In a specialized hernia center, laparoendoscopic single-site surgery was found to be safe and effective for many types of abdominal wall hernias including parastomal hernias.
Keywords: Laparoendoscopic single-site surgery, Multiply recurrent inguinal hernia, Ventral hernia, Parastomal hernia, Modified Sugarbaker technique
Abstract
Background:
Laparoendoscopic single-site surgery has rapidly progressed from the animal laboratory to clinical use since mass production of multichannel ports began in 2007. Indeed, it has now been shown to be feasible and safe for many commonly performed operations.
Methods:
This study cohort comprised 22 unselected patients with abdominal wall hernias of varying types: multiply recurrent inguinal (n=2), suprapubic (n=1), ventral/incisional (n=17), and parastomal hernias (n=2), who underwent laparoendoscopic single-site ventral hernia repair between December 2009 and February 2011. Standard dissecting instruments and a 52cm/5.5mm/30°angle laparoscope were used.
Results:
Patients included 14 men and 8 women, with a median age of 56 (range, 32 to 78) years and a mean body mass index of 31.5±4.7kg/m2. The mean mesh size was 460cm2 (range, 225 to 884cm2). Mean operation time was 125 minutes for ventral/incisional hernias and 270 minutes for parastomal hernias. No conversions to multiport or open surgeries were necessary. There was no mortality or morbidity, and no recurrence at 6- to 18-month follow-up. The mean satisfaction score was 2.7 (range, 2 to 3) with no patients reporting dissatisfaction with the procedure.
Conclusion:
This series, though relatively small, represents a diverse group of patients with varying abdominal wall hernias, including parastomal hernias. These successful laparoendoscopic single-site surgeries, with no complications, demonstrate safety and efficacy, albeit in a specialized hernia center. This study is a prelude to the eventual validation of laparoendoscopic single-site hernia surgery with prospective randomized controlled trials.
INTRODUCTION
Minimal access surgery has progressed in leaps and bounds since the first laparoscopic cholecystectomy was reported by Mühe in 1987.1,2 This procedure became widely accepted as the gold standard within a few years of its introduction, well before the prevalence of evidence-based medicine, the accepted means to judge surgical protocols today. In its defense, multiport laparoscopy made perfect sense, as compared with large and morbid incisions, which were then the norm. It was not surprising then that the uptake of minimally invasive surgery was swift, though not without controversies, as inadequately trained surgeons had significant complication rates, including common bile duct injuries.3–5 However, it has taken over 2 decades for the next exciting innovation in laparoscopic surgery, namely, laparoendoscopic single-site surgery (LESS), to make its debut.6
Although LESS has often been attributed to Wittmoser,7 who used a single port for the insertion of a thorascope and dissecting instruments to perform cervical sympathectomy, this was strictly speaking not the LESS as we know it today, because of the airtight seal around the single port, which defines part of the LESS technique. Although there have been multiple reports on using multiple instruments via different fascial punctures through the same skin incision, the first commercially available single port was the SILS port, which addressed the problem of providing an air seal while also providing separate channels for introducing blunt trocars to accommodate the laparoscope and dissecting instruments. Many other single ports are now available, including the Triport and X-cone. All of these ports have the common feature of direct visualization of insertion and deployment, and in the case of the SILS port, blunt trocars, which can abolish trocar-induced bowel/vascular injuries. While the latter are rare, or rather, rarely reported in the literature, but common in the malpractice courts, they often cause a great deal of harm, and even death, to our patients.
The push for LESS is seen as a natural progression from laparotomy to multiport to single- port surgery for the same procedure, as it is our belief, which has subsequently been borne out by exhaustive research, that multiple small incisions are better than a long muscle-cutting incision, and therefore one small incision must be better than multiple incisions for the same procedure, assuming that each will have equal safety and efficacy. Of course this is an assumption that awaits validation by good clinical trials. With the rapid evolution of LESS, it is no longer being investigated in the light of feasibility, but rather of safety and efficacy, as multiple series of more than 100 cases in a variety of surgical procedures including cholecystectomy, prostatectomy, and total extraperitoneal inguinal hernia repair have confirmed feasibility.8–10 Our specialized hernia center began performing LESS hernia repair in October 2009. After the initial 2 months, all patients with abdominal wall hernias, irrespective of site (groin, ventral/incisional, and parastomal) and number were routinely offered LESS. This study aims to assess the safety and efficacy of LESS in an unselected patient population with a diverse range of ventral hernias common to any general surgical practice.
MATERIALS AND METHODS
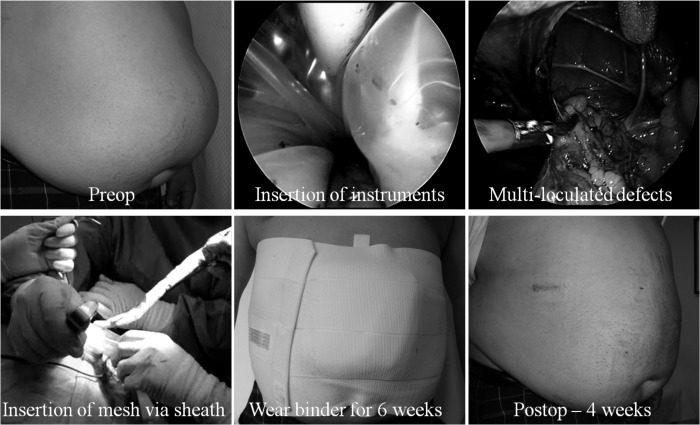
This prospective nonrandomized study was approved by the Holroyd Private Hospital Medical Advisory Committee. Our unit began performing LESS total extraperitoneal (TEP) repair in October 2009. From October to November 2009, patients were selected for LESS based on time availability, as we anticipated that the initial learning curve would not permit the smooth and timely running of our operating list. With the acquisition of two 52cm/5.5mm/30°angle laparoscopes (Karl Storz, Tuttlingen, Germany) in December 2009, we were able to offer LESS hernia repair to all patients (Figure 1). This study includes all patients who were referred with multiply recurrent inguinal hernias (after failed open and laparoscopic repair), ventral/incisional, and parastomal hernias from December 2009 to February 2011.
Figure 1.
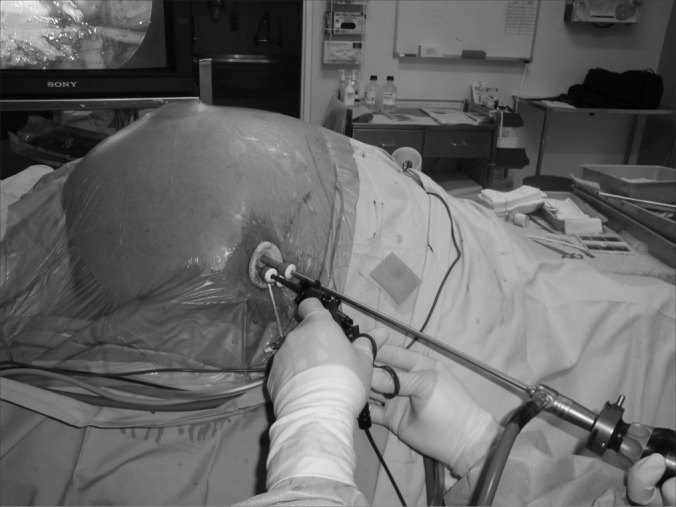
Laparo-endoscopic single site surgery for ventral hernia using SILS™ port. Note the side arm of the 52 cm/5.5 mm/30° angle laparoscope staying well clear of the heads of dissecting instruments.
Patients were informed of our practice of using a single incision in the upper outer quadrant, and that there would be a small possibility that additional lateral incisions may be needed, as per conventional multiport laparoscopy. In addition, patients were further informed of a possibility of a minilaparotomy to divide bowel adhesions that are deemed unsafe to treat laparoscopically, but that in our experience, the procedure could then revert to the laparoscopic approach, once safe adhesiolysis has been achieved. We have not had to perform any open ventral hernia repair since we introduced laparoscopic ventral hernia repair (LVHR) in February 2003.
All patients were catheterized after induction of anesthesia and received 1g of cephazolin. Patients were prepped from mid-thighs to nipples and were draped from epigastrium to suprapubic areas with iodine-impregnated plastic drapes. Having infiltrated the skin with 20mL of 0.5% Marcaine with 1:200 000 adrenaline, a 3-cm transverse skin crease incision was made in Palmer's point with the side chosen to be opposite to the hernia. The subcutaneous tissue was dissected down to the muscle layers, which were in turn sequentially split perpendicular to their fibers before the peritoneum was entered under direct vision. A SILS/Triport was placed intraperitoneally. For the SILS port, three 5-mm trocars were then inserted. For the Triport, the sleeve and ribbon were sequentially withdrawn until the inner ring was felt snugly against the anterior abdominal wall; the outer locking ring was then applied and further locked in place with sharp towel clips. Pneumoperitoneum was achieved with CO2 insufflations to a pressure of 12mm Hg. Our standard procedures were then performed, with several special considerations described.
The most difficult part of any LVHR is the safe division of adhesions; the following principles apply: inserting instruments under direct vision; avoiding directly manipulating bowels; judicious use of electrocautery, especially in the presence of bowel adhesions; using Ligaclips for hemostasis when possible; using sharp dissection, especially in dividing bowel adhesions; using saline jet dissection to better demarcate tissue planes, especially when bowels are involved; and complete reduction of hernia sac contents and dissection of the midline in LVHR to expose any incidental incisional hernias.
Loss of Triangulation
Because all the instruments are in the same port, severe clashing of these will occur unless steps are taken to minimize it. First, during routine dissection only a 52cm/5.5mm/30° angle laparoscope is used. This increases the range of movements of instruments within the port, especially when the SILS port is used (because of its relative rigidity over the Triport), and more important, because of its length, it also prevents the side arm of the scope from clashing with the heads of the dissecting instruments. Furthermore, dissection techniques must be modified to accomplish safe and efficient LESS LVHR. These are the “inline” and “vertical/chopsticks” dissection techniques.10 In the former, dissection is achieved with the tips grasping the tissues, and the instruments move in the opposite direction “inline.” In the latter, the instruments move vertically opposite each other on either side of the scope, with the pivot being at the port, thus avoiding clashing with it. In practice, these 2 techniques are combined to achieve the most efficient dissection.
Special Considerations
Multiply recurrent inguinal and suprapubic hernias.
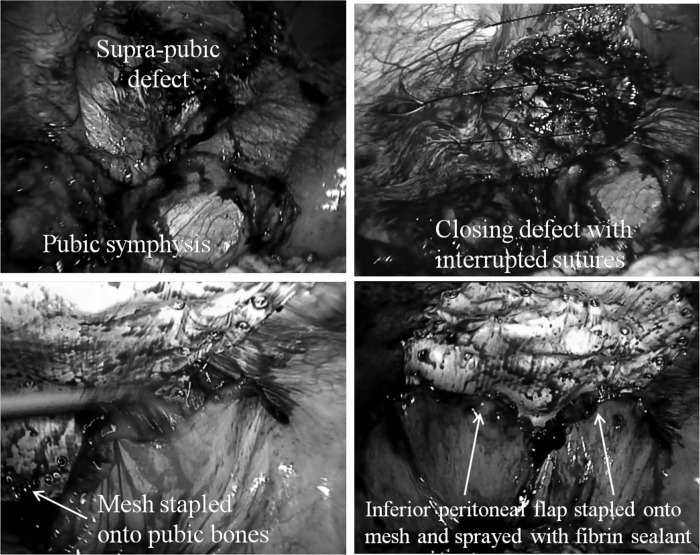
The dissection usually starts with identifying the pubic symphysis while the peritoneum is incised transversely and is reflected proximally and continued laterally with care taken when dissecting over the iliac vessels. In this way, an inferior peritoneal flap is created and the pubic bones are exposed to allow bony fixation of the inferomedial aspect of the mesh (Gore-tex Dualmesh Plus, WL Gore & Associates, Flagstaff, AZ, USA) with Protack staples (Covidien, Norwalk, Connecticut, USA). The inferolateral aspect of the mesh (over the iliac vessels) is glued with fibrin sealant (Tisseel Duo, Baxter AG, Vienna, Austria). The superior and lateral aspects of the mesh are then fixed with staples before transfascial sutures are placed superiorly and laterally. The inferior peritoneal flap is then reflected superiorly and stapled onto the mesh with care being taken to avoid any significant defects that would allow bowel herniation and cause bowel obstruction (Figure 2).
Figure 2.
Techniques for laparoscopic repair of supra-pubic hernias: Dissect peritoneum off pubic bones, close defect, staple mesh onto pubic bones, staple inferior peritoneal fold onto mesh and spray fibrin sealant along peritoneal fold and staples
Ventral/incisional hernias.
All the contents of the hernia sac must be completely reduced. Intransigent bowel adhesions, especially if a previous mesh is involved, can be taken down with a sleeve of the old mesh. If laparoscopic adhesiolysis is considered unsafe, then a minilaparotomy, immediately opposite the densest adhesions, can be performed as a last resort, Once safely carried out, the midline wound is then formally closed before pneumoperitoneum is recommenced to complete the operation laparoscopically. Defects (including suprapubic hernias) <6cm in diameter are routinely closed, via 2-mm incisions, transversely and extracorporeally interrupted with 1 nylon suture. The ligamentum teres is taken down sufficiently for unhindered mesh placement with a margin of at least 5cm from the hernia defect. Patients whose defects are too big to close routinely wear an abdominal binder for 6 weeks postoperatively to decrease the risks of seroma formation.
Parastomal hernias.
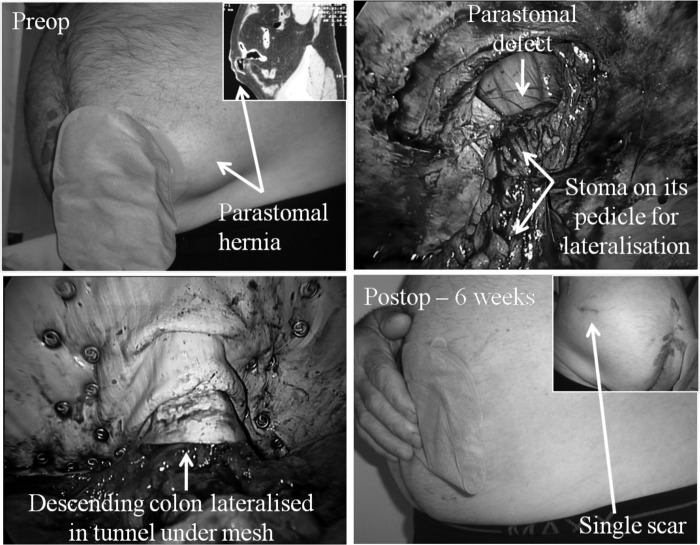
Because these are probably the most difficult of all abdominal wall hernias to repair, patients must be adequately informed of all the potential risks. These patients routinely see a stoma nurse preoperatively to mark the site of a possible contralateral stoma as a last resort. In addition, a urinary catheter is placed in the stoma with the balloon being insufflated with 5mL of water to aid identification of the stoma intraperitoneally. A sterile stoma bag is placed over the stoma, and a piece of clear plastic is placed over it before the iodine-impregnated plastic drape is placed. This allows access to the stoma during the operation without affecting the sterility of the main operative field. The stoma (colon or ileum) must be dissected down to a pedicle, with care being taken not to affect its viability. The pedicle must be mobilized sufficiently to allow lateralization, without tension, against the anterior abdominal wall before placement of the mesh as seen in a laparoscopic modified Sugarbaker technique for parastomal hernia repair. Parastomal hernias, being a special case in that the bowel/stoma must lie anterior to the mesh in a tunnel, require that placement of staples along the stoma defect must take place under direct vision to avoid stapling into the bowel. To aid this, the mesh is rolled up and the roll is maintained by sutures at both ends every 5cm. This allows orderly unrolling and stapling of the mesh. The mesh is fixed on either side of the bowel, working progressively more anterior with the inner layer of staples being placed first. As with all incisional hernias, incidental midline incisional hernias can be found in over 50% of cases. It is important that the previous midline laparotomy wound is covered by the mesh by at least 5cm across the midline. Transfascial sutures are placed last superiorly, inferiorly, and medially. Fibrin sealant is sprayed along the mesh/bowel interface to minimize the risk of bowel herniation under the mesh (Figure 3).
Figure 3.
Successful laparo-endoscopic single site surgery for recurrent ventral incisional hernia using Triport™.
Although conventional straight dissecting instruments were used in all cases, often, longer instruments need to be used for very lateral hernias or in big patients. In addition, instruments of different lengths will also minimize clashing of the heads. Irrespective of the type of abdominal wall hernias repaired, fibrin sealant (Tisseel Duo, Baxter AG, Vienna, Austria) is sprayed along the periphery of the mesh and along staples in an attempt to reduce the risks of subsequent adhesions.11 For mesh sizes ≤15cm to 19cm, 2mLof fibrin sealant is used, and for bigger meshes, 4mL is used.
RESULTS
Between December 2009 and February 2011, 23 patients were referred for LVHR. Of these, 22 underwent LESS LVHR: 2 with multiply recurrent inguinal hernias, 1 with a suprapubic hernia, 17 with ventral/incisional (2 ventral, 6 incisional, and 9 recurrent incisional) hernias, and 2 with parastomal hernias (with these being performed in the last 3 months of the study period). One patient with recurrent parastomal hernia was advised to have conventional multiport laparoscopic parastomal hernia repair with a modified Sugarbaker technique. This was the first laparoscopic parastomal hernia repair the author had performed. The mean body mass index was 31.5±4.7kg/m2. The median age was 56 years (range, 32 to 78). There were 14 men and 8 women. The mean mesh size was 460cm2 (range, 225 to 884). The operation time was 125 minutes for all hernias, excluding parastomal, (range, 85 to 270); the operation times for the parastomal hernias were 240 minutes and 300 minutes, reflecting the severe degree of bowel adhesions and the extra time required for complete adhesiolysis. There was no conversion to either open or conventional multiport repair. Two patients went home on the same day, while the remaining except for one, went home on day 1 postoperatively. One patient (with a ventral incisional hernia) stayed in the hospital for 3 days because she lived 5 hours away in a rural area. The average analgesic requirements were 16 tablets of dextropropoxyphene hydrochloride with paracetamol (range, 4 to 20). There was no recurrence with a follow-up of 6 months to 18 moonths. There were no wound complications. There was no evidence of seroma formation at 6-month follow-up (Figure 4). The mean satisfaction score was 2.7 (range, 2 to 3), with no patient being dissatisfied with single incision laparoscopic ventral hernia repair.
Figure 4.
Laparoendoscopic single site surgery for parastomal hernia (arising after abdomino-perineal resection of the rectum) with modified Sugarbaker technique.
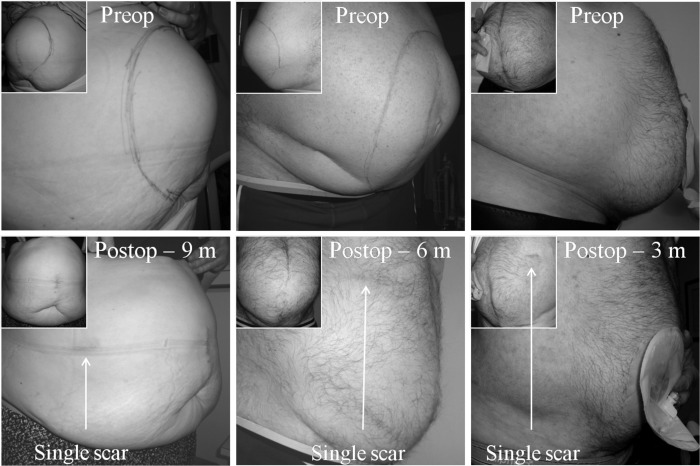
Figure 5.
Laparo-endoscopic single site surgery for very large ventral incisional (left), recurrent ventral incisional (middle) and recurrent parastomal hernia (right) – barely visible scar post-operatively (m=months).
DISCUSSION
After the first successful laparoscopic cholecystectomy by Mühe in 1987, open cholecystectomy has become virtually obsolete, and is currently performed in <5% of all cases, usually as a result of conversion secondary to severe inflammation. Indeed, within a decade of this seminal event, many previously open procedures have followed the same course. The rapid rise to becoming a gold standard procedure was, however, not without problems, as there was a transient but noticeable rise in the rates of common bile duct injuries in cases of laparoscopic cholecystectomy.3–5
Parallel with these developments was the introduction and popularization of laparoscopic hernia repair, which began in 1989.12 In some Western countries such as Australia, in 2010, laparoscopic (total extraperitoneal and transabdominal preperitoneal) inguinal hernia repair represented 44% of all inguinal hernias repaired (www.medicareaustralia.gov.au), and this therefore represents the most commonly performed procedure, compared with other open procedures, including the Lichenstein, Bassini, Shouldice, Kugel, mesh plug, and others. Indeed, nationally 48% of surgeons in Australia profess to performing laparoscopic inguinal hernia repair. In fact, in the states of New South Wales and Queensland, the proportion of surgeons performing laparoscopic inguinal hernia repair is currently 51%. While reasonably accurate data exist for laparoscopic inguinal hernia repair, because of specific item numbers assigned to open and laparoscopic inguinal hernia repair, there are no accurate data for laparoscopic ventral hernia repair in the Western world, including Australia. However, it has been estimated that LVHR is being used for only 10% of ventral/incisional hernias being repaired. One of the reasons for the poor uptake for LVHR is the feared complication of enterotomy, which can occur among up to 2% of all patients, even in high-volume centers.13,14 At least some of the unrecognized enterotomies are due to inadvertent perforation by dissecting instruments, which are usually introduced “out-of-sight” during LVHR, because the surgeon forgets to look at the secondary trocar sites with each instrument insertion. Furthermore, some of these injuries are due to the introduction of sharp secondary trocars. Indeed, in a study of 37 000 gynecologic procedures,15 0.03% of all bowel injuries were caused by sharp secondary trocars. This equates to an excess of 120 cases of avoidable bowel injuries. Such injuries should no longer occur, with the introduction of single ports like SILS and Triport, because these involve no sharp trocars. Furthermore, because the dissecting instruments and the laparoscope are parallel, this should negate the risks of bowel injuries from intraperitoneal introduction of instruments.
The main objection to the use of LESS procedures is the relative loss of triangulation. However, this has largely been overcome by the use of a smaller and longer laparoscope, which, apart from increasing the range of movements within the port, will also minimize the clashing of the heads of the dissecting instruments with the side arm of the scope. In addition, modified dissection techniques, such as “inline” and “vertical/chopsticks,” will further enhance safe and efficient dissection, even with standard straight dissecting instruments.10 Unlike the dissection repertoire of a TEP repair where the maneuvers can be quite broad in distance between the tips of the dissecting instruments, adhesiolysis, especially when it involves bowels, occurs in millimeters, a scale quite suited to LESS dissection techniques, which further diminish the argument regarding relative loss of triangulation.
There is no doubt that LESS represents the most significant innovation in the field of laparoscopic surgery for the last 2 decades. In the area of hernia surgery, enormous efforts have been made in the area of mesh prosthetics and these have resulted in the current situation where currently some 270 different meshes are available. The introduction of LESS has reinvigorated the industry to participate in an exciting area of surgical innovations in the field of optics (eg, flexible laparoscopes), in instrumentation (eg, angulated/roticulated dissecting instruments) and, increasingly, in robotics (eg, robotic Freehand), with the primary focus being on optimizing LESS and hence make it more surgeon friendly and hopefully increase patient safety and acceptability.16–18 In the end, one should never forget the fact that the best instruments are the surgeon's hands.
There are currently very few reports on LESS LVHR, with most reports consisting of single-case reports.19 Roy et al20 reported a series of 4 selected cases of LESS LVHR, with defect sizes measuring 4cm to 5cm in diameter in half of the cases (umbilical hernias) and Swiss cheese defects measuring up to 6cm to 7cm maximum. This would mean that the mesh size based on a 5-cm clearance of the defect of some 225cm2 for the umbilical hernias repaired and 289cm2 for the largest of incisional hernias repaired. We have concluded that LESS LVHR is feasible, based on this study of 22 unselected patients, representing one of the largest series of LESS LVHR reported to date.
Our patients presented with a spectrum of abdominal wall hernias, including multiply recurrent inguinal, suprapubic, ventral/incisional, and parastomal hernias. Although the number is relatively small, compared with published series on conventional LVHR, our operative time of 125 minutes, compared favorably with these series, given that the mean mesh size for our patients is 460cm2, or the equivalent of a 20cm to 23cm mesh. Given the unselected nature (except for 1 patient) in the cohort, the mean body mass index of 31.5kg/m2 represents more closely the general obesity of the Australian population, which was made worse by the inability of our patients to engage in vigorous physical activities to reduce their weight because of the hernia. In fact, we have seen anecdotal evidence of significant weight loss, in some of our patients, after their operation. Two of the patients, whose operations occurred early in the morning, went home on the same day, while the remaining except for 1, went home 1 day after their operation, even for the 2 patients with LESS parastomal hernia repair with the modified Sugarbaker technique. This is impressive given that their mean operation time was 270minutes. There was no mortality, no morbidities, and no recurrence with a follow-up of 6 months to 18 months. All of our patients were highly satisfied with their operation, with a mean satisfaction score of 2.7, with no patients being dissatisfied with the procedure.
Due to single ports being relatively new, and their acceptance limited to a few centers, the cost of the ports represents an additional $150 per procedure relative to conventional ports. However, compared with LESS inguinal hernia repair, LESS LVHR is potentially and usually much more complex, time-consuming, and expensive, overall; therefore, this additional cost of the single port would only represent a small percentage of the overall cost. Furthermore, like most new devices, the price always comes down with increasing competition and popularity. This is based on the assumption that it can be shown that LESS outperforms conventional multiport laparoscopy in terms of cost effectiveness, safety, efficacy, patient acceptability, and improved cosmesis. However, the exploding development of ancillary devices, such as roticulated instruments and flexible endoscopes, may threaten the viability and propagation of a potentially beneficial technique by making LESS procedures economically “unviable”, at worst, or confined to few centers, at best. This is the case with the radical prostatectomy performed with the da Vinci Robotic system (Intuitive Surgical, Sunnyvale, CA).20
While a few studies have demonstrated the feasibility of LESS LVHR in select cases,18,19 this study is the first to demonstrate the safety and efficacy of LESS LVHR in an unselected patient population with various types of ventral hernias in a general surgical practice. The general applicability of LESS in LVHR, demonstrated in this study, should serve as an important milestone in the quest to demonstrate whether LESS is superior to conventional multiport hernia repair. This awaits evidence from large, multicenter, prospective, randomized controlled studies.
CONCLUSION
Like LESS inguinal hernia repair, LESS LVHR has now passed beyond the feasibility phase into the demonstrated safety and efficacy phase. However, these good results have been obtained by one dedicated hernia specialist who performs LESS hernia repair routinely, in a specialized hernia center. This success must not be extrapolated to the general surgical community. Such propagation can only be made by exhaustive prospective randomized controlled trials comparing LESS to conventional multiport laparoscopic procedures. However, given the recent enthusiasm, if not expectation, of surgeons as scientists, it should not take more than a few years to come to a consensus statement, hopefully positive, on LESS procedures. Novice surgeons should therefore avoid attempts at LESS hernia surgery until they are highly competent with the multiport laparoscopic hernia repair.
References:
- 1. Reynolds W. The first laparoscopic cholecystectomy. JSLS. 2001;5(1):89–94 [PMC free article] [PubMed] [Google Scholar]
- 2. Mouret P. How I developed laparoscopic cholecystectomy. Ann Acad Med Singapore. 1996;25:744–747 [PubMed] [Google Scholar]
- 3. Gadacz TR. U.S. Experience with laparoscopic cholecystectomy. Am J Surg. 1993;165(4):450–454 [DOI] [PubMed] [Google Scholar]
- 4. Dunn D, Nair R, Fowler S, McCloy R. Laparoscopic cholecystectomy in England and Wales: results of an audit by the Royal College of Surgeons of England. Ann R Coll Engl. 1994;76(4):269–275 [PMC free article] [PubMed] [Google Scholar]
- 5. Alkhaffaf B, Decadt B. 15 years of litigation following laparoscopic cholecystectomy in England. Ann Surg. 2010;251(4):682–685 [DOI] [PubMed] [Google Scholar]
- 6. Gill IS, Advincula AP, Aron M, et al. Consensus statement of the consortium for laparoendoscopic single-site surgery. Surg Endosc. 2010;24:762–768 [DOI] [PubMed] [Google Scholar]
- 7. Schurr MO, Buess G. Wittmoser's technique of thoracoscopic sympathectomy and vagotomy. Endosc Surg Allied Technol. 1993;1(5-6):266–267 [PubMed] [Google Scholar]
- 8. Erbella J, Jr., Bunch GM. Single-incision laparoscopic cholecystectomy: The first 100 outpatients. Surg Endosc. 2010;24:1958–1961 [DOI] [PubMed] [Google Scholar]
- 9. White WM, Haber GP, Goel RK, Crouzet S, Stein RJ, Kaouk JH. Single-port urological surgery: Single-centre experience with the first 100 cases. Urology. 2009;74:801–804 [DOI] [PubMed] [Google Scholar]
- 10. Tran HM. Demonstrated safety and efficacy of single incision laparoscopic surgery for total extraperitoneal inguinal hernia repair. JSLS. 2011;15(1):47–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tran H, Salita L, Chandratnam E, Juringan I, Hawthorne W. Strategies to minimize adhesions to intraperitoneally placed mesh in laparoscopic ventral hernia repair. JSLS. 2012;16(1):89–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Filipovic-Cugura J, Kirac I, Kulis T, Jankovic, Benkavac-Beslin M. Single incision laparoscopic surgery (SILS) for total extraperitoneal (TEP) inguinal hernia repair: first case. Surg Endosc. 2009;23:920–921 [DOI] [PubMed] [Google Scholar]
- 13. LeBlanc KA, Booth WV, Whitaker JM, Bellanger DE. Laparoscopic incisional and ventral herniorrhaphy: Our initial 100 patients. Hernia. 2001;5:41–45 [DOI] [PubMed] [Google Scholar]
- 14. Heniford BT, Park A, Ramshaw BJ, Voeller G. Laparoscopic repair of ventral hernias: Nine years' experience with 850 consecutive hernias. Ann Surg. 2003;238(3):391–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin P, Grow DR. Complications of laparoscopy. Obstet Gynecol Clin North Am. 1999;26(1):23–38 [DOI] [PubMed] [Google Scholar]
- 16. Allemann P, Leroy, Asakuma M, Abeidi FA, Dallemagne B, Marescaux J. Robotics may overcome technical limitations of single-trocar surgery – An experimental prospective study of Nissen fundoplication. Arch Surg. 2010;145:267–271 [DOI] [PubMed] [Google Scholar]
- 17. Tran HM. Robotic single-port hernia surgery. JSLS. 2011;15(3):309–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cuschieri A. Single-incision laparoscopic surgery. J Minim Access Surg. 2011;7(1):3–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bucher P, Pugin F, Morel P. Single port laparoscopic repair of primary and incisional ventral hernia repair. Hernia. 2009;13:569–570 [DOI] [PubMed] [Google Scholar]
- 20. Roy P, De A. Single-incision laparoscopic intraperitoneal onlay mesh hernioplasty for anterior abdominal wall hernia: a safe and feasible approach. J Minim Access Surg. 2011;7(1):37–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. White MA, Haber GP, Kaouk JH. Robotic single-site surgery. Curr Opin Urol. 2010;20:86–91 [DOI] [PubMed] [Google Scholar]