BA, SA, NAA or water, respectively. The samples were placed under a vacuum for 1min. The vials were kept at 23 °C for 24h for ethylene and CO2 measurements. Four biological replications were performed for each treatment or sampling time point.
Measurement of ethylene
Ethylene was analysed with a Hewlett Packard 5890 Series II gas chromatograph equipped with a Hewlett-Packard 3394A integrator. The column was 0.318cm stainless steel packed with Alumina F-1, 60/80 mesh. Injector port, flame ionization detector, and column temperatures were 230, 245, and 200 °C, respectively. N2, H2, and air flow rates were 30, 30, and 230ml min–1, respectively.
Measurement of respiration
CO2 was measured using a model 910 Buck Scientific gas chromatograph with a thermal conductivity detector (TCD). The oven temperature was 100 °C and the detector temperature was 250 °C. The carrier gas was helium, set to a flow of 10ml min–1. The computer software used was PeakSimple version 3.85. The column was 0.91 m × 0.318cm stainless steel packed with silica gel.
Determination of chlorophyll content
Chlorophyll was extracted by placing silique walls into 1.5-ml microfuge tubes containing 80% acetone and shaking overnight in the dark. The chlorophyll a/b contents were measured using a Beckman spectrophometer and were calculated as described previously (He and Gan, 2002).
RNA extraction and RT-qPCR analyses of gene expression
Isolating DNA-free RNA from Arabidopsis siliques ready for the reverse transcriptase quantitative PCR (RT-qPCR) applications was performed according to Protocol 2 as described by Onate-Sanchez and Vicente-Carbajosa (2008). cDNA was synthesized using the iScripTM cDNA Synthesis Kit (Bio-Rad Product #170–8890). qPCR analyses of individual genes were performed with primers listed in Supplementary Table S1at JXB online using the iQTM SYBR Green Supermix (Bio-Rad Product #170–8880) according to the manufacturer’s protocol. Briefly, for each qPCR reaction, 1 µl of each diluted sample was used as a template in a 25 µl reaction containing 12.5 µl Bio-Rad® 2× SYBR green supermix (Bio-Rad, Hercules, CA, USA), 8.5 µl ddH2O, and 1 µl of each primer. All qPCR reactions were performed on a Bio-Rad® IQ-5 thermocycler with 40 cycles and an annealing temperature of 55 °C. Cycle threshold (Ct) values were determined by the IQ-5 Bio-Rad software assuming 100% primer efficiency. Four cDNA samples derived from four samples at each time point were used. Arabidopsis Actin2 (At3g18780) was used as an internal control. Data were expressed according to normalized expression (ddCT method). The expression levels of individual genes in the 4 DPA siliques of WT were set to 1.
Statistical methods
Expression levels of genes were subjected to t test analyses, with P=0.05 as the cutoff. All other data were subjected to ANOVA by using SPSS. The overall least significant difference (LSD) (P=0.05) was calculated and used to separate means.
Results
Phenotypic changes in silique development and senescence in WT and atnap knockout plants
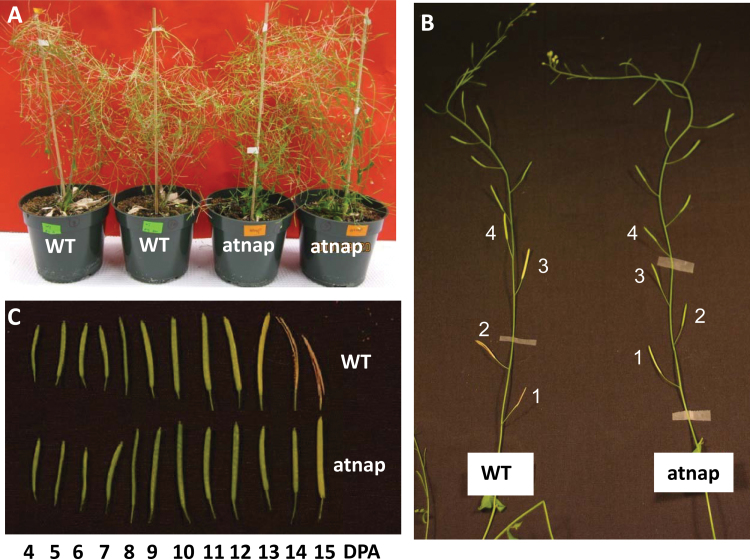
The atnap knockout mutant carries a T-DNA insert in the 2nd exon of AtNAP (At1g69490) that encodes a NAC family transcription factor (Guo and Gan, 2006). The atnap null mutant and WT plants are developmentally indistinguishable in terms of bolting and flowering times. However, silique senescence is dramatically delayed. As shown in Fig. 1B, the 1st siliques of the primary inflorescences of WT and the atnap plants were age-matched, but the WT silique became senescent and shattered while the mutant silique remained intact. Senescence had already progressed to the 4th silique of the WT while the 2nd silique of the atnap mutant remained green. Fig. 1C shows siliques with different ages (DPA). The senescence progression in the atnap siliques was markedly delayed compared with that in the WT.
Fig. 1.
Delayed silique senescence in the atnap knockout mutant. (A) Senescence of siliques of age-matched atnap mutant and wild-type (WT) plants. The plants were approximately 70 d after germination. (B) Senescence in siliques of main shoots of the atnap mutant and WT plants. The 1st, 2nd …siliques are age-matched. Note the 1st WT silique became completely senescent and shattered. DPA, days post-anthesis. (C) Age-dependent progression of silique senescence in the atnap mutant and WT plants.
Chlorophyll changes during development and senescence in silique walls of WT and the atnap mutant plants
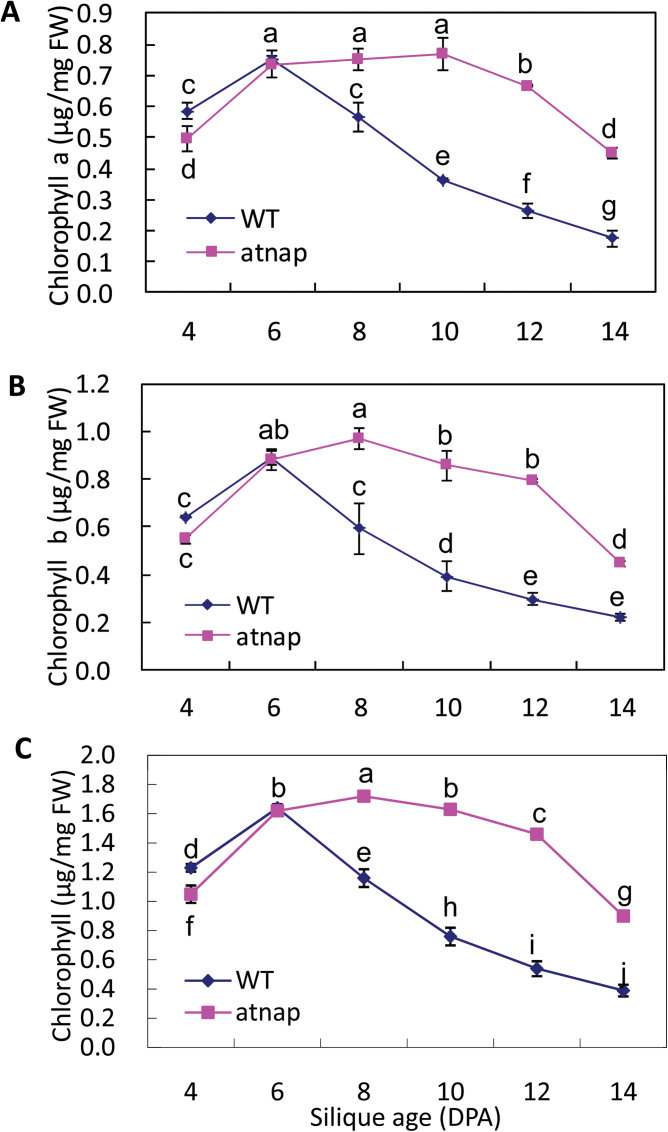
Chlorophyll a, chlorophyll b, and chlorophyll a+b concentrations in the silique walls of the WT and the atnap mutant were similar until 6 DPA (Fig. 2A–C). Chlorophyll concentrations then declined over time in WT siliques, while in the atnap remained high and then declined relatively slowly. The levels of chlorophyll a, b, and a+b were higher in the atnap silique walls than in WT silique walls after 6 DPA.
Fig. 2.
Chlorophyll a (A), b (B), and a+b (C) in silique walls (excluding seeds) of the WT and atnap mutant. Bars are means ±SD (n=4). Significant (P=0.05) differences between means are indicated by different letters.
Changes in ethylene production and respiration during silique development and senescence
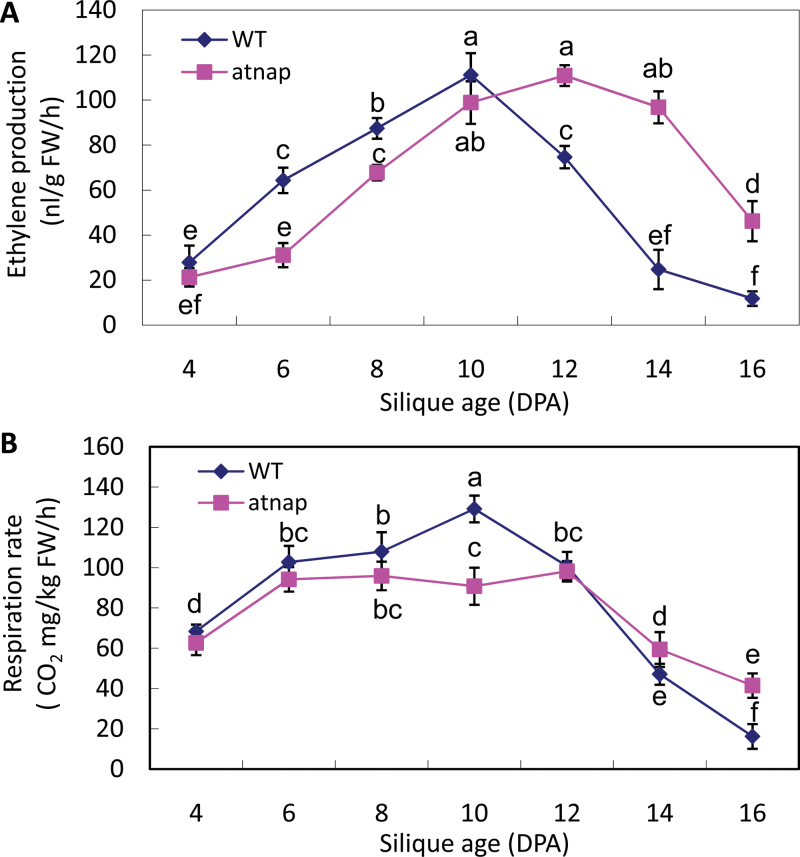
The changes in the rates of ethylene production and respiration during fruit development and senescence in WT and atnap siliques were measured. Ethylene production increased in the WT siliques until 10 DPA and then decreased rapidly (Fig. 3A). Ethylene production by the atnap siliques also increased and then decreased, but these changes were delayed by about 2 d compared with that of WT. However, maximum ethylene production rates were similar between the two silique types.
Fig. 3.
Ethylene production (A) and respiration rate (B) of the WT and atnap mutant during silique development and senescence. Bars are means ±SD (n=4). Significant (p=0.05) differences between means are indicated by different letters.
The changes in respiration rate of the WT siliques (Fig. 3B) had a similar pattern to that of the ethylene production. By contrast, however, respiration rates of the atnap siliques increased to the maximum (approximately 70% of the maximum of that of WT) at 6 DPA, then remained steady until 12 DPA when the respiration rates started to decrease (Fig. 3B). These data suggest that AtNAP may be required for the typical respiration surge in response to endogenous ethylene.
Effect of exogenous ethylene on respiration and endogenous ethylene production of siliques during development and senescence in WT and the atnap mutant plants
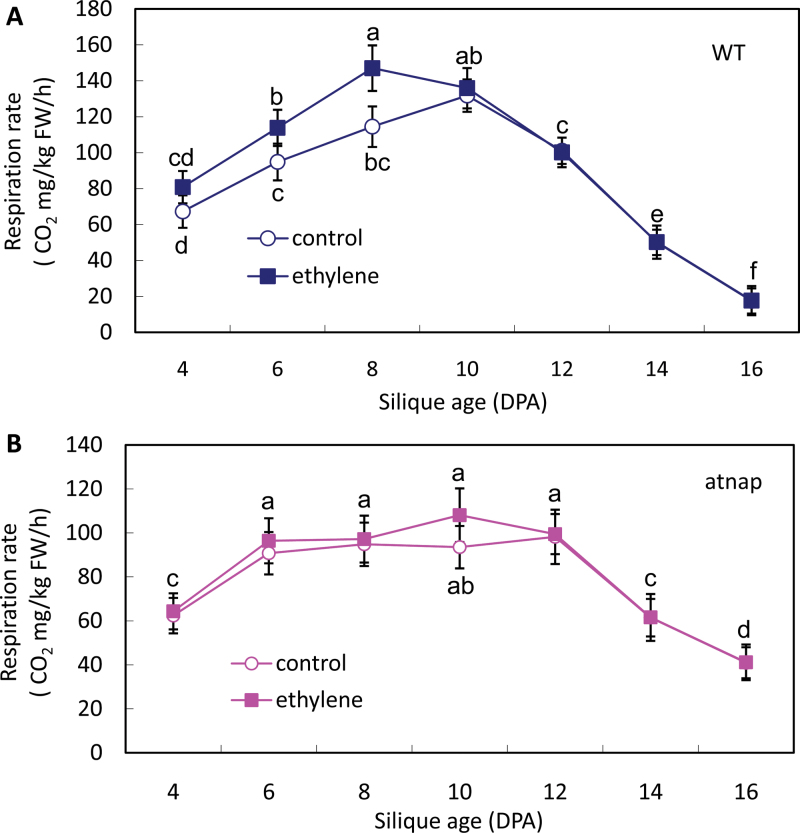
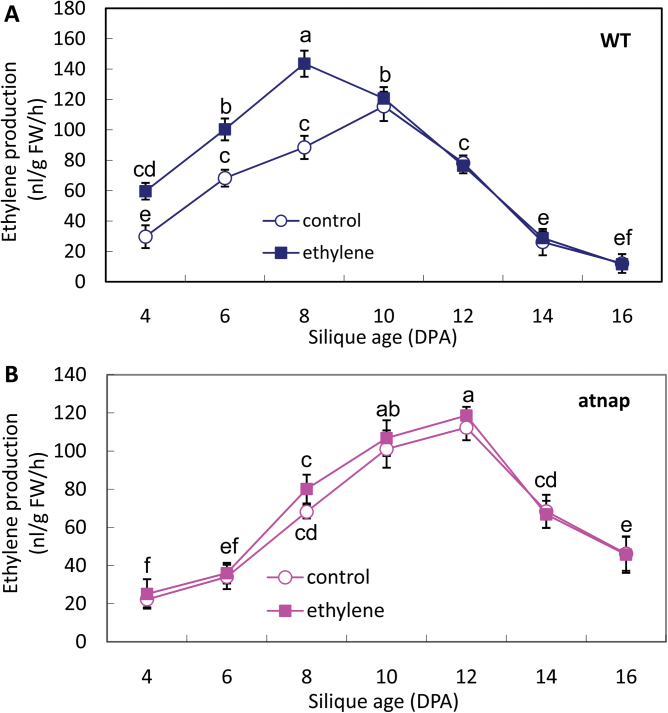
Exogenous ethylene will generally result in an earlier respiratory peak in climacteric fruits. Therefore, the respiration patterns of WT and mutant fruits treated with 10 µl l–1 ethylene were investigated further. As shown in Fig. 4A, external application of ethylene stimulated the respiratory intensity in WT fruits and the climacteric peak was observed at 8 DPA, approximately 2 d earlier than that of the control. By contrast, the same ethylene concentration appeared to have little effect on the respiration of ethylene-treated and untreated siliques of the atnap mutant (Fig. 4B). These data again suggest that AtNAP may be required for the respiration surge in response to both endogenous and exogenous ethylene.
Fig. 4.
Effect of exogenous ethylene on respiration rate of WT and the atnap siliques. (A) Respiration rate of WT siliques after 10 µl l–1 exogenous ethylene treatment. (B) Respiration rate of the atnap mutant siliques after 10 µl l–1 exogenous ethylene treatment. Bars are means ±SD (n=4). Significant (P=0.05) differences between means are indicated by different letters.
Endogenous ethylene production in siliques after exogenous ethylene treatment was also measured. Exogenous ethylene also stimulated endogenous ethylene production in the WT siliques (Fig. 5A) but had little effect in the atnap mutant siliques (Fig. 5B).
Fig. 5.
Effect of exogenous ethylene on ethylene production in WT and the atnap siliques. (A) Ethylene production of WT siliques after treatment with 10 µl l–1 ethylene. (B) Ethylene production of the atnap mutant siliques after treatment with 10 µl l–1 ethylene. Bars are means ±SD (n=4). Significant (P=0.05) differences between means are indicated by different letters.
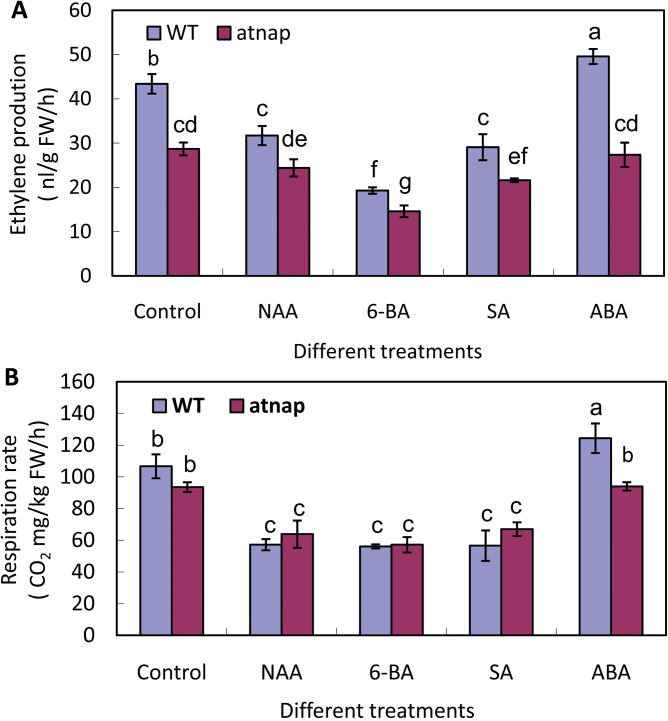
Effects of exogenous ABA, SA, 6-BA and NAA on the ethylene production and respiration in WT and the atnap mutant siliques
Ethylene production and respiration rates of the WT and atnap mutant siliques in response to the plant hormones ABA, 6-BA, NAA, and SA were also measured. Ethylene production was lower in the mutant siliques than the WT in the control and after all hormone treatments (Fig. 6A), but different responses to the treatments were detected. Production of WT siliques decreased after the treatments with 6-BA, SA, and NAA, respectively, but was increased by ABA treatment (P <0.05). In the atnap mutant siliques, ethylene production was similar in the control, NAA, and ABA treatments, with no increased production resulting from ABA treatment. 6-BA caused the greatest reduction of ethylene production.
Fig. 6.
Effect of various exogenous plant hormones on ethylene production and respiration rate in WT and the atnap mutant siliques. (A) Ethylene production of WT and atnap mutant siliques after exogenous plant hormone treatments. (B) Respiration rate of WT and atnap mutant siliques after exogenous plant hormone treatments. Significant (P=0.05) differences between means are indicated by different letters.
Respiration rates were highest in the ABA-treated WT siliques, but, in general, little difference in rates were detected between the WT and the atnap mutant siliques in response to the plant hormones (Fig. 5B). Overall rates were lower in NAA, 6-BA, and SA treatments.
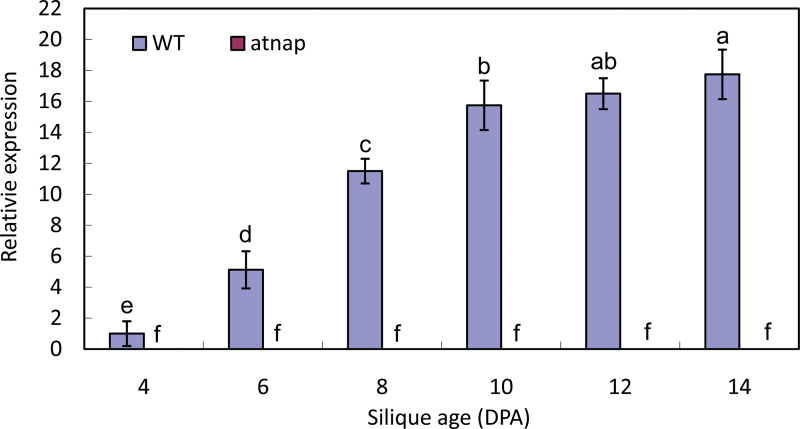
AtNAP transcript levels increase with progression of fruit senescence
Because the loss-of-function of AtNAP had a profound impact on Arabidopsis silique senescence, we investigated if AtNAP is up-regulated during fruit senescence. qPCR showed that the transcript levels of AtNAP increased with the progression of fruit senescence in the WT (Fig. 7), but the AtNAP transcripts were not detectable in the atnap mutant siliques (Fig. 7).
Fig. 7.
AtNAP gene expression in WT and the atnap siliques during development and senescence. qPCR analyses were performed. Relative expression levels were calculated and normalized with respect to Actin 2 (ACT2) transcripts. Four independently isolated RNA samples at each time point were used. Bars are means ±SD (n=4). Note the expression levels in the atnap knockout siliques were almost non-detectable. Significant (P=0.05) differences between means are indicated by different letters.
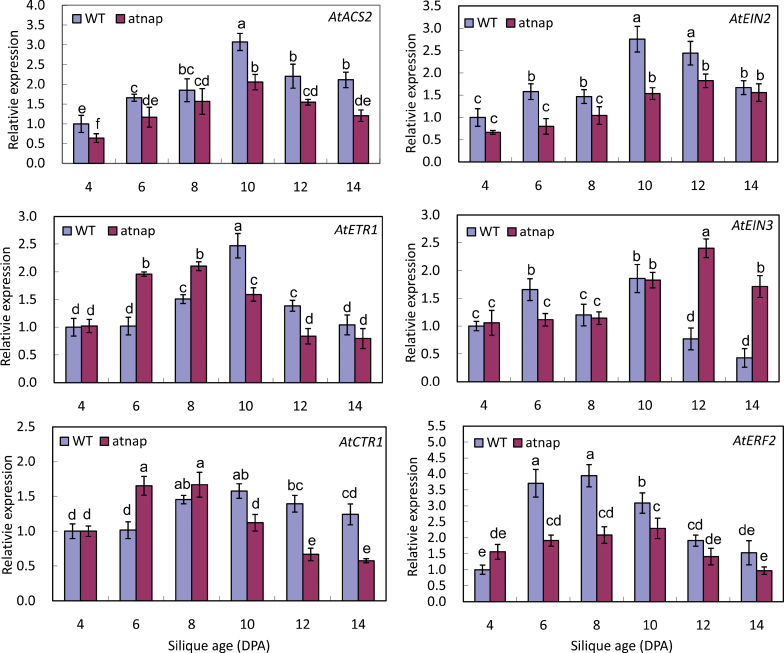
Altered expression levels of genes involved in ethylene biosynthesis, perception, and signalling in the atnap fruits
The expression patterns of selected genes related to ethylene biosynthesis (ACS), perception (ETR1), and signal transduction (CTR1, EIN2, ERF1/2, and EIN3) were analysed.
AtACS2 encodes an ACC synthase that catalyzes the formation of ACC in the ethylene biosynthesis pathway. The expression levels of AtACS2 increased then decreased in both the WT and atnap mutant siliques but the expression levels in the atnap mutant siliques were lower than those in developmentally matched WT siliques (Fig. 8).
Fig. 8.
Expression levels of genes involved in ethylene biosynthesis, perception, and signal transduction in siliques of WT and the atnap mutant during development and senescence. qPCR analyses were performed. Relative expression levels were calculated and normalized with respect to Actin 2 (ACT2) transcripts. Four independently isolated RNA samples at each time point were used. Bars are means ±SD (n=4). Significant (P=0.05) differences between means are indicated by different letters.
AtETR1 encodes an ethylene receptor (Chang et al., 1993; Bleecker et al., 1998). The changes in AtETR1 transcript levels in developing and senescing WT siliques were very similar to the pattern of AtACS2 in WT siliques. However, the pattern of AtETR1 expression in the atnap background was different from that of AtACS2; the expression levels of AtETR1 in 6- and 8-DPA mutant siliques were higher than in age-matched WT siliques (Fig. 8).
AtCTR1 is a gene encoding an ethylene signalling component that is immediately downstream of AtETR1 (Kieber et al., 1993). The expression patterns in both the WT and atnap mutant fruits are very similar to those of AtETR1 (Fig. 8).
AtEIN2 (Alonso et al., 1999) encodes an ethylene signal transducer downstream of AtCTR1. The expression of AtEIN2 in the atnap siliques was down-regulated compared with that in age-matched WT siliques (Fig. 8).
AtERF1 encodes an Arabidopsis EIN3-binding F-box protein (Binder et al., 2007). It functions downstream of AtEIN2 and upstream of AtEIN3. As shown in Fig. 8, the expression pattern of AtERF1 in WT siliques is different from the other genes tested; its transcript levels reached the peak at 8 DPA, approximately 2 d earlier than the other genes tested. The expression of this gene in the atnap mutant siliques was, however, less dramatically changed during development and senescence (Fig. 8).
AtEIN3 is a nuclear protein gene (Chao et al., 1997) that functions immediately after AtERF1 in ethylene signalling (Binder et al., 2007). The null mutation of this gene resulted in accelerated senescence, among others (Chao et al., 1997). The AtEIN3 expression was sharply down-regulated in the WT siliques that were 12 DPA or older but was highly up regulated in the age-matched atnap mutant siliques (Fig. 8).
Discussion
The Arabidopsis silique has characteristics of a climacteric fruit
Much progress has been made in the molecular regulation of leaf senescence in Arabidopsis (Gan, 2003) and fruit ripening in tomato (Giovannoni, 2004, 2007). However, such fundamental questions as whether Arabidopsis fruit is climacteric and how its senescence is regulated are largely unknown. In this study, the patterns of ethylene production and respiration (Fig. 3), as well as silique responses to exogenous ethylene (Figs 4, 5) indicates that they have characteristics of climacteric fruits.
AtNAP plays an important role in fruit senescence
AtNAP, a plant-specific transcription factor gene, was shown to have a pivotal role in controlling leaf senescence, leaf senescence being delayed for up to 10 d in the atnap knockout plants (Guo and Gan, 2006). It is revealed here that AtNAP also plays a critical role in regulating silique senescence in Arabidopsis. Similar to its up-regulation during leaf senescence (Guo and Gan, 2006; Zhang and Gan, 2012), AtNAP transcript levels were elevated with the progression of silique senescence in Arabidopsis (Fig. 7), and silique senescence was delayed for 4–5 d when AtNAP was knocked out (Figs 1, 2). Interestingly, a NAC transcriptional factor in tomato (with a protein ID AAU43923) encoded by the tomato nonripening (nor) locus (Giovannoni, 2007) appears to be an orthologue of AtNAP (Guo and Gan, 2006); nor displays a significant delay in tomato fruit ripening (Tigchelaar et al., 1973).
AtNAP is required for ethylene stimulation of respiration during fruit senescence
It is widely accepted that ethylene is a major factor that promotes senescence of the stigma and ovule (Carbonell-Bejerano et al., 2011), fruits (Oeller et al., 1991; Picton et al., 1993; Wilkinson et al., 1995; Watkins, 2002; Giovannoni, 2007) as well as leaves (Jing et al., 2005). However, the link between ethylene and respiration is not well understood. Our research presented here showed that the ethylene surge and the respiratory surge in the atnap knockout mutant plants were de-coupled. While the onset of the ethylene climacteric was delayed for approximately 2 d when compared with that of WT siliques, and occurred during fruit senescence in the atnap mutant plants (Fig. 3A), the respiratory surge was suppressed in the atnap mutant siliques (Fig. 3B). Similarly, exogenous ethylene treatment promoted earlier occurrence of the surge in respiration in the WT siliques (Fig. 4A). By contrast, the same treatment did not alter the respiration pattern in the atnap mutant siliques (Fig. 4B). These data suggest that AtNAP is required for ethylene to stimulate the respiration in senescing siliques in Arabidopsis, but further analysis is required.
Physiological and molecular bases of AtNAP-regulated silique senescence
The reduced effect of endogenously produced and exogenously applied ethylene to induce corresponding respiratory surges in the atnap mutant siliques (Figs 3, 4) suggests that the sensitivity of siliques to ethylene may be reduced in the mutant plants. AtNAP as a transcription factor can bind to the promoter regions of its immediate target genes to regulate their expression at the transcriptional levels, and the expression of these target genes may subsequently alter the expression and function of other genes. Changes in expression of several genes that are involved in ethylene biosynthesis, perception, and signal transduction pathways were therefore investigated (Fig. 8). For simplicity, the six genes can be arranged in the following order: AtACS2→ AtETR1→AtCTR1→AtEIN2→AtERF1→AtEIN3, with AtACS2 in ethylene production and AtEIN3 as the most downstream component of the ethylene signal transduction. As shown in Fig. 8, the first five genes were indeed suppressed to various extents in the atnap knockout mutant fruits whereas the expression of AtEIN3 was significantly enhanced after 12 DPA. Ethylene production by the atnap fruits reached maximum levels at 12 DPA (Fig. 5). The expression of EIN3-like genes in tomato was shown to be associated with fruit ripening (Tieman et al., 2001; Yokotani et al., 2003). It is still unclear how the changes in expression of AtEIN3 and other genes affect silique senescence in the atnap mutant plants.
Recently, AtNAP was shown to regulate the expression of SAG113 directly, a PP2C family protein phosphatase gene (Zhang and Gan, 2012; Zhang et al., 2012). The AtNAP-PP2C regulatory node mediates ABA to inhibit stomata from closing so that leaves lose water dramatically; the loss of water triggers and promotes leaf senescence (Zhang and Gan, 2012; Zhang et al., 2012). However, it is unknown whether this mechanism may apply to fruit senescence although stomata do develop with the growth and development of siliques in Arabidopsis (Carbonell-Bejerano et al., 2010). The ABA levels also increase during fruit ripening in fruit such as tomato and avocado (Adato et al., 1976; Zhang et al., 2009) although the ABA levels during silique development and senescence in Arabidopsis are yet to be determined. One plausible explanation is that AtNAP, via the ABA-AtNAP-SAG113 PP2C regulatory chain, will prevent stomata from closing so that enough oxygen can diffuse into the silique tissue for ethylene-stimulated fast respiration in the WT siliques. In the atnap knockout siliques, however, the closure of the stomata would be promoted, this would result in less oxygen for respiration as observed in Fig. 3B. This model is supported by the fact that the ABA treatment promoted respiration in the WT siliques (presumably by inhibiting stomata from closing such that more oxygen could get into the silique tissues for respiration) but had no effect on the respiration of the atnap mutant siliques (Fig. 6B). This model is also supported by observations in tomato that ABA precedes ethylene and may regulate it through the NAC gene (Giovanni Giuliano and James Giovannoni, personal communication). Further research is needed to test this stomatal model.
Supplementary data
Supplementary data can be found at JXB online.
Supplementary Table S1. Primers used for qPCR.
Acknowledgements
We are very grateful to Dr James Giovannoni (Cornell University) for critical reading of the manuscript and for sharing unpublished results, Jacqueline Nock (Cornell University) for help in measuring ethylene production and respiration rate, and Dr Yongfeng Guo for the atnap seeds. We also thank Dr Xiuying Xia, Kai Hou, Dr Jiankang Cao, and other members in the Watkins’ and Gan’s laboratories for useful discussions. Xiaohong Kou was a recipient of a scholarship from the China Scholarship Council. This work was supported by a US Department of Energy grant no. DE–FG02–02ER15341 (to S-SG), US National Institute of Food and Agriculture regional project NE1036 (to CBW), and the National Nature Science Foundation of China no. 31171769 (to X-HK).
References
- Adato I, Gazit S, Blumenfeld A. 1976. Relationship between changes in abscisic acid and ethylene production during ripening of avocado fruits Australian Journal of Plant Physiology 3 555–558 [Google Scholar]
- Alexander L, Grierson D. 2002. Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening Journal of Experimental Botany 53 2039–2055 [DOI] [PubMed] [Google Scholar]
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR. 1999. EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis Science 284 2148–2152 [DOI] [PubMed] [Google Scholar]
- Binder BM, Walker JM, Gagne JM, Emborg TJ, Hemmann G, Bleecker AB, Vierstra RD. 2007. The Arabidopsis EIN3 binding F-box proteins EBF1 and EBF2 have distinct but overlapping roles in ethylene signaling The Plant Cell 19 509–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker AB, Esch JJ, Hall AE, Rodríguez FI, Binder BM. 1998. The ethylene-receptor family from Arabidopsis: structure and function Philosophical Transactions of the Royal Society of London. Series B Biological Sciences 353 1405–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonell-Bejerano P, Urbez C, Carbonell J, Granell A, Perez-Amador MA. 2010. A fertilization-independent developmental program triggers partial fruit development and senescence processes in pistils of Arabidopsis Plant Physiology 154 163–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonell-Bejerano P, Urbez C, Granell A, Carbonell J, Perez-Amador MA. Ethylene is involved in pistil fate by modulating the onset of ovule senescence and the GA-mediated fruit set in Arabidopsis . BMC Plant Biology. 2011;11:84. doi: 10.1186/1471-2229-11-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Kwok SF, Bleecker AB, Meyerowitz EM. 1993. Arabidopsis ethylene-response gene ETR1: similarity of product to two-component regulators Science 262 539–544 [DOI] [PubMed] [Google Scholar]
- Chao Q, Rothenberg M, Solano R, Roman G, Terzaghi W, Ecker JR. 1997. Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins Cell 89 1133–1144 [DOI] [PubMed] [Google Scholar]
- Fang S-C, Fernandez DE. 2002. Effect of regulated overexpression of the MADS domain factor AGL15 on flower senescence and fruit maturation Plant Physiology 130 78–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan S-S. 1995. Molecular characterization and genetic manipulation of plant senescence. PhD dissertation, University of Wisconsin-Madison, Madison, WI, USA
- Gan S-S. Mitotic and postmitotic senescence in plants. Science of Aging Knowledge Environment. 2003;2003:Re7. doi: 10.1126/sageke.2003.38.re7. [DOI] [PubMed] [Google Scholar]
- Giovannoni JJ. 2004. Genetic regulation of fruit development and ripening The Plant Cell 16 S170–S180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni JJ. 2007. Fruit ripening and its manipulation. In: Gan S-S, ed, Senescence processes in plants Oxford, UK: Blackwell Publishing Ltd; 278–303 [Google Scholar]
- Guo Y, Gan S-S. 2006. AtNAP, a NAC family transcription factor, has an important role in leaf senescence The Plant Journal 46 601–612 [DOI] [PubMed] [Google Scholar]
- He Y, Gan S-S. 2002. A gene encoding an acyl hydrolase is involved in leaf senescence in Arabidopsis The Plant Cell 14 805–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing H-C, Schippers JHM, Hille J, Dijkwel PP. 2005. Ethylene-induced leaf senescence depends on age-related changes and OLD genes in Arabidopsis Journal of Experimental Botany 56 2915–2923 [DOI] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR. 1993. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of protein kinases Cell 72 427–441 [DOI] [PubMed] [Google Scholar]
- Klee HJ, Giovannoni JJ. 2011. Genetics and control of tomato fruit ripening and quality attributes Annual Review of Genetics 45 41–59 [DOI] [PubMed] [Google Scholar]
- Oeller PW, Lu MW, Taylor LP, Pike DA, Theologis A. 1991. Reversible inhibition of tomato fruit senescence by antisense RNA Science 254 437–439 [DOI] [PubMed] [Google Scholar]
- Onate-Sanchez L, Vicente-Carbajosa J. 2008. DNA-free RNA isolation protocols for Arabidopsis thaliana, including seeds and siliques BioMed Central Research Notes 1, 93 (doi:10.1186/1756-0500-1181-1193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton S, Barton SL, Bouzayen M, Hamilton AJ, Grierson D. 1993. Altered fruit ripening and leaf senescence in tomatoes expressing an antisense ethylene-forming enzyme transgene The Plant Journal 3 469–481 [Google Scholar]
- Tieman DM, Ciardi JA, Taylor MG, Klee HJ. 2001. Members of the tomato LeEIL (EIN3-like) gene family are functionally redundant and regulate ethylene responses throughout plant development The Plant Journal 26 47–58 [DOI] [PubMed] [Google Scholar]
- Tigchelaar EC, Tomes M, Kerr EA, Barman RJ. 1973. A new fruit ripening mutant, non-ripening (nor) Report of the Tomato Genetic Cooperative 23 33–34 [Google Scholar]
- Vrebalov J, Ruezinsky D, Padmanabhan V, White R, Medrano D, Drake R, Schuch W, Giovannoni J. 2002. A MADS-box gene necessary for fruit ripening at the tomato ripening-inhibitor (rin) locus Science 296 343–346 [DOI] [PubMed] [Google Scholar]
- Wagstaff C, Yang TJ, Stead AD, Buchanan-Wollaston V, Roberts JA. 2009. A molecular and structural characterization of senescing Arabidopsis siliques and comparison of transcriptional profiles with senescing petals and leaves The Plant Journal 57 690–705 [DOI] [PubMed] [Google Scholar]
- Watkins CB. 2002. Ethylene synthesis, mode of action, consequences and control. In: Knee M, ed, Fruit quality and its biological basis Sheffield, UK: Sheffield Academic Press; 180–224 [Google Scholar]
- Wilkinson JQ, Lanahan MB, Yen H-C, Giovannoni JJ, Klee HJ. 1995. An ethylene-inducible component of signal transduction encoded by never-ripe Science 270 1807–1809 [DOI] [PubMed] [Google Scholar]
- Yokotani N, Tamura S, Nakano R, Inaba A, Kubo Y. 2003. Characterization of a novel tomato EIN3-like gene (LeEIL4) Journal of Experimental Botany 54 2775–2776 [DOI] [PubMed] [Google Scholar]
- Zhang K, Gan S-S. 2012. An abscisic acid-AtNAP transcription factor-SAG113 protein phosphatase 2C regulatory chain for controlling dehydration in senescing Arabidopsis leaves Plant Physiology 158 961–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Xia X, Zhang Y, Gan S-S. 2012. An ABA-regulated and Golgi-localized protein phosphatase controls water loss during leaf senescence in Arabidopsis The Plant Journal 69 667–678 [DOI] [PubMed] [Google Scholar]
- Zhang M, Yuan B, Leng P. 2009. The role of ABA in triggering ethylene biosynthesis and ripening of tomato fruit Journal of Experimental Botany 60 1579–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.