Background: VX-770 (ivacaftor), approved for therapy in CF patients bearing the G551D mutation, has an unknown mode of action.
Results: Potentiation of purified WT and mutant CFTR by VX-770 did not require the normal activating ligand ATP.
Conclusion: VX-770 binds WT and mutant CFTR channels directly to induce a nonconventional mode of gating.
Significance: These findings will enable discovery of the VX-770-binding site.
Keywords: ABC Transporter, ATP, CFTR, Chloride Channels, Cystic Fibrosis, Ion-sensitive Electrodes, Membrane Proteins, Protein Phosphorylation, Protein Purification
Abstract
The cystic fibrosis transmembrane conductance regulator (CFTR) acts as a channel on the apical membrane of epithelia. Disease-causing mutations in the cystic fibrosis gene can lead to CFTR protein misfolding as in the case of the F508del mutation and/or channel dysfunction. Recently, a small molecule, VX-770 (ivacaftor), has shown efficacy in restoring lung function in patients bearing the G551D mutation, and this has been linked to repair of its channel gating defect. However, these studies did not reveal the mechanism of action of VX-770 in detail. Normally, CFTR channel activity is regulated by phosphorylation, ATP binding, and hydrolysis. Hence, it has been hypothesized that VX-770 modifies one or more of these metabolic events. In this study, we examined VX-770 activity using a reconstitution system for purified CFTR protein, a system that enables control of known regulatory factors. We studied the consequences of VX-770 interaction with CFTR incorporated in planar lipid bilayers and in proteoliposomes, using a novel flux-based assay. We found that purified and phosphorylated CFTR was potentiated in the presence of Mg-ATP, suggesting that VX-770 bound directly to the CFTR protein, rather than associated kinases or phosphatases. Interestingly, we also found that VX-770 enhanced the channel activity of purified and mutant CFTR in the nominal absence of Mg-ATP. These findings suggest that VX-770 can cause CFTR channel opening through a nonconventional ATP-independent mechanism. This work sets the stage for future studies of the structural properties that mediate CFTR gating using VX-770 as a probe.
Introduction
Cystic fibrosis (CF)3-causing mutations in CFTR lead to loss of the functional expression of cyclic AMP-regulated CFTR chloride channel activity on the surface of the epithelium lining multiple organs, most notably the airways, intestines, pancreatic ducts, and reproductive tracts (1). The most common CF-causing mutation is F508del-CFTR, and this leads to CFTR protein misfolding and retention in the endoplasmic reticulum (2). Hence, this mutation results in the net loss of CFTR channel function at the cell surface. Partial restoration of normal processing, by low temperature culture conditions, improves overall CFTR-mediated chloride conduction in cell culture, yet the opening of the channel gate and the stability of the protein remain impaired (3–5). Therefore, effective therapies for patients bearing F508del-CFTR would act to improve folding, channel gating and cell surface stability. Conversely, there are numerous rarer mutations that do not lead to misfolding but rather impair normal channel gating activity. G551D-CFTR is included in this latter class of mutations, and recently there has been tremendous excitement surrounding a new drug (VX-770) that ameliorates the gating defect of this mutant. As it also improves gating by the major mutant F508del-CFTR (after biosynthetic rescue), there is optimism that in combination with a chemical “corrector” such as VX-809, which partially rescues the processing defect (6), VX-770 will exhibit therapeutic efficacy in patients with F508del-CFTR.
VX-770 (also known as ivacaftor or KalydecoTM) was recently approved by the Food and Drug Administration for treatment of CF patients bearing the G551D mutation because it improves respiratory health of these patients (7, 8). This compound was identified as a potentiator in cell-based assays of VX-770 activity on normal F508del-CFTR (after biosynthetic rescue) and G551D-CFTR, because of the absolute requirement for previous cellular activation by agonists of cAMP (9–11). However, its mechanism of action has not yet been fully described. Until now, it was not clear whether VX-770 interacted directly with the CFTR protein or with an associated kinase or phosphatase, which in turn would modify the phosphorylation status of CFTR. It was also unclear whether VX-770 modified phosphorylation-dependent gating or ATP-dependent gating. It was particularly intriguing that VX-770 is effective in patients bearing G551D, as this mutation is known to be defective in ATP-dependent gating (12).
Our understanding of the molecular basis for CFTR channel gating is still evolving, but it is well known that the protein must be phosphorylated for its channel activity (13). Furthermore, one well tested model suggests that ATP binding to the canonical catalytic site conferred at the dimerization interface of nucleotide binding domain 1 (NBD1) and NBD2 promotes opening of the channel gate (14–16). The availability of new compounds such as VX-770, which modify channel gating of WT-CFTR, F508del-CFTR, and G551D-CFTR, provide novel and important tools with which to understand the molecular basis for gating, the molecular defects caused by mutation, and the molecular mechanisms required for mutant protein repair.
EXPERIMENTAL PROCEDURES
Expression, Purification, and Reconstitution of CFTR
CFTR (WT, G551D, and F508del) was overexpressed with a C-terminal His10 tag in Sf9 cells and was purified with a method significantly different from that described previously in our laboratory. Protein overexpressed from 0.5 liters of culture was homogenized in the presence of protease inhibitors using an Emulsiflex C3 high pressure homogenizer (Avenstin, Ottawa, Ontario, Canada) at 15,000 p.s.i. Crude membrane pellets were isolated by ultracentrifugation for 1 h at 100,000 × g after a low speed centrifugation step to remove large debris, or fractions enriched in plasma membranes were isolated on a 35% sucrose cushion by ultracentrifugation at 137,000 × g for 1 h. 1/5th to 1/20th of the membrane pellet was solubilized by the presence of 2% fos-choline 14 (Anatrace, Maumee, OH) for 1 h with resuspension by a 30-gauge needle, and insoluble material was removed by ultracentrifugation. The sample was bound to Ni-NTA beads (Qiagen Inc., Mississauga, Ontario, Canada) for 1 h on ice. Beads were washed with 10 mm imidazole buffer containing 1 mm dodecyl maltoside (DDM) and a second wash step employed DDM and 50 mm imidazole. Protein was eluted with 600 mm imidazole buffer containing 1 mm DDM. Lower molecular weight contaminants and imidazole were removed by centrifugation with an Amicon ultracentrifugal filter device (Millipore Corp., Billerica, MA). Where applicable, CFTR was phosphorylated with 200 nm protein kinase A catalytic subunit (PKA; Promega Corp., Madison, WI) and 5 mm Mg-ATP for 20 min while attached to the Ni-NTA resin after the initial wash with 10 mm imidazole and then subjected to an additional 10 mm imidazole wash after the phosphorylation step. CFTR was reconstituted into 5 mg of phosphatidylethanolamine (PE)/phosphatidylserine (PS)/phosphatidylcholine (PC)/ergosterol, 5:2:1:1 (w/v) or 5 mg of egg PC by incubation in the presence of DDM and lipid for 30 min, followed by passage through an Extracti-Gel D detergent-binding column (Pierce). Purified CFTR was quantitated using ELISA described below and mixed at a ratio of ∼1:1200 (protein/lipid, w/w) with egg PC and DDM.
ELISA for CFTR
A high protein-binding 96-well plate was coated with anti-CFTR M3A7 (Millipore Corp., Billerica, MA) antibody, 0.5 μg/ml, in PBS overnight at 4 °C. The plate was blocked with 2% BSA in PBS for 2 h, and CFTR samples and standards (0–5 nm) of purified CFTR quantitated by amino acid analysis were incubated for 1 h at 37 °C in 1% BSA in PBS with 0.05% Tween 20. The plate was washed three times with 0.05% Tween 20 in PBS before and after incubation of the plate for 1 h with an anti-PentaHis-HRP conjugate (Qiagen, Mississauga, Ontario, Canada) at a dilution of 1:2000 in 2% BSA in PBS at room temperature with shaking. The plate was developed using 100 μl of the 3,3′,5,5′-tetramethylbenzidine substrate (Thermo Scientific, Rockford, IL) for 5–7 min followed by acidification with 100 μl of 0.18 m H2SO4. Absorbance was measured at 450 nm.
Iodide Flux Assay
Proteoliposomes were reconstituted in the presence of 75 mm KI. The external iodide was exchanged for 75 mm potassium glutamate by gel filtration chromatography. External iodide concentrations were monitored continuously using an iodide-selective electrode (Lazar Research Laboratories, Los Angeles, CA) interfaced to the Digidata 1320A data acquisition system and controlled by Clampex 8 software (Axon Instruments, Sunnyvale, CA), as described previously (17, 18).
ATPase Assay
The ATPase activity of purified CFTR in DDM solution was monitored by release of [32P]Pi from 5 μCi of [γ-32P]ATP (PerkinElmer Life Sciences) in the presence of 0.5 mm or increasing concentrations of nonradioactive Mg-ATP (0–5 mm) for 2 h at 37 °C, essentially as described previously (19, 20). The reaction was stopped by addition of 1 m formic acid, and 1-μl samples were spotted on a 20 × 20-cm PEI-cellulose TLC plate with UV indicator (Merck). The TLC plate was run in 1 m formic acid, 0.5 m LiCl, and the [32P]Pi and [γ-32P]ATP spots were quantitated using a Molecular Dynamics PhosphorImager and ImageQuant software (GE Healthcare). Data (n = 3 ± S.E.) were fitted to the Michaelis-Menten equation to yield the kinetic parameters Km and Vmax using GraphPad Prism software (version 4.00; GraphPad, San Diego). CFTR protein concentrations were determined using ELISA as described above.
Single Channel Studies
Planar bilayer studies of purified CFTR single channel conductance were performed at room temperature (22 °C) essentially as described previously (12, 20). Planar lipid bilayers were formed by painting a 10 mg/ml solution of egg PC, or PE/PS, 1:1 (w/w) in n-decane over a 200-μm aperture in a bilayer chamber (Warner Instruments, Hamden, CT). Bilayer formation was monitored electrically by observation of an increase in membrane capacitance (>200 picofarads for all experiments). Purified, phosphorylated CFTR was reconstituted at a protein/lipid ratio of ∼1:3000 (w/w), as described above, into a mixture of PE/PS/PC/ergosterol, 5:2:1:1 (w/v), in the presence of nystatin, and added to the cis-compartment. Fusion of proteoliposomes with the bilayer was potentiated with the establishment of an osmotic gradient across the bilayer (300 mm KCl in the cis-compartment and 50 mm in the trans-compartment). After addition of 1 mm ATP to the cis-compartment, CFTR channel activity was detected using a custom bilayer amplifier (M. Shen, University of Alabama), and data were recorded, digitally filtered at 100 Hz, and analyzed using pCLAMP 6.0.2 (Axon Instruments, Burlingame, CA). For experiments lacking ATP, VX-770 was added at a concentration of 2–10 μm, and then 1 mm ATP was added later to confirm the activity of the channel, or CFTRinh-172 was added to specifically inhibit CFTR channel activity.
SDS-PAGE/Western Blotting
One μg of purified CFTR in DDM solution or a small quantity as indicated of purification fractions or protein eluted from Ni-NTA beads by sample buffer were run on a 4–12% gradient SDS-polyacrylamide gel and developed by the silver stain procedure. Approximately 0.1 μg of purified CFTR was subjected to SDS-PAGE on a 4–12% gradient gel as above and then transferred to a nitrocellulose membrane. The membrane was probed with the M3A7 antibody at a concentration of 0.1 mg/ml. Following a final wash, the blot was developed by chemiluminescence with ECL reagent (GE Healthcare) using HyBlot CL autoradiography film (Denville Scientific, Metuchen, NJ).
RESULTS
Novel Reconstitution System Reports Regulated Channel Activity of Purified WT-CFTR
Our goal was to monitor the consequences of small molecule interaction on the activity of WT or mutant CFTR proteins in a system where multiple conditions could be compared simultaneously and the contribution of other proteins, including kinases and phosphatases, minimized. Such studies benefit from activity assays that report the function of a population of purified and reconstituted CFTR proteins. Whereas single channel studies provide exquisite resolution of CFTR channel gating, by definition they do not report the activity of a population of molecules and do not permit rapid comparative studies. Toward this goal, we developed a new method that enables purification of WT-CFTR and the reconstitution and functional analysis in phospholipid liposomes within 4 h. We employed the detergent fos-choline 14 to solubilize WT-CFTR (bearing a polyhistidine tag) from Sf9 membranes and captured the tagged protein by virtue of its affinity to Ni-NTA. A rapid, stepwise elution protocol (using different imidazole concentrations as described under “Experimental Procedures”) was employed to quickly remove CFTR from the affinity matrix in DDM micelles. For functional studies, we reconstituted purified CFTR into unilamellar liposomes competent for measurements of regulated channel activity. This new purification protocol and subsequent reconstitution is much more rapid than our previously published methods (20–23). In the older method, CFTR was solubilized using the detergent perfluoro-octanoate (PFO), bound to the Ni-NTA matrix, and eluted by the application of a pH gradient. This PFO-based purification protocol together with lipid reconstitution required ∼3 days.
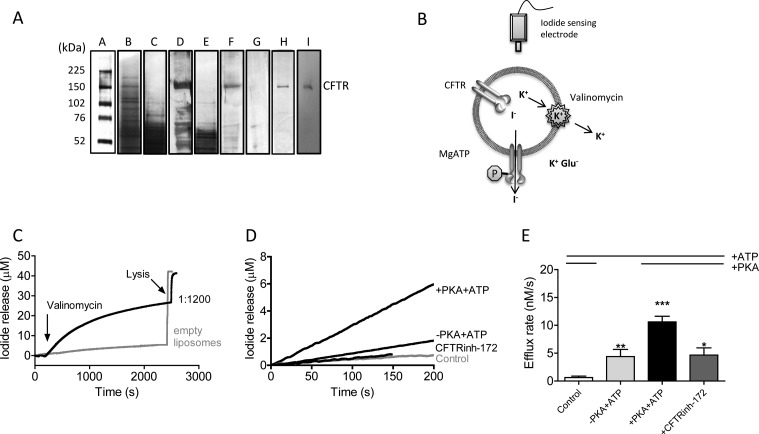
In Fig. 1A, we show that the new fos-choline 14-based method was effective in purifying CFTR to near-homogeneity. Membrane fractions contain multiple protein bands by silver-stained SDS-polyacrylamide gel (Fig. 1A, lane B). After solubilization of membrane protein with 2% fos-choline 14 detergent and binding to Ni-NTA resin, washing the resin with 10 mm imidazole and 1 mm DDM removed a variety of lower molecular weight bands from the gel (Fig. 1A, lane C), and a significant band of full-length CFTR as well as lower molecular weight bands remained associated with the resin (lane D). Upon washing with 50 mm imidazole and 1 mm DDM, lower molecular weight contaminants were removed from the resin (Fig. 1A, lanes E and F), and following 600 mm imidazole, 1 mm DDM washing, full-length CFTR was eluted from the resin (lane G, loss of 150-kDa band). After a further washing and concentration step for the eluted protein, the sample preparation was highly pure (Fig. 1A, lane H) and reacted with the M3A7 anti-CFTR antibody (lane I). This purified CFTR protein was functional as a channel following PKA-mediated phosphorylation and reconstitution into phospholipid liposomes (Fig. 1, C–E). We determined that ∼50% of the protein was oriented in the inside-out orientation (data not shown). As in our previous studies, we confirmed that the fusion of proteoliposomes containing phosphorylated CFTR with planar bilayers conferred the appearance of stepwise changes in current following the addition of 1 mm Mg-ATP (Fig. 2B). The properties of this anion-selective channel activity recapitulated those documented for CFTR channel activity in biological membranes (13, 24–29). Specifically, the channel activity of phosphorylated CFTR exhibited a unitary conductance of 10 pS with an open probability of ∼0.47 in the presence of 1 mm Mg-ATP (Fig. 2B). This compares favorably with a mean open probability of 0.68 previously reported by our group for CFTR purified by the PFO method (30). We also confirmed that the CFTR protein, purified, phosphorylated, and reconstituted using these new methods, exhibited intrinsic ATPase activity, similar to that reported using our previous protocol work (12, 31, 32). Purified CFTR protein displayed a phosphorylation-activated ATPase activity with a Km of 600 μm and Vmax of 68 nmol/mg protein/min (data not shown), whereas we previously reported values of 407 μm and 63 nmol/mg protein/min, respectively, for the PFO purification procedure (30).
FIGURE 1.
Purification and functional reconstitution of WT-CFTR. A, silver-stained gel lanes are as follows: lane A, prestained marker with the associated molecular mass indicated at left; lane B, 1/80th of the membrane fraction; lane C, 1% of the 10 mm imidazole wash fraction; lane D, 1/8th of the protein remaining associated with the beads after the 10 mm wash step; lane E, 0.4% of the 50 mm imidazole wash step; lane F, 1/8th of the protein remaining associated with the beads after the 50 mm wash step; lane G, 1/8th of the protein remaining associated with the beads after the 600 mm wash step; lane H, 1 μg of human CFTR purified, concentrated, and quantified as described under “Experimental Procedures”; lane I, Western blot for 0.1 μg of purified CFTR protein using the M3A7 anti-CFTR antibody. Gel and blot data are representative of three purifications. B, diagrammatic representation of the flux assay using CFTR-proteoliposomes. See under “Experimental Procedures” and “Results” for further details on the flux assay. C, sample flux assay traces. Purified CFTR was reconstituted into egg PC liposomes at a protein/lipid ratio of 1:1200 by mass. Control vesicles were prepared identically but in the absence of CFTR (empty or control; gray trace). Proteoliposomes were treated with 200 nm PKA and 1 mm Mg-ATP for 5 min, and flux was initiated by the addition of valinomycin at a concentration of 20 nm. Vesicles were lysed by addition of 0.5% Triton X-100 to verify that iodide was trapped (as indicated by the instantaneous rise in iodide near the end of the traces). D, initial iodide release (or flux) rates mediated by CFTR determined immediately after valinomycin addition. The efflux measured in the presence of PKA and Mg-ATP was significantly greater than that measured in the absence of PKA (also see Fig. 2A). The control trace (gray line) shows the lack of flux by liposomes lacking CFTR. CFTRinh-172 (20 μm) prevented the flux by PKA- and ATP-treated CFTR. E, bars represent the mean of 5–7 trials ± S.E. * indicates treatment with CFTRinh-172 causes significantly lower flux than in its absence (+PKA+ATP bar), p = 0.02. ** indicates efflux activity in proteoliposomes (−PKA+ATP) is significantly higher than control vesicles, p = 0.02, but significantly less than when treated with PKA, p = 0.005. *** indicates samples containing phosphorylated CFTR (+PKA+ATP) had efflux activity significantly higher than control vesicles, p < 0.0001.
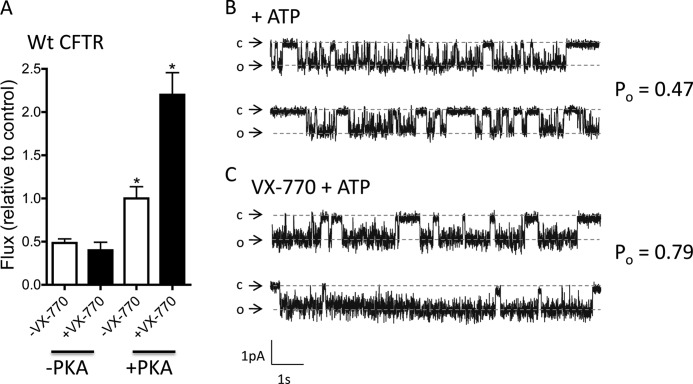
FIGURE 2.
Channel activity of purified and reconstituted phosphorylated WT-CFTR is directly modified by VX-770. A, PKA phosphorylation increased the initial rate of flux mediated by purified and reconstituted WT-CFTR in the presence of Mg-ATP (1 mm; white bars, *, p = 0.027). The flux rate exhibited by unphosphorylated CFTR (in the presence of Mg-ATP and normalized to the flux by PKA and Mg-ATP protein, i.e. control) is unchanged in the absence and presence of VX-770 (black bar, 10 μm). However, the flux rate exhibited by phosphorylated CFTR (in the presence of Mg-ATP) is significantly increased by 10 μm VX-770 addition (black bar, *, p = 0.039). Bars represent the mean of three (unphosphorylated) or four (phosphorylated) trials ± S.E. B, proteoliposomes containing purified and phosphorylated WT-CFTR were fused to planar lipid bilayers. Single channel current steps were recorded at −50 mV in the presence of 1 mm Mg-ATP following fusion to a planar lipid bilayer containing 300 and 50 mm KCl on the cis- and trans-compartments of the apparatus, respectively. c and o indicate closed channel and open channel current levels, respectively. The current-voltage relationships yielded a unitary conductance of ∼10 pS. Open probability measurements were possible by combining channel events lists from different trials. The trace shown in B is representative of five trials (or five different protein preparations with 874 events analyzed to yield a Po of 0.47). C, single channel current steps measured as described in B but in the presence of 2 μm VX-770 and 1 mm Mg-ATP. These open probability measurements were possible by combining channel events lists from seven different trials (or seven different protein preparation with 855 events analyzed to yield a Po of 0.79).
A proteoliposomal halide flux assay for reconstituted CFTR was developed to enable assessment of the activation of a population of CFTR channels, adopting methods that were previously employed to study other purified channels and transporters (Fig. 1B) (33, 34). An inside-out anion gradient was established across the proteoliposome bilayer such that the activation of the conduction pathway could be reported as the efflux of that anion. As expected, in liposomes lacking reconstituted CFTR protein, the rate of iodide efflux was minimal (Fig. 1C), with almost all of the iodide remaining trapped until the addition of detergent to mediate liposomal lysis. However, proteoliposomes containing WT-CFTR (reconstituted in a protein/lipid mass ratio of ∼1:1200) and pretreated with PKA (200 nm) and Mg-ATP (1 mm) mediated the rapid efflux of iodide after addition of the potassium-selective ionophore valinomycin (20 nm). This valinomycin-dependent response confirms that iodide efflux was previously limited by charge build up, a phenomenon expected for proteoliposomes exclusively bearing purified CFTR anion channels (Fig. 1C).
It is well known that PKA phosphorylation is required for the CFTR channel to be activated by Mg-ATP in biological membranes (35, 36). Therefore, if the initial rate of iodide efflux truly reflects channel opening, we expect that there will be a significant difference between iodide efflux rates by reconstituted and “unphosphorylated” CFTR (not subjected to phosphorylation by exogenous PKA) in the presence of 1 mm Mg-ATP, relative to the efflux rate mediated by CFTR in the presence of added PKA catalytic subunit (200 nm) plus 1 mm Mg-ATP. As shown in Fig. 1, D and E, the rate of iodide efflux mediated by “highly” phosphorylated CFTR was approximately 2–3-fold the rate mediated by CFTR protein not subjected to treatment with exogenous PKA. These findings support the idea that the initial rate of efflux reflects CFTR channel activation. We found that the rate of efflux mediated by PKA- and Mg-ATP-activated CFTR was significantly reduced by pretreatment with the specific CFTRinh-172 (20 μm, Fig. 1, D and E) in our reconstitution system, supporting the utility of this system for monitoring the activity of modulators that act directly on CFTR.
VX-770 Interacts Directly to Modulate Channel Activation of Phosphorylated WT-CFTR
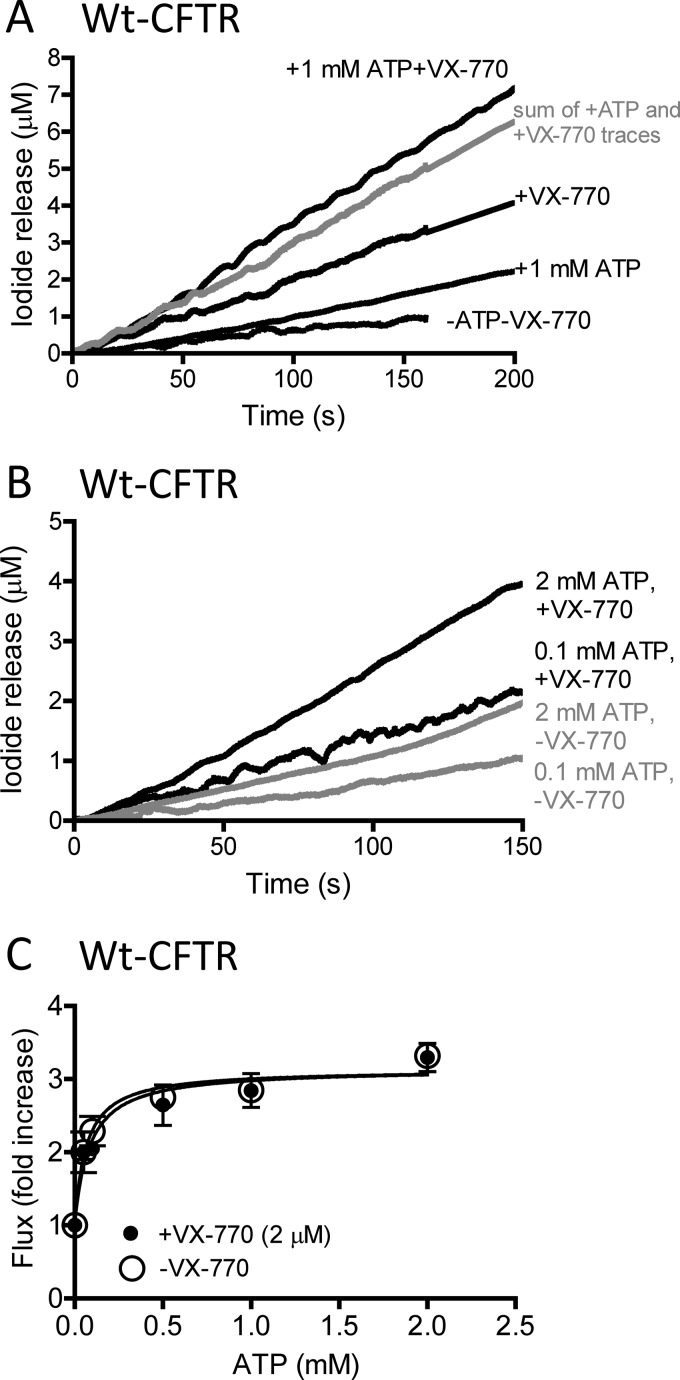
Although VX-770 will be used therapeutically to “repair” the gating defect of CFTR caused b the G551D mutation, it also increases the open probability of WT-CFTR (9). Therefore, we first asked several fundamental questions regarding VX-770 mode of action, using reconstituted WT-CFTR protein. We determined whether VX-770 acts directly on purified CFTR and whether its potentiating activity was regulated by the phosphorylation status of CFTR. Under assay conditions employing purified WT-CFTR lacking PKA-mediated phosphorylation, we did not observe any change in the rate of iodide flux activity after VX-770 (10 μm) addition (Fig. 2A). However, if the purified CFTR is pre-phosphorylated by PKA (200 nm), there is a 2-fold increase in the rate of flux upon VX-770 addition (Fig. 2A). Hence, VX-770 binds CFTR directly, and its role in potentiating channel activity depends on the phosphorylation status of CFTR. Potentiation of purified and PKA-phosphorylated CFTR was also observed at the single channel level in planar lipid bilayer studies (Fig. 2, B and C). Bilayer studies of purified, reconstituted, and phosphorylated WT CFTR show that its open probability (Po = 0.79) after addition of both 1 mm Mg-ATP plus VX-770 (2 μm, Fig. 2C) is approximately twice that measured for WT-CFTR in the presence of 1 mm Mg-ATP alone (Fig. 2B). Although bilayer fusion of proteoliposomes generally led to the appearance of multiple CFTR channels, thereby limiting detailed single channel kinetic studies, open probability measurements were possible by combining channel events lists from different trials. The traces shown in Fig. 2, B and C, are representative of five trials (with 874 events analyzed) and seven different trials (with 855 events analyzed), respectively. Together, the data obtained using the proteoliposomal assay and the planar bilayer studies show that VX-770 binds directly to potentiate the channel activity of purified and phosphorylated WT-CFTR.
VX-770 Mediates ATP-independent Gating by Phosphorylated WT-CFTR
As mentioned previously, a well tested model for the mechanism of action of CFTR suggests that activation is mediated by ATP binding to the canonical catalytic site of the phosphorylated channel. Interestingly, however, certain small molecules are thought to open the channel gate in an ATP-independent manner, a notable example being that of curcumin (37). Therefore, we were prompted to test whether VX-770 can also mediate ATP-independent gating using the current reconstitution system. In this set of experiments, purified CFTR was phosphorylated with added PKA (200 nm) and Mg-ATP (1 mm) while tethered to the Ni-NTA affinity matrix (via its polyhistidine tag) and then washed extensively to remove Mg-ATP prior to reconstitution into liposomes. These conditions optimize the phosphorylation status of CFTR and minimize the amount of bound Mg-ATP.
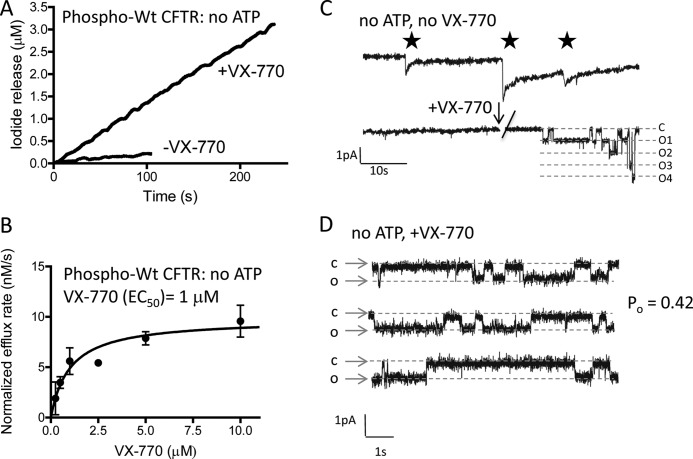
Interestingly, in this ATP-depleted state of the phosphorylated CFTR protein, the addition of VX-770 led to a significant increase in the initial rate of halide flux (Fig. 3A). The dose dependence was fitted using a hyperbolic and single-site binding algorithm to obtain an EC50 of ∼1 μm (Fig. 3B, r = 0.84).
FIGURE 3.
ATP-independent opening of phosphorylated CFTR by VX-770. A, VX-770 (10 μm) stimulates the initial rate of iodide flux in reconstituted pre-phosphorylated WT-CFTR in the nominal absence of ATP. B, VX-770 dependence of the ATP-independent flux mediated by pre-phosphorylated WT-CFTR. Data points shown represent the mean of five independent dose-response trials and are expressed relative to a sample treated with DMSO alone. Data were fitted to a hyperbolic single site binding equation, yielding an EC50 of ∼1 μm (see “Results”). C, planar lipid bilayer studies of purified and PKA-phosphorylated WT-CFTR in the nominal absence of ATP. As expected, despite multiple proteoliposome fusion events (nystatin spikes indicated with stars), there were no single channel events detected in the absence of ATP (representative of eight studies). At the point indicated with the arrow, the bilayer was treated with 2 μm VX-770, which induced multiple channel current steps ∼10–20 s after the addition artifact. In this experiment, ∼4 CFTR channels were activated. D, in studies (n = 2 protein preparations) showing single channel activity after VX-770 addition, the unitary current steps corresponded to the low single channel conductance expected for WT-CFTR (10 pS). Single channel current steps were recorded at −50 mV in a lipid bilayer chamber containing 300 and 50 mm KCl on the cis- and trans-compartments of the apparatus, respectively. Po of 0.42 was determined from a combined events list of 2625 events.
To confirm that this ATP-independent effect of VX-770 in the flux assay was conferred by the CFTR protein, we performed single channel studies. Proteoliposomes containing PKA-phosphorylated, ATP-depleted CFTR (prepared as described above) were rendered fusogenic with the addition of ergosterol and nystatin (24), and fusion with planar lipid bilayers was detected as transient nystatin-mediated current spikes (Fig. 3C, indicated with stars). As expected, no channel activity could be detected for ATP-depleted, phosphorylated CFTR despite the fusion of multiple proteoliposomes. Importantly, ATP-depleted CFTR channels (with the signature unitary conductance of 10 pS) could be activated with the addition of 2 μm VX-770 (Fig. 3C, +VX-770). This result was reproducible for multiple distinct protein preparations (n = 8 trials). Typically, multiple channels were stimulated by VX-770 (because of spontaneous multiple proteoliposome fusions), preventing assessment of single channel gating for most experiments. Combination of event lists from these trials permitted estimation of channel open probability at 0.42 (event = 2625). Single channel activity was apparent for two trials (Fig. 3D). These data confirm that VX-770 is capable of evoking channel openings by PKA-phosphorylated CFTR in the nominal absence of ATP.
These findings prompted us to test the hypothesis that VX-770 mediates its gating effect by binding to the canonical ATP catalytic site, as this is how ATP is thought to exert its effect on CFTR gating. According to a current model (14–16), ATP binding to the canonical catalytic site (sandwiched between the signature motif in NBD1 and the Walker A and B motifs of NBD2, Fig. 7) mediates opening of the phosphorylated CFTR channel. In Fig. 4, we show that the initial rates of halide flux evoked by saturating concentrations of VX-770 (10 μm) or Mg-ATP (1 mm) in liposomes bearing PKA-phosphorylated WT-CFTR were additive (Fig. 4A, with summary bars shown in Fig. 6C). These findings suggest that the two ligands (VX-770 and ATP) bind to different sites to modify CFTR channel activity. The rate of flux through CFTR increases ∼1.5–2-fold as the ATP concentration increases from 0.1 to 2 mm (Fig. 4B). Addition of 2 μm VX-770 to samples resulted in higher initial slopes, but the change in slope upon addition of 2 mm ATP versus 0.1 mm ATP appeared to be consistent (representative traces shown in Fig. 4B). We investigated this further for a range of ATP concentrations and show that pretreatment of phosphorylated WT-CFTR with VX-770 (2 μm) led to higher absolute flux rates in the presence of VX-770, but when normalized, there was no change in the dose response for ATP-mediated halide flux (Fig. 4C). Hence, these data suggest that VX-770 does not compete for binding to the canonical ATP-binding catalytic site of CFTR.
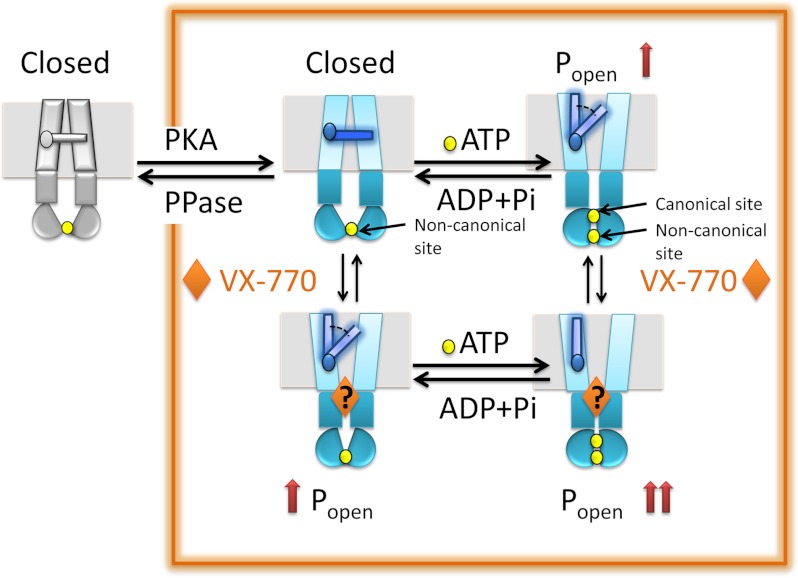
FIGURE 7.
Schematic showing potential mechanism of action of VX-770. According to this model, the phosphorylated form of the CFTR channel (enclosed in the orange rectangle), but not the unphosphorylated form (gray), is capable of ATP and/or VX-770-mediated opening. The closed state of the CFTR channel is shown with the gate (thin blue bar) in the membrane domain in the horizontal position. We speculate in this model that the gate is closed when the canonical catalytic site between the NBDs (half-moons) is not occupied by ATP (yellow sphere), and the NBDs are separated at this site. Normally, ATP binding to the catalytic site induces a tight NBD dimer which in turn leads to conformational changes along the loop regions (dark teal rectangles) to the membrane domains (cyan rectangles) inducing their conformational change and opening of the channel gate. Our results suggest that in the absence of ATP the interaction of VX-770 (at an unknown site) also induces conformational changes effective in opening the channel gate. Together, the consequences of VX-770 binding and ATP binding induce an additive effect to stabilize the open conformation of the channel gate and enhance open probability.
FIGURE 4.
Additive effect of VX-770 and ATP in regulation of phosphorylated WT CFTR. A, representative iodide efflux traces mediated by proteoliposomes containing pre-phosphorylated WT-CFTR in the absence or presence of 1 mm Mg-ATP and the absence or presence of 10 μm VX-770 as indicated, also see summary bar graphs in Fig. 6C. Data in gray indicate the sum of the efflux rates +VX-770 and +ATP traces. B, representative iodide efflux traces mediated by proteoliposomes containing pre-phosphorylated WT-CFTR in the absence (gray) or presence (black) of 2 μm VX-770 and in the presence of 0.1 or 2 mm ATP. C, no effect of VX-770 on ATP dependence of WT-CFTR flux activity in the presence or absence of VX-770 (2 μm). Data points represent initial rates mediated by PKA-phosphorylated WT-CFTR proteoliposomes pretreated in the presence of 2 μm VX-770 (+VX-770; closed circles) or in its absence but in the presence of DMSO vehicle (−VX-770; open circles) at a range of Mg-ATP concentrations. Although treatment with VX-770 significantly increased flux activity (p < 0.0001), to 1.4-fold the value in its absence, data were normalized to 1 in the absence of ATP in both cases to illustrate similarity of the ATP dependence. Data represent the mean of three traces ± S.E.
FIGURE 6.
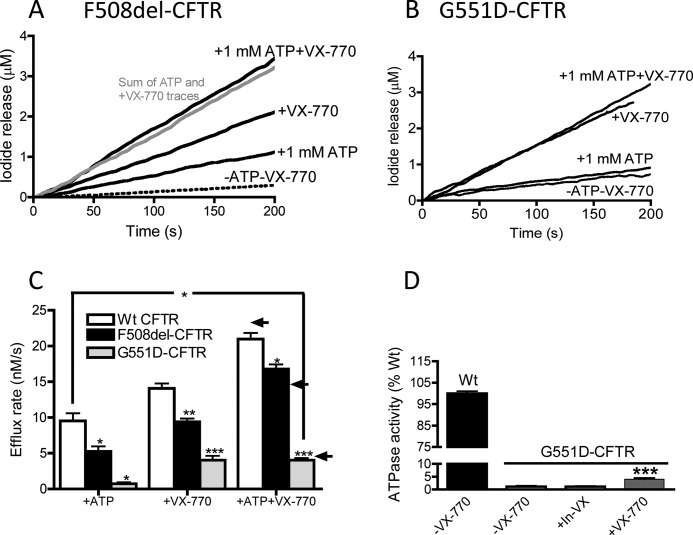
Additive effect of VX-770 and ATP in regulation of phosphorylated F508del-CFTR and phosphorylated G551D-CFTR. Representative iodide efflux traces are from proteoliposomes containing F508del-CFTR (A) or G551D-CFTR (B). Additive effect of ATP (1 mm) and VX-770 (10 μm) on F508del-CFTR. In contrast to F508del-CFTR (and WT-CFTR), G551D-CFTR proteoliposomes are not activated Mg-ATP but do show robust response to VX-770 alone. C, bar graph shows summary data for initial flux rates by the WT-CFTR and mutant CFTR proteins. Data are means of three replicates ± S.E., except for WT +ATP and WT +ATP VX-770, which are means of 10 ± S.E., and the G551D-CFTR data, which are means of 5–7, respectively, ± S.E. Samples were normalized to their respective untreated sample (−ATP-VX-770). Asterisks indicate samples that are significantly different from values for WT-CFTR treated similarly; * 0.05>p > 0.01; ** 0.01>p > 0.001; ***, p < 0.001. Arrows to the right indicate the mathematical addition of VX-770 alone flux and ATP alone flux values for upper WT-, middle F508del-, and lower G551D-CFTR. D, VX-770 modestly increases the ATPase activity of G551D-CFTR. Significantly more ATPase activity was measured for PKA-phosphorylated G551D-CFTR samples treated with VX-770 versus control (***, p < 0.0001) or the inactive analog (In-VX, p = 0.0027). Bars represent the mean of 3 (WT-CFTR), 22 (−VX-770), 17 (+VX-770), or 7 (In-VX) trials ± S.E.
VX-770 Mediates ATP-independent Gating by Phosphorylated F508del-CFTR and G551D-CFTR
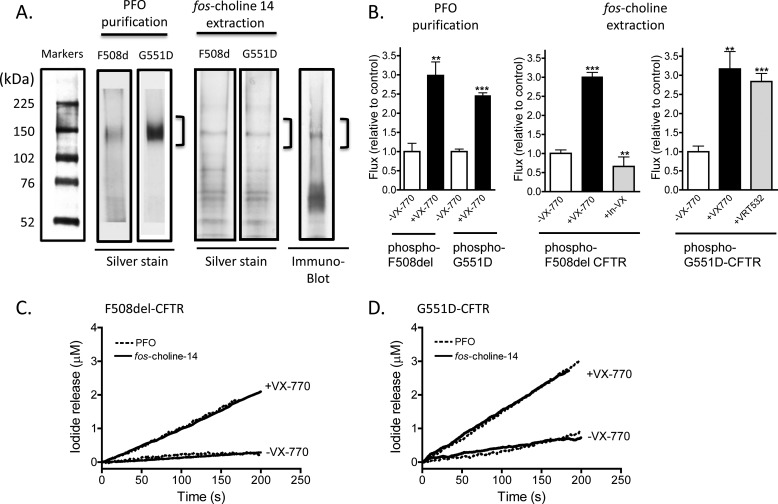
We then tested the hypothesis that VX-770 also interacts directly to potentiate purified and reconstituted CFTR mutants, F508del-CFTR and G551D-CFTR. As in the case of the WT-CFTR, we published our methods for purifying and reconstituting two clinically relevant CFTR mutants from baculovirus-infected Sf9 cells using the detergent PFO. This method is effective in purifying and yielding functional mutant CFTR protein after reconstitution (Fig. 5, A–D), (14, 18, 19). However, the process is slow, requiring 3 days. Hence, we were prompted to test the efficacy of our new, rapid purification method (employing the detergent fos-choline 14 and effective in purifying WT-CFTR, Figs. 1–4). We found that although the new method was effective in purifying full-length WT-CFTR, the fos-choline 14-based method extracted but did not purify the full-length F508del-CFTR and G551D-CFTR mutant proteins. In Fig. 5A, it is clear that there are multiple protein bands in addition to the 150-kDa band (corresponding to the full-length CFTR) in silver-stained gels (Fig, 5A). As many of these are detected using CFTR-specific antibody (M3A7, the adjacent immunoblot of F508del-CFTR protein), we reasoned that most of the other bands correspond to mutant CFTR degradation products.
FIGURE 5.
VX-770 acts directly to potentiate phosphorylated F508del-CFTR and phosphorylated G551D-CFTR in the absence of Mg-ATP. A, SDS-PAGE analysis and silver stain of purified F508del-CFTR and purified G551D-CFTR protein isolated using the PFO detergent extraction method. In this method, the mutant CFTR proteins are solubilized in PFO and applied to a Ni-NTA affinity column. The full-length mutant protein is eluted by the application of a continuous pH gradient (FPLC). The PFO method requires 3 days and yields functional protein as published previously (12, 18–23, 47–49). The full-length PFO-purified proteins run as expected as broad 150-kDa bands in overloaded gels as expected for Sf9-expressed proteins (21). Data are representative of 10 separate purifications. However, full-length (150 kDa) mutant proteins plus other proteins are evident in silver-stained gels after the fos-choline 14-based methods (a method wherein CFTR protein solubilized in fos-choline is eluted from a Ni-NTA affinity column in a batchwise method described for the first time in this paper and requiring half a day). Immunoblots using a CFTR-selective antibody shows that other bands likely correspond to CFTR fragments. Hence, the fast batchwise method employing fos-choline purifies the full-length mutants plus degradation products. Data are representative of three separate purifications. B, initial iodide efflux rates mediated by phosphorylated F508del-CFTR or phosphorylated G551D-CFTR are potentiated by VX-770 (10 μm) in the presence of 1 mm ATP, regardless of the purification method used (PFO or fos-choline). These data show that the activity mediated by reconstituted fos-choline-extracted and Ni-NTA-purified mutant CFTR is conferred by the full-length mutant protein. An inactive analog of VX-770 (V-09-1188, labeled In-VX, provided by Vertex Pharmaceuticals) fails to mediate potentiation of F508del-CFTR, whereas P1 (or VRT-532) is effective in potentiating G551D-CFTR. Means ± S.E. of five and four studies for the PFO purification and three and five studies for the fos-choline method for F508del-CFTR and G551D-CFTR, respectively, are shown. **, p = 0.0034 for PFO-purified F508del-CFTR in the presence versus absence of VX-770; p = 0.001 for fos-choline purified F508del-CFTR in the presence of In-VX versus sample treated with VX-770; and p = 0.0019 for fos-choline purified G551D in the presence of VX-770 versus its absence. ***, p ≤ 0.0001, when compared with the associated control sample in the absence of VX-770. PKA-phosphorylated F508del-CFTR (C) or PKA phosphorylated G551D-CFTR (D) purified using either method exhibits potentiation by VX-770 in the nominal absence of Mg-ATP. We show traces representative (n = 3) for mutant proteins extracted either using fos-choline 14 or PFO detergent (B and C). Samples were phosphorylated with 200 nm and 1 mm ATP and treated with 10 μm VX-770, In-VX, or VRT-532 where indicated. The similarity of these responses support the idea that the activities shown for fos-choline-extracted mutant proteins report the intrinsic activities of the full-length mutants and support the utility of this rapid process for studying mutant CFTR proteins in a cell-free reconstitution system that enables excellent control of ligand concentrations.
In Fig. 5B, we show that the addition of VX-770 (10 μm) to liposomes containing phosphorylated F508del-CFTR or phosphorylated G551D-CFTR (purified using the PFO based method) enhanced iodide-mediated flux in the presence of Mg-ATP (1 mm). These findings show that VX-770 is acting directly on purified and phosphorylated F508del-CFTR and G551D-CFTR (Fig. 5B). The same potentiation was observed in proteoliposomes bearing mutant protein extracted using the fos-choline 14-based method (Fig. 5B, right panels). These comparative data support the idea that both reconstitution methods are effective in reporting the consequence of ligand interaction with the full-length mutant CFTR protein (10, 11, 38–40). This potentiating effect of VX-770 is shared by another well studied modulator of CFTR channel activity, VRT-532 (described as P1 in the compound collection of CF Foundation Therapeutics (41)) (Fig. 5B), but importantly, not by an inactive analog of VX-770 (V-09-1188, In-VX, Fig. 5B), pointing to the specificity of the response.
Our studies shown in Fig. 3A revealed the novel finding that VX-770 potentiated WT-CFTR channel activity independent of added Mg-ATP. We used our reconstitution system to test this mode of action for VX-770 on the mutants F508del-CFTR and G551D-CFTR. Importantly, regardless of the extraction method used, the iodide flux mediated by phosphorylated F508del-CFTR (Fig. 5C) or phosphorylated G551D-CFTR (Fig. 5D) was enhanced by the addition of VX-770 (10 μm) alone, in the complete absence of added Mg-ATP. These findings support the idea that VX-770 binds directly to potentiate ATP-independent channel activity by purified and phosphorylated F508del-CFTR and G551D-CFTR (purified using the PFO-based method). Furthermore, the superposition of the data for both mutants regardless of the extraction method employed justifies the subsequent use of the novel, fast reconstitution methods to study the regulation of the mutant proteins in a cell-free system wherein the concentration of Mg-ATP and other ligands can be controlled.
Next, we were prompted to study the interaction between ATP and VX-770-mediated channel activity by phosphorylated F508del-CFTR and G551D-CFTR. In Fig. 6A, we show that the initial rates of halide flux evoked by saturating concentrations of VX-770 (10 μm) and Mg-ATP (1 mm) from liposomes bearing PKA-phosphorylated F508del-CFTR were additive (Fig. 6A, with summary bars shown in Fig. 6C). These findings suggest that the two ligands (VX-770 and ATP) bind to different sites to modify F508del-CFTR channel activity. As expected, phosphorylated G551D-CFTR (bearing a “mutated” signature motif in NBD1 and a “disrupted” canonical catalytic site) lacks ATP-dependent channel function as measured in this reconstitution assay (Fig. 6, B and C). However, as shown in Fig. 5, G551D-CFTR exhibits a robust response to VX-770 alone (Fig, 6, B and C). Together, these data argue that VX-770 acts to “promote” channel activation in G551D-CFTR by binding to a site on the CFTR protein that is distinct from the catalytic site.
To test this idea further, we studied the intrinsic ATPase activity of purified and phosphorylated G551D-CFTR. As shown in Fig. 6D, the G551D-CFTR mutant is defective in ATPase activity relative to WT-CFTR. This low level of intrinsic ATPase activity increased slightly upon addition of VX-770 (10 μm) to ∼4% of the wild type CFTR protein (Fig. 6D). The disparity between the increase in ATPase activity in G551D-CFTR upon VX-770 treatment (to 4% WT alone) and the increase in CFTR-mediated halide flux caused by VX-770 in G551D-CFTR (to 30% WT in the presence of ATP alone; Fig. 6C) further suggests that the efficacy of VX-770 in enhancing CFTR-mediated flux is not mediated by a change in the binding and hydrolysis of ATP at the canonical catalytic site.
DISCUSSION
These studies provide insights into the molecular basis for CFTR chloride channel activity and the mechanism whereby VX-770, a clinically effective small molecule, can rescue defective channel activity caused by mutation. Our observations show that PKA-phosphorylated CFTR itself, rather than associated regulatory proteins, is the target of VX-770. Furthermore, our results suggest that this compound induces channel opening via a nonconventional mechanism that does not require ATP binding and hydrolysis. These findings support the idea that phosphorylated CFTR can be stimulated by an ATP-independent mechanism (in addition to its ATP-dependent mechanism) and provide an explanation for the efficacy of this compound in clinical trials of patients bearing the G551D mutation, a mutation that disrupts the binding site through which ATP-dependent gating normally occurs.
The novel finding that VX-770 binds directly to phosphorylated WT-CFTR to open its channel gate independent of Mg-ATP was facilitated using a new rapid purification and reconstitution method (employing the detergent fos-choline 14). This new method is much faster (requiring 4 h) and potentially more accessible to most laboratories studying WT-CFTR than our previously published PFO-based purification and functional reconstitution method (requiring 3 days) (19–21). Direct evidence for the binding of VX-770 to mutant CFTR proteins to open their channel gates in a Mg-ATP-independent manner required the more laborious and previously published PFO-based method (17, 18, 22, 23). This need for the PFO-based method was due to the preponderance of mutant CFTR degradation products that must be separated from the full-length mutants by chromatography. However, the new rapid method for reconstitution of the mutants has utility for studying the regulation of a population of mutant CFTR proteins in a cell-free system, wherein protein interactions are minimized and ligand concentrations are well controlled (17).
Insights into the Nature of the Binding Site for VX-770
We found that both VRT-532 (18) and VX-770 (this study) enhanced ATPase activity of G551D-CFTR. Interestingly, the increase in ATPase mediated by both compounds was insufficient to rescue the mutant ATPase activity to levels greater than 4% of that mediated by WT-CFTR. The novel proteoliposomal flux assay allowed comparison of the effect of VRT-532 with VX-770 on the channel activity of a population of purified G551D-CFTR proteins. Both potentiators caused an increase in channel activity approaching 30–50% of WT-CFTR activity. Together, our findings show that both small molecules exert similar effects on both activities of the mutant protein. However, there is clearly a disparity between the effect of both compounds on the catalytic activity of G551D-CFTR and their effect on its channel opening. We speculate that these potentiators modify the gating properties of this mutant not by directly modifying the catalytic site at the NBD interface but rather by modifying a distinct structural element that is important in gating. The minor increase in ATPase activity induced by both compounds may report a secondary consequence of potentiator binding. Clearly, these small molecules provide a tremendous opportunity to understand novel mechanisms underlying CFTR channel gating requiring future study.
Future studies will be required to define the molecular properties of the VX-770-binding site. However, our functional studies provide certain clues regarding properties of this interaction. As discussed previously, our findings suggest that VX-770 binds to an allosteric site, which is distinct from the canonical, catalytic site. This hypothesis is supported by our observation that VX-770 binding does not alter the ATP dependence of WT-CFTR channel activity in our proteoliposome flux studies. Clearly, future patch clamp studies of single WT and mutant CFTR channels will be required to provide detailed insight into the interaction of VX-770 and ATP in channel gating.
However, we speculate that VX-770 may bind at an intramolecular interface along the long axis of the molecule (see model in Fig. 7). Stabilization of such interface(s) could then facilitate opening of the gate channel as suggested in recent studies and hypotheses proposed by Kirk and co-workers (37, 42–44). The VX-770-binding site will be probed directly by generating novel chemical probes based on the VX-770 structure that covalently modify interacting residues in the CFTR protein, similar to the strategy that we recently described for the potentiator VRT-532 (17).
It remains formally possible that this small molecule could also exert its effects indirectly, possibly via a change in the physical properties of the bilayer. For example, Lundbaek et al. (45) noted that amphiphiles affect the inactivation of voltage-dependent sodium channels, and the studies by Artigas et al. (46) suggested that the modification of CFTR by the small molecule 2,3-butanedione monoxime was mediated in part by alterations in membrane properties. However, an inactive analog of VX-770 (10 μm) was ineffective in stimulating channel activity by mutant CFTR reconstituted in phospholipid liposomes (Fig. 5B) suggesting that the VX-770 potentiation effect is mediated via specific binding to the CFTR protein. Future work will examine whether VX-770 may also directly affect the physical properties of the bilayer.
Allosteric Modulators as Drugs
There are several potential advantages of compounds that act through an allosteric binding site relative to those that directly target the active site (i.e. one or both of the ATP-binding sites). One major advantage relates to the potential for greater selectivity. Given the conservation of ATP-binding sites across members of the ABC family of membrane proteins, a compound that targets these orthosteric or active sites may exhibit cross-reactivity among multiple family members. We speculate that VX-770 may exhibit its clinical efficacy and lack of side effects (as documented in recent trials) (7) because it interacts with a region unique to CFTR and away from the canonical catalytic site. Interestingly, the channel activity of murine CFTR is not modified by VX-770 (9), suggesting that its binding occurs at a site (or interface) that is poorly conserved between mouse and human. The primary sequence of murine CFTR is only 70% identical to human CFTR, and there are several regions exhibiting relatively weak conservation that could constitute the binding site, including the “regulatory insertion” in NBD1, the PKA regulated “R” domain, and regions within the intracellular loops extending from the membrane to the nucleotide binding domains. The critical role for PKA phosphorylation of CFTR in modifying the efficacy of VX-770 implicates the potential importance of the phospho-regulated regions in comprising or facilitating access of this compound to its binding site.
Finally, this study has provided insight into the mechanism of action of the first of a class of small molecule interventions that targets the defect caused by mutation in CFTR channel activity. Our progress was enabled by the use of purified and functionally reconstituted CFTR and CFTR mutant proteins. This reconstitution system allowed us to isolate individual metabolic states of the protein, i.e. its PKA-phosphorylated state either in the ATP-bound form or its apo-form to probe the properties of the ligand-receptor interaction with greater precision. Our findings provide optimism that the larger spectrum of CF-causing mutations, defective in ATP-dependent gating, can be repaired by the delivery of small molecules that act allosterically to modify the channel gate. Furthermore, the pivotal role for PKA phosphorylation in regulating the VX-770 activity may provide clues for enhancing its efficacy in vivo.
Acknowledgments
Studies of the effect of VX-770 on the ATPase activity of G551D-CFTR were supported by a sponsored research agreement from Vertex Pharmaceuticals (Fig. 6D). Vertex Pharmaceuticals provided VX-770 and its inactive analog for this study. We also thank Ling Jun Huan, Steven Molinski, and Stan Pasyk for their assistance with ATPase studies and discussions regarding interpretation. Stan Pasyk also assisted in the single channel analyses. We acknowledge the Cystic Fibrosis Foundation Therapeutics (CFFT) and Dr. Robert Bridges, Rosalind Franklin University, for providing CFTRinh-172 and other CFTR modulator compounds.
This work was supported in part by operating grants (to C. E. B.) by Cystic Fibrosis Canada and the Canadian Institutes of Health Research (supporting studies of channel function of purified and reconstituted WT-CFTR, F508del-CFTR, and G551D-CFTR). Vertex Pharmaceuticals supported the ATPase studies in Fig. 6D with a sponsored research agreement.
This article was selected as a Paper of the Week.
- CF
- cystic fibrosis
- CFTR
- cystic fibrosis transmembrane conductance regulator
- DDM
- dodecyl maltoside
- Ni-NTA
- nickel-nitrilotriacetic acid
- PC
- phosphatidylcholine
- PE
- phosphatidylethanolamine
- PS
- phosphatidylserine
- NBD
- nucleotide binding domain
- PFO
- perfluoro-octanoate
- pS
- picosiemens.
REFERENCES
- 1. Proesmans M., Vermeulen F., De Boeck K. (2008) What's new in cystic fibrosis? From treating symptoms to correction of the basic defect. Eur. J. Pediatr. 167, 839–849 [DOI] [PubMed] [Google Scholar]
- 2. Cheng S. H., Gregory R. J., Marshall J., Paul S., Souza D. W., White G. A., O'Riordan C. R., Smith A. E. (1990) Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell 63, 827–834 [DOI] [PubMed] [Google Scholar]
- 3. Denning G. M., Anderson M. P., Amara J. F., Marshall J., Smith A. E., Welsh M. J. (1992) Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature 358, 761–764 [DOI] [PubMed] [Google Scholar]
- 4. Roxo-Rosa M., Xu Z., Schmidt A., Neto M., Cai Z., Soares C. M., Sheppard D. N., Amaral M. D. (2006) Revertant mutants G550E and 4RK rescue cystic fibrosis mutants in the first nucleotide binding domain of CFTR by different mechanisms. Proc. Natl. Acad. Sci. U.S.A. 103, 17891–17896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Okiyoneda T., Barrière H., Bagdány M., Rabeh W. M., Du K., Höhfeld J., Young J. C., Lukacs G. L. (2010) Peripheral protein quality control removes unfolded CFTR from the plasma membrane. Science 329, 805–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Van Goor F., Hadida S., Grootenhuis P. D., Burton B., Stack J. H., Straley K. S., Decker C. J., Miller M., McCartney J., Olson E. R., Wine J. J., Frizzell R. A., Ashlock M., Negulescu P. A. (2011) Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc. Natl. Acad. Sci. U.S.A. 108, 18843–18848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Accurso F. J., Rowe S. M., Clancy J. P., Boyle M. P., Dunitz J. M., Durie P. R., Sagel S. D., Hornick D. B., Konstan M. W., Donaldson S. H., Moss R. B., Pilewski J. M., Rubenstein R. C., Uluer A. Z., Aitken M. L., Freedman S. D., Rose L. M., Mayer-Hamblett N., Dong Q., Zha J., Stone A. J., Olson E. R., Ordoñez C. L., Campbell P. W., Ashlock M. A., Ramsey B. W. (2010) Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N. Engl. J. Med. 363, 1991–2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ramsey B. W., Davies J., McElvaney N. G., Tullis E., Bell S. C., Dřevínek P., Griese M., McKone E. F., Wainwright C. E., Konstan M. W., Moss R., Ratjen F., Sermet-Gaudelus I., Rowe S. M., Dong Q., Rodriguez S., Yen K., Ordoñez C., Elborn J. S., and VX08–770-102 Study Group (2011) A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N. Engl. J. Med. 365, 1663–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van Goor F., Hadida S., Grootenhuis P. D., Burton B., Cao D., Neuberger T., Turnbull A., Singh A., Joubran J., Hazlewood A., Zhou J., McCartney J., Arumugam V., Decker C., Yang J., Young C., Olson E. R., Wine J. J., Frizzell R. A., Ashlock M., Negulescu P. (2009) Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc. Natl. Acad. Sci. U.S.A. 106, 18825–18830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Drumm M. L., Wilkinson D. J., Smit L. S., Worrell R. T., Strong T. V., Frizzell R. A., Dawson D. C., Collins F. S. (1991) Chloride conductance expressed by ΔF508 and other mutant CFTRs in Xenopus oocytes. Science 254, 1797–1799 [DOI] [PubMed] [Google Scholar]
- 11. Bompadre S. G., Sohma Y., Li M., Hwang T. C. (2007) G551D and G1349D, two CF-associated mutations in the signature sequences of CFTR, exhibit distinct gating defects. J. Gen. Physiol 129, 285–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li C., Ramjeesingh M., Wang W., Garami E., Hewryk M., Lee D., Rommens J. M., Galley K., Bear C. E. (1996) ATPase activity of the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 271, 28463–28468 [DOI] [PubMed] [Google Scholar]
- 13. Tabcharani J. A., Chang X. B., Riordan J. R., Hanrahan J. W. (1991) Phosphorylation-regulated Cl− channel in CHO cells stably expressing the cystic fibrosis gene. Nature 352, 628–631 [DOI] [PubMed] [Google Scholar]
- 14. Vergani P., Lockless S. W., Nairn A. C., Gadsby D. C. (2005) CFTR channel opening by ATP-driven tight dimerization of its nucleotide binding domains. Nature 433, 876–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baukrowitz T., Hwang T. C., Nairn A. C., Gadsby D. C. (1994) Coupling of CFTR Cl− channel gating to an ATP hydrolysis cycle. Neuron 12, 473–482 [DOI] [PubMed] [Google Scholar]
- 16. Hwang T. C., Sheppard D. N. (2009) Gating of the CFTR Cl− channel by ATP-driven nucleotide binding domain dimerization. J. Physiol. 587, 2151–2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alkhouri B., Denning R. A., Kim Chiaw P., Eckford P. D., Yu W., Li C., Bogojeski J. J., Bear C. E., Viirre R. D. (2011) Synthesis and properties of molecular probes for the rescue site on mutant cystic fibrosis transmembrane conductance regulator. J. Med. Chem. 54, 8693–8701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pasyk S., Li C., Ramjeesingh M., Bear C. E. (2009) Direct interaction of a small molecule modulator with G551D-CFTR, a cystic fibrosis-causing mutation associated with severe disease. Biochem. J. 418, 185–190 [DOI] [PubMed] [Google Scholar]
- 19. Ramjeesingh M., Ugwu F., Stratford F. L., Huan L. J., Li C., Bear C. E. (2008) The intact CFTR protein mediates ATPase rather than adenylate kinase activity. Biochem. J. 412, 315–321 [DOI] [PubMed] [Google Scholar]
- 20. Ramjeesingh M., Garami E., Galley K., Li C., Wang Y., Eckford P. D. W., Bear C. E. (2010) in Reliable Lab Solutions: Essential Ion Channel Methods (Conn P. M., ed) pp. 337–357, Academic Press, Burlington, MA [Google Scholar]
- 21. Ramjeesingh M., Li C., Garami E., Huan L. J., Hewryk M., Wang Y., Galley K., Bear C. E. (1997) A novel procedure for the efficient purification of the cystic fibrosis transmembrane conductance regulator (CFTR). Biochem. J. 327, 17–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wellhauser L., Kim Chiaw P., Pasyk S., Li C., Ramjeesingh M., Bear C. E. (2009) A small molecule modulator interacts directly with ΔPhe-508-CFTR to modify its ATPase activity and conformational stability. Mol. Pharmacol. 75, 1430–1438 [DOI] [PubMed] [Google Scholar]
- 23. Kim Chiaw P., Wellhauser L., Huan L. J., Ramjeesingh M., Bear C. E. (2010) A chemical corrector modifies the channel function of F508del-CFTR. Mol. Pharmacol. 78, 411–418 [DOI] [PubMed] [Google Scholar]
- 24. Anderson M. P., Gregory R. J., Thompson S., Souza D. W., Paul S., Mulligan R. C., Smith A. E., Welsh M. J. (1991) Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science 253, 202–205 [DOI] [PubMed] [Google Scholar]
- 25. Anderson M. P., Berger H. A., Rich D. P., Gregory R. J., Smith A. E., Welsh M. J. (1991) Nucleoside triphosphates are required to open the CFTR chloride channel. Cell 67, 775–784 [DOI] [PubMed] [Google Scholar]
- 26. Anderson M. P., Rich D. P., Gregory R. J., Smith A. E., Welsh M. J. (1991) Generation of cAMP-activated chloride currents by expression of CFTR. Science 251, 679–682 [DOI] [PubMed] [Google Scholar]
- 27. Bear C. E., Duguay F., Naismith A. L., Kartner N., Hanrahan J. W., Riordan J. R. (1991) Cl− channel activity in Xenopus oocytes expressing the cystic fibrosis gene. J. Biol. Chem. 266, 19142–19145 [PubMed] [Google Scholar]
- 28. Kartner N., Hanrahan J. W., Jensen T. J., Naismith A. L., Sun S. Z., Ackerley C. A., Reyes E. F., Tsui L. C., Rommens J. M., Bear C. E. (1991) Expression of the cystic fibrosis gene in nonepithelial invertebrate cells produces a regulated anion conductance. Cell 64, 681–691 [DOI] [PubMed] [Google Scholar]
- 29. Bear C. E., Li C. H., Kartner N., Bridges R. J., Jensen T. J., Ramjeesingh M., Riordan J. R. (1992) Purification and functional reconstitution of the cystic fibrosis transmembrane conductance regulator (CFTR). Cell 68, 809–818 [DOI] [PubMed] [Google Scholar]
- 30. Ramjeesingh M., Li C., Garami E., Huan L. J., Galley K., Wang Y., Bear C. E. (1999) Walker mutations reveal loose relationship between catalytic and channel-gating activities of purified CFTR (cystic fibrosis transmembrane conductance regulator). Biochemistry 38, 1463–1468 [DOI] [PubMed] [Google Scholar]
- 31. Kogan I., Ramjeesingh M., Bear C. E. (2004) ATPase assay of purified, reconstituted CFTR protein. J. Cyst. Fibros. 3, 133–134 [DOI] [PubMed] [Google Scholar]
- 32. Ramjeesingh M., Li C., Kogan I., Wang Y., Huan L. J., Bear C. E. (2001) A monomer is the minimum functional unit required for channel and ATPase activity of the cystic fibrosis transmembrane conductance regulator. Biochemistry 40, 10700–10706 [DOI] [PubMed] [Google Scholar]
- 33. Walden M., Accardi A., Wu F., Xu C., Williams C., Miller C. (2007) Uncoupling and turnover in a Cl−/H+ exchange transporter. J. Gen. Physiol. 129, 317–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee S. Y., Letts J. A., MacKinnon R. (2009) Functional reconstitution of purified human Hv1 H+ channels. J. Mol. Biol. 387, 1055–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chang X. B., Tabcharani J. A., Hou Y. X., Jensen T. J., Kartner N., Alon N., Hanrahan J. W., Riordan J. R. (1993) Protein kinase A (PKA) still activates CFTR chloride channel after mutagenesis of all 10 PKA consensus phosphorylation sites. J. Biol. Chem. 268, 11304–11311 [PubMed] [Google Scholar]
- 36. Gadsby D. C., Nairn A. C. (1999) Regulation of CFTR Cl− ion channels by phosphorylation and dephosphorylation. Adv. Second Messenger Phosphoprotein Res. 33, 79–106 [DOI] [PubMed] [Google Scholar]
- 37. Wang W., Bernard K., Li G., Kirk K. L. (2007) Curcumin opens cystic fibrosis transmembrane conductance regulator channels by a novel mechanism that requires neither ATP binding nor dimerization of the nucleotide binding domains. J. Biol. Chem. 282, 4533–4544 [DOI] [PubMed] [Google Scholar]
- 38. Li C., Ramjeesingh M., Reyes E., Jensen T., Chang X., Rommens J. M., Bear C. E. (1993) The cystic fibrosis mutation (ΔF508) does not influence the chloride channel activity of CFTR. Nat. Genet. 3, 311–316 [DOI] [PubMed] [Google Scholar]
- 39. Cai Z., Taddei A., Sheppard D. N. (2006) Differential sensitivity of the cystic fibrosis (CF)-associated mutants G551D and G1349D to potentiators of the cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channel. J. Biol. Chem. 281, 1970–1977 [DOI] [PubMed] [Google Scholar]
- 40. Jih K. Y., Li M., Hwang T. C., Bompadre S. G. (2011) The most common cystic fibrosis-associated mutation destabilizes the dimeric state of the nucleotide binding domains of CFTR. J. Physiol. 589, 2719–2731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Van Goor F., Straley K. S., Cao D., González J., Hadida S., Hazlewood A., Joubran J., Knapp T., Makings L. R., Miller M., Neuberger T., Olson E., Panchenko V., Rader J., Singh A., Stack J. H., Tung R., Grootenhuis P. D., Negulescu P. (2006) Rescue of ΔF508-CFTR trafficking and gating in human cystic fibrosis airway primary cultures by small molecules. Am. J. Physiol. Lung Cell Mol. Physiol. 290, L1117–L1130 [DOI] [PubMed] [Google Scholar]
- 42. Kirk K. L., Wang W. (2011) A unified view of cystic fibrosis transmembrane conductance regulator (CFTR) gating. Combining the allosterism of a ligand-gated channel with the enzymatic activity of an ATP-binding cassette (ABC) transporter. J. Biol. Chem. 286, 12813–12819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang W., Wu J., Bernard K., Li G., Wang G., Bevensee M. O., Kirk K. L. (2010) ATP-independent CFTR channel gating and allosteric modulation by phosphorylation. Proc. Natl. Acad. Sci. U.S.A. 107, 3888–3893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bernard K., Wang W., Narlawar R., Schmidt B., Kirk K. L. (2009) Curcumin cross-links cystic fibrosis transmembrane conductance regulator (CFTR) polypeptides and potentiates CFTR channel activity by distinct mechanisms. J. Biol. Chem. 284, 30754–30765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lundbaek J. A., Koeppe R. E., 2nd, Andersen O. S. (2010) Amphiphile regulation of ion channel function by changes in the bilayer spring constant. Proc. Natl. Acad. Sci. U.S.A. 107, 15427–15430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Artigas P., Al'aref S. J., Hobart E. A., Díaz L. F., Sakaguchi M., Straw S., Andersen O. S. (2006) 2,3-Butanedione monoxime affects cystic fibrosis transmembrane conductance regulator channel function through phosphorylation-dependent and phosphorylation-independent mechanisms. The role of bilayer material properties. Mol. Pharmacol. 70, 2015–2026 [DOI] [PubMed] [Google Scholar]
- 47. Kogan I., Ramjeesingh M., Li C., Bear C. E. (2002) Studies of the molecular basis for cystic fibrosis using purified reconstituted CFTR protein. Methods Mol. Med. 70, 143–157 [DOI] [PubMed] [Google Scholar]
- 48. Ramjeesingh M., Garami E., Galley K., Li C., Wang Y., Bear C. E. (1999) Purification and reconstitution of epithelial chloride channel cystic fibrosis transmembrane conductance regulator. Methods Enzymol. 294, 227–246 [DOI] [PubMed] [Google Scholar]
- 49. Bear C. E., Li C., Galley K., Wang Y., Garami E., Ramjeesingh M. (1997) Coupling of ATP hydrolysis with channel gating by purified, reconstituted CFTR. J. Bioenerg. Biomembr. 29, 465–473 [DOI] [PubMed] [Google Scholar]