These investigators found no significant evidence suggesting a potential role of Helicobacter pylori infection in the development of erosive esophagitis.
Keywords: Gastroesophageal reflux, Helicobacter pylori
Abstract
Background and Objectives:
Helicobacter pylori infection represents one of the most common and medically prominent infections worldwide. Gastroesophageal reflux disease (GERD) has a multifactorial etiology. The nature of the relationship between Helicobacter pylori infection (HP) and reflux esophagitis is still not clear. This study is designed to find the influence of HP on GERD.
Patients and Methods:
The study was conducted retrospectively at Sakarya Newcity Hospital between January 2006 and January 2009. Data were collected on patient's age, sex, weight, the grade of GERD and the severity of HP.
Results:
There were 1,307 women and 1,135 men in this review with a mean age of 39,54 (range, 17 to 70) years. Helicobacter pylori positive (1 to 3 severity) was frequently seen in patients with GERD. A statistically significant relationship was found between HP positivity and the grade of GERD. The Helicobacter pylori infection (1 to 3 severity) was found in 1,437 (82.5%) of patients with GERD in our series.
Conclusions:
Controversy still exists about the association between GERD and HP infection. Based on our findings, significant evidence suggests the potential role of HP infection in the development of GERD. Also, the current data provide sufficient evidence to define the relationship between GERD and HP infection.
INTRODUCTION
Helicobacter pylori (HP) infection represents one of the most common and medically prominent infections worldwide. Infection with this micro-aerobic, gram-negative bacteria has been shown as an causal factor in the development of peptic ulcer disease. The nature of the relationship between HP and gastroesophageal reflux disease (GERD) is still not clear.1,2 GERD is a chronic disease that rarely resolves spontaneously, and it is associated with frequent recurrences. Several studies have investigated the prevalence of GERD in elderly people, though few have specifically targeted this age group.1 GERD is defined as an increased frequency or duration of exposure of the distal esophagus to gastric content. GERD is a multifactorial disease where the environmental factors, diet, and host physiological factors may have a role. The association between GERD and HP infection is still controversial.3,4 This study was designed to investigate the relationship between HP infection and the grade of GERD in patients with reflux symptoms.
MATERIALS AND METHODS
A retrospective analysis was performed of 2,442 patients who underwent upper gastrointestinal endoscopy over a 3-year period (from January 2006 to January 2009) at Sakarya Newcity Hospital. The relationship between HP and GERD was analyzed. Patients were divided into 2 groups according to HP infection effect on GERD: HP positive and negative. Demographic data (age, sex, weight), severity of HP infection and endoscopic appearance of distal esophageal mucosa were compared.
Endoscopic Procedure
After a fasting period of 6 hours, patients were placed in the left lateral decubitus position, and upper endoscopy was performed with a standard forward-viewing endoscope. The patient's oropharynx was anesthetized with topical lidocaine. Intubation of the esophagus was usually performed under direct vision, and then the esophagus, stomach, and the duodenum were inspected. The gastric fundus was also seen by retroverting the tip of the gastroscope. After inspection of the entire gastric mucosa, multiple biopsies were taken from the gastric antrum and other areas if necessary. The specimens were preserved in formaldehyde solution (10%) for histopathologic evaluation.
Endoscopic appearance of distal esophageal mucosa were described in 4 categories, according to the Los Angeles classification5:
Grade I: One or more mucosal breaks no longer than 5mm, none of which extends between the tops of the mucosal folds (Figure 1).
Figure 1.
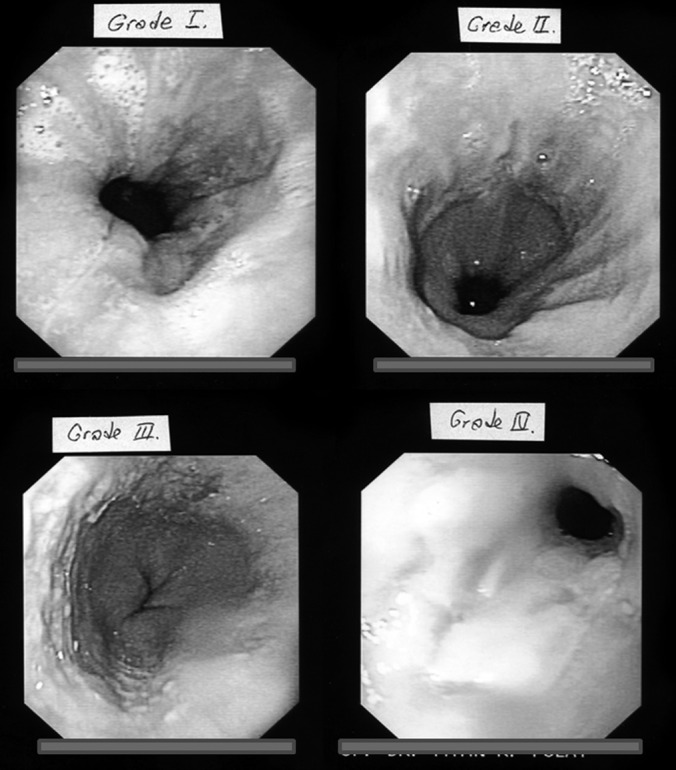
Endoscopic appearance of different grades of gastroesophageal reflux disease.
Grade II: One or more mucosal breaks more than 5mm, none of which extends between the tops of the mucosal folds (Figure 1).
Grade III: Mucosal breaks that extends between the tops of 2 or more mucosal folds, but which involve less than 75% of the esophageal circumference (Figure 1).
Grade IV: Mucosal breaks that involve at least 75% of the esophageal circumference (Figure 1).
The specimens were (hematoxylin-eosin staining and Giemsa staining were performed) evaluated for HP, inflammatory activity, chronic inflammation, intestinal metaplasia, and malignancy.
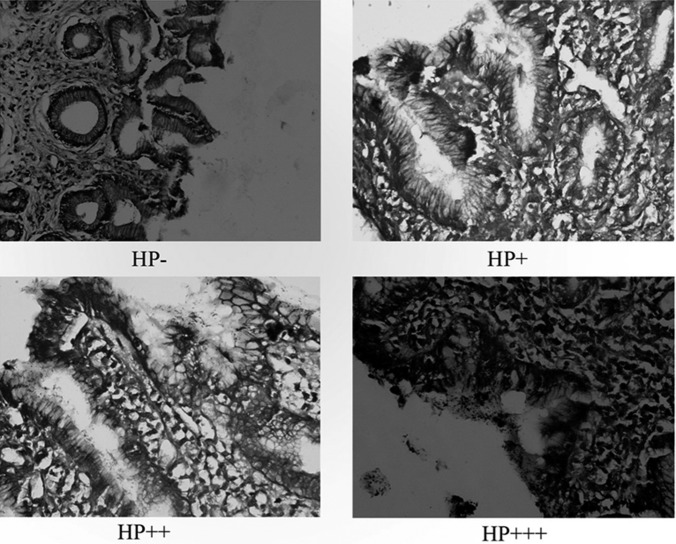
The severity of HP infection was classified as follows (Figure 2):
HP – (negative); no HP bacterium is present in the evaluated area,
HP + (mild positive); 1 to 10 HP bacteria per area,
HP ++ (medium positive); 10 to 30 HP bacteria per area,
HP +++ (severe positive); more than 30 HP bacteria per area.
Figure 2.
Histologic appearance of HP in the antrum (hematoxylin-eosin, ×40).
Statistical Analysis
Statistical analysis was performed with the Student t test, the chi-square, and Fisher's exact test for categorical factors. Statistical significance was assumed for P<.05.
RESULTS
Patient demographics, such as sex, age and weight were compared, and no statistically significant differences were found between the 2 groups of patients (Table 1). No mortalities or complications occurred in either group during the process.
Table 1.
Patient Demographics
| No GERD | GERD (Grade 1–4) | P Value | |
|---|---|---|---|
| Sex (M/F) | 0.87 | 0.80 | .7 |
| Age | 34.6 ±5.5 | 40.2 ±4 | .1 |
| Weight | 60 ±5.5 | 65 ±8.5 | .6 |
Grading of the GERD status and HP severity was performed, and the results are shown in Table 2. HP negative was compared, and statistically significant differences were found between both groups of patients (P<.05). Among the patients with grade IV GERD, 39 of 66 patients (59%) were HP + (mild positive). In 15 (0.6%) patients, there was neither GERD nor HP. HP positive (of any degree) was present in 1,437 (82.5%) of the patients in the GERD group (Table 3). HP positive was compared, and statistically significant differences were found between the groups (P<.05). Also, in the patients with GERD of Grades III and IV; the HP positivity of any severity was obviously higher than in the patients with GERD Grades I and II (P<.05).
Table 2.
Relationship Between Severity of HP and Grades of GERD
| No GERD | GERD Grade I | GERD Grade II | GERD Grade III | GERD Grade IV & BE | Total | |
|---|---|---|---|---|---|---|
| HP− | 15 | 54 | 84 | 84 | 81 | 318 |
| HP+ | 276 | 192 | 249 | 60 | 39 | 816 |
| HP++ | 312 | 327 | 309 | 61 | 21 | 1020 |
| HP+++ | 99 | 108 | 60 | 15 | 6 | 288 |
| Total | 702 | 681 | 702 | 210 | 147 | 2442 |
Table 3.
Relationship Between HP and GERD
| No GERD | GERD (Grade 1–4) | Total | P Value | |
|---|---|---|---|---|
| HP Negative | 15 | 303 | 318 | <.0001 |
| HP Positive (1–3) | 687 | 1437 | 2124 | <.0007 |
| Total | 702 | 1740 | 2442 |
DISCUSSION
Esophagogastroduodenoscopy is usually the first study of choice for investigation of the dysphagia, odynophagia, dyspepsia, gastroesophageal reflux, and/or recurrent vomiting.6,7 The procedure also allows interventions such as biopsy. Endoscopy is relatively insensitive for making the diagnosis of GERD. However, the presence of erosive esophagitis and/or Barret esophagitis (BE) is strongly suggestive of GERD. The presence of normal mucosa at endoscopy does not rule out the diagnosis of GERD.1,8,9 The presence of HP infection in gastric mucosa may play a protective role. It appears that the influence of HP infection on GERD depends on the localization of inflammation.
GERD starts in the stomach.10,11 It is caused by gastric distention due to overeating or ingestion of fried foods. Signs of injury to the exposed squamous epithelium are erosions, ulceration, fibrosis, and columnar metaplasia. This process results in the loss of muscle function, and the sphincter becomes mechanically defective, allowing free reflux with progressively higher degrees of mucosal injury.10,11 Initially, the symptoms of GERD were associated with a hiatal hernia. This led to the conclusion that the hernia itself was the cause of the symptoms.10 A hiatal hernia can also contribute to an esophageal propulsion defect due to loss of anchorage of the esophagus in the abdomen.11 It seemed reasonable to attempt to correct these symptoms by surgically reducing the hernia with simple closure of the crura. The problem was that in 50% of patients, the symptoms recurred.10
The contrary epidemiological trends of an increase of GERD and a decrease of HP infection have induced the suggestion that HP is a possible etiologic factor contributing to the increase of prevalence of GERD.1 HP infection has different effects on gastric physiology that need to be further investigated.1,6
In patients with reflux symptoms or esophagitis, a lower prevalence of HP infection was found in some studies, suggesting a possible protective effect of HP infection.1,6,7 In a review of 20 studies, the average prevalence of HP infection in patients with GERD was found to be 38%.8 In this study, the prevalence of HP with GERD was found in 1,437 patients (82.5%). The pooled estimation of the odds ratio for the prevalence of HP in patients with GERD was 0.60. However, this protective role is still equivocal. A lower prevalence of HP infection was found among an Asian population with GERD. But this effect is less prominent in the Caucasian population.8,9
The role of HP in relation to GERD symptoms and pathogenesis remains controversial. A recent prospective study that compared the symptoms before and after HP eradication therapy in 95 patients showed that the symptoms remained unchanged and were independent of HP status.10 In a different study, it was found that neither the diagnosis nor the severity of peptic esophagitis in HP infected patients was influenced by HP eradication itself.12
Outcomes for HP in this study are comparable to those reported in the literature. Thrifit and coworkers13 explored the relationship between H. pylori infection and Barret's esophagitis (BE) and sought to identify potential modifiers. They compared the prevalence of positive H. pylori serology among 217 adults with simple BE (without dysplasia), 95 with dysplastic BE and 398 population controls. Liu and coworkers14 studied the effect of H. pylori infections in the stomach, and reductions on the severity of inflammation. However, when it colonizes in the esophagus, H. pylori increases the severity of esophageal inflammation and the incidence of BE and GERD.14 Based on the findings of this study, it seems that a statistically significant relationship exists between the severity of HP infection and the grade of GERD patients (P<.05). Also, we found that in the patients with GERD of Grades III and IV, the HP positivity of any severity were obviously higher then in the patients with GERD of Grades I and II (P<.05).
CONCLUSION
Controversy still exists about the association between gastroesophageal reflux disease and Helicobacter pylori infection. Based on the findings of this study, significant evidence suggests the potential role of Helicobacter pylori infection in the development of gastroesophageal reflux disease.
Contributor Information
Fatin R. Polat, Department of Surgery, New City State Hospital, Sakarya, Turkey..
Sabriye Polat, Department of Pathology, New City State Hospital, Sakarya, Turkey..
References:
- 1. Vaezi MF, Swoger J. Gastro-oesophageal reflux disease in the elderly. In: Granderath FA, Pointner KT, eds. Gastro-oesophageal Reflux Disease. Wien: Springer; 2006;23–45 [Google Scholar]
- 2. Grande M, Cadeddu F, Villa M, et al. Helicobacter pylori and gastroesophageal reflux disease. World J Surg Oncol. 2008;6:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chourasia D, Ghoshal UC. Pathogenesis of gastro-oesophageal reflux disease: what role do Helicobacter pylori and host genetic factors play? Trop Gastroenterol. 2008;29(1):13–19 [PubMed] [Google Scholar]
- 4. Kwon JH, Chung IS, Son HS, et al. The relationship of gastrin, pepsinogen, and Helicobacter pylori in erosive reflux esophagitis. Korean J Gastroenterol. 2008;51(3):159–166 [PubMed] [Google Scholar]
- 5. Lundell LR, Dent J, Bennett JR. Endoscopic assessment of oesophagitis: clinic and functional correlates and further validation of the Los Angeles Classification. Gut. 1999;45:172–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koike T, Ohara S, Sekine H. Helicobacter pylori infection prevents erosive reflux esophagitis by decreasing gastric acid secretion. Gut. 2001;1:330–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fallone CA, Barkun AW, Friedman G. Is Helicobacter pylori eradication associated with gastroesophageal reflux disease? Am J Gastroenterol. 2000;95:914–920 [DOI] [PubMed] [Google Scholar]
- 8. Wu JCY, Sung Jy, Ng EK. Prevalence and distribution of Helicobacter pylori in gastroesophageal reflux disease -a study from the East. Am J Gastroenterol. 1999;94:1790–1794 [DOI] [PubMed] [Google Scholar]
- 9. O'Connor HJ. Helicobacter pylori and gastroesophageal reflux disease- clinical implications and management. Aliment Pharmacol Ther. 1999;13:117–127 [DOI] [PubMed] [Google Scholar]
- 10. Peters JH, Demeeste TR. Gastroesophageal reflux and hiatal hernia. Michael J., Zinner MJ, ed. Abdominal Operations. Tenth edition London: Prentice Hall International ınc, 1997;787–842 [Google Scholar]
- 11. Jeffery H. Peters, Tom R. Demeeste. Esophagus and diaphragmatic hernia. Schwartz SI, ed. Principles of Surgery. 7th ed New York: McGraw-Hill International Inc; 2006;1081–1179 [Google Scholar]
- 12. Levine A, Milo T, Broide E. Influence of HP eradication on gastroesophageal reflux symptoms and epigastric pain in children and adolescent. Pediatrics. 2004;113:54–58 [DOI] [PubMed] [Google Scholar]
- 13. Thrift AP, Pandeya N, Smith KJ, et al. Helicobacter pylori infection and the risks of Barrett's esophagus: a population-based case-control study. Int J Cancer. 2011. June 16 doi: 10.1002/ijc.26242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu FX, Wang WH, Wang J, Li J, Gao PP. Effect of Helicobacter pylori infection on Barrett's esophagus and esophageal adenocarcinoma formation in a rat model of chronic gastroesophageal reflux. Helicobacter. 2011. February;16(1):66–77 doi: 10.1111/j.1523-5378.2010.00811.x [DOI] [PubMed] [Google Scholar]