Background: Aβ peptide, implicated in Alzheimer disease, occurs in various lengths.
Results: Non-abundant Aβ1–38 and Aβ1–43 affect oligomerization, cytotoxicity, and aggregation of Aβ1–40 and Aβ1–42.
Conclusion: Small amounts of Aβ lengths other than Aβ1–40 or Aβ1–42 significantly alter the behavior of the total Aβ pool.
Significance: Drug strategies targeting APP processing to affect Aβ1–38 levels need careful consideration.
Keywords: Aggregation, Alzheimer Disease, Amyloid, Biophysics, Neurodegeneration, C-terminal Heterogeneity, Cytotoxicity, Amyloid-β Peptide, γ-Secretase Modulating Therapy, Molecular Plasticity
Abstract
Current therapeutic approaches under development for Alzheimer disease, including γ-secretase modulating therapy, aim at increasing the production of Aβ1–38 and Aβ1–40 at the cost of longer Aβ peptides. Here, we consider the aggregation of Aβ1–38 and Aβ1–43 in addition to Aβ1–40 and Aβ1–42, in particular their behavior in mixtures representing the complex in vivo Aβ pool. We demonstrate that Aβ1–38 and Aβ1–43 aggregate similar to Aβ1–40 and Aβ1–42, respectively, but display a variation in the kinetics of assembly and toxicity due to differences in short timescale conformational plasticity. In biologically relevant mixtures of Aβ, Aβ1–38 and Aβ1–43 significantly affect the behaviors of Aβ1–40 and Aβ1–42. The short timescale conformational flexibility of Aβ1–38 is suggested to be responsible for enhancing toxicity of Aβ1–40 while exerting a cyto-protective effect on Aβ1–42. Our results indicate that the complex in vivo Aβ peptide array and variations thereof is critical in Alzheimer disease, which can influence the selection of current and new therapeutic strategies.
Introduction
Extracellular deposits containing the amyloid-β peptide (Aβ)3 represent one of the hallmarks of Alzheimer disease (AD) (1). Aβ is generated from the transmembrane amyloid precursor protein (APP) by β- and γ-secretase-mediated cleavage (2–4). This action primarily results in the production of the 40-amino acid Aβ1–40 peptide and smaller amounts of the 42-amino acid Aβ1–42 peptide in addition to minute quantities of other Aβ peptides ranging in length from 27 to 43 amino acids (5, 6). The observed variation at the Aβ C terminus is a consequence of the heterogeneous γ-secretase processing pattern (7) that first generates Aβ1–48 and Aβ1–49 peptides through cleavage of APP at the ϵ-site followed by successive trimming of every three to four residues (8, 9). Hence, the preferential Aβ1–40 production pathway involves the intermediate formation of Aβ1–43, Aβ1–46, and Aβ1–49 (10). The array of Aβ peptides that is produced in this way can be affected by clinical mutations in APP (8, 11) or in the presenilin-1 active site subunit of the γ-secretase complex (8, 11, 12). Mutations of presenilin-1 potently shift the ϵ-cleavage site on APP toward the Aβ1–38 production pathway with intermediate formation of Aβ1–42, Aβ1–45, and Aβ1–48 (10).
Generally, longer Aβ peptides are more hydrophobic as the C termini progressively form part of the transmembrane domain of APP and are, therefore, considered more aggregation-prone (13–15). Consistent with this finding, senile plaques have been found to be primarily composed of Aβ1–42 and Aβ1–43, whereas shorter Aβ peptides remain largely undetected (16–18). Based on these observations, γ-secretase inhibitors were developed that aimed at lowering the activity of γ-secretase and reducing Aβ production. However, the multifunctionality of the γ-secretase enzyme, including a critical role in the Notch signaling process, led to the recognition that total inhibition of this enzyme is an undesired approach. This observation served as a starting point for the generation of γ-secretase modulators (GSMs) that fine-tune the action of γ-secretase to shift the production of Aβ peptides toward shorter variants while leaving the total Aβ peptide production and activity of γ-secretase unchanged (19, 20). Given the finding that specifically aggregated Aβ peptide can lead to a neurotoxic response, GSMs offered in this way a promising perspective as a potential agent to slow down the progress of senile plaque deposition in AD by decreasing the production of Aβ1–42 while increasing that of Aβ1–38. The first generation of GSMs was classified as non-steroidal anti-inflammatory drugs and derivatives thereof. Administration of these drugs to healthy individuals showed positive effects on cognitive function that could be entirely attributed to the cyclo-oxygenase (COX) inhibitory action of the compound without displaying GSM action (21, 22). Clinical trials with non-COX inhibitory non-steroidal anti-inflammatory drugs did not display protective effects on AD disease progress, possibly as a result of the low potency and poor brain penetrance of the compounds, inhibition of Notch processing, or accumulation of APP C-terminal fragments (23). A next generation of Notch-sparing GSMs and non-steroidal anti-inflammatory drug-derived compounds with improved potency and brain penetration are currently being developed but yet await clinical trials (22).
The reported neurotoxicity of Aβ1–43 (12) as well as the observed increasing (24) and decreasing Aβ1–38 (25) levels in cerebrospinal fluid upon AD progress, indicate that the contributions of Aβ1–38 and Aβ1–43 to AD progression require further elucidation. By comparing their pathogenicity, aggregation profiles, and biophysical properties with that of well studied Aβ1–40 and Aβ1–42, we show that Aβ1–38 and Aβ1–43 both form aggregates that differ in cytotoxic potential. We further show that inclusion of Aβ1–38 and Aβ1–43 into complex mixtures containing Aβ1–40 and Aβ1–42 substantially affects the behavior of total Aβ and that Aβ1–38 and Aβ1–40, previously considered non-amyloidogenic, can unexpectedly become toxic in these mixtures. These findings have been related to conformational plasticity of the respective peptides and highlight the relevance of understanding the role of C-terminal variation of Aβ peptides and their potential as therapeutic targets.
EXPERIMENTAL PROCEDURES
Preparation of Aβ Peptides and Peptide Ratios
Aβ peptides (rPeptide) were dissolved and mixed as described before (26, 27). Briefly, Aβ was dissolved into 1,1,1,3,3,3-hexafluor-2-propanol, evaporated with an N2 stream, and redissolved in dimethyl sulfoxide. Solvents were removed by elution over a 5-ml HiTrap desalting column (GE Healthcare) into a 50 mm Tris buffer, pH 7.4, containing 1 mm EDTA. Peptide concentrations were measured using the Coomassie (Bradford) Protein Assay kit (Thermo Scientific) against a bovine serum albumin standard (Thermo Scientific). Aβ peptide concentrations were diluted to a concentration of 50 μm in 50 mm Tris buffer, pH 7.4, containing 1 mm EDTA and incubated at 25 °C under quiescent conditions for further experiments.
Thioflavin T Fluorescence
Fibrillation kinetics of Aβ in the presence of 12 μm thioflavin T (ThT) were followed in situ at 25 °C using a Fluostar OPTIMA fluorescence plate reader (BMG LABTECH GmbH) at an excitation wavelength of 440 nm and an emission wavelength of 480 nm. Readings were recorded in triplicate every 5 min for a period of 10 h and corrected by subtracting the intensity obtained for buffer containing 12 μm ThT. The end of the lag phase was determined manually. Elongation rate was fitted to the central region of the exponential phase. Final fluorescence was determined at 10 h of incubation.
Dot Blot
At various time points a volume of 5-μl sample was spotted onto Protran BA85 nitrocellulose blotting membrane (Whatman). The membranes were blocked at 25 °C in phosphate-buffered saline containing 0.2% Tween 20 (PBST XL) for 1 h and incubated for 1 h at 25 °C with primary rabbit polyclonal anti-oligomer antibody (A11) (Invitrogen), diluted 1:4000 in 100 mm Hepes, pH 7.0 (28). After incubation with secondary anti-rabbit-HRP-tagged antibody (Promega) diluted 1:5000 in phosphate-buffered saline containing 0.05% Tween 20 (PBST) for 0.5 h at 25 °C, the membranes were visualized using the ImmobilonTM Western chemiluminescent HRP substrate system (Millipore).
Transmission Electron Microscopy
A volume of 5 μl of Aβ was adsorbed to carbon-coated Formvar film on 400-mesh copper grids (Agar Scientific Ltd) for 1 min. The grids were washed in ultrapure water (Merck) and stained with 1% (w/v) uranyl acetate (VWR). Samples were studied using a JEOL JEM-2100 microscope at an accelerating voltage of 200 kV or a JEOL JEM-1400 microscope at an accelerating voltage of 80 kV (JEOL Ltd.).
Atomic Force Microscopy (AFM)
AFM imaging was performed on a custom-built instrument using Si3N4 tips (Veeco Instruments, Woodbury NY; type MSCT-AUHW) with a spring constant of 0.5 newtons/m and a nominal tip radius of 10 nm. The measurements were made in tapping mode in air, with a tapping amplitude of less than 4 nm. The AFM scan settings were optimized to minimum force interaction with the sample. AFM samples were prepared by placing 5 μl of sample on freshly cleaved mica. After 4 min adsorption time, unbound Aβ was washed off twice with 100 μl of ultrapure water (Merck) and dried using a gentle N2 stream. The images are represented in three-dimensions after removal of height discontinuities between subsequent scan lines and compensation for piezo drift using SPIP software (Image Metrology A/S, Lyngby, Denmark).
Far-UV Circular Dichroism (CD)
After 1.5 h of incubation, Aβ was diluted to 15 μm and placed in a quartz cuvette with an optical path of 3 mm, and far-UV circular dichroism spectra were recorded in a Jasco J-715 spectrometer. The wavelength range was set from 260 to 190 nm with 0.2-nm resolution, 2.0-s response time, 2.0-nm bandwidth at a scanning speed of 50 nm/min. Data were collected as averages of eight scans. The spectra obtained were corrected by subtracting the spectrum obtained for buffer only.
Attenuated Total Reflection Fourier Transform Infrared Spectroscopy (ATR FTIR)
Using a Bruker Tensor 27 infrared spectrophotometer equipped with a Bio-ATR II accessory, infrared spectra of aggregating Aβ (220 μm, 25 °C, in 50 mm Tris buffer, pH 7.4, containing 1 mm EDTA) were recorded. The samples were applied to the FTIR sample holder and incubated for 1.5 h. Spectra were recorded in the range of 900–3500 cm−1 at a spectral resolution of 4 cm−1 at the beginning (time 0) and the end (time 1.5 h) of the experiment. Each measurement consisted of 120 accumulations. The spectrophotometer was continuously purged with dried air. The obtained spectra were corrected for atmospheric interference, baseline-subtracted, and rescaled in the amide I area (1700 to 1600 cm−1). Changes of secondary structure over 1.5 h of incubation were analyzed by subtraction of the spectrum recorded at time 0.
In Silico Predictions
The statistical mechanics algorithm TANGO (29) was used to predict aggregation-prone regions in the Aβ peptide sequence (30). TANGO provides an aggregation propensity (0–100%) per residue as output. An aggregating region is defined as a continuous stretch of residues with an individual TANGO score higher than 5% and a total score for the region higher than 50%. Total TANGO scores are calculated as the sum of the individual residual TANGO scores for a given sequence. TANGO calculations were performed using the online TANGO calculator with the following parameters: pH of 7.0, a temperature of 298.15 K, and 0.02 m ionic strength without N- or C- terminal protection.
Molecular Dynamics (MD) Simulations
The NMR structure (Protein Data Bank entry 1IYT) was used as starting structure of Aβ1–42 and as a template to generate the other Aβ species studied here. All MD simulations were performed with GROMACS 4.5.3 using the OPLS/AA force field (31). Experimental details are described in the supplemental materials. The LINCS algorithm (32) was used for bond-length constraining. The non-bonded pair list was updated every 10 fs. The simulation of each system was repeated at least 10 times and then individually analyzed, and their averaged properties are reported here. Programs included in the GROMACS package as well as some in-house scripts were used to perform the analysis of the trajectories. Molecular graphics images were produced using the UCSF Chimera package from the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco (supported by National Institutes of Health Grant P41 RR001081) (33).
Neuroblastoma Cells and Cytotoxicity
All tissue culture reagents were obtained from Invitrogen. The human neuroblastoma cell line SH-SY5Y (ATCC number CRL-2266) was cultured in DMEM/F-12 supplemented with 10% fetal bovine serum (HyClone, ThermoScientific). The cells were incubated at 37 °C in a humidified 5% CO2 atmosphere. Cytotoxicity assays were performed in 96-well plates after plating 25,000 cells per well in serum-deprived DMEM/F-12. After pre-aggregation for 1.5 h, Aβ was diluted in DMEM/F-12 and added to the cells. After 24 h of treatment, cell viability was analyzed using the Cell Titer Blue Cell Viability assay (Promega). After 4 h, color conversion was analyzed by measuring the fluorescence intensity of the samples at an excitation wavelength of 544 nm and an emission wavelength of 590 nm using a Fluostar OPTIMA fluorescence plate reader. Values are percent of cell viability ± S.D., and buffer signal was normalized to 100%.
Statistical Analysis
Results from ThT fluorescence and cytotoxicity experiments were analyzed using two-tailed unpaired t tests for significance. Significance is indicated by *** (p < 0.0001), ** (p < 0.0005), and * (p < 0.005). MD results were analyzed using two-way analysis of variance with repeated measures to determine whether each group differs significantly from each other and multivariate analysis to determine at which specific point the groups significantly differed. Bonferroni post hoc analysis was applied. Significance is indicated by ## (p < 0.005). All properties determined by MD techniques are reported as the average property of 10 simulations ± S.E.
RESULTS
Aβ Peptide Length Determines Aggregation, Oligomerization, and Toxicity
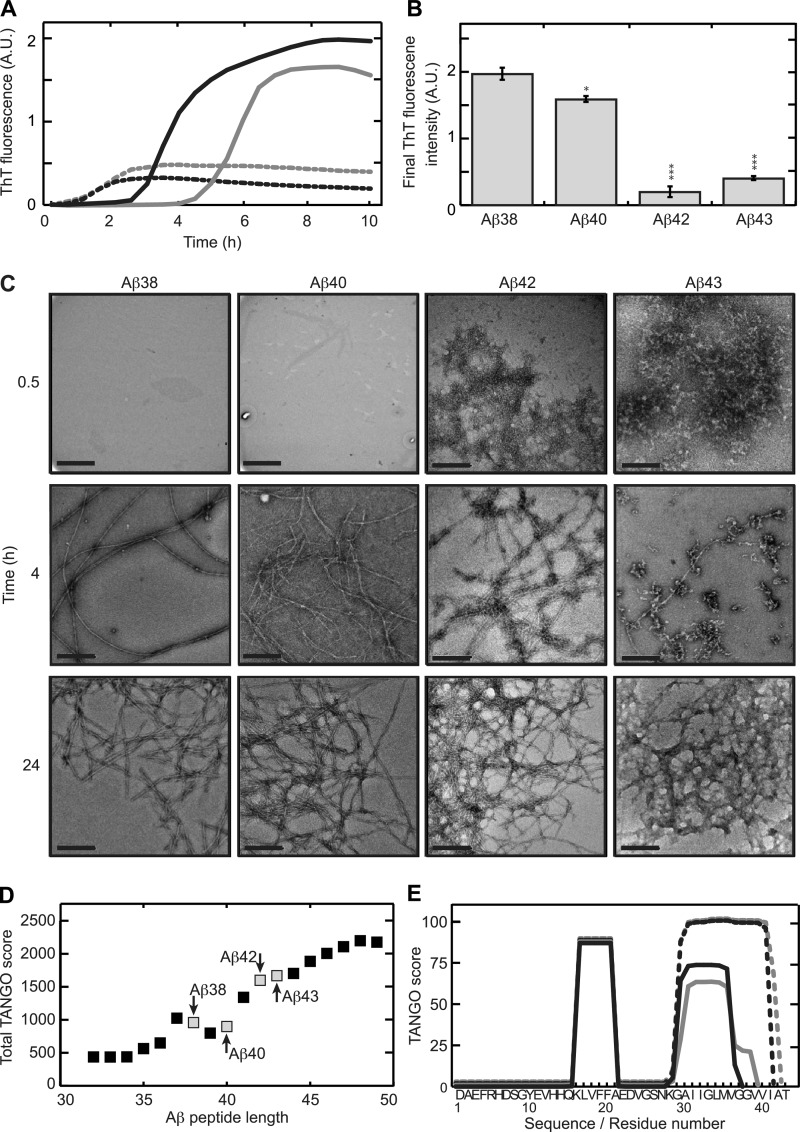
It has been reported before that Aβ1–40 and Aβ1–42 display different aggregation kinetics (13, 34). Consistent with these data, we also observed substantial differences in aggregation kinetics as a function of peptide length (Fig. 1A) when comparing Aβ1–38, Aβ1–40, Aβ1–42, and Aβ1–43 using ThT fluorescence. Although Aβ1–38 and Aβ1–40 showed a delayed onset of aggregation, Aβ1–42 and Aβ1–43 rapidly aggregated, as suggested by the immediate rise in ThT fluorescence signal. Even though the aggregation regimes of Aβ1–38 and Aβ1–40 are generally alike, with a distinct lag phase and significant and sigmoidal development of ThT signal after 10 h of incubation, Aβ1–38 showed a more rapid onset of aggregation compared with Aβ1–40. The final (10 h) ThT fluorescence intensity of both Aβ1–42 and Aβ1–43 aggregates was very low compared with Aβ1–38 and Aβ1–40 (Fig. 1B) and has been reported to correlate with the weight concentration and the morphology of the formed fibrils (35). Transmission electron microscopy showed that 0.5 h of incubation of Aβ1–42 and Aβ1–43 resulted in networks of intertwined fibrils, whereas for Aβ1–38 and Aβ1–40 aggregates were absent (Fig. 1C). Upon incubation for 4 h, the fibrillar network observed for Aβ1–43 had progressed into polymorphous clusters interconnected by mature fibrils. Aβ1–42 showed a similar organization, yet short and aligned fibrils seemed more prevalent compared with Aβ1–43. In contrast, Aβ1–38 and Aβ1–40 both formed long, negatively stained and regularly twisted fibrils with a diameter of 8–12 nm that is typically observed for amyloid-like fibrils (36). All Aβ peptides formed extensive fibrillar networks upon incubation for 24 h. To establish by which mechanism C-terminal variation affected the observed aggregation characteristics of Aβ, the statistical thermodynamics algorithm TANGO was used to predict aggregating stretches in the various Aβ peptides tested. TANGO scores further showed that, in general, increasing aggregation propensity could be observed with increasing peptide length with the exception of Aβ varying in length from 37 to 40 amino acids (Fig. 1D). A per-residue analysis of the aggregation propensity showed that all Aβ sequences contain a common aggregating stretch ranging from residue 16 to residue 22 (37) (Fig. 1E). A second aggregating region starts at residue 28 and spans the remaining C-terminal part of the sequence and showed strong variation with Aβ length due predominantly to the presence of two subsequent glycine residues, which disfavor aggregation but which are compensated by additional aggregation promoting residues in the longer forms. Our analysis shows that differences in aggregation propensity directly stem from C-terminal variation. In line with our observation that Aβ1–38 aggregates faster than Aβ1–40 (Fig. 1A), TANGO predicted a slightly higher aggregation propensity for Aβ1–38 than for Aβ1–40 (Fig. 1, D and E).
FIGURE 1.
C-terminal heterogeneity affects aggregation kinetics of the Aβ peptide. A, ThT fluorescence was recorded in situ every 5 min at 25 °C. Aβ1–38 (continuous black line) and Aβ1–40 (continuous gray line) display a lag phase, whereas Aβ1–42 (dotted black line) and Aβ1–43 (dotted gray line) induce ThT fluorescence almost immediately. The values represent the means of three experiments. A.U., absorbance units. B, final (10 h) ThT fluorescence intensities was derived from panel A. Statistical significance (unpaired 2-tailed t test) compared with the Aβ1–38 value is indicated by *** (p < 0.0001), ** (p < 0.0005), or * (p < 0.005). C, after 0.5 h of incubation Aβ1–42 and Aβ1–43 formed networks, whereas Aβ1–38 and Aβ1–40 do not show visible aggregates. After 4 h of incubation, Aβ1–38 and Aβ1–40 formed 8–12-nm wide, extended, negatively stained fibrils. Aβ1–42 organized into a network of rigid 14–16-nm wide fibrils. For Aβ1–43, a mixture of protofibrils and fibrils was observed. After 24 h all Aβs formed similar fibrillar networks. Scale bar, 200 nm. D, total TANGO scores indicated an increasing overall aggregation propensity of Aβ with increasing peptide length. 37–40 amino acid-long peptides deviated from this trend. Peptides studied in this manuscript are marked. E, sequence-based prediction of aggregation prone stretches by the TANGO algorithm suggests a common aggregating region in the core of the peptide and a second aggregating region at the C terminus. Differences in total TANGO score (D) are exclusively due to the C-terminal aggregating region. Aβ1–38 (continuous black line) and Aβ1–40 (continuous gray line) display similar predicted aggregation propensity, whereas that of Aβ1–42 (dotted black line) and Aβ1–43 (dotted gray line) were higher.
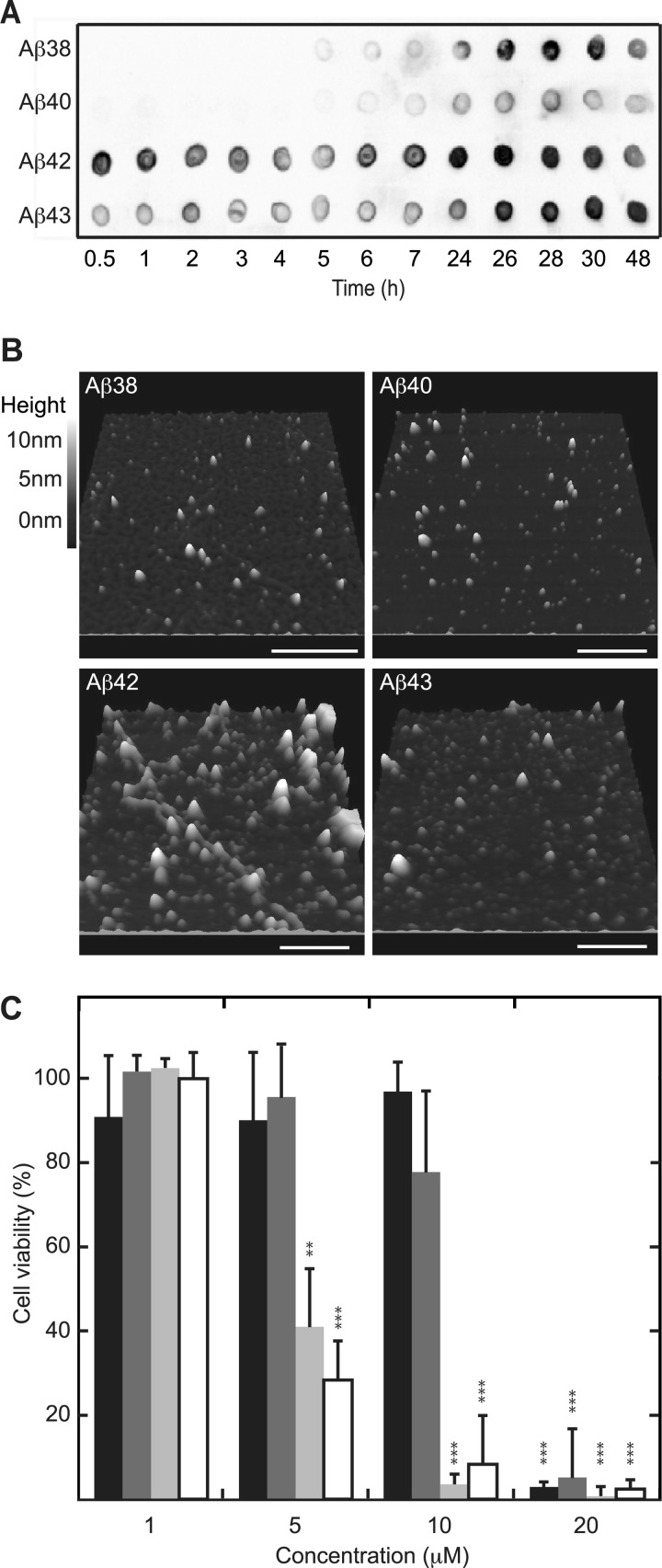
ThT does not interact strongly with oligomeric Aβ (38), whereas soluble oligomeric Aβ is generally considered to represent the toxic species (39–41). We used complementary oligomer-sensitive techniques such as the A11 oligomer-specific antibody (28) and AFM to obtain information on the lifetime of oligomeric Aβ as a function of C-terminal variation. Aβ peptides were allowed to aggregate and were tested for A11 reactivity at various time points of incubation (Fig. 2A). Aβ1–42 and Aβ1–43 formed A11-positive oligomers already after 0.5 h of incubation, whereas Aβ1–38 and Aβ1–40 only interacted with the A11-antibody after an incubation time of 5 and 6 h, respectively, with Aβ1–38 exhibiting substantially stronger staining with the antibody than Aβ1–40. Complementary to A11 reactivity, AFM imaging of samples upon 1.5 h of incubation showed the presence of small oligomeric species for all Aβ peptides tested, including Aβ1–38 and Aβ1–40 (Fig. 2B). Even though oligomers were present for all Aβ peptides tested, Aβ1–38 and Aβ1–40 oligomers only developed into A11-positive oligomers at a later stage compared with Aβ1–42 and Aβ1–43. Cytotoxicity of oligomeric Aβ upon C-terminal variation was assessed using neuroblastoma cell line SH-SY5Y (Fig. 2C). A11-positive oligomers derived from Aβ1–42 and Aβ1–43 induced loss of cell viability at a concentration of 5 μm, whereas those derived of Aβ1–38 and Aβ1–40 affected cell viability only at a significantly higher concentration of 20 μm.
FIGURE 2.
Differences in aggregation kinetics due to C-terminal heterogeneity are reflected at toxic oligomer levels. Aβ at 50 μm was allowed to aggregate at 25 °C under quiescent conditions. A, analysis with the A11 oligomer-specific antibody detected oligomeric Aβ1–42 and Aβ1–43 after as little as 0.5 h of incubation, whereas Aβ1–38 and Aβ1–40 became A11-positive after 5–6 h of incubation. B, imaging using AFM indicated the presence of oligomers for all Aβ samples incubated for 1.5 h. The length of the bar represents 500 nm. C, preincubated (1.5 h) Aβ was added to cultured SH-SY5Y cells and incubated for 24 h before probing cytotoxicity using Cell Titer Blue viability assay. Aβ1–38 (black) and Aβ1–40 (dark gray) only cause cytotoxicity at a concentration of 20 μm, whereas Aβ1–42 (light gray) and Aβ1–43 (white) are significantly cytotoxic at a concentration of 5 μm. Values are expressed as the percent of cell viability ± S.D. (n = 4), and buffer signal was normalized to 100%. Statistical significance (unpaired 2-tailed t test) compared with buffer control values (normalized to 100%) is indicated by p value analysis similar to Fig. 1B.
Aβ Lengths Display Conformational Differences
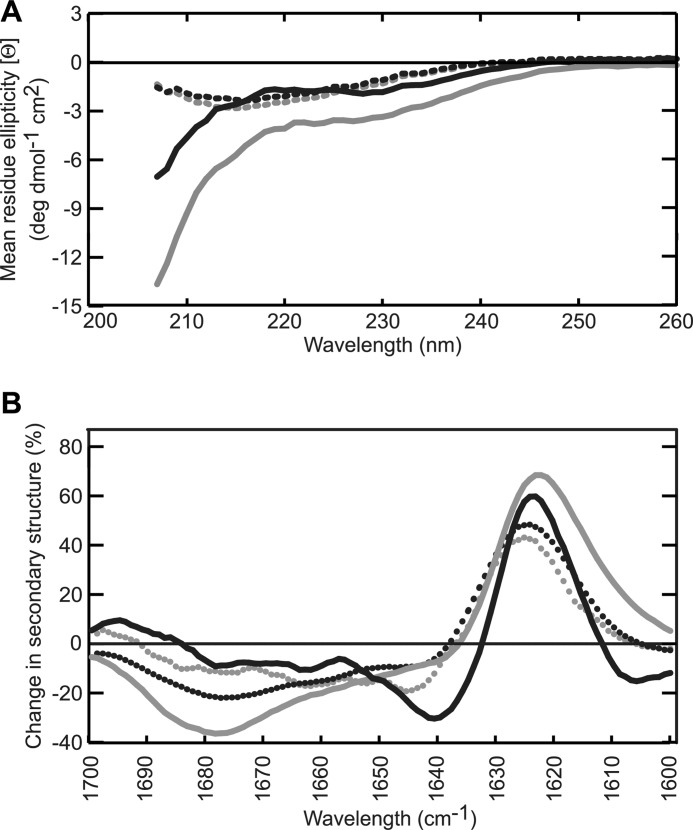
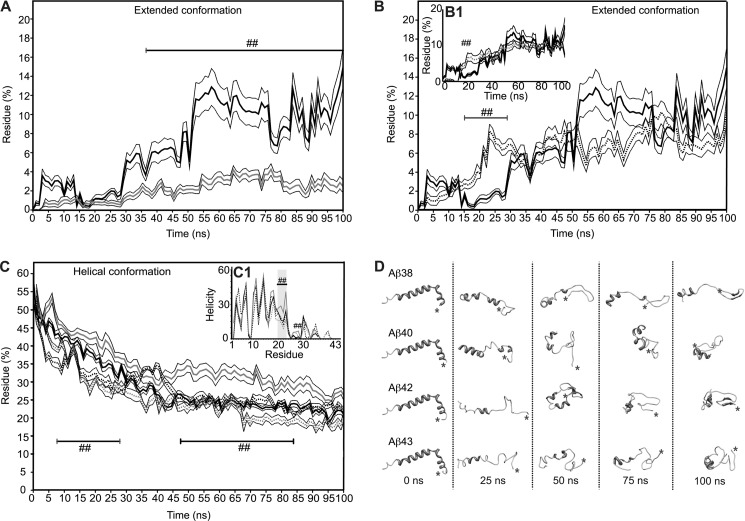
CD and FTIR spectroscopy were used to evaluate Aβ structure after 1.5 h of incubation. The spectra recorded for Aβ1–38 and Aβ1–40 using CD were very similar and displayed typical characteristics of a largely unstructured protein, whereas the spectra of Aβ1–42 and Aβ1–43 showed pronounced β-sheet formation with a minimum intensity at a wavelength of 218 nm (Fig. 3A). As FTIR is more sensitive to β-sheet formation than CD and can distinguish between parallel and anti-parallel β-sheet arrangements, FTIR measurements were performed complementary to CD. Fig. 3B shows difference spectra obtained by subtraction of the spectrum of non-aggregated Aβ (time 0) from the spectrum recorded after 1.5 h of incubation. The strong increase of absorbance at a wavelength of 1627 cm−1, concurrent with a loss of signal between 1650–1655 cm−1 and 1680 cm−1 for all four peptides tested, indicated that β-sheet formation took place during the 1.5 h incubation time at the cost of random coil and β-turn structure. The more narrow peak for Aβ1–38 suggests the formation of a more stable β-sheet as a result of more extensive H-bonding compared with the other peptide lengths investigated, although Aβ1–40 was found to form most β-sheet judging from a higher signal intensity at a wavelength of 1627 cm−1. The small increase at 1695 cm−1 seen here for Aβ1–38 and Aβ1–43 in addition to the increase at a wavelength of 1627 cm−1 reveals the formation of an antiparallel oriented β-sheet that has been typically used as a fingerprint for oligomer formation (42). Variation in evolution of these regions is observed between the Aβ isoforms. This observation suggests that the various Aβ isoforms display small structural differences during aggregation. Even though CD and FTIR provide useful structural information in terms of an average of the entire protein sequence, they do not provide insight into the behavior of individual residues in the sequence and, hence, are not able to address the question of why the addition of two valines in Aβ1–40 rendered this peptide less aggregation-prone than Aβ1–38. Also, CD and FTIR spectroscopy were unable to report on short-time scale conformational flexibility of peptides potentially required to trigger the onset of aggregation. We used MD simulations to establish whether individual residues contributed to changes in peptide conformation that could explain the observed results. We focused our observations on definition of secondary structure of proteins (DSSP) analysis (43). Here, we report the DSSP results as: coil (unstructured conformation), extended conformation (β-bridge plus β-sheet structures), loop (bend plus turns), and helical conformation (α-helix plus 310-helix plus π-helix). All peptides presented a general trend to possess a mixture of a collapsed coil structure and helical conformation for residues 1–20. N-terminal helical structure was partially retained over time, whereas C-terminal helicity, if any, was rapidly lost (supplemental Fig. 1). Also as a general observation, all extended conformations occurred between residues 21–28 and residues 32 to the C-terminal amino acid. A loop section comprising residues 29–31 formed flexible links between these extended portions. Contrary to what was expected, Aβ1–38 conformation behavior most closely resembled that of Aβ1–42 rather than Aβ1–40 (Fig. 4, A and B, supplemental Fig. 1). Over the 100-ns time scale of the simulations, Aβ1–38 showed a marked tendency to form extended conformations (Fig. 4, A and B), comparable with Aβ1–42 and Aβ1–43 (Fig. 4, B and B1). Aβ1–40, on the other hand, exhibited low tendency to form extended conformations (Fig. 4A). Interestingly, during the first 10s of nanoseconds the behavior of Aβ1–38 was erratic and fluctuated between resembling Aβ1–40 and Aβ1–42/Aβ1–43. Only after 50 ns of simulation did the content of extended conformation invariably increase (Fig. 4, A and D). Aβ1–42 and Aβ1–43 seemed to accumulate and stabilize better than their extended conformations, from earlier simulation times onwards (Fig. 4, B and B1, supplemental Fig. 2). The same behavior was reflected in the overall helicity of the peptides (Fig. 4C) revealing a slight, yet statistically significant, higher tendency to retain its helical conformation for Aβ1–38 in comparison to Aβ1–42 and Aβ1–43. A marked increase in the helicity of residues 20–23 and 28 was uniquely observed for Aβ1–40 (Fig. 4, C and D). Collectively, these data showed that the behaviors of Aβ1–42 and Aβ1–43 were remarkably similar in terms of their high tendency to form an extended β-sheet conformation, whereas Aβ1–40 retained helicity longer. The Aβ1–38 peptide showed very interesting behavior in terms of its highly fluctuating tendency to form extended β-sheet conformation. Over time Aβ1–38 conformation alternated rapidly between Aβ1–42/Aβ1–43-like conformation and Aβ1–40-like conformation before forming a stable, extended β-sheet.
FIGURE 3.
Aggregating Aβ peptides show differences on a secondary structural level. A, CD measurements were performed with preincubated (1.5 h) Aβ at 15 μm. Spectra recorded for Aβ1–38 (continuous black line) and Aβ1–40 (continuous gray line) were characteristic of peptides with a large degree of disorder, whereas Aβ1–42 (dotted black line) and Aβ1–43 (dotted gray line) displayed curves with a single minimum at 217 nm, suggesting β-sheet formation. High buffer interference was observed at wavelengths <207 nm. B, FTIR absorbance was measured of monomeric and preincubated (1.5 h) Aβ at 200 μm, and the difference between both spectra was plotted. The difference spectra showed an intensity increase at a wavelength of 1627 cm−1 indicating that all four peptides were converted into a β-sheet conformation. For Aβ1–38 (continuous black line) and Aβ1–43 (dotted gray line) an additional increase in intensity around 1695 cm−1 was observed implying an antiparallel oriented β-sheet. Aβ1–40 (continuous gray line) and Aβ1–42 (dotted black line) data were both characterized by a loss of β-turn as observed by the decrease in intensity at 1680 cm−1.
FIGURE 4.
Aβ peptides show conformational fluctuations at short time scales that vary upon C-terminal elongation. Secondary structure composition was determined by the DSSP method: extended conformation (β-bridge plus β-sheet structures) and helical conformation (α-helix plus 310-helix plus π-helix). Results were averaged over 10 independent simulations. The extended conformation content in function of time for Aβ1–38 (continuous black line) is compared with Aβ1–40 (continuous gray line) (A) and Aβ1–42 (dotted black line) (B); the inset plot (B1), Aβ1–43 (dotted gray line), reveals a similar profile compared with Aβ1–42. Statistically significant differences (S.E.) are denoted by (p < 0.005 (##)). C, the helical content in the function of time is shown for all peptides. Grayscale code is the same as for the previous panels. Regions of the simulation time wherein there are statistically significant differences are denoted by (p < 0.005 (##)) compared with Aβ1–42. The inset (C1) shows helicity per residue for all Aβ peptides (##, p < 0.005, compared with all Aβ peptides). Helicity is reported as the percentage of simulation time that a given amino acid residue presented α-helix conformation. D, shown are snapshots for all Aβ peptides at 0, 25, 50, 75, and 100 ns of simulation time. C termini positions are denoted with an asterisk (*).
Mixtures of Aβ Show Complex Aggregation Behavior
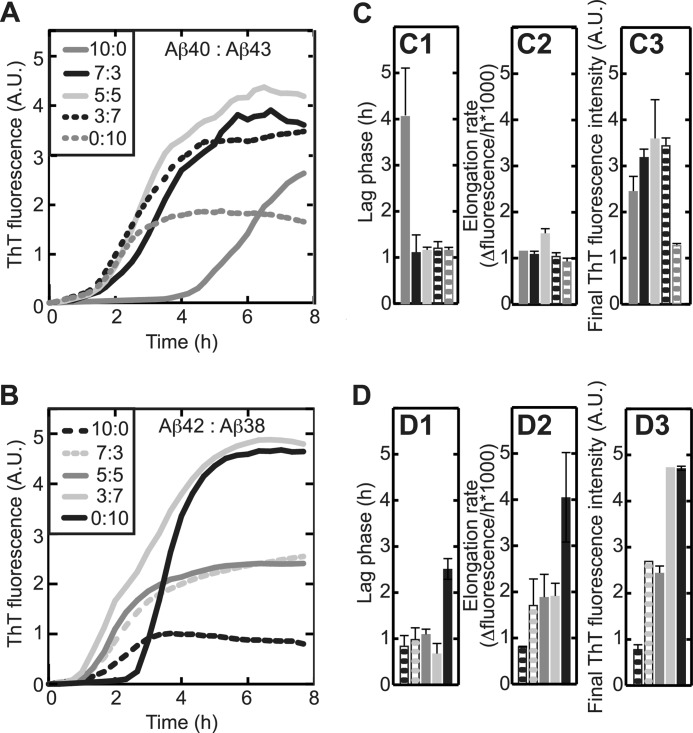
To evaluate the influence of the observed differences between Aβ isoforms in a more biologically relevant setting, we mimicked the complex pool of various Aβ peptide lengths as observed in vivo by preparing Aβ peptide mixtures containing Aβ1–40, Aβ1–42 and Aβ1–38, or Aβ1–43. Increased levels of Aβ1–38 in the cerebrospinal fluid of AD patients are reported (24) as well as an increased generation of this peptide due to presinilin-1 mutations (44). Some forms of familial AD display increased generation of Aβ1–43 (44), a peptide length frequently present in amyloid plaques (18). Aβ1–40 and Aβ1–43 were shown to directly interact using electrospray ionization-MS (supplemental Fig. 4C), although these dimeric species only accumulated at a population of ∼1% (supplemental Fig. 3C). Effective but low accumulation of mixed dimers was also observed upon mixing Aβ1–38 and Aβ1–42 (supplemental Figs. 3B and 4B). Mixed dimeric complex formation was further detected for Aβ1–38·Aβ1–40 and Aβ1–42·Aβ1–43 (supplemental Fig. 3 and 4). Along the lines of the earlier-identified processing pathways of APP toward the formation of either Aβ1–40 and Aβ1–38 with Aβ1–43 and Aβ1–42 as intermediates, respectively (10), we mapped dose-response curves of the presence of Aβ1–43 and Aβ1–38 on the aggregation kinetics of Aβ1–40 and Aβ1–42, respectively, using ThT fluorescence (Fig. 5). We reported earlier that Aβ1–42·Aβ1–40 mixtures behave differently according to their proportional presence (26). Titration of Aβ1–40 with increasing concentrations of Aβ1–43 substantially reduced the lag phase of aggregation to become similar to that of Aβ1–43 alone, whereas final fluorescence intensities were not affected (Fig. 5, A, and C). These observations suggest that Aβ1–43 dominantly influences the nucleation process of Aβ1–40, whereas aggregate morphology or mass were presumably determined by Aβ1–40 (Fig. 5C). Titration of Aβ1–38 with Aβ1–42 similarly led to a decreased nucleation rate but, in addition, gradually increased the elongation rate and final ThT fluorescence intensity (Fig. 5, B and D). The complex aggregation characteristics compared with each of these peptides in isolation are highly suggestive of interaction of the Aβ peptides in mixtures.
FIGURE 5.
Little prevalent Aβ peptides strongly affect the behavior of predominant Aβ. ThT fluorescence was recorded in situ every 5 min at 25 °C with 50 μm Aβ and 12 μm ThT. Values represent the means of two experiments. A and C, irrespective of its concentration, Aβ1–43 (dotted gray line) reduced the lag phase for aggregation (C1) of Aβ1–40 (continuous gray line) without affecting elongation rates (C2). Aβ1–40·Aβ1–43 mixtures displayed higher fluorescence intensity after 8 h incubation (C3) than Aβ1–40 and Aβ1–43 alone. Grayscale code is as in A. B and D, titration of Aβ1–42 (dotted black line) with Aβ1–38 (continuous black line) reduced lag phase (D1) and elongation rate (D2) but increased fluorescence intensity at plateau (D3). Grayscale code is as in Fig. B. A.U., absorbance units.
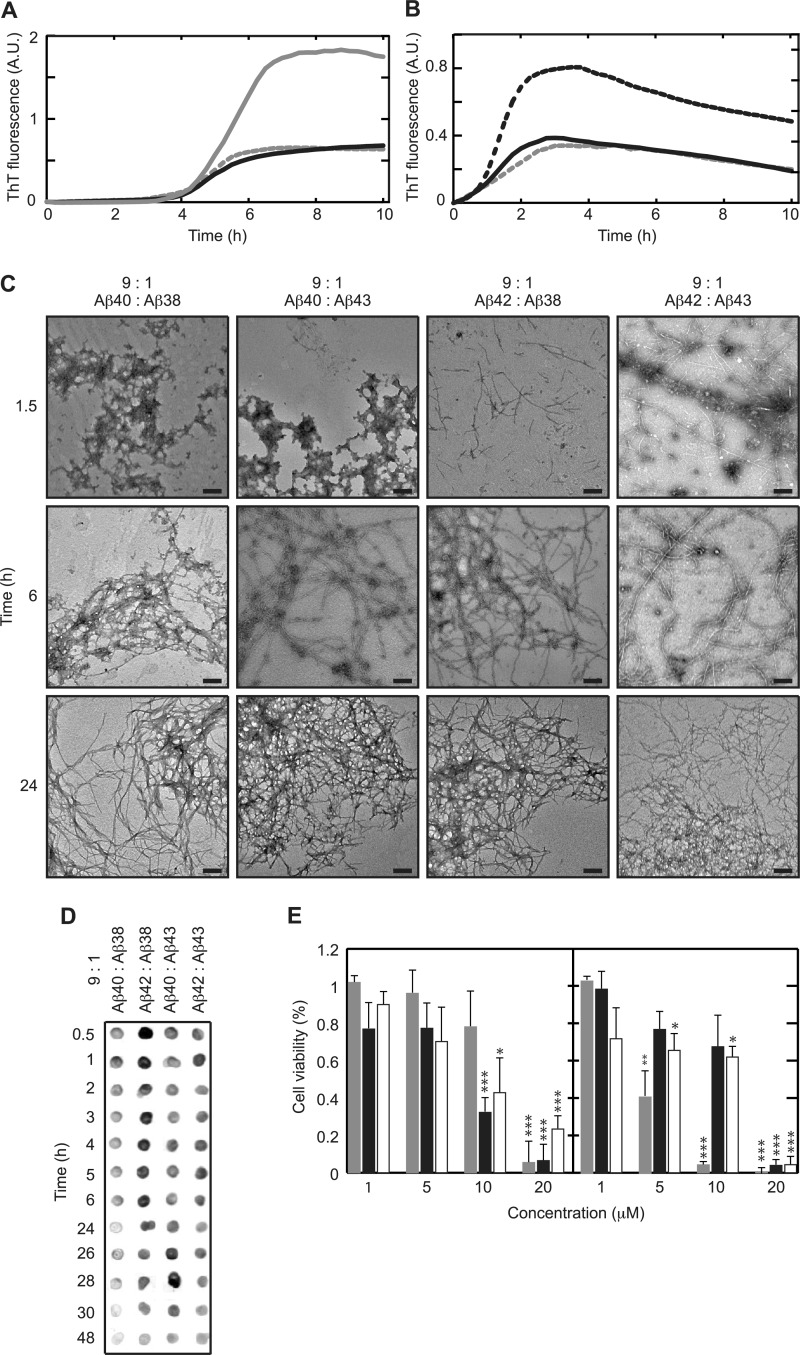
Aβ1–40 is the most predominant species recovered from cerebrospinal fluid (6, 45). Aβ1–38 has been reported to be present in cerebrospinal fluid at concentrations of 1.26 to 2.78 ng/ml, whereas concentrations of Aβ1–42 were 0.46 to 2.07 ng/ml (6, 45, 46). Quantitative detection of Aβ1–43 has only been performed in brain plaques and, as suitable antibodies are not available, can generally not be distinguished from that of Aβ1–42. Although quantitative information on the released amounts of the four Aβ isoforms of interest from APP is only available for FAD mutations based on in vitro observations (11, 44, 47, 48), we performed titration assays (Fig. 5). Results indicated that 30% of Aβ1–38 or Aβ1–43 already caused a significant alteration of the aggregation profile of Aβ1–42 and of the lag phase of Aβ1–40. Many FAD-related mutations accumulate Aβ1–42, whereas it can be assumed that in sporadic AD Aβ1–40 is predominantly produced (49). We, therefore, decided to evaluate the effect of small concentrations (10%) of Aβ1–38 and Aβ1–43 on predominantly present (90%) Aβ1–40 and Aβ1–42 to monitor more subtle influences of the presence of peptides in mixtures. In summary, we evaluated oligomerization, cytotoxicity, and aggregation of 9:1 mixtures of Aβ1–40·Aβ1–38, Aβ1–40·Aβ1–43, Aβ1–42·Aβ1–38, and Aβ1–42·Aβ1–43. At these low concentrations the effect of the addition of Aβ1–38 and Aβ1–43 to either Aβ1–40 or Aβ1–42 was limited to a significant decrease in final fluorescence intensity while leaving the nucleation phase unchanged (Fig. 6, A and B). Visualization of aggregate morphology by transmission electron microscopy further rationalized the observed change in final fluorescence intensity (Figs. 1C and 6C). Even though aggregate formation could not be established at early time points for Aβ1–38 and Aβ1–40 in isolation, a 9:1 mixture of these peptides showed the formation of extensive ThT-negative but A11-positive aggregates that were present for extended periods of time (Fig. 6, A, C, and D). Also, at early time points of incubation the morphologies of both Aβ1–40 and Aβ1–42 in mixtures (Fig. 6C) appear different from these peptides in isolation (compare with Fig. 1C). Upon extended incubation, all mixtures aggregated into morphologically similar networks of long, interacting fibrils, similar to those observed for peptides in isolation (Fig. 6C). The observed differences in A11 interaction and aggregate morphology further led us to investigate the cytotoxic response of Aβ mixtures using cultured SH-SY5Y cells. Interestingly, even though Aβ1–38 or Aβ1–40 in isolation induced no cytotoxic response below a concentration of 20 μm, the addition of Aβ1–38 to Aβ1–40 resulted in a pronounced and significant loss of cell viability at a total peptide concentration of 10 μm, consistent with the A11-positive response for this mixture (Fig. 6, D and E). Strikingly, the addition of Aβ1–38 to Aβ1–42 instead exerted a cytoprotective effect despite showing the formation of A11-positive oligomers, preventing loss of cell viability up to a total Aβ concentration of 10 μm. In addition to this, even though both Aβ1–42 and Aβ1–43 are similarly cytotoxic at a concentration of 10 μm, the mixture of these two peptides alleviates the cytotoxic response, whereas Aβ1–43 induces cytotoxicity in the presence of Aβ1–40.
FIGURE 6.
Aβ peptides in mixtures display complex aggregation behavior and toxicity. A and B, the addition of 10% (=5 μm) Aβ1–38 (continuous black line) or Aβ1–43 (dotted gray line) to 90% (=45 μm) Aβ1–40 (continuous gray line) or Aβ1–42 (dotted black line) decreased the final (10 h) ThT fluorescence compared with Aβ1–40 or Aβ1–42 alone. Values represent the means of three experiments (C). After 1.5 h of incubation, mixtures containing Aβ1–38 formed amorphous aggregates, whereas mixtures containing Aβ1–43 formed short fibrillar structures. Longer incubation for 6 h resulted in fibrillar networks for all mixtures, which extended into dense, highly intertwined, stained networks after 24 h of incubation. The length of the scale bar is 200 nm. D, all Aβ mixtures intensively reacted with A11 oligomer-specific antibody after 0.5 h of incubation, which gradually decreased upon longer incubation dependent on the Aβ mixture. E, Aβ was added to cultured SH-SY5Y cells and incubated for 24 h before probing cytotoxicity using the Cell Titer Blue viability assay. Note that non-toxic Aβ1–40 (left, gray) became highly toxic upon mixing with non-toxic Aβ1–38 (left, black) and Aβ1–43 (left, white) at a concentration of 10 μm. Toxicity of Aβ1–42 (right, gray) is reduced upon the addition of Aβ1–38 (right, black) or Aβ1–43 (right, white). Values are the percent of cell viability ± S.D. (n = 4), and buffer signal was normalized to 100%. Statistical significance (unpaired 2-tailed t test) compared with buffer control is indicated by *** (p < 0.0001), ** (p < 0.0005), and * (p < 0.005). A.U., absorbance units.
Collectively, our data suggest that, apart from distinct propensities to form cytotoxic oligomers and aggregates for individual Aβ peptides, mixtures of various Aβ peptides do not behave in a predictable manner according to a simple additive effect but can actively modulate the behavior of other isoforms present in the mixture to either induce or prevent toxicity or modify their aggregation propensities.
DISCUSSION
Aβ aggregation is a complex process during which a monomeric population progressively self-assembles first into oligomers and finally into mature fibrils. We show that biologically relevant mixtures of Aβ peptides containing Aβ1–38, Aβ1–40, Aβ1–42, and Aβ1–43 behave in a more complex manner than can be anticipated from their behaviors in isolation with direct consequences for their oligomerization, aggregation, and cytotoxic behavior. We also report that co-occurring Aβ peptides can affect each other by conformational modulation of the C-terminal region that, in turn, is a function of C-terminal flexibility to adopt various conformations. For example, Aβ1–38 in isolation exhibited little cytotoxic potential, similar to Aβ1–40. At the same time, cytotoxic oligomers accumulated rapidly for Aβ1–42 and Aβ1–43. Nevertheless, mixtures of Aβ1–38 and Aβ1–40 were highly toxic, whereas the addition of Aβ1–38 to Aβ1–42 surprisingly induced a cytoprotective effect. The A11 reactivity of the individual Aβ peptides in isolation correlated with the observed cytotoxicity, although all isoforms were found to form oligomers. This observation suggests a conformational difference between oligomers of different isoforms. The presence of both toxic A11-positive and non-toxic A11-negative oligomers has been reported recently for Aβ as well as for the yeast-sup35 protein (50, 51). The cytotoxicity-A11 reactivity correlation for peptides in isolation could not be extended to mixtures of Aβ isoforms. This lack of correlation can be explained by the polyclonal nature of the antibody to recognize non-toxic oligomers in addition to toxic species. Another possibility is that non-toxic and toxic oligomers are both formed and that the variation in signal intensities and cytotoxicity arises from the variation in the distribution between these oligomers. At high concentrations when the level of toxic oligomers is sufficiently high, the shorter Aβ isoforms become similar cytotoxic to the longer Aβ isoforms. Although not being able to reveal distinct accumulation of specific conformations using CD, FTIR revealed small structural differences during a 1.5-h incubation time. Even though all tested Aβ peptides showed substantial β-sheet formation over time, only for Aβ1–38 and Aβ1–43 anti-parallel β-sheet formation could be identified that has been interpreted previously as typical for oligomer formation (42). As both FTIR and CD are only informative on an ensemble level, we used MD to elucidate the short-time scale dynamic behavior of the peptides. MD simulations revealed that both Aβ1–38 and Aβ1–42 gained extended β-sheet conformation rapidly, whereas helicity in Aβ1–40 was retained for longer, which is in good agreement with earlier reports (52). In line with our findings, it has previously been reported that stabilization of the central α-helical region of Aβ by ligands or mutations results in a significant delay of aggregation (53–55) and that inhibition of unfolding of the central α-helical region increases longevity in a Drosophila model of AD (54). At the same time, rapid induction of extended β-sheet formation has been found to have a strong predictive power in terms of toxic potential rationalizing the development of so-called β-sheet breakers as a therapeutic approach (for review, see Ref. 56). Interestingly, Aβ1–38 showed behavior that could be explained by rapid gain and loss of extended β-sheet conformation, fluctuating in behavior between Aβ1–40 and Aβ1–42/Aβ1–43, respectively. Rapid conformational switching between distinct conformations has been observed before for synaptically confined proteins SNAP-25 and synaptobrevin and was proposed to characterize a specific class of intrinsically disordered proteins (57). Conformational flexibility was suggested to allow for fast ligand interaction and conformational selection, which potentially has functional implications for the findings we report on Aβ1–38 but which warrant further investigation. The presence of other peptides with higher preference of one over another conformation may drive Aβ1–38 to rapidly recognize these as a potential ligand and template for conformational selection, which in turn either induces or inhibits aggregation. However, this may be an oversimplification of the actual situation as Aβ1–42 in the presence of Aβ1–38 is in fact less toxic, which does not comply with this suggestion. Further research is required to precisely underpin the molecular mechanism of this observation. It has been previously reported that Glu22, Asp23, and Lys28 play a critical role in the aggregation process (58–61). Presumably, Asp23 (and Lys28), although residing in a helical conformation, may not be able to trigger the formation of extended conformations, at least at early time points, thus retarding the aggregation of Aβ1–40. Therefore, not only the unfolding rate of the C-terminus, as has been previously suggested, can dictate the potential event to trigger aggregation and toxicity of Aβ (60), but also the capability of a given Aβ peptide to retain its helical conformation may be considered to induce such events. Based on the outcome of our MD simulations, we suggest that the plastic behavior of Aβ1–38, inducing toxicity for Aβ1–40 while eliminating response to Aβ1–42, plays a key role to these observations. Hence, the presence of other peptides may direct the self-assembly process toward at least two possible pathways, one leading to toxic oligomers and the second leading to non-toxic intermediates. Aβ1–43 does not display fluctuating secondary structures and rapidly forms extended β-sheets. The self-assembly process in the presence of other peptides is, therefore, probably more dependent on the flexibility of the structure of the second peptide. Indeed, it has been shown for the N-terminal domain of the HypF protein from Escherichia coli that both toxic and non-toxic oligomers can be formed (62).
Overall, our results can be of major importance for the further development of therapeutic strategies. The current approach of modulating γ-secretase activity to decrease Aβ1–42 generation results in increased Aβ1–38 levels at the same time. This approach is based on the observation that longer Aβ isoforms are more aggregation-prone. Hence, establishing an increased Aβ1–38 level is considered a suitable and non-toxic approach to inhibit AD disease progress without compromising the important multi-substrate processing by γ-secretase. However, clinical studies still have to confirm their disease-modulating capacity. Whether this is due to the low brain-barrier penetrating potency of the compounds being tested requires further investigation. Together with previously published data (44), our results possibly indicate that another explanation for the lack of clinical evidence to place GSMs firmly on the map as AD therapy may be the induction of adverse, unexpected events as a result of the increased Aβ1–38 levels in combination with other Aβ peptides. In this view, Golde et al. (63) recently argued that the efficiency of different GSMs to shift Aβ release toward shorter isoforms could determine their therapeutic potential. We propose that peptide conformational flexibility may confer toxic properties to its oligomers and underline the importance of understanding the interplay between various Aβ isoforms.
Acknowledgments
We thank Maja Debulpaep for instructions and technical assistance with transmission electron microscopy and Kim Sweers and Ine Segers-Nolten for support during AFM measurements.
This work was supported by the Agency for Innovation by Science and Technology in Flanders (IWT), a project grant from the “Stichting voor Alzheimer Onderzoek” (SAO), an Odysseus grant from the National Funds for Scientific Research Flanders (FWO), an Alzheimer Research UK (ARUK) grant, and a European Molecular Biology Organization Short-term Fellowship.

This article contains supplemental Experimental Procedures and Figs. 1–4.
- Aβ
- amyloid-β peptide
- AD
- Alzheimer disease
- APP
- amyloid precursor protein
- GSM
- γ-secretase modulator
- ThT
- thioflavin T
- AFM
- atomic force microscopy
- MD
- molecular dynamics
- DSSP
- definition of secondary structure of proteins.
REFERENCES
- 1. Glenner G. G., Wong C. W. (1984) Alzheimer disease. Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 120, 885–890 [DOI] [PubMed] [Google Scholar]
- 2. Haass C., Schlossmacher M. G., Hung A. Y., Vigo-Pelfrey C., Mellon A., Ostaszewski B. L., Lieberburg I., Koo E. H., Schenk D., Teplow D. B. (1992) Amyloid β-peptide is produced by cultured cells during normal metabolism. Nature 359, 322–325 [DOI] [PubMed] [Google Scholar]
- 3. De Strooper B., Saftig P., Craessaerts K., Vanderstichele H., Guhde G., Annaert W., Von Figura K., Van Leuven F. (1998) Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature 391, 387–390 [DOI] [PubMed] [Google Scholar]
- 4. Yan R., Bienkowski M. J., Shuck M. E., Miao H., Tory M. C., Pauley A. M., Brashier J. R., Stratman N. C., Mathews W. R., Buhl A. E., Carter D. B., Tomasselli A. G., Parodi L. A., Heinrikson R. L., Gurney M. E. (1999) Membrane-anchored aspartyl protease with Alzheimer disease β-secretase activity. Nature 402, 533–537 [DOI] [PubMed] [Google Scholar]
- 5. Vigo-Pelfrey C., Lee D., Keim P., Lieberburg I., Schenk D. B. (1993) Characterization of β-amyloid peptide from human cerebrospinal fluid. J. Neurochem. 61, 1965–1968 [DOI] [PubMed] [Google Scholar]
- 6. Wiltfang J., Esselmann H., Bibl M., Smirnov A., Otto M., Paul S., Schmidt B., Klafki H. W., Maler M., Dyrks T., Bienert M., Beyermann M., Rüther E., Kornhuber J. (2002) Highly conserved and disease-specific patterns of carboxyl-terminal-truncated Aβ peptides 1–37/38/39 in addition to 1–40/42 in Alzheimer disease and in patients with chronic neuroinflammation. J. Neurochem. 81, 481–496 [DOI] [PubMed] [Google Scholar]
- 7. Gu Y., Misonou H., Sato T., Dohmae N., Takio K., Ihara Y. (2001) Distinct intramembrane cleavage of the β-amyloid precursor protein family resembling γ-secretase-like cleavage of Notch. J. Biol. Chem. 276, 35235–35238 [DOI] [PubMed] [Google Scholar]
- 8. Sato T., Dohmae N., Qi Y., Kakuda N., Misonou H., Mitsumori R., Maruyama H., Koo E. H., Haass C., Takio K., Morishima-Kawashima M., Ishiura S., Ihara Y. (2003) Potential link between amyloid β-protein 42 and C-terminal fragment γ 49–99 of β-amyloid precursor protein. J. Biol. Chem. 278, 24294–24301 [DOI] [PubMed] [Google Scholar]
- 9. Qi-Takahara Y., Morishima-Kawashima M., Tanimura Y., Dolios G., Hirotani N., Horikoshi Y., Kametani F., Maeda M., Saido T. C., Wang R., Ihara Y. (2005) Longer forms of amyloid β protein. Implications for the mechanism of intramembrane cleavage by γ-secretase. J. Neurosci. 25, 436–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Takami M., Nagashima Y., Sano Y., Ishihara S., Morishima-Kawashima M., Funamoto S., Ihara Y. (2009) γ-Secretase. Successive tripeptide and tetrapeptide release from the transmembrane domain of β-carboxyl terminal fragment. J. Neurosci. 29, 13042–13052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bentahir M., Nyabi O., Verhamme J., Tolia A., Horré K., Wiltfang J., Esselmann H., De Strooper B. (2006) Presenilin clinical mutations can affect γ-secretase activity by different mechanisms. J. Neurochem. 96, 732–742 [DOI] [PubMed] [Google Scholar]
- 12. Saito T., Suemoto T., Brouwers N., Sleegers K., Funamoto S., Mihira N., Matsuba Y., Yamada K., Nilsson P., Takano J., Nishimura M., Iwata N., Van Broeckhoven C., Ihara Y., Saido T. C. (2011) Potent amyloidogenicity and pathogenicity of Aβ43. Nat. Neurosci. 14, 1023–1032 [DOI] [PubMed] [Google Scholar]
- 13. Jarrett J. T., Lansbury P. T. (1993) Seeding “one-dimensional crystallization” of amyloid. A pathogenic mechanism in Alzheimer disease and scrapie? Cell 73, 1055–1058 [DOI] [PubMed] [Google Scholar]
- 14. Bitan G., Kirkitadze M. D., Lomakin A., Vollers S. S., Benedek G. B., Teplow D. B. (2003) Amyloid β-protein (Aβ) assembly. Aβ40 and Aβ42 oligomerize through distinct pathways. Proc. Natl. Acad. Sci. U.S.A. 100, 330–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Benseny-Cases N., Cócera M., Cladera J. (2007) Conversion of non-fibrillar β-sheet oligomers into amyloid fibrils in Alzheimer disease amyloid peptide aggregation. Biochem. Biophys. Res. Commun. 361, 916–921 [DOI] [PubMed] [Google Scholar]
- 16. Gravina S. A., Ho L., Eckman C. B., Long K. E., Otvos L., Jr., Younkin L. H., Suzuki N., Younkin S. G. (1995) Amyloid β protein (Aβ) in Alzheimer disease brain. Biochemical and immunocytochemical analysis with antibodies specific for forms ending at Aβ 40 or Aβ42(43). J. Biol. Chem. 270, 7013–7016 [DOI] [PubMed] [Google Scholar]
- 17. Iwatsubo T., Saido T. C., Mann D. M., Lee V. M., Trojanowski J. Q. (1996) Full-length amyloid-β(1–42 (43)) and amino-terminally modified and truncated amyloid-β42 (43) deposit in diffuse plaques. Am. J. Pathol. 149, 1823–1830 [PMC free article] [PubMed] [Google Scholar]
- 18. Welander H., Frånberg J., Graff C., Sundström E., Winblad B., Tjernberg L. O. (2009) Aβ43 is more frequent than Aβ40 in amyloid plaque cores from Alzheimer disease brains. J. Neurochem. 110, 697–706 [DOI] [PubMed] [Google Scholar]
- 19. Weggen S., Eriksen J. L., Das P., Sagi S. A., Wang R., Pietrzik C. U., Findlay K. A., Smith T. E., Murphy M. P., Bulter T., Kang D. E., Marquez-Sterling N., Golde T. E., Koo E. H. (2001) A subset of NSAIDs lower amyloidogenic Aβ42 independently of cyclooxygenase activity. Nature 414, 212–216 [DOI] [PubMed] [Google Scholar]
- 20. Eriksen J. L., Sagi S. A., Smith T. E., Weggen S., Das P., McLendon D. C., Ozols V. V., Jessing K. W., Zavitz K. H., Koo E. H., Golde T. E. (2003) NSAIDs and enantiomers of flurbiprofen target γ-secretase and lower Aβ42 in vivo. J. Clin. Invest. 112, 440–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Laino C. (2009) In follow-up analysis of clinical trial, NSAIDs seem to preserve cognitive function in patients with healthy brains. Neurol. Today 9, 21–22 [Google Scholar]
- 22. Oehlrich D., Berthelot D. J., Gijsen H. J. (2011) γ-Secretase modulators as potential disease modifying anti-Alzheimer drugs. J. Med. Chem. 54, 669–698 [DOI] [PubMed] [Google Scholar]
- 23. Imbimbo B. P., Giardina G. A. (2011) γ-Secretase inhibitors and modulators for the treatment of Alzheimer disease. Disappointments and hopes. Curr. Top. Med. Chem. 11, 1555–1570 [DOI] [PubMed] [Google Scholar]
- 24. Lewczuk P., Esselmann H., Meyer M., Wollscheid V., Neumann M., Otto M., Maler J. M., Rüther E., Kornhuber J., Wiltfang J. (2003) The amyloid-β (Aβ) peptide pattern in cerebrospinal fluid in Alzheimer disease. Evidence of a novel carboxyl-terminal-elongated Aβ peptide. Rapid Commun. Mass Spectrom. 17, 1291–1296 [DOI] [PubMed] [Google Scholar]
- 25. Maddalena A. S., Papassotiropoulos A., Gonzalez-Agosti C., Signorell A., Hegi T., Pasch T., Nitsch R. M., Hock C. (2004) Cerebrospinal fluid profile of amyloid β peptides in patients with Alzheimer disease determined by protein biochip technology. Neurodegener. Dis. 1, 231–235 [DOI] [PubMed] [Google Scholar]
- 26. Kuperstein I., Broersen K., Benilova I., Rozenski J., Jonckheere W., Debulpaep M., Vandersteen A., Segers-Nolten I., Van Der Werf K., Subramaniam V., Braeken D., Callewaert G., Bartic C., D'Hooge R., Martins I. C., Rousseau F., Schymkowitz J., De Strooper B. (2010) Neurotoxicity of Alzheimer disease Aβ peptides is induced by small changes in the Aβ42 to Aβ40 ratio. EMBO J. 29, 3408–3420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Broersen K., Jonckheere W., Rozenski J., Vandersteen A., Pauwels K., Pastore A., Rousseau F., Schymkowitz J. (2011) A standardized and biocompatible preparation of aggregate-free amyloid β peptide for biophysical and biological studies of Alzheimer disease. Protein Eng. Des. Sel. 24, 743–750 [DOI] [PubMed] [Google Scholar]
- 28. Kayed R., Head E., Thompson J. L., McIntire T. M., Milton S. C., Cotman C. W., Glabe C. G. (2003) Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300, 486–489 [DOI] [PubMed] [Google Scholar]
- 29. Fernandez-Escamilla A. M., Rousseau F., Schymkowitz J., Serrano L. (2004) Prediction of sequence-dependent and mutational effects on the aggregation of peptides and proteins. Nat. Biotechnol. 22, 1302–1306 [DOI] [PubMed] [Google Scholar]
- 30. Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. (1987) The precursor of Alzheimer disease amyloid A4 protein resembles a cell-surface receptor. Nature 325, 733–736 [DOI] [PubMed] [Google Scholar]
- 31. Jorgensen W. L., Tirado-Rives J. (1988) The OPLS potential functions for proteins, energy minimizations for crystals of cyclic peptides and crambin. J. Am. Chem. Soc. 110, 1657–1666 [DOI] [PubMed] [Google Scholar]
- 32. Hess B., Bekker H., Berendsen H. J. C., Fraaije J. G. E. M. (1997) LINCS. A linear constraint solver for molecular simulations. J. Comput. Chem. 18, 1463–1472 [Google Scholar]
- 33. Pettersen E. F., Goddard T. D., Huang C. C., Couch G. S., Greenblatt D. M., Meng E. C., Ferrin T. E. (2004) UCSF Chimera. A visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 [DOI] [PubMed] [Google Scholar]
- 34. Harper J. D., Lansbury P. T. (1997) Models of amyloid seeding in Alzheimer disease and scrapie. Mechanistic truths and physiological consequences of the time-dependent solubility of amyloid proteins. Annu. Rev. Biochem. 66, 385–407 [DOI] [PubMed] [Google Scholar]
- 35. Biancalana M., Koide S. (2010) Molecular mechanism of thioflavin-T binding to amyloid fibrils. Biochim. Biophys. Acta 1804, 1405–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stine W. B., Jr., Snyder S. W., Ladror U. S., Wade W. S., Miller M. F., Perun T. J., Holzman T. F., Krafft G. A. (1996) The nanometer-scale structure of amyloid-β visualized by atomic force microscopy. J. Protein Chem. 15, 193–203 [DOI] [PubMed] [Google Scholar]
- 37. Röhrig U. F., Laio A., Tantalo N., Parrinello M., Petronzio R. (2006) Stability and structure of oligomers of the Alzheimer β peptide Aβ16–22. From the dimer to the 32-mer. Biophys. J. 91, 3217–3229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. LeVine H., 3rd (1993) Thioflavine T interaction with synthetic Alzheimer disease β-amyloid peptides. Detection of amyloid aggregation in solution. Protein Sci. 2, 404–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lambert M. P., Barlow A. K., Chromy B. A., Edwards C., Freed R., Liosatos M., Morgan T. E., Rozovsky I., Trommer B., Viola K. L., Wals P., Zhang C., Finch C. E., Krafft G. A., Klein W. L. (1998) Diffusible, nonfibrillar ligands derived from Aβ1–42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. U.S.A. 95, 6448–6453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lesné S., Koh M. T., Kotilinek L., Kayed R., Glabe C. G., Yang A., Gallagher M., Ashe K. H. (2006) A specific amyloid-β protein assembly in the brain impairs memory. Nature 440, 352–357 [DOI] [PubMed] [Google Scholar]
- 41. Ahmed M., Davis J., Aucoin D., Sato T., Ahuja S., Aimoto S., Elliott J. I., Van Nostrand W. E., Smith S. O. (2010) Structural conversion of neurotoxic amyloid-β1–42 oligomers to fibrils. Nat. Struct. Mol. Biol. 17, 561–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cerf E., Sarroukh R., Tamamizu-Kato S., Breydo L., Derclaye S., Dufrêne Y. F., Narayanaswami V., Goormaghtigh E., Ruysschaert J. M., Raussens V. (2009) Antiparallel β-sheet. A signature structure of the oligomeric amyloid β-peptide. Biochem. J. 421, 415–423 [DOI] [PubMed] [Google Scholar]
- 43. Kabsch W., Sander C. (1983) Dictionary of protein secondary structure. Pattern recognition of hydrogen-bonded and geometrical features. Biopolymers 22, 2577–2637 [DOI] [PubMed] [Google Scholar]
- 44. Chávez-Gutiérrez L., Bammens L., Benilova I., Vandersteen A., Benurwar M., Borgers M., Lismont S., Zhou L., Van Cleynenbreugel S., Esselmann H., Wiltfang J., Serneels L., Karran E., Gijsen H., Schymkowitz J., Rousseau F., Broersen K., De Strooper B. (2012) The mechanism of γ-secretase dysfunction in familial Alzheimer disease. EMBO J. 31, 2261–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bibl M., Mollenhauer B., Wolf S., Esselmann H., Lewczuk P., Kornhuber J., Wiltfang J. (2007) Reduced CSF carboxyl-terminal-truncated Aβ peptides in frontotemporal lobe degenerations. J. Neural. Transm. 114, 621–628 [DOI] [PubMed] [Google Scholar]
- 46. Mann D. M., Iwatsubo T., Ihara Y., Cairns N. J., Lantos P. L., Bogdanovic N., Lannfelt L., Winblad B., Maat-Schieman M. L., Rossor M. N. (1996) Predominant deposition of amyloid-β42(43) in plaques in cases of Alzheimer disease and hereditary cerebral hemorrhage associated with mutations in the amyloid precursor protein gene. Am. J. Pathol. 148, 1257–1266 [PMC free article] [PubMed] [Google Scholar]
- 47. Scheuner D., Eckman C., Jensen M., Song X., Citron M., Suzuki N., Bird T. D., Hardy J., Hutton M., Kukull W., Larson E., Levy-Lahad E., Viitanen M., Peskind E., Poorkaj P., Schellenberg G., Tanzi R., Wasco W., Lannfelt L., Selkoe D., Younkin S. (1996) Secreted amyloid β-protein similar to that in the senile plaques of Alzheimer disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer disease. Nature Med. 2, 864–870 [DOI] [PubMed] [Google Scholar]
- 48. Citron M., Westaway D., Xia W., Carlson G., Diehl T., Levesque G., Johnson-Wood K., Lee M., Seubert P., Davis A., Kholodenko D., Motter R., Sherrington R., Perry B., Yao H., Strome R., Lieberburg I., Rommens J., Kim S., Schenk D., Fraser P., St George Hyslop P., Selkoe D. J. (1997) Mutant presenilins of Alzheimer disease increase production of 42-residue amyloid β-protein in both transfected and transgenic mice. Nature Med. 3, 67–72 [DOI] [PubMed] [Google Scholar]
- 49. Younkin S. G. (1998) The role of Aβ42 in Alzheimer disease. J. Physiol. Paris 92, 289–292 [DOI] [PubMed] [Google Scholar]
- 50. Krishnan R., Goodman J. L., Mukhopadhyay S., Pacheco C. D., Lemke E. A., Deniz A. A., Lindquist S. (2012) Conserved features of intermediates in amyloid assembly determine their benign or toxic states. Proc. Natl. Acad. Sci. U.S.A. 109, 11172–11177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ladiwala A. R., Litt J., Kane R. S., Aucoin D. S., Smith S. O., Ranjan S., Davis J., Van Nostrand W. E., Tessier P. M. (2012) Conformational differences between two amyloid β oligomers of similar size and dissimilar toxicity. J. Biol. Chem. 287, 24765–24773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shen L., Ji H. F., Zhang H. Y. (2008) Why is the C-terminus of Aβ(1–42) more unfolded than that of Aβ(1–40)? Clues from hydrophobic interaction. J. Phys. Chem. B. 112, 3164–3167 [DOI] [PubMed] [Google Scholar]
- 53. Päiviö A., Nordling E., Kallberg Y., Thyberg J., Johansson J. (2004) Stabilization of discordant helices in amyloid fibril-forming proteins. Protein Sci. 13, 1251–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nerelius C., Sandegren A., Sargsyan H., Raunak R., Leijonmarck H., Chatterjee U., Fisahn A., Imarisio S., Lomas D. A., Crowther D. C., Strömberg R., Johansson J. (2009) α-Helix targeting reduces amyloid-β peptide toxicity. Proc. Natl. Acad. Sci. U.S.A. 106, 9191–9196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ito M., Johansson J., Strömberg R., Nilsson L. (2011) Unfolding of the amyloid β-peptide central helix. Mechanistic insights from molecular dynamics simulations. PLoS ONE 6, e17587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wisniewski T., Sadowski M. (2008) BMC Neurosci. 9, S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Choi U. B., McCann J. J., Weninger K. R., Bowen M. E. (2011) Beyond the random coil. Stochastic conformational switching in intrinsically disordered proteins. Structure 19, 566–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Borreguero J. M., Urbanc B., Lazo N. D., Buldyrev S. V., Teplow D. B., Stanley H. E. (2005) Folding events in the 21–30 region of amyloid β-protein (Aβ) studied in silico. Proc. Natl. Acad. Sci. U.S.A. 102, 6015–6020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lazo N. D., Grant M. A., Condron M. C., Rigby A. C., Teplow D. B. (2005) On the nucleation of amyloid β-protein monomer folding. Protein Sci. 14, 1581–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Baumketner A., Bernstein S. L., Wyttenbach T., Lazo N. D., Teplow D. B., Bowers M. T., Shea J. E. (2006) Structure of the 21–30 fragment of amyloid β-protein. Protein Sci. 15, 1239–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Masman M. F., Eisel U. L., Csizmadia I. G., Penke B., Enriz R. D., Marrink S. J., Luiten P. G. (2009) In silico study of full-length amyloid β 1–42 tri- and penta-oligomers in solution. J. Phys. Chem. B. 113, 11710–11719 [DOI] [PubMed] [Google Scholar]
- 62. Campioni S., Mannini B., Zampagni M., Pensalfini A., Parrini C., Evangelisti E., Relini A., Stefani M., Dobson C. M., Cecchi C., Chiti F. (2010) A causative link between the structure of aberrant protein oligomers and their toxicity. Nat. Chem. Biol. 6, 140–147 [DOI] [PubMed] [Google Scholar]
- 63. Golde T. E., Ran Y., Felsenstein K. M. (2012) Shifting a complex debate on γ-secretase cleavage and Alzheimer disease. EMBO J. 31, 2237–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]