Abstract
We examined the neuroprotective efficacy associated with post-ischemic vascular adhesion protein-1 (VAP-1) blockade in rats subjected to transient (1h) middle cerebral artery occlusion (MCAo). We compared saline-treated control rats to rats treated with a highly-selective VAP-1 inhibitor, LJP-1586 [Z-3-fluoro-2-(4-methoxybenzyl) allylamine hydrochloride]. Initial intraperitoneal LJP-1586 (or saline control) treatments were delayed until 6h or 12h reperfusion. At 72h reperfusion, LJP-1586-treated rats displayed 51% and 33% smaller infarct volumes, relative to their controls, in the 6- and 12h-treatment groups, respectively. However, only in the 6h-treatment group was the infarct volume reduction significant (p<0.05). On the other hand, we observed significantly improved neurologic functions in both 6- and 12h-treatment groups, versus their matched controls (p<0.05). Also, the effect of 6h LJP-1586 treatment on post-ischemic leukocyte trafficking in pial venules overlying the ischemic cortex was evaluated using intravital microscopy. These experiments revealed that: 1) LJP-1586 did not affect intravascular leukocyte (largely neutrophil) adhesion, at least out to 12h reperfusion; and 2) the onset of neutrophil extravasation, which occurred between 6-8h reperfusion in control rats, was prevented by LJP-1586-treatment. In conclusion, in rats subjected to transient MCAo, selective VAP-1 pharmacologic blockade provided neuroprotection, with a prolonged therapeutic window of 6 to 12h reperfusion.
Keywords: semicarbazide-sensitive amine oxidase, leukocyte trafficking, neutrophil, adhesion, extravasation, neuroprotection
Introduction
Stroke is a leading cause of death and disability worldwide. Yet, despite a substantial research effort, covering many years, effective therapies remain limited. One of the few treatment strategies arising from this work relates to the use of thrombolytics. This approach derives from the rationale that interventions promoting early recanalization of blocked cerebral arteries can yield benefits. Unfortunately, thrombolytic strategies have a temporally limited therapeutic utility of approximately 3 – 4.5 h and a potential for intracranial hemorrhage. Moreover, in patients subjected to thrombolysis, slow clinical recovery is often observed despite complete recanalization (Warach and Latour 2004). In addition, spontaneous recanalization of large cerebral arteries has been estimated to occur within hours of stroke onset in ~17% of patients (Kassem-Moussa and Graffagnino 2002). Initiation of post-ischemic reperfusion, whether occurring spontaneously or induced by thrombolysis, is often accompanied by inflammatory activity (Barone and Feuerstein 1999;Huang et al. 2006). One such inflammatory cascade involves increased leukocyte trafficking at venular sites within reperfused brain regions (e.g., (Becker et al. 2001;Stevens et al. 2002;Xu et al. 2006). Although this may promote expansion of brain tissue damage, it also can provide opportunities for palliative interventions.
One largely ignored potential participant in post-ischemic, leukocyte-related inflammation is the endothelial protein, vascular adhesion protein-1 (VAP-1), which is also called semicarbazide-sensitive amine oxidase (SSAO). The VAP-1/SSAO protein is thought to play an important role in promoting adhesion as well as transmigration of multiple classes of leukocytes—i.e., polymorphonuclear leukocytes (PMNLs), monocytes, and lymphocytes. At sites of injury and inflammation, VAP-1/SSAO is acutely mobilized to the luminal surface of endothelial cells and chronically upregulated. It can exacerbate and prolong the inflammatory process by attracting additional leukocytes to the site of injury (Salmi and Jalkanen 2001). It is important to note that mobilization and concentration of VAP-1/SSAO at the vascular luminal surface will render it more accessible to circulating drugs that selectively block SSAO/VAP-1 actions, thereby focusing the beneficial actions of such blockers to sites of inflammation (Salter-Cid et al. 2005). Furthermore, in VAP-1/SSAO knockout mice, the inflammatory response to nonmicrobial stimuli is repressed, but antimicrobial reactivity remains generally intact (Stolen et al. 2005). The latter has strong potential clinical relevance, since a major contributing factor to acute post-stroke mortality in patients is the onset of immuno-incompetence and an increased incidence of peripheral infection (e.g., pneumonia) (Meisel et al. 2004;Meisel et al. 2005). Thus, by preserving anti-microbial function, blocking VAP-1/SSAO may be associated with less patient risk than more generalized anti-inflammatory interventions.
LJP-1586 [Z-3- fluoro-2-94-methoxybenzyl) allylamine hydrochloride] is a non-hydrazine compound that has substantial specificity for inhibition of VAP-1/SSAO. With oral administration, LJP-1586 was reported to sustain its pharmacodynamic effect for up to 72 hours despite a fairly short elimination half-life (~90 min) (O'Rourke et al. 2008). Its ability to restrict inflammatory mediator production and leukocyte transmigration in the brain was recently revealed in a mouse intracerebral hemorrhage model (Ma et al. 2011).
To date, no investigations have examined the possible neuroprotective effects of selective VAP-1 inhibition in association with transient focal cerebral ischemia. In this study, we employed a reversible middle cerebral artery occlusion (MCAo) model. Present experiments were designed to examine the therapeutic window associated with post-ischemic VAP-1 inhibition, where initiation of LJP-1586 administration was delayed by up to 12h following the onset of reperfusion.
Materials and Methods
Animal Preparation
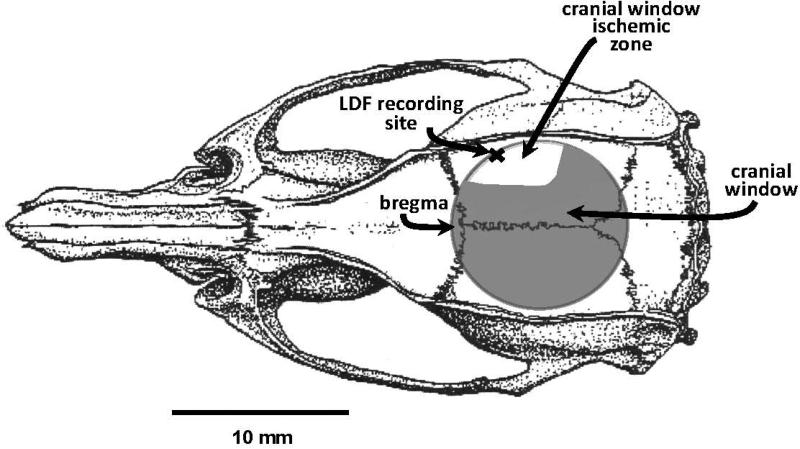
The study protocol was approved by the Institutional Animal Care and Use Committee. Male Sprague-Dawley rats (250-290g) were used. All animals were fasted overnight before the procedure. Rats were anesthetized with isoflurane. The trachea was intubated, and the lungs were mechanically ventilated. Anesthesia was maintained with 1-1.5% isoflurane in a mixture of 70% N2O and 30% O2. The tail artery was cannulated for monitoring of blood pressure and blood sampling. Blood pressure and blood gases were kept within normal ranges. Rectal temperature was servo-controlled at 37°C. Regional cerebral blood flow (rCBF) was continuously monitored by Laser-Doppler flowmetry (LDF, PeriMed, Jarfälla, Sweden) before and during MCAo, and for 15 min after onset of reperfusion. The LDF probe was attached to a thinned portion of skull over the MCA territory (4-5 mm lateral and 1-2 mm posterior to the bregma Fig. 1) (Hungerhuber et al. 2006).
Fig. 1.
Placement of the cranial window over a 10 mm diameter craniotomy. The bregma is shown as a point of reference, along with the LDF recording site. Also depicted in the figure is the approximate location of the cortical surface region under the cranial window (rostral-lateral right hemisphere) from which pial venules were chosen for examination of post-MCAo leukocyte trafficking. Illustration was modified from (Paxinos and Watson 1997).
Ischemia Model
MCAo was induced as previously described (Longa et al. 1989), but with minor modifications. Briefly, a midline ventral cervical skin incision was made, and the right and left common carotid arteries (CCA) were identified. The right external carotid artery (ECA) was isolated, ligated, and divided. The right internal carotid artery (ICA) was isolated and separated from the adjacent vagus nerve, and the pterygopalatine artery was ligated close to its origin. A 4-0 silicone-coated occlusion monofilament (Doccol Co., Redlands, CA) was inserted into the ECA stump and gently advanced 19-20 mm from the carotid artery bifurcation into the ICA until a slight resistance was felt. In order to minimize the blood flow derived from collateral circulation, the left CCA was then clamped. After 60 min of MCAo, the microclip on the left CCA was removed to limit the risk of hemorrhage at reperfusion. Ten minutes later, the filament was removed and the clip on the right CCA was taken off to allow for reperfusion. The arterial catheter was removed, the wounds were closed, and anesthesia was discontinued. When spontaneous breathing was re-established, the rats were extubated and returned to their cages.
Experimental Groups
Recommendations regarding LJP-1586/vehicle (saline) treatment protocols were provided by the drug supplier and were based upon published data. Previous findings established that LJP-1586 effects on leukocyte trafficking associated with peripheral inflammation were similar, irrespective of whether a parenteral (ip or iv) or oral route of delivery was used (O'Rourke et al. 2008). These authors also reported that, despite a plasma elimination half-life in the range of 1.5-2h, LJP-1586 retained its in vivo inhibitory potency toward VAP-1 activity and leukocyte trafficking for at least 24h following a single 10 mg/kg treatment in rodents. Data supporting the substantial selectivity of LJP-1586, with respect to inhibition of VAP-1/SSAO vs monoamine oxidases, as well as other enzymes linked to leukocytes (e.g., matrix metalloproteinase-9, cyclooxygenases, myeloperoxidase, cathepsin-B, iNOS, and xanthine oxidase), can be found in a number of published reports (Dunkel et al. 2011;O'Rourke et al. 2008;Xu et al. 2006). In fact, non-VAP-1/SSAO actions of LJP-1586 have only been reported at doses several orders of magnitude beyond those employed in the current study (O'Rourke et al. 2008). In the present investigation, rats were randomized into two experimental series. Series A consisted of 4 groups. Group 1 received 10 mg/kg of LJP-1586, at 6, 30, and 54 h of reperfusion after ischemia. Group 2 was given LJP-1586 at 12, 36, and 60 h of reperfusion after ischemia. Groups 3-4 were appropriate time-matched controls. Series B rats were used to monitor leukocyte trafficking over 4-12h following onset of reperfusion (see below).
Neurologic Outcome Evaluation
Neurobehavioral function assessments, as modified from approaches described in previous reports from our laboratory and others (Garcia et al. 1995;Wang et al. 1999), were performed by a blinded observer at 72 h of reperfusion. The scores, therefore, could range from 0 (normal function) to 8. This battery of tests primarily examines integrated motor function, and is sensitive to striatal and sensorimotor cortex injury. Several neurobehavioral categories were monitored. The first was consciousness, for which scores range from 0 (normal) to 1 (lethargy and/or reduced tail-pinch response). The second neurobehavioral category was limb asymmetry, which included two subcategories, with the first being walking behavior (0=normal; 1=adduction of left paw; 2=circling to paretic [left] side. The 2nd subcategory was limb tone, where scores ranged from 0 (normal, symmetric response) to 1 (reduced left side limb tone). The third neurobehavioral category was labeled motor coordination, which also included two tests (e.g., see Garcia et al. 1995;Wang et al. 1999). These were a 90° ladder climb to platform, with the following scoring system (0—climbs to platform; 1—pulls the ipsilateral hindlimb onto the ladder (left side is weak); and 2—does not climb. The 2nd subcategory involved the use of a rotating screen, with scores ranging from 0 (grasps screen rotated to 180° for >5 sec) to 1 (grasps screen at 180° for <5 sec) or 2 (cannot grasp at 180°).
Image Analysis and Infarct Size Determinations
All rats surviving the full 72 h were euthanized by isoflurane overdose. The brains were quickly removed, sectioned coronally into 2 mm slices and stained with 1% 2,3,5-triphenyl-tetrazolium chloride (TTC) solution at 37 °C for 10 min, followed by a solution of 10% formaldehyde. Slice images were acquired by scanner (CanoScan LiDE 70) and processed on PC-compatible computers with image analysis software (MetaMorph, Universal Imaging Corp). The infarct volume measurements were obtained as described by Goldlust et al. (Goldlust et al. 1996), and edema of the ipsilateral hemisphere was taken into consideration.
Post-MCAo pial venular leukocyte trafficking (Series B)
These rats were prepared with closed cranial windows. The procedure for placement of cranial windows, described in a previous paper from our laboratory (Xu et al. 2001), was initiated at 15min following onset of reperfusion. Series B rats were further divided into 2 principal subgroups—one receiving ip saline and the other receiving ip LJP-1586 at 6h reperfusion (see above). Additional rats were included as sham-operated (non-ischemic) time controls (n=4) or rendered neutropenic prior to MCAo (n=4). For the latter, rats were injected iv with 0.2 ml of a rabbit anti-rat PMNL antibody (from Research Diagnostics, Inc., Flanders, NJ, diluted 1:1) 24h prior to ischemia. That specific approach was previously shown to elicit a substantial and significant selective reduction in circulating neutrophils (Xu et al. 2006). In the present study, we measured an 81 ± 4% reduction in the blood neutrophil count (Advia 120 Hematology Analyzer, Siemens, Tarrytown, NY). The space under the window was filled with artificial cerebrospinal fluid (aCSF) that was equilibrated with 10% O2/ 5%CO2 with balance of N2 (Santizo et al. 2002). The 37 °C aCSF was suffused at 0.5 ml/min. The leukocyte activity of pial venules was monitored using a rhodamine-filtered digital video camera (Photometrics, Fryer Co. Inc., Huntley, IL), attached to a microscope. We limited our selection of pial venules, for examination of leukocyte trafficking, to the rostral-lateral portion of the right brain cortical surface exposed under the cranial window (as depicted in Fig. 1). This area, which includes the LDF recording site, lies within the MCA distribution territory and is anticipated to become ischemic when the MCA is occluded. Leukocytes were labeled with rhodamine 6G (200 μg/ml in 0.9% saline). The rhodamine was given initially as an iv bolus (l ml), followed by continuous infusion at a rate of l ml/hr (Lindauer et al. 1996;Santizo and Pelligrino 1999;Santizo et al. 2000). Images of leukocyte behavior were captured and saved, using the MetaMorph software system (Universal Imaging Corp., Downingtown, PA), and displayed on a computer monitor. Leukocyte dynamics were monitored at 4, 6, 8, 10, and 12h of reperfusion. Illumination was limited to <60 seconds at a time to avoid photoquenching. Leukocyte adherence was measured in all experiments as the area percentage of firmly adherent leukocytes overlapping the viewed venular area. The viewed venular area was determined from frames captured following switching from fluorescence to standard light microscopy. The value of the viewed venular area was expressed as a percentage of the total area exposed in the captured frame. Expressing leukocyte adherence (and infiltration—see below) as a fractional area, rather than the number of rhodamine-positive cells per unit venular area was necessitated by the two-dimensional view generated by our video/microscopy system. Thus, we could not readily distinguish overlapping firmly adherent (or extravasated) cells from one another. Such overlap became increasingly prominent with time. Leukocyte infiltration was seen in all of the saline-treated controls, starting at time points >6h. When extravasation was observed, the measurement of leukocyte behavior was modified. Thus, in addition to the measurement of leukocyte adherence described above, the total area of rhodamine-positive cells was counted, including cells that were distinctly extravascular. In both instances, the denominator was viewed venular area. An additional calculation was then made, where the percentage of “adherent” leukocytes was subtracted from the total leukocyte percentage. The result provided us with an “index of infiltration”.
Statistics
For parametric data, statistical analyses were performed using an unpaired t-test (infarct volumes—vehicle controls vs LJP-1586-treated); a repeated measures one-way analysis of variance (peri-ischemic blood flow); or a one-way ANOVA, with a post hoc Duncan's method for multiple comparisons (leukocyte trafficking). For nonparametric data analyses (neurologic outcomes, comparing time-equivalent vehicle- to LJP-treated rats), we used a Mann-Whitney rank sum test. A level of p<0.05 was considered significant in all statistical tests. For all parametric data, values are presented as means ± SEM, or, in the case of non-parametric data, medians and ranges (boxplot design).
Results
Peri-ischemic rCBF changes
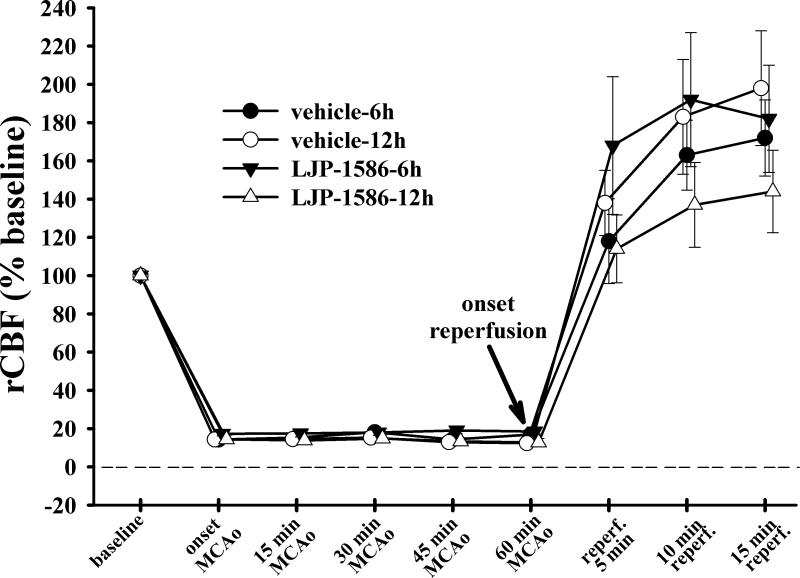
Upon insertion of the suture into the ICA, we observed an almost immediate drop in the rCBF (as monitored in the right MCA territory by LDF), quickly achieving a level that was 8-18% of the basal value, when the contralateral CCA was clamped. At the onset of reperfusion, rCBF began to rise rapidly (within 1 min) to a level 1.5-2x greater than the baseline level (Fig. 2). The rCBF changes were similar in all groups.
Fig. 2.
Peri-ischemic regional CBF (rCBF) changes, expressed as percentages of the preischemic baseline flow (in perfusion units), obtained from a laser-Doppler flow probe positioned over the MCA territory, 4-5 mm lateral and 1-2 mm posterior to the bregma (see Fig. 1). Values are means ± SEM. Data was obtained in rats treated with LJP-1586 starting at 6h (n=13) and 12h (n=13) of reperfusion, and in rats given ip saline vehicle at 6h (n=11) and 12h (n=7) post-MCAo. No significant differences were observed when comparing time-specific intra-ischemic or post-ischemic CBF values in saline-treated vs LJP-1586-treated rats. ANOVA results for reperfusion data revealed no significance (F=1.364; p=0.202)
Effects of LJP-1586 treatments on infarct volume
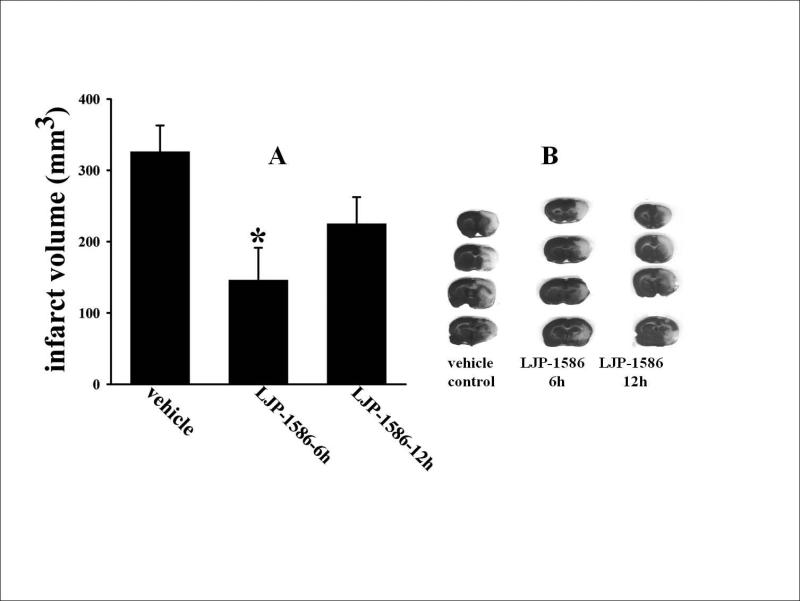
Infarct volumes, at 72h reperfusion (Fig. 3A), in the LJP-1586-6h (146 ± 49 mm3; n=13) and LJP-1586-12h (225 ± 37 mm3; n=13) treatment groups were reduced (by 51% and 33%) in relation to the values obtained in their time-appropriate vehicle control groups (298 ± 54 mm3 [n=11] and 334 ± 52 mm3 [n=7], respectively). However, only the 6h LJP-1586 vs 6h vehicle treatment group values were significantly different. Representative TTC-stained sections are provided in Fig. 3B.
Fig. 3.
Effects of treatments with LJP-1586 and vehicle (initiated at 6 or 12h post-MCAo) on brain infarct size evaluated after 3 days of reperfusion following 1 h of MCAo. A. Infarct volumes obtained in rats treated with LJP-1586 starting at 6h (n=13) and 12h (n=13) of reperfusion, along with their respective controls given ip saline vehicle at 6h (n=11) and 12h (n=7) post-MCAo. Values are means ± SEM; *p<0.05 versus vehicle-treated control. B. Representative TTC-stained coronal sections obtained from the 6h and 12h LJP-1586-treatment groups and a vehicle control rat. Only a single control rat is represented, since the infarct volumes in the two control groups were not significantly different.
Effects of LJP-1586 treatments on neurologic outcomes
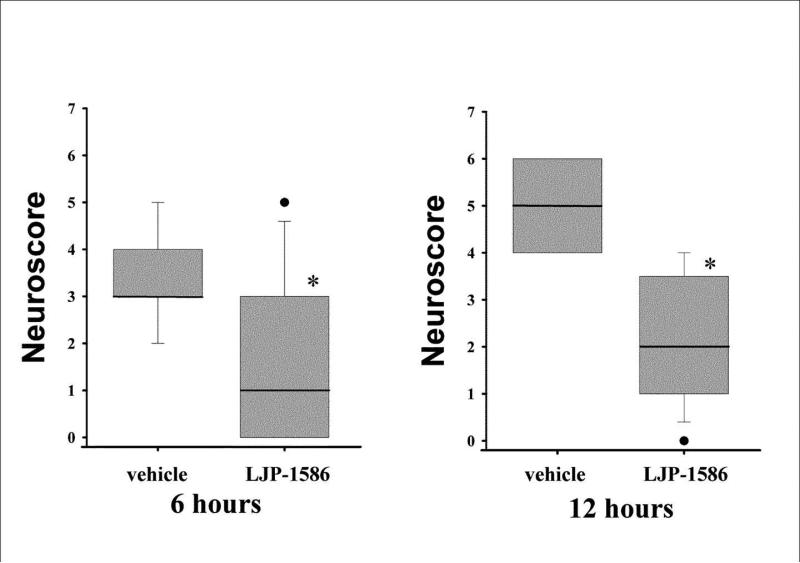
The neurologic outcome scores (measured at 72h reperfusion) for the LJP-1586-treated groups and their time-matched vehicle control groups are summarized in Fig 4, with the 6 hour treatments shown in the left panel and the 12 hour treatments in the right panel. The nonparametric dataset is presented in boxplot form. The median values are depicted by the bold lines within the boxes. Statistically significant differences (Mann-Whitney test) were found when comparing each LJP-1586-treated rat group to its respective vehicle control.
Fig. 4.
Neurologic outcome scores in LJP-1586-treated rats in comparison to their appropriate time-matched vehicle controls. Results for the 6 hour post-MCAo initial treatment groups are displayed in the left panel; while the 12 hour initial treatment results are shown in the right panel. The scoring system (see Methods) was weighted toward assessments of sensorimotor function, with some emphasis on limb asymmetry. The data, presented in boxplot form, represents scores obtained at 3 days of reperfusion, where the upper and lower box edges represent the 25th and 75th percentiles, and the whiskers represent the 95% confidence intervals. The median values are depicted by the bold lines within the boxes and outliers are represented by closed circles. * p<0.05 versus the time-matched vehicle-treated control (Mann-Whitney). n values are provided in legend to Fig. 3.
Intravital microscopy: Leukocyte behavior
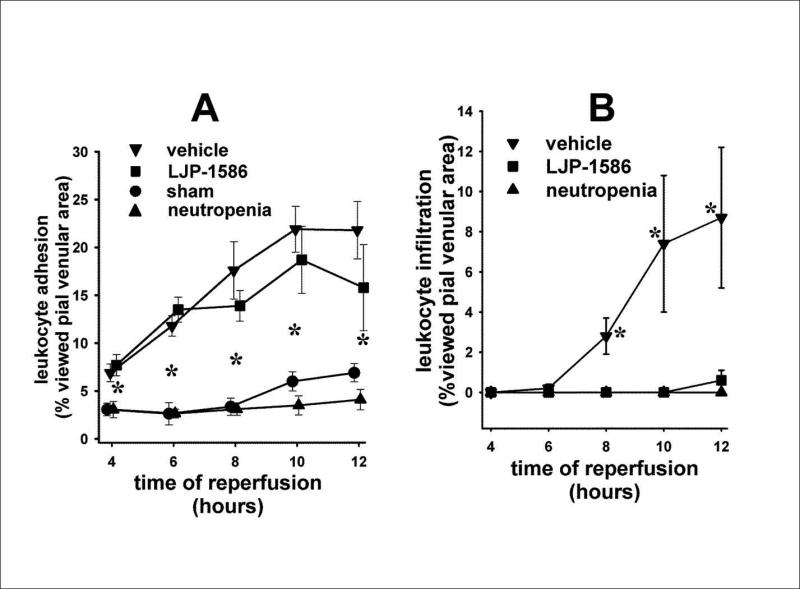
The pial venules selected for analysis of leukocyte trafficking were located within the rostral-lateral aspect of the brain surface tissue (in the ischemic right hemisphere) exposed under the cranial window (see fig. 1). Adhesion and infiltration of rhodamine 6G-labeled leukocytes was monitored over the time span of 4-12h reperfusion following MCAo. In both control and LJP-1586-treated rats, intravascular leukocyte adhesion was already well underway at 4h reperfusion, gradually increasing out to 12h reperfusion. When comparing the two groups, the levels of intravascular adhesion were not significantly different, over the 4-12h time interval (Fig. 6A; see also Fig. 5). All control rats (n=4) exhibited infiltration starting at 6h-8h reperfusion; whereas, in rats administered LJP-1586 at 6h reperfusion (n=4), infiltration was essentially absent out to 12h reperfusion; although intravascular adhesion in the vehicle and LJP-1586-treated rats were quite similar (Fig. 6A; Fig. 5). The level of extravascularly-expressed rhodamine 6G was calculated according to a procedure described in the Methods section and previous papers (e.g., (Xu et al. 2006). That data is shown in Fig. 6B. Statistically significant differences, when comparing extravasation in vehicle vs LJP-1586-treated rats, were observed at 8h - 12h reperfusion. To confirm that the leukocytes observed during this post-ischemic time period largely consisted of PMNL/neutrophils, one group of rats (n=4) was rendered neutropenic via treatment with an anti-rat PMNL antibody given iv at 24h prior to ischemia. Compared to vehicle- and LJP-1586-treated rats, we observed a significantly diminished presence of adherent rhodamine-6G-postive cells, in the neutropenic group, over the 4-12h post-MCAo reperfusion interval (Fig. 6A). In contrast to vehicle-treated controls, no infiltration was detected neutropenic rats (Fig. 6B). In a previous study (Xu et al. 2006), in rats subjected to transient forebrain ischemia (TFI), we reported a comparable pattern of reduction in adherent leukocytes, and an absence of infiltration, at 10h reperfusion in anti-rat PMNL-treated animals. Similar to neutropenic animals, sham-treated rats (n=4) displayed significantly diminished levels of leukocyte adhesion in relation to vehicle- and LJP-1586-treated animals (Fig.6A) and an absence of infiltration (data not shown).
Fig. 6.
Intravascular adhesion of rhodamine 6G-labeled leukocytes (A), in vehicle-treated, LJP-1586-treated, sham-treated, and anti-PMNL antibody-treated rats over the 4-12 h time period following onset of reperfusion. .The values for leukocyte adhesion were derived from the intravascular area of rhodamine 6G expression relative to the viewed venular area (see Fig. 5). The level of extravasation (B) was calculated as the percentage of the area of rhodamine 6G expression measured outside of the pial venule relative to the viewed venular area (see Xu et al. 2006). Values are means ± SEM. Asterisks in panel A represent significance (p<0.05) when comparing vehicle and LJP-1586 groups to sham and neutropenic groups; whereas asterisks in panel B indicate significant differences between vehicle-treated and the remaining groups. n = 4, in each group. Since all extravasation values in the neutropenia and sham groups were zero, only the neutropenic group was included in panel B.
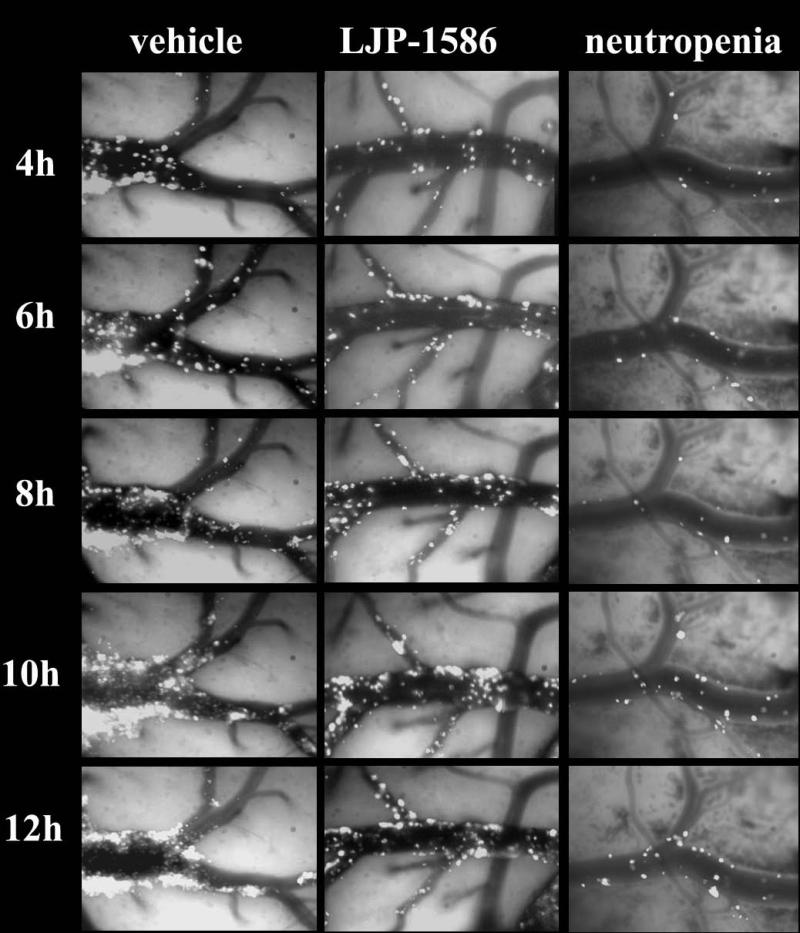
Fig. 5.
Captured images from 3 individual rats showing neutrophil presence at 4, 6, 8, 10, and 12h post-MCAo . The “vehicle-” and “LJP-1586” rats were treated at 6h post MCAo. The “neutropenic” rat was given anti-rat PMNL antibody iv 24h prior to MCAo, using a previously-described protocol (Xu et al. 2006). There are three features to note. First, in the LJP-1586-treated rat, the adherent leukocytes remained confined to the intravascular compartment out to 12h. Second, in the vehicle control, extravascular leukocytes appeared at ≥ 6h reperfusion. Third, based upon the limited expression of adherent leukocytes in neutropenic rats (right-hand panels), the rhodamine 6G reactive cells in the left-hand and middle panels mostly represent adherent and extravasated PMNLs/neutrophils.
Discussion
A key observation in this investigation was that post-MCAo treatment with a selective pharmacologic inhibitor of VAP-1 (LJP-1586), starting at 6h reperfusion, yielded significant neuroprotection, with some benefit being seen even when the initiation of drug administration was delayed until 12h of reperfusion. Additional, albeit circumstantial, evidence suggested that the neuroprotective action of LJP-1586 may have been related to, at least in part, limiting VAP-1-mediated PMNL/neutrophil extravasation that is initiated at 6-8h reperfusion and continues to 12h and probably beyond.
There is sufficient evidence to support the postulate that VAP-1 is a principal “convergence site” in pathologies associated with both acute and chronic cerebral inflammation (Hernandez-Guillamon et al. 2010;Ma et al. 2011;Mao 2008;O'Rourke et al. 2007;Unzeta et al. 2007;Xu et al. 2006). Thus, it represents a potentially important target for therapeutic intervention. Indeed, direct VAP-1 blocking strategies avoid some of the limitations arising from treatments designed to interfere with other upstream mediators of intravascular leukocyte adhesion (e.g., P- and E-selectin), which may not necessarily possess a sufficient influence on transmigration (Engelhardt et al. 1997). Also, owing to the presence of multiple downstream effectors of the damage caused by leukocyte infiltration into the brain (e.g., matrix metalloproteinases [MMPs], reactive O2 species, cytokines, granzymes and other pro-apoptotic substances), inhibiting just one of those effectors may not be nearly as effective as intervening at an upstream process (e.g., transmigration) common to the cytotoxicity caused by multiple leukocyte subsets.
The mechanisms through which VAP-1 acts to promote the transmigration of leukocytes remain unsettled. It is commonly thought that the products of VAP-1's amine oxidase function, especially hydrogen peroxide, contribute to the diapedesis process (Salmi and Jalkanen 2001). There has been some recent progress in the identification of leukocyte subset-specific counterreceptors for endothelial VAP-1 (i.e., sialic acid-binding Ig-like lectins (Aalto et al. 2011;Kivi et al. 2009). The enzymatic function of VAP-1 has also been linked to upregulation of E- and P-selectins and ICAM-1 on vascular endothelium (Jalkanen et al. 2007;Ma et al. 2011). However, the general role of those adhesion molecules in the transmigration process remains unclear and further study is warranted.
Previous findings from our laboratory (Xu et al. 2006) demonstrated the neuroprotective efficacy of pharmacologic blockade of VAP-1 in rats subjected to TFI. In that report, evidence was obtained indicating that the benefits of VAP-1 inhibitor treatment were largely the result of preventing post-ischemic neutrophil infiltration into the brain. The observation of an onset of neutrophil transmigration at ~6h reperfusion provided the rationale, in that study, for waiting until the 6h post-ischemic time point before initiating VAP-1 inhibitor treatment. This had the intended effect of maintaining the elevated levels of intravascular neutrophil adhesion, while preventing the appearance of neutrophils outside of cerebral venules, over the remainder of the 10h post-ischemic observation period. The above strategy was effective in providing significant neuroprotection, giving us a therapeutic window of at least 6h. However, an important limitation of these findings arises from the fact that the rats studied in that report (diabetic, ovariectomized females given estrogen replacement) are particularly susceptible to enhanced post-TFI neutrophil trafficking, perhaps permitting a more robust response to VAP-1 inhibition. Therefore, in the present investigation, we sought to examine rats exposed to a more “conventional” ischemic insult. To that end, we studied non-diabetic male rats subjected to temporary MCAo.
Whether or not neutrophils contribute to post-MCAo neuropathology has been the subject of debate. That is, some indicate an absence (Beray-Berthat et al. 2003b;Harris et al. 2005;Liesz et al. 2011;Martin et al. 2006), while others report the presence (Beray-Berthat et al. 2003a;Justicia et al. 2003;Morrison et al. 2011) of a contributory neutrophil role. Results obtained in the present study suggest that infiltrating neutrophils contribute to the brain damage elicited by transient MCAo. That conclusion derives from a couple of key experimental findings. First, we showed that rhodamine 6G-labeled leukocyte trafficking (in pial venules), over the initial 12 h of reperfusion, is markedly repressed in association with anti-PMNL antibody treatment. This suggests that the rhodamine 6G-labeled adherent cells we observed post-MCAo were, indeed, mostly neutrophils. Second, the absence of any extravasation of these cells over 4-12h reperfusion in the presence of VAP-1 blockade, as opposed to the extravasation seen in control rats subjected to MCAo, supports a key role for VAP-1 in promoting acute post-ischemic neutrophil infiltration.
An involvement of monocytes in neuropathologies arising during post-MCAo reperfusion has been proposed (Gelderblom et al. 2009). However, the nature of the participation of these cells appears to be complex, and may include contributions from resident macrophages and microglial cells during the early phase of reperfusion, as well as a role for blood-derived monocytes at much later stages (Jin et al. 2010;Stevens et al. 2002). In contrast, there is evidence against monocyte contributions in the neuropathology accompanying transient MCAo (Justicia et al. 2006). A possible benefit arising from VAP-1 inhibitor (LJP-1586) treatment on monocyte contributions to the neuropathology accompanying hemorrhagic stroke in mice was recently reported (Ma et al. 2011). This included suppression of macrophage/microglial activation and prevention of monocyte chemoattractant protein-1 (MCP-1) upregulation. Whether a similar benefit can be realized in ischemic stroke models awaits additional experimentation.
In studies examining lymphocyte contributions, despite evidence to the contrary (Justicia et al. 2006), current information favors a significant role. This includes results from lymphocyte-deficient murine models (Hurn et al. 2007;Liesz et al. 2011) and mice treated with a blocking antibody toward lymphocyte α4-integrin (Liesz et al. 2011). On the other hand, the findings of recent reports indicated that not all interventions designed to limit lymphocyte trafficking following MCAo are beneficial. For example, while evidence indicates a neurotoxic influence arising from increased inflammatory T-cell trafficking in the post-ischemic brain, there is some disagreement regarding whether regulatory T-cells are protective or not. Regulatory B cells, on the other hand, appear to play a neuroprotective role (Liesz et al. 2011;Ren et al. 2011b;Ren et al. 2011a). Similarly, there is some evidence to indicate that natural killer (NK) lymphocytes may have a beneficial counter-inflammatory influence in acute cerebral ischemia (Marsh et al. 2009). Nevertheless, little or nothing is known regarding whether VAP-1 plays any role in modulating interactions among these lymphocyte subsets. This needs to be addressed in future investigations.
In conclusion, present findings showed that treatment with a highly-selective blocker of VAP-1 provided significant neuroprotection in rats subjected to temporary MCAo. Some protection was still evident even when initial treatment was delayed until 12h after the onset of reperfusion. Thus, the neuropathology accompanying focal ischemia and reperfusion includes significant contributions from VAP-1. In the brain, this protein is reported to be primarily found in microvascular cells (endothelium and smooth muscle) (Jiang et al. 2008), but absent from neurons and glia (Unzeta et al. 2007), and converts primary amines into products (e.g., H2O2; aldehydes) that are thought to facilitate leukocyte trafficking and promote cytotoxicity in pro-inflammatory conditions (Salmi and Jalkanen 2001).
Acknowledgements
We acknowledge the expert technical assistance of Susan Anderson and Shuhua Ye. We also wish to thank LaJolla Pharmaceutical Co., San Diego, CA for their generous donation of LJPLJP-1586. The authors report no conflicts of interest.
Sources of Funding
This work was supported by National Institutes of Health Grants NS63279 and HL88259.
Definitions of abbreviations
- aCSF
artificial cerebrospinal fluid
- CCA
common carotid artery
- ECA
external carotid artery
- ICA
internal carotid artery
- ICAM-1
intercellular adhesion molecule-1
- LDF
laser-Doppler flowmetry
- MCAo
middle cerebral artery occlusion
- MCP-1
monocyte chemoattractant protein-1
- MMP
matrix metalloproteinase
- PMNL
polymorphonuclear leukocyte
- rCBF
regional cerebral blood flow
- SSAO
semicarbazide-sensitive amine oxidase
- TFI
transient forebrain ischemia
- TTC
2,3,5-triphenyl-tetrazolium chloride
- VAP-1
vascular adhesion protein-1
References
- Aalto K, Autio A, Kiss EA, Elima K, Nymalm Y, Veres TZ, Marttila-Ichihara F, Elovaara H, Saanijoki T, Crocker PR, Maksimow M, Bligt E, Salminen TA, Salmi M, Roivainen A, Jalkanen S. Siglec-9 is a novel leukocyte ligand for vascular adhesion protein-1 and can be utilized in PET-imaging of inflammation and cancer. Blood. 2011;118:3725–3733. doi: 10.1182/blood-2010-09-311076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone FC, Feuerstein GZ. Inflammatory mediators and stroke: new opportunities for novel therapeutics. J Cereb. Blood Flow Metab. 1999;19:819–834. doi: 10.1097/00004647-199908000-00001. [DOI] [PubMed] [Google Scholar]
- Becker K, Kindrick D, Relton J, Harlan J, Winn R. Antibody to the alpha 4 integrin decreases infarct size in transient focal cerebral ischemia in rats. Stroke. 2001;32:206–211. doi: 10.1161/01.str.32.1.206. [DOI] [PubMed] [Google Scholar]
- Beray-Berthat V, Croci N, Plotkine M, Margaill I. Polymorphonuclear neutrophils contribute to infarction and oxidative stress in the cortex but not in the striatum after ischemia-reperfusion in rats. Brain Res. 2003a;987:32–38. doi: 10.1016/s0006-8993(03)03224-4. [DOI] [PubMed] [Google Scholar]
- Beray-Berthat V, Palmier B, Plotkine M, Margaill I. Neutrophils do not contribute to infarction, oxidative stress, and NO synthase activity in severe brain ischemia. Exp. Neurol. 2003b;182:446–454. doi: 10.1016/s0014-4886(03)00106-7. [DOI] [PubMed] [Google Scholar]
- Dunkel P, Balogh B, Meleddu R, Maccioni E, Gyires K, Matyus P. Semicarbazide-sensitive amine oxidase/vascular adhesion protein-1: a patent survey. Expert. Opin. Ther. Pat. 2011;21:1453–1471. doi: 10.1517/13543776.2011.594040. [DOI] [PubMed] [Google Scholar]
- Engelhardt B, Vestweber D, Hallmann R, Schulz M. E- and P-selectin are not involved in the recruitment of inflammatory cells across the blood-brain barrier in experimental autoimmune encephalomyelitis. Blood. 1997;90:4459–4472. [PubMed] [Google Scholar]
- Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26:627–634. doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA, Arumugam TV, Orthey E, Gerloff C, Tolosa E, Magnus T. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40:1849–1857. doi: 10.1161/STROKEAHA.108.534503. [DOI] [PubMed] [Google Scholar]
- Goldlust EJ, Paczynski RP, He YY, Hsu CY, Goldberg MP. Automated measurement of infarct size with scanned images of triphenyltetrazolium chloride-stained rat brains. Stroke. 1996;27:1657–1662. doi: 10.1161/01.str.27.9.1657. [DOI] [PubMed] [Google Scholar]
- Harris AK, Ergul A, Kozak A, Machado LS, Johnson MH, Fagan SC. Effect of neutrophil depletion on gelatinase expression, edema formation and hemorrhagic transformation after focal ischemic stroke. BMC. Neurosci. 2005;6:49. doi: 10.1186/1471-2202-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Guillamon M, Garcia-Bonilla L, Sole M, Sosti V, Pares M, Campos M, Ortega-Aznar A, Dominguez C, Rubiera M, Ribo M, Quintana M, Molina CA, Alvarez-Sabin J, Rosell A, Unzeta M, Montaner J. Plasma VAP-1/SSAO activity predicts intracranial hemorrhages and adverse neurological outcome after tissue plasminogen activator treatment in stroke. Stroke. 2010;41:1528–1535. doi: 10.1161/STROKEAHA.110.584623. [DOI] [PubMed] [Google Scholar]
- Huang J, Upadhyay UM, Tamargo RJ. Inflammation in stroke and focal cerebral ischemia. Surg. Neurol. 2006;66:232–245. doi: 10.1016/j.surneu.2005.12.028. [DOI] [PubMed] [Google Scholar]
- Hungerhuber E, Zausinger S, Westermaier T, Plesnila N, Schmid-Elsaesser R. Simultaneous bilateral laser Doppler fluxmetry and electrophysiological recording during middle cerebral artery occlusion in rats. J Neurosci. Methods. 2006;154:109–115. doi: 10.1016/j.jneumeth.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Hurn PD, Subramanian S, Parker SM, Afentoulis ME, Kaler LJ, Vandenbark AA, Offner H. T- and B-cell-deficient mice with experimental stroke have reduced lesion size and inflammation. J Cereb. Blood Flow Metab. 2007;27:1798–1805. doi: 10.1038/sj.jcbfm.9600482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalkanen S, Karikoski M, Mercier N, Koskinen K, Henttinen T, Elima K, Salmivirta K, Salmi M. The oxidase activity of vascular adhesion protein-1 (VAP-1) induces endothelial E- and P-selectins and leukocyte binding. Blood. 2007;110:1864–1870. doi: 10.1182/blood-2007-01-069674. [DOI] [PubMed] [Google Scholar]
- Jiang ZJ, Richardson JS, Yu PH. The contribution of cerebral vascular semicarbazide-sensitive amine oxidase to cerebral amyloid angiopathy in Alzheimer's disease. Neuropathol. Appl. Neurobiol. 2008;34:194–204. doi: 10.1111/j.1365-2990.2007.00886.x. [DOI] [PubMed] [Google Scholar]
- Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc. Biol. 2010;87:779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justicia C, Martin A, Rojas S, Gironella M, Cervera A, Panes J, Chamorro A, Planas AM. Anti-VCAM-1 antibodies did not protect against ischemic damage either in rats or in mice. J Cereb. Blood Flow Metab. 2006;26:421–432. doi: 10.1038/sj.jcbfm.9600198. [DOI] [PubMed] [Google Scholar]
- Justicia C, Panes J, Sole S, Cervera A, Deulofeu R, Chamorro A, Planas AM. Neutrophil infiltration increases matrix metalloproteinase-9 in the ischemic brain after occlusion/reperfusion of the middle cerebral artery in rats. J Cereb. Blood Flow Metab. 2003;23:1430–1440. doi: 10.1097/01.WCB.0000090680.07515.C8. [DOI] [PubMed] [Google Scholar]
- Kassem-Moussa H, Graffagnino C. Nonocclusion and spontaneous recanalization rates in acute ischemic stroke: a review of cerebral angiography studies. Arch. Neurol. 2002;59:1870–1873. doi: 10.1001/archneur.59.12.1870. [DOI] [PubMed] [Google Scholar]
- Kivi E, Elima K, Aalto K, Nymalm Y, Auvinen K, Koivunen E, Otto DM, Crocker PR, Salminen TA, Salmi M, Jalkanen S. Human Siglec-10 can bind to vascular adhesion protein-1 and serves as its substrate. Blood. 2009;114:5385–5392. doi: 10.1182/blood-2009-04-219253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesz A, Zhou W, Mracsko E, Karcher S, Bauer H, Schwarting S, Sun L, Bruder D, Stegemann S, Cerwenka A, Sommer C, Dalpke AH, Veltkamp R. Inhibition of lymphocyte trafficking shields the brain against deleterious neuroinflammation after stroke. Brain. 2011;134:704–720. doi: 10.1093/brain/awr008. [DOI] [PubMed] [Google Scholar]
- Lindauer U, Dreier J, Angstwurm K, Rubin I, Villringer A, Einhaupl KM, Dirnagl U. Role of nitric oxide synthase inhibition in leukocyte- endothelium interaction in the rat pial microvasculature. J Cereb. Blood Flow Metab. 1996;16:1143–1152. doi: 10.1097/00004647-199611000-00008. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Ma Q, Manaenko A, Khatibi NH, Chen W, Zhang JH, Tang J. Vascular adhesion protein-1 inhibition provides antiinflammatory protection after an intracerebral hemorrhagic stroke in mice. J Cereb. Blood Flow Metab. 2011;31:881–893. doi: 10.1038/jcbfm.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L. Post-ischemic treatment with the SSAO inhibitor, LJP-12078 or LJP-1586, reduces infarct volumes and improves neurologic outcomes in a rat reversible middle cerebral artery occlusion model. Soc. Neurosci. 2008 Abst. 512.5. [Google Scholar]
- Marsh BJ, Stevens SL, Hunter B, Stenzel-Poore MP. Inflammation and the emerging role of the toll-like receptor system in acute brain ischemia. Stroke. 2009;40:S34–S37. doi: 10.1161/STROKEAHA.108.534917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Rojas S, Chamorro A, Falcon C, Bargallo N, Planas AM. Why does acute hyperglycemia worsen the outcome of transient focal cerebral ischemia? Role of corticosteroids, inflammation, and protein O-glycosylation. Stroke. 2006;37:1288–1295. doi: 10.1161/01.STR.0000217389.55009.f8. [DOI] [PubMed] [Google Scholar]
- Meisel C, Prass K, Braun J, Victorov I, Wolf T, Megow D, Halle E, Volk HD, Dirnagl U, Meisel A. Preventive antibacterial treatment improves the general medical and neurological outcome in a mouse model of stroke. Stroke. 2004;35:2–6. doi: 10.1161/01.STR.0000109041.89959.4C. [DOI] [PubMed] [Google Scholar]
- Meisel C, Schwab JM, Prass K, Meisel A, Dirnagl U. Central nervous system injury-induced immune deficiency syndrome. Nat. Rev. Neurosci. 2005;6:775–786. doi: 10.1038/nrn1765. [DOI] [PubMed] [Google Scholar]
- Morrison H, McKee D, Ritter L. Systemic neutrophil activation in a mouse model of ischemic stroke and reperfusion. Biol. Res. Nurs. 2011;13:154–163. doi: 10.1177/1099800410384500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke AM, Wang EY, Miller A, Podar EM, Scheyhing K, Huang L, Kessler C, Gao H, Ton-Nu HT, Macdonald MT, Jones DS, Linnik MD. Anti-inflammatory effects of LJP 1586 [Z-3-fluoro-2-(4-methoxybenzyl)allylamine hydrochloride], an amine-based inhibitor of semicarbazide-sensitive amine oxidase activity. J Pharmacol. Exp. Ther. 2008;324:867–875. doi: 10.1124/jpet.107.131672. [DOI] [PubMed] [Google Scholar]
- O'Rourke AM, Wang EY, Salter-Cid L, Huang L, Miller A, Podar E, Gao HF, Jones DS, Linnik MD. Benefit of inhibiting SSAO in relapsing experimental autoimmune encephalomyelitis. J Neural Transm. 2007;114:845–849. doi: 10.1007/s00702-007-0699-3. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1997. [Google Scholar]
- Ren X, Akiyoshi K, Dziennis S, Vandenbark AA, Herson PS, Hurn PD, Offner H. Regulatory B cells limit CNS inflammation and neurologic deficits in murine experimental stroke. J Neurosci. 2011a;31:8556–8563. doi: 10.1523/JNEUROSCI.1623-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Akiyoshi K, Vandenbark AA, Hurn PD, Offner H. CD4+FoxP3+ regulatory T-cells in cerebral ischemic stroke. Metab Brain Dis. 2011b;26:87–90. doi: 10.1007/s11011-010-9226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmi M, Jalkanen S. VAP-1: an adhesin and an enzyme. Trends Immunol. 2001;22:211–216. doi: 10.1016/s1471-4906(01)01870-1. [DOI] [PubMed] [Google Scholar]
- Salter-Cid LM, Wang E, O'Rourke AM, Miller A, Gao H, Huang L, Garcia A, Linnik MD. Anti-inflammatory effects of inhibiting the amine oxidase activity of semicarbazide-sensitive amine oxidase. J Pharmacol. Exp. Ther. 2005;315:553–562. doi: 10.1124/jpet.105.089649. [DOI] [PubMed] [Google Scholar]
- Santizo R, Pelligrino DA. Estrogen reduces leukocyte adhesion in the cerebral circulation of female rats. J. Cereb. Blood Flow Metab. 1999;19:1061–1065. doi: 10.1097/00004647-199910000-00001. [DOI] [PubMed] [Google Scholar]
- Santizo RA, Koenig HM, Pelligrino DA. Estrogen and leukocyte adhesion following transient forebrain ischemia in rats. Stroke. 2000;31:2231–2235. doi: 10.1161/01.str.31.9.2231. [DOI] [PubMed] [Google Scholar]
- Santizo RA, Xu HL, Galea E, Muyskens S, Baughman VL, Pelligrino DA. Combined endothelial nitric oxide synthase upregulation and caveolin-1 downregulation decrease leukocyte adhesion in pial venules of ovariectomized female rats. Stroke. 2002;33:613–616. doi: 10.1161/hs0202.102363. [DOI] [PubMed] [Google Scholar]
- Stevens SL, Bao J, Hollis J, Lessov NS, Clark WM, Stenzel-Poore MP. The use of flow cytometry to evaluate temporal changes in inflammatory cells following focal cerebral ischemia in mice. Brain Res. 2002;932:110–119. doi: 10.1016/s0006-8993(02)02292-8. [DOI] [PubMed] [Google Scholar]
- Stolen CM, Marttila-Ichihara F, Koskinen K, Yegutkin GG, Turja R, Bono P, Skurnik M, Hanninen A, Jalkanen S, Salmi M. Absence of the endothelial oxidase AOC3 leads to abnormal leukocyte traffic in vivo. Immunity. 2005;22:105–115. doi: 10.1016/j.immuni.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Unzeta M, Sole M, Boada M, Hernandez M. Semicarbazide-sensitive amine oxidase (SSAO) and its possible contribution to vascular damage in Alzheimer's disease. J Neural Transm. 2007;114:857–862. doi: 10.1007/s00702-007-0701-0. [DOI] [PubMed] [Google Scholar]
- Wang Q, Santizo R, Baughman VL, Pelligrino DA. Estrogen provides neuroprotection in transient forebrain ischemia through perfusion-independent mechanisms in rats. Stroke. 1999;30:630–637. doi: 10.1161/01.str.30.3.630. [DOI] [PubMed] [Google Scholar]
- Warach S, Latour LL. Evidence of reperfusion injury, exacerbated by thrombolytic therapy, in human focal brain ischemia using a novel imaging marker of early blood-brain barrier disruption. Stroke. 2004;35:2659–2661. doi: 10.1161/01.STR.0000144051.32131.09. [DOI] [PubMed] [Google Scholar]
- Xu HL, Galea E, Santizo RA, Baughman VL, Pelligrino DA. The key role of caveolin-1 in estrogen-mediated regulation of endothelial nitric oxide synthase function in cerebral arterioles in vivo. J. Cereb. Blood Flow Metab. 2001;21:907–913. doi: 10.1097/00004647-200108000-00002. [DOI] [PubMed] [Google Scholar]
- Xu HL, Salter-Cid L, Linnik MD, Wang EY, Paisansathan C, Pelligrino DA. Vascular adhesion protein-1 plays an important role in postischemic inflammation and neuropathology in diabetic, estrogen-treated ovariectomized female rats subjected to transient forebrain ischemia. J Pharmacol. Exp. Ther. 2006;317:19–29. doi: 10.1124/jpet.105.096958. [DOI] [PubMed] [Google Scholar]