Background: GoLoco (GL) motif binds to Gα and inhibits its guanine nucleotide dissociation.
Results: Crystal structures of LGN-GL3(4)·Gαi1(3) complexes reveal a double Arg finger-mediated GDP recognition mechanism, which is distinct from that shown in the RGS14·Gαi1 complex.
Conclusion: LGN-GL/Gαi interaction might represent a general binding mode between GoLoco motifs and Gαi.
Significance: Our findings shed new light on the GoLoco motif-mediated G protein signaling regulation.
Keywords: Cell Division, Crystal Structure, G Proteins, Protein Complexes, Protein-Protein Interactions, GDI, GoLoco, LGN
Abstract
GoLoco (GL) motif-containing proteins regulate G protein signaling by binding to Gα subunit and acting as guanine nucleotide dissociation inhibitors. GLs of LGN are also known to bind the GDP form of Gαi/o during asymmetric cell division. Here, we show that the C-terminal GL domain of LGN binds four molecules of Gαi·GDP. The crystal structures of Gαi·GDP in complex with LGN GL3 and GL4, respectively, reveal distinct GL/Gαi interaction features when compared with the only high resolution structure known with GL/Gαi interaction between RGS14 and Gαi1. Only a few residues C-terminal to the conserved GL sequence are required for LGN GLs to bind to Gαi·GDP. A highly conserved “double Arg finger” sequence (RΨ(D/E)(D/E)QR) is responsible for LGN GL to bind to GDP bound to Gαi. Together with the sequence alignment, we suggest that the LGN GL/Gαi interaction represents a general binding mode between GL motifs and Gαi. We also show that LGN GLs are potent guanine nucleotide dissociation inhibitors.
Introduction
The α subunit of the heterotrimeric G proteins (Gα) is a critical component of the G protein signaling pathway, in which Gα cycles between the GDP- and GTP-bound states (1). In the canonical signaling model, ligand-mediated activation of G protein-coupled receptors (GPCRs)4 catalyzes the exchange of GDP for GTP in binding to Gα and subsequently results in the dissociation of Gα·GTP from Gβγ heterodimer (2, 3). The dissociated Gα·GTP binds to and activates downstream effectors, thus transducing signals from GPCR (4–6). Because Gα has intrinsic GTPase activity, the Gα subunit subsequently returns to the Gα·GDP form, which marks the termination of the GPCR signaling. Many proteins have been discovered as regulators of the GTP- and GDP-bound forms of the Gα reaction cycle. Among these, GoLoco motif proteins were discovered to bind specifically to GDP-loaded Gαi or Gαo and inhibit the spontaneous release of GDP from Gα. These GoLoco proteins are referred to as guanine nucleotide dissociation inhibitors (GDIs) (7–11).
The GoLoco motif (8, 12, 13) was first identified as a conserved sequence of 19 amino acids, occurring singly or as tandem repeats in a variety of signaling proteins across the animal kingdom (7). Our understanding of the molecular mechanism of the GDI function of GoLoco proteins is mainly based on the crystal structure of RGS14 GoLoco bound to Gαi1·GDP (14), which shows that the conserved GoLoco motif and its variable C-terminal tail interact with the Ras-like and all-helical domains of Gαi1, respectively. A so-called “arginine finger” formed by the highly conserved (D/E)QR triad in the conserved GoLoco motif extends into the GDP-binding pocket and directly contacts the α- and β-phosphates of GDP (14). This structure and the subsequent mutagenesis and structural studies (14–18) suggested an appealing hypothesis: the highly variable C-terminal sequences following the conserved GoLoco motifs and the all-helical domain of Gα subunits are likely the specificity determinants of interactions between GoLoco motifs and different Gα subunits. However, because there no structures of GoLoco motifs in complex with Gα other than the Gαi1·RGS14 complex are available to date, the above hypothesis remains untested.
LGN is a multidomain scaffolding protein containing eight tetratricopeptide (TPR) repeats in its N-terminal region, a flexible linker sequence in the middle, and four GoLoco motifs in the C-terminal end (19, 20). LGN is an evolutionarily conserved protein (Pins in Drosophila, and GPR1/2 in Caenorhabditis elegans) that plays crucial roles in regulating spindle orientations during asymmetric cell division (19, 21) and can be considered as an example member of the multiple GoLoco motif protein family. It forms a ternary protein complex with nuclear mitotic apparatus protein NuMA (Mud in Drosophila and Lin5 in C. elegans) and cortical membrane-bound Gαi/o via its TPR repeats and GoLoco motifs, respectively (22–28). The central linker of LGN binds to the guanylate kinase domain of the DLG family scaffold protein in a phosphorylation-dependent manner (29–31). In Drosophila neuroblast, loss of Pins or Gαi affects cell polarity as well as mitotic spindle orientation (32). In mammals, overexpression or removal of LGN results in dramatic spindle rocking in metaphase and improper spindle pole organization (19, 21, 33). The binding of Gαi through the GoLoco motifs was shown to regulate the cortical localization of LGN (33). Thus, the LGN GoLoco motifs can be viewed as scaffolding modules in tethering the TPR repeat partners (e.g. NuMA/Mud and mInsc/Insc) of LGN to the cell cortex via binding to membrane-attached Gαi. Interestingly, the GoLoco motifs of LGN can directly bind to TPR repeats intramolecularly, thus keeping LGN in an autoinhibited conformation (22). Gαi·GDP binding to GoLoco motifs releases the autoinhibited conformation of LGN and renders LGN TPR repeats capable of binding to NuMA (22, 34), although the mechanistic basis of the LGN autoinhibition is unknown.
In this study, we performed detailed biochemical and structural analyses of the interactions between LGN GoLoco motifs and Gαi·GDP. We demonstrate that in contrast to the RGS14/Gαi·GDP interaction, only a few residues of the highly variable sequences C-terminal to the conserved GoLoco motifs of LGN are involved in binding to Gαi·GDP. The structures of two LGN GoLoco motifs in complex with Gαi reveal a double Arg finger sequence (RΨ(D/E)(D/E)QR) within the GoLoco motif that is specifically involved in the GDP coordination. We further show that the LGN GoLoco·Gαi·GDP interaction observed in this study likely represents a general mode of GoLoco motif-mediated Gα binding. We further demonstrate that the LGN GoLoco motifs are potent GDIs. Thus, the LGN GoLoco motifs can function as a Gα/LGN/NuMA/Insc scaffold as well as a regulator of Gα signaling in asymmetric cell division.
EXPERIMENTAL PROCEDURES
Protein Expression and Purification
The human Gαi3, Gαi1, mouse LGN GL fragments were individually cloned into a modified version of pET32a vector. All the mutations were created using the standard PCR-based method and confirmed by DNA sequencing. Recombinant proteins were expressed in Escherichia coli BL21 (DE3) host cells at 16 or 37 °C and were purified by using a Ni2+-nitrilotriacetic acid-agarose affinity chromatography followed by size exclusion chromatography. For in vitro biochemical analysis, LGN GLs were expressed as the GST-fused proteins and purified by GSH-Sepharose affinity chromatography.
Isothermal Titration Calorimetry Measurements
ITC measurements were performed on an ITC200 Micro calorimeter (MicroCal) at 25 °C. All protein samples were dissolved in the buffer containing 50 mm Tris, pH 8.0, 100 mm NaCl, and 1 mm EDTA. The titrations were carried out by injecting 40 μl of Gαi3·GDP aliquots (0.2 mm) into LGN GLs fragments fused to the C-terminal end of thioredoxin (0.02 mm) at time intervals of 2 min to ensure that the titration peak returned to the base line. The titration data were analyzed using the program Origin7.0 from MicroCal.
Fluorescence Polarization Assay
Fluorescence polarization assay were performed on a PerkinElmer LS-55 fluorimeter equipped with an automated polarizer at 25 °C. Commercial synthesized peptides were labeled with fluorescein 5-isothiocyanate (Invitrogen) at the N termini. In a typical assay, the FITC-labeled peptide (∼1 μm) was titrated with binding partners in a 50 mm Tris pH 8.0 buffer containing 100 mm NaCl, 1 mm DTT, and 1 mm EDTA. The KD values were obtained by fitting the titration curves with the classical one-site binding model, with or without invoking the Hill coefficient model.
GST Pulldown Assay
For GST pulldown assay, GST or GST-tagged proteins (60 μl from 1 mg/ml stock solutions) were first loaded to 40 ml GSH-Sepharose 4B slurry beads in an assay buffer (50 mm Tris, pH 8.0, 100 mm NaCl, 1 mm DTT, and 1 mm EDTA). The GST fusion protein-loaded beads were then mixed with potential binding partners, and the mixtures were incubated for 1 h at 4 °C. After three times washing, proteins captured by affinity beads were eluted by boiling, resolved by 15% SDS-PAGE, and detected by Coomassie Blue staining.
Analytical Gel Filtration Chromatography
Analytical gel filtration studies were carried out on an AKTA FPLC system (GE Healthcare). Proteins at concentration of 10–20 μm in a volume of 100 μl were loaded on a Superose 12 10/300 GL column 20 (GE Healthcare) equilibrated with the buffer containing 50 mm Tris, pH 8.0, 100 mm NaCl, 1 mm DTT, and 1 mm EDTA. Protein elution was detected by absorbance at 280 nm.
GDI Activity Assay
Measurements of AlF4−-induced increase of intrinsic tryptophan fluorescence were performed on the PerkinElmer LS-55 spectrometer with excitation at 292 nm and emission at 342 nm. Purified Gαi3 protein was diluted in 2-ml cuvettes to 200 nm in a preactivation buffer (100 mm NaCl, 100 μm EDTA, 2 mm MgCl2, 20 μm GDP, 20 mm Tris-HCl, pH 8.0) and incubated at 30 °C. At the time points 400 and 500 s after Gαi3 dilution, 2 mm NaF and 30 μm AlCl3 (final concentrations), respectively, were added to the reaction mixture, and fluorescence intensity changes as a function of time were recorded. The GDI activities of GL peptides were assayed by repeating the above procedure except that the reaction mixtures contained defined concentrations of specific peptides.
The measurements of GTPγS binding were also performed on PerkinElmer LS-55 spectrometer with excitation at 485 nm and emission at 530 nm (slit widths each at 2.5 nm). BODIPY FL-GTPγS was diluted to 1 μm in buffer (20 mm Tris-HCl, pH 8.0, 1 mm EDTA, and 10 mm MgCl2) and equilibrated to 30 °C in 2-ml cuvettes. Purified Gαi3 was diluted to 100 nm in the buffer (100 mm NaCl, 100 μm EDTA, 2 mm MgCl2, 20 μm GDP, 20 mm Tris-HCl, pH 8.0) and preincubated with GL peptides (with different concentrations) at 30 °C for 10 min before addition to the cuvette. Relative fluorescence levels were set to 0 at the average fluorescence reading over the first 70 s, and Gαi3/GL mixtures were added at the time point of 100 s.
Crystallography
Crystals of the Gαi1(3) in complex with GL3/4 (diluted to 7.5 mg/ml in 50 mm Tris, pH 8.0, 100 mm NaCl, 1 mm EDTA, 1 mm DTT, 10 mm Mg2+, 20 μm GDP buffer) were obtained by the hanging drop vapor diffusion method at 18 °C. The crystals were grown in buffer containing 0.5 m ammonium sulfate, 1.0 m lithium sulfate monohydrate, 0.1 m sodium citrate tribasic dehydrate, pH 5.6. Crystals were soaked in crystallization solution containing a higher concentration (1.5 m) of lithium sulfate for cryo-protection. All the diffraction data were collected at Shanghai Synchrontron Radiation Facility BL17U at a wavelength of 0.9793 Å using a single crystal of each complex. The diffraction data were processed and scaled using HKL2000 (35). Molecular replacement was used to solve the structure of Gαi1(3)·GL4(3) with the program Molrep (36). The crystal structure of RGS14·Gαi1 complex (Protein Data Bank code 1KJY) was used as a search model by removing the RGS14 peptide. The initial model was rebuilt manually and then refined using REFMAC (37) and PHENIX (38) against the whole data set. Further manual model building and adjustment were completed using COOT (39). The final refinement statistics are summarized in Table 1.
TABLE 1.
Statistics of x-ray crystallographic data collection and model refinement
The numbers in parentheses represent the value for the highest resolution shell.
| Data sets | Gαi3QtoL_GL4 | Gαi1_GL4 | Gαi3_GL4 | Gαi3_GL3 |
|---|---|---|---|---|
| Space group | P6122 | P6122 | P6122 | P6122 |
| Unit cell (Å) | a = 207.3, c = 236.6 | a = 207.4, c = 236.7 | a = 209.6, c = 237.2 | a = 209.7, c = 235.5 |
| No. of unique reflections | 66,825 | 66,971 | 39,388 | 35,265 |
| Resolution limit (Å) | 50.00–2.90 (2.95–2.90) | 50.00–2.90 (2.95–2.90) | 50.00–3.50 (3.56–3.50) | 50.00–3.60 (3.66–3.60) |
| Redundancy | 10.8 (11.2) | 9.4 (9.7) | 4.4 (4.5) | 9.2 (9.4) |
| Completeness (%) | 100 (100) | 100 (100) | 98.8 (99.9) | 99.7 (100) |
| I/σI | 27.5 (3.6) | 25.1 (3.3) | 14.7 (1.8) | 37.6 (5.8) |
| Rmerge (%)a | 9.8 (76.2) | 9.6 (73.5) | 11.0 (75.7) | 7.3 (39.6) |
| Structure refinement | ||||
| Resolution range (Å) | 43.04–2.90 (3.00–2.90) | 47.69–2.90 (3.00–2.90) | 49.65–3.50 (3.61–3.48) | 39.63–3.62 (3.75–3.62) |
| Rcryst/Rfree (%)b | 22.0/25.0 (32.6/42.4) | 22.0/24.5 (31.9/36.1) | 22.6/26.1 (30.1/35.8) | 22.8/25.3 (27.7/31.0) |
| Root mean square deviation bonds (Å)/angle (°) | 0.010/1.36 | 0.010/1.41 | 0.011/1.63 | 0.009/1.26 |
| Average B factor (Å2)c | 67.90 | 66.50 | 106.40 | 123.50 |
| No. of atoms | ||||
| Protein atoms | 10907 | 10921 | 10601 | 10141 |
| Water molecules | 12 | 35 | 0 | 0 |
| Ligands | 25 | 25 | 11 | 11 |
| No. of reflections | ||||
| Working set | 63256 (6246) | 63457 (6234) | 37222 (3584) | 33318 (3272) |
| Test set | 3375 (313) | 3385 (343) | 1963 (195) | 1754 (151) |
| Ramachandran plotc | ||||
| Favored (%) | 95.5 | 95.8 | 92.2 | 90.1 |
| Allowed (%) | 4.5 | 4.2 | 7.4 | 8.2 |
| Outliers (%) | 0 | 0 | 0.4 | 1.7 |
a Rmerge = Σ|Ii − <I>|/ ΣIi, where Ii is the intensity of measured reflection, and <I> is the mean intensity of all symmetry-related reflections.
b Rcryst = Σ||Fcalc| − |Fobs||/ΣFobs, where Fobs and Fcalc are observed and calculated structure factors. Rfree = ΣT||Fcalc| − |Fobs||/ΣFobs, where T is a test data set of ∼5% of the total unique reflections randomly chosen and set aside prior to refinement.
c B factors and Ramachandran plot statistics are calculated using MOLPROBITY (45).
RESULTS
Mapping the Minimal Gαi·GDP Binding Sequences in LGN GoLoco Motifs
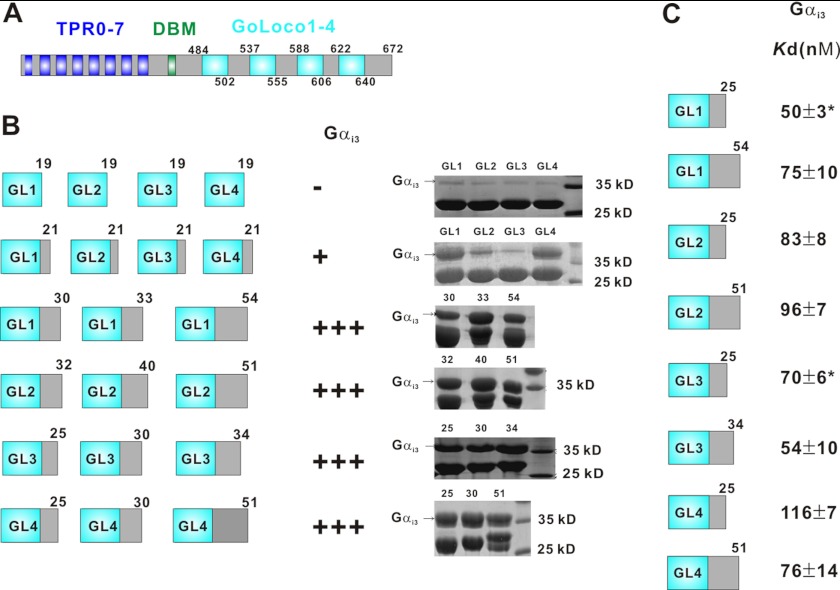
The C-terminal region of LGN contains four GoLoco motifs, each of which consists of a conserved 19-residue fragment followed by a stretch of variable amino acid residues with different lengths (Fig. 1A). We define the full-length GoLoco motif to be the conserved 19-residue fragment plus all of the following C-terminal sequence before the start of the next GoLoco motif core. With this definition, each GL1, 2, 3, and 4 motif of LGN consists of 54, 51, 34, and 51 residues, respectively (Fig. 1A). Previous structural study of the Gαi1·RGS14-GoLoco complex showed that the 16-residue sequence C-terminal to the GoLoco core motif make extensive contacts with Gαi1 and thus are essential for the interaction between Gαi1 and RGS14 (14). To understand the interaction between LGN and Gαi, we set out to map the minimal Gαi·GDP binding sequence of each LGN GL. We first used GST-fused LGN GL with different lengths to pull down purified Gαi3·GDP in our binding assay. This assay showed that each GL containing only the 19-residue core displayed only a background level of binding to Gαi3·GDP (Fig. 1B). Obvious binding of Gαi3·GDP to GL1 and GL4 was observed by extending the conserved 19-residue GL core by two residues (Fig. 1B). Any one of LGN GLs with length equal to or longer than 25 residues displayed comparable binding to their corresponding full-length motifs (Fig. 1B). We next measured the quantitative binding affinities of each of the four GLs to Gαi3·GDP using isothermal titration calorimetry or fluorescence spectroscopy. Such quantitative binding assays revealed that the four full-length GLs share similar affinities (KD = 54–96 nm) in binding to Gαi3·GDP (Fig. 1C). In agreement with the results derived from the pulldown binding assay, each LGN GL with a length of 25 residues has an essentially same binding affinity compared with the corresponding full-length motif (Fig. 1C), indicating that each of the 25-residue LGN GL contains the complete Gαi·GDP binding sequence. This finding is in sharp contrast to the interaction between Gαi·GDP and the RGS14 GoLoco motif, which requires a total length of 35 residues (14). Consistent with earlier studies (40), the LGN GLs bind to Gαi3·GTPγS with a ∼100-fold weaker affinity than to Gαi3·GDP (data not shown).
FIGURE 1.
Characterization of the binding between Gαi·GDP and the four LGN GLs. A, schematic diagram of the domain organization of LGN. DBM denotes the DLG-binding domain of LGN. B, GST pulldown assay of the binding between LGN GLs with variable lengths (indicated by the number at the top of each GL) with Gαi3·GDP. C, ITC and fluorescence-based (denoted with asterisks) measurements of the binding affinities of Gαi3·GDP with LGN GLs of different lengths.
Gαi·GDP Can Simultaneously Bind to All Four LGN GLs
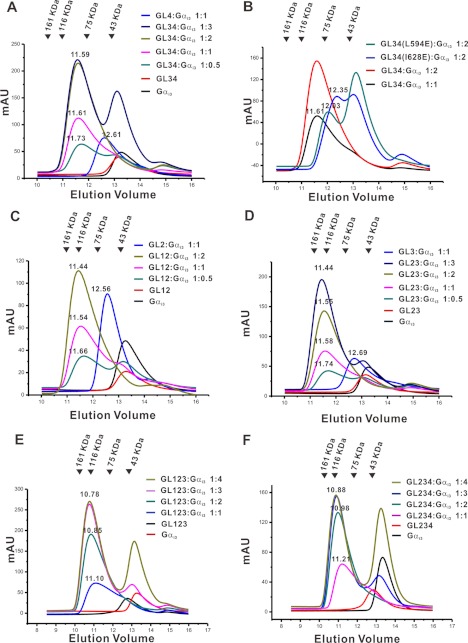
We next asked whether Gαi·GDP can simultaneously bind to the multiple GLs of LGN. We first tested the interaction between Gαi·GDP and the LGN GL34 tandem (aa 587–650), because the intervening sequence between the core sequences of GL3&4 is the shortest (15 residues to be exact; Fig. 1A). According to the structure of Gαi1·RGS14 complex (14), two successive GL core sequences separated by a 15-residue linker cannot bind to two Gαi because the bound Gαi molecules would crash into each other. We examined the binding stoichiometry between LGN-GL34 and Gαi3·GDP using analytical gel filtration chromatography. Upon addition of 2 or 3 molar ratios of Gαi3 to GL34, a peak corresponding to a (Gαi3·GDP)2·GL34 complex was detected (Fig. 2A), indicating that the two GLs in GL34 can simultaneously bind to Gαi3·GDP. To further substantiate that the elution peak at ∼11.60 ml in Fig. 2A represents the 2:1 stoichiometric complex formed between Gαi3·GDP and GL34, we used two GL34 mutants (L594E and I628E), in which either the Gαi3·GDP binding site on GL3 (the L594E mutant) or on GL4 (the I628E mutant) was disrupted. On the gel filtration column, the 1:2 mixtures of the two GL34 mutants with Gαi3·GDP were eluted at a volume significantly larger than the wild type GL34, and a large portion of free Gαi3·GDP was also detected (Fig. 2B); presumably the GL34 mutants only formed 1:1 stoichiometric complex with Gαi3·GDP. This result also confirms that the wild type GL34 can form a 1:2 stoichiometric complex with Gαi3·GDP. Further lengthening of the linker between GL3 and GL4 by inserting 10 flexible residues (five GS repeats, referred to as GL34Ins5GS) did not alter the elution profile of its complex with Gαi3·GDP (data not shown), indicating that the 15-residue intervening sequence between GL3 and GL4 is sufficiently long for two molecules of Gαi3·GDP to bind simultaneously to GL34. Similarly, two molecules of Gαi3·GDP are capable of binding to LGN-GL12 (aa 483–586) or GL23 (aa 537–620). (Fig. 2, C and D). Additionally, three molecules of Gαi3·GDP were found to bind simultaneously to GL123 (aa 483–620) or GL234 (aa 537–650) of LGN (Fig. 2, E and F).
FIGURE 2.
Gαi·GDP binding to multiple GL containing fragments of LGN analyzed by analytical gel filtration chromatography. A, the binding of LGN-GL34 to different molar ratios of Gαi3·GDP. B, the binding of LGN-GL34(L594E) and LGN-GL34(I628E) to Gαi3·GDP. C, the binding of LGN-GL12 to Gαi3·GDP. D, the binding of LGN-GL23 to Gαi3·GDP. E, the binding of LGN-GL123 to Gαi3·GDP. F, the binding of LGN-GL234 to Gαi3·GDP.
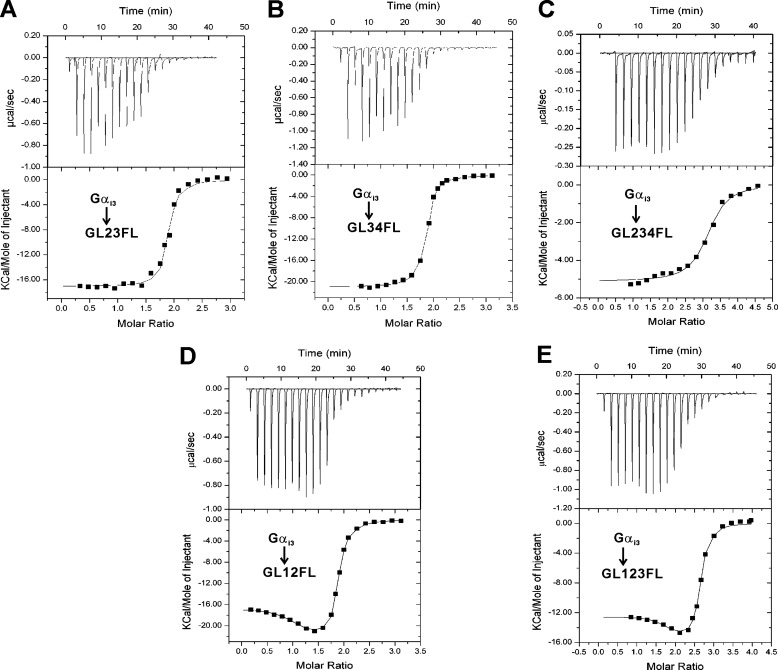
To characterize the binding stoichiometry more precisely, ITC analyses were performed. The titration profiles of Gαi3·GDP to GL23 and GL34 can be well fitted with the model using one set of identical sites, yielding overall stoichiometries of 1.9:1 and 1.8:1, respectively (Fig. 3, A and B, and Table 2), consistent with the binding stoichiometry derived from the gel filtration analyses. The apparent binding affinity of GL23 was similar to those of the individual GoLocos, whereas GL34 had a weaker binding affinity than that of GL3 or GL4 (Table 2 and Fig. 1C). The titration profile of Gαi3·GDP to the triple-GoLoco-containing protein GL234 was also fitted with the model with one set of binding sites, giving a weaker binding affinity of ∼358 nm and a binding stoichiometry of 3.1:1 (Fig. 3C and Table 2). The purified GL234 protein underwent slight degradation, which might affect the accuracy of the binding affinity measurement. The titration profile of Gαi3·GDP to GL12, however, was best fitted with the model that assumes two sets of binding sites (Fig. 3D), yielding one strong site (KD = ∼11 nm) and one weak site (KD = ∼188 nm) (Table 2). The ITC titration profile of Gαi3·GDP to GL123 was also fitted with the ‘two sets of binding sites’ model, giving rise to two strong sites (KD ∼ 4 nm) and one weak site (KD ∼ 186 nm) (Fig. 3E and Table 2). Similar atypical profiles of ITC titrations were also observed in the analyses of AGS3-GLs/Gαi·GDP interaction (41). It is worth noting that these data analyses do not represent the complete description of the thermodynamics of the interactions between tandem LGN-GoLoco repeats and Gαi3·GDP, in which intersite cooperativity likely exists. Because the full-length GoLoco region of LGN, i.e., GL1234, suffers from severe degradation, we did not analyze the binding property of GL1234 directly. However, the ITC titration data, consistent with the gel filtration analyses, strongly suggested that the full-length LGN binds Gαi3·GDP with a stoichiometry of 1:4. The four GoLoco motifs of LGN have intrinsically similar binding affinities to Gαi3·GDP. To explore the molecular details of the binding, we proceeded to determine the crystal structure of the Gαi·LGN-GoLoco complex.
FIGURE 3.
ITC analyses of the binding of tandem GoLoco motifs to Gαi3·GDP. ITC measurements of binding of Gαi3·GDP to LGN-GL23 (A), LGN-GL34 (B), LGN-GL24 (C), LGN-GL12 (D), and LGN-GL13 (E). The titration data were fitted with the models with one set of binding sites and two sets of binding sites. The derived thermodynamic parameters are shown in Table 2.
TABLE 2.
Thermodynamic parameters of the bindings of LGN GoLoco motifs to Gαi3·GDP determined by ITC titration
The titration data of GL12 and GL123 were fitted with the two sets of binding sites model, whereas the other data were fitted with the one set of binding sites model. N denotes the number of binding sites in each model.
| N | KD | ΔH | ΔS | ΔG | |
|---|---|---|---|---|---|
| nm | kcal mol−1 | cal mol−1 K−1 | kcal mol−1 | ||
| GL12 | |||||
| Site 1 | 0.97 | 11.27 | −16.69 | −18.7 | −11.12 |
| Site 2 | 0.89 | 188.32 | −22.80 | −44.4 | −9.57 |
| GL123 | |||||
| Site 1 | 1.63 | 4.69 | −12.55 | −3.97 | −11.37 |
| Site 2 | 1.0 | 186.22 | −15.46 | −21.0 | −9.20 |
| GL23 | 1.86 | 87.72 | −17.05 | −24.0 | −9.90 |
| GL34 | 1.81 | 173.61 | −20.97 | −37.2 | −9.88 |
| GL234 | 3.08 | 358.42 | −5.12 | 11.9 | −8.67 |
Overall Crystal Structures of GL3 and GL4 in Complex with Gαi·GDP
Extensive efforts have been put to screen various constructs of the four LGN GLs in complex with GDP-loaded Gαi3 or Gαi1, and we succeeded in obtaining well diffracting crystals for synthetic GL4 (621DEDFFSLILRSQAKRMDEQRVLLQRD645) and GL3 (587DEDFFDILVKCQGSRLDDQRCAPPS611) peptides in complex with Gαi1/3·GDP. The Gαi1·GL4, Gαi3·GL4, and Gαi3·GL3 complexes diffracted to 2.9, 3.5, and 3.6 Å resolutions, respectively (Table 1). According to a previous structure-based protein design study, point mutations on Gαi (E116L, Q147L, and E245L, respectively) can enhance its binding affinity to various GLs (15). We therefore constructed such three Gαi3 mutants, hoping that the mutants might have higher affinities in binding to LGN GLs and thus yield better quality complex crystals. Opposite to our expectation, none of these mutants showed obviously enhanced binding to LGN GLs (data not shown). Nonetheless, the Q147L-Gαi3 mutant·GL4 complex yielded better diffracting crystals (2.9 Å) than the wild type Gαi3·GL4 complex.
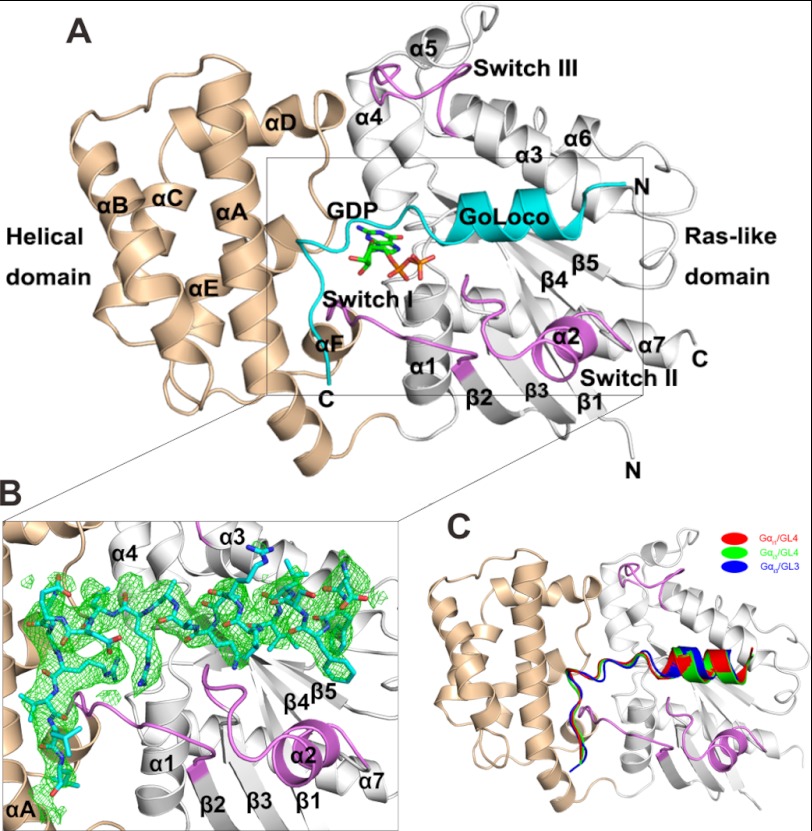
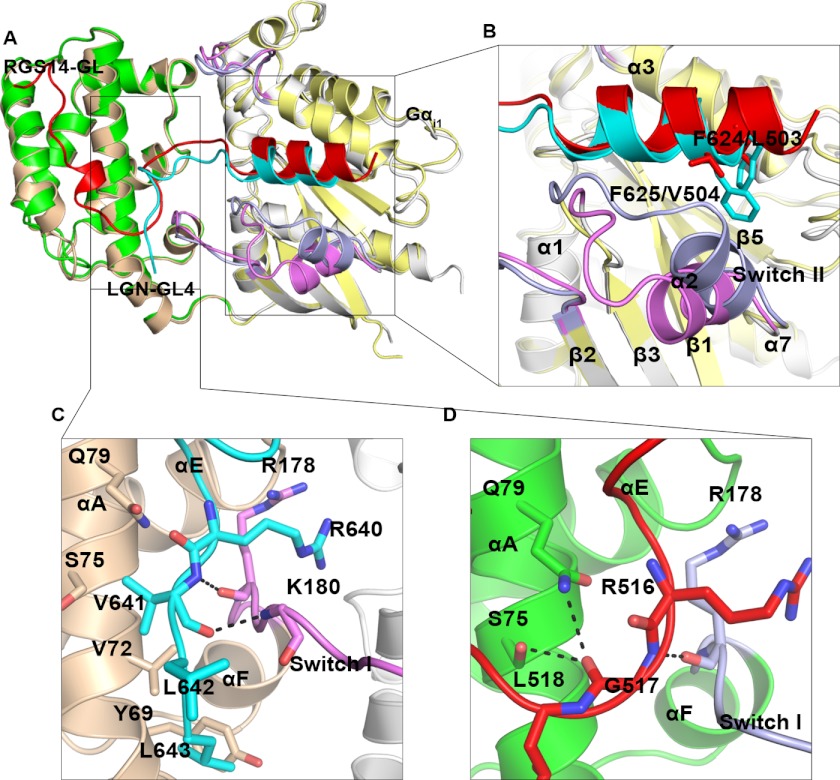
The structures of Gαi1(3)·GL4 and Gαi3·GL3 were solved by molecular replacement using the Gαi1·RGS14 structure as the search model (Protein Data Bank code 1KJY) (14). The Gαi·GDP structure is well defined, and 21–22 amino acids of the GL3 or GL4 peptide are ordered in the structures of complexes (Fig. 4, A and B). The structures of Gαi in the Gαi1(3)·GL4 and Gαi3·GL3 complexes are highly similar to that in the Gαi1·RGS14 complex (root mean square deviation of 0.67 Å), except for the Switch II region, which is shifted further away from the LGN-GL peptides because of the presence of two bulky hydrophobic residues in the GL peptides (Fig. 5, A and B). The GL peptides in the three complexes adopt highly similar structures (Fig. 4C). The N-terminal 10 residues of each LGN GL peptide (aa 623–632 of GL4 and aa 589–598 of GL3), which corresponds to the first half of the conserved 19-residue GL core, forms an α-helix that occupies the cleft between Switch II and α3 of Gαi (Fig. 4A). The following eight residues of the GL core (aa 633–640 of GL4 and aa 599–606 of GL3) forms a “lid” in covering GDP. Only three or four residues C-terminal to the GL core (aa 641–643 of GL4 and aa 607–610 of GL3) were found to bind to the all-helical domain of Gαi (Fig. 4). The structures of the LGN GL peptides in complex with Gαi are entirely consistent with our biochemical data, showing that extending of the conserved GL core at the C-terminal end by three or four residues is necessary and sufficient for LGN GLs to bind to Gαi (Figs. 1 and 2). The structures of the complexes also indicate that LGN GLs should function as GDIs by directly stabilizing the bound GDP as well as the interaction between the Ras-like domain and the all-helical domain of Gαi (Fig. 4A).
FIGURE 4.
Crystal structures of Gαi3 in complex with GL4 and GL3, respectively. A, ribbon diagram showing the crystal structure of LGN-GL4 in complex with Gαi1·GDP. GDP is shown in the ball-and-stick model. All-helical domain and Ras-like domain of Gαi1 is shown in wheat and light gray, respectively. The three switches are shown in violet, and the GL4 peptide is shown in cyan. B, the Fo − Fc density map of GL4 peptide is shown in green and contoured at 3.0 σ. C, comparison of the structures of the Gαi1·GL4, Gαi3·GL4, and Gαi3·GL3 complexes by superimposing the backbone atoms in the three structures. Gαi1 is shown the same as in A, whereas Gαi3 in complex with GL3 (root mean square deviation of 0.76 Å) and GL4 (root mean square deviation of 0.51 Å) is not shown. GL3 peptide is shown in blue, and GL4 peptides bound to Gαi1 and Gαi3 are shown in red and green, respectively.
FIGURE 5.
Comparison of the crystal structures of Gαi1·GL4 and Gαi1·RGS14 complexes. A, comparison of the crystal structure of Gαi1·GL4 (cyan) with that of Gαi1·RGS14 (green). The all-helical domain and Ras-like domain of Gαi1 are colored wheat and light gray, respectively. The Switch I, II, and III regions of Gαi1 in complex with LGN-GL4 and with RGS14 are highlighted with violet and light blue, respectively. B, comparison of the structural details of the α-helical region of LGN-GL4 and RGS14-GL, showing that the larger hydrophobic side chains of LGN-GL4 result in the shift of the Switch II of Gαi1. C, structure details of the C terminus of LGN-GL4, showing that two backbone hydrogen bonds stabilize the C-terminal conformation. D, structure details of the sharp turn at Gly517 of RGS14 peptide. Hydrogen bonds formed between RGS14 and Gαi1 are shown with dashed lines.
A General Interaction Mode Revealed by the LGN GLs in Complex with Gαi
Although the structures of Gαi bound to the GLs of RGS14 and LGN are highly similar, the conformation of Gαi-bound GLs of RGS14 and LGN are distinctly different (Fig. 5). First, a 16-residue fragment C-terminal to the conserved GL core of RGS14 is required for binding to Gαi, and this 16-residue fragment forms ordered structure and has extensive interactions with the all-helical domain of Gαi1 (14). In LGN-GL4/GL3, in contrast, only three or four residues C-terminal to the GL core are required for binding to Gαi (Fig. 4A). Second, the orientation of the variable C-terminal tail of the RGS14 GL peptide is opposite to that of the LGN GL peptides (Fig. 5A). In the LGN GL4·Gαi complex, the hydrophobic side chains of Val641, Leu642, and Leu643 interact with Val72 and Tyr69 from the αA helix of the Gαi all-helical domain; thus the C-terminal end of GL4 extends toward the N-terminal end of Gαi αA (Fig. 5C). The residue corresponding to Val641 in the RGS14 peptide is Gly517 (Fig. 5D and Fig. 6A). The backbone carbonyl oxygen of Gly517 forms two hydrogen bonds with side chains of Ser75 and Gln79 from Gαi αA. The unique backbone dihedral angles (φ = 78°, ψ = −171°) of Gly517, which are not allowed by other amino acids, enable the C-terminal tail of the RGS14 GL peptide to take a sharp turn at this position and extend to the C-terminal end of Gαi αA (Fig. 5, A and D). Sequence alignment of all known GLs from mammals reveals that only the GLs of RGS14 and RGS12 contain a Gly right after the conserved core motif, and the C-terminal residues of these two GLs share the identical sequence (Fig. 6A). The above structure-based amino acid sequence analysis suggests that the LGN GL/Gαi interactions observed in this study represent the general mode of the interactions between GoLoco proteins and Gαi. RGS14 and RGS12, instead, may represent a special subclass of GoLoco proteins in terms of Gαi binding.
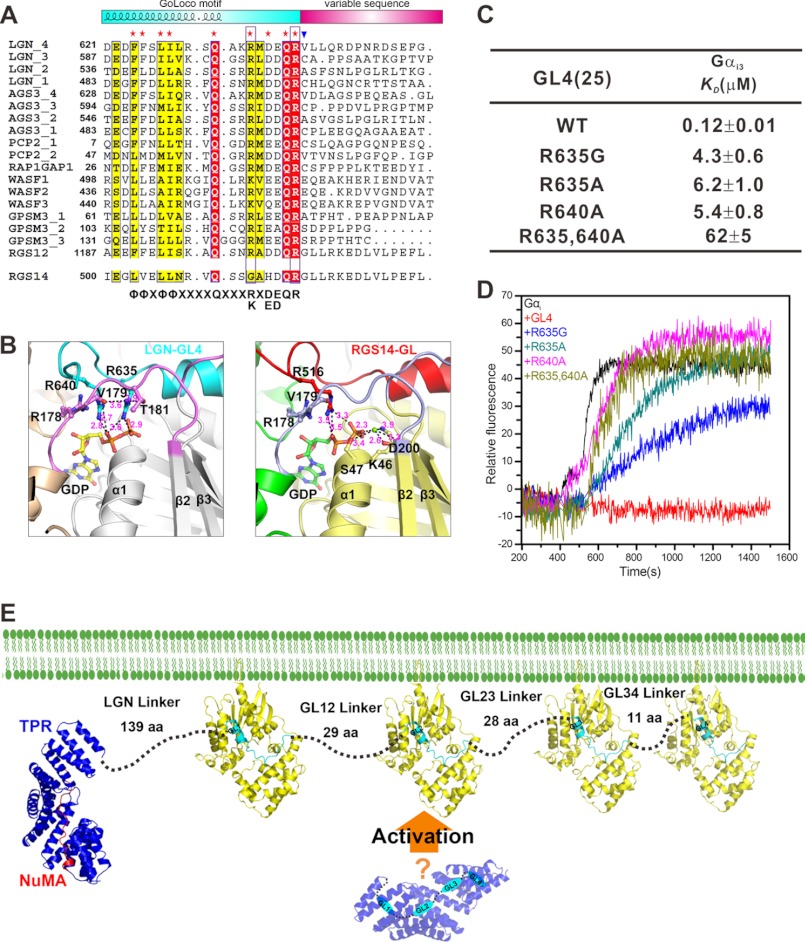
FIGURE 6.
The double arginine fingers of the LGN GLs play a crucial role in GDP coordination and GDI activity. A, sequence alignment of the GLs in mammalian GoLoco proteins. Absolutely and highly conserved residues are highlighted in red and yellow, respectively. The residue right behind (D/E)QR, which determines the C-terminal direction, is highlighted with a blue triangle. The residues involved in the interactions with Gαi are labeled with red stars at the top. The di-arginine fingers are highlighted with black boxes. B, structural details of the GDP-binding pocket in the Gαi1·GL4 complex and Gαi1·RGS14 complex. Polar interactions are shown with dashed lines. Distances of polar interactions are shown with magenta numbers (Å). The color scheme is the same as in Fig. 4A. C, binding affinities of the GL4 mutants with single or double substitutions of its two arginine residues to Gαi3·GDP derived from fluorescence-based assays. D, GDI activities of the wild type and mutant GL4 peptides measured with AlF4−-induced increase of intrinsic tryptophan fluorescence. E, structural model of the LGN·Gαi·GDP complex. The TPR domain, the TPR-binding NuMA peptide, and the GLs responsible for Gαi·GDP binding are shown in blue, red, and cyan, respectively.
The Double Arg Finger-mediated GDP Binding of LGN GLs
The structure of the Gαi1·RGS14 GL complex shows that a highly conserved (D/E)QR triad at the C-terminal end of the conserved GL core plays a critical role in binding to Mg2+-GDP (14). Similar to the Gαi1·RGS14 GL interaction, the side chain of Arg640 (Arg606) of GL4 (GL3) in the (D/E)QR triad, which is equivalent to Arg516 of RGS14, is inserted into the GDP-binding pocket and binds to α-phosphate of GDP (Fig. 6B). However, there is a distinct feature of GL4/GL3 in GDP binding with respect to RGS14 GL. Another highly conserved Arg five residues upstream of the Arg in the (D/E)QR triad in LGN GL peptides (Arg635 in GL4 and Arg601 in GL3) binds to the α and β phosphates of GDP (Fig. 6B). In RGS14 GL, the residue corresponding to this second Arg is a Gly, and a Mg2+ ion was found to be necessary to stabilize the β phosphates of GDP (14). Therefore, different from RGS14, LGN GLs use two Arg residues instead of one to bind to and stabilize GDP. The structures of the LGN GLs in complex with Gαi further indicate that the LGN GLs can bind to GDP-bound Gαi independent of the presence of Mg2+. This structure-based prediction is confirmed by direct binding experiment (data not shown). Sequence alignment analysis reveals that, except for RGS14 GL, the rest of GLs all contain a (R/K)X(D/E)(D/E)QR GDP-binding sequence (Fig. 6A), and we refer to this sequence as the double Arg finger. This sequence analysis further supports that the LGN GL/Gαi interaction represents the general mode of GL-mediated binding to Gα.
The Double-arginine Fingers Are Critical to the GDI Activities of LGN-GLs
To confirm the functional importance of the two Arg in the double-arginine finger in LGN GLs, we performed point mutations of the two arginines and tested the Gαi·GDP binding affinities and GDI activities of these mutants. Single substitution mutations (R635G, R635A, and R640A) caused ∼50-fold decrease in GL4 binding to Gαi·GDP, and the double mutation (R635A/R640A) led to ∼500-fold Gαi·GDP binding affinity decrease (Fig. 6C). Similar results were also obtained from the other LGN GLs, indicating that the two conserved arginine fingers are critical for binding of Gαi·GDP to LGN-GLs. This finding is in contrast to the RGS14 GL, in which the substitution of the Arg in the finger with Ala or Leu did not decrease the binding affinity of RGS14 to Gαi1·GDP (14). Careful examination of the crystal structures of Gαi in complex with LGN GL peptides revealed that the side chains of the two Arg residues also form hydrogen bonds with Val179 and Thr181 from Gαi (Fig. 6B). In contrast, the side chain of Arg516 in RGS14 GL interacts exclusively with GDP (14).
The GDI activities of LGN GLs were evaluated by AlF4−-induced increase of intrinsic tryptophan fluorescence of Gαi and by direct binding of BODIPY-GTPγS to Gαi. In agreement with the previous studies (40), the four GLs exhibited similar GDI activities (data not shown). Moreover, comparison of the GDI activities of GL peptides with different lengths showed that the 25-residue minimal Gαi-binding GL fragments shown in Fig. 1 are also sufficient for their GDI activities (data not shown). Further quantification of the GDI activities using the association rate of BODIPY-GTPγS binding revealed IC50 values of a few μm for LGN GLs, which is slightly weaker than that of RGS14 GL (data not shown). At a saturated concentration of GL peptide (GL, 200 μm; Gαi3, 0.2 μm), the wild type LGN-GL4 showed a complete inhibition of GDP dissociation from Gαi3 (Fig. 6D). The R635G-GL4 or the R635A-GL4 displayed obviously weakened GDI activities, whereas the R640A-GL4 and R635,640A-GL4 had essentially no detectable GDI activity (Fig. 6D). Substitution of the first Arg (Arg601) in the double-arginine finger of GL3 with Ala or Gly also diminished its GDI activity (data not shown). Thus, we conclude that both arginines in the double-arginine finger of LGN GoLoco motifs are important for their GDI activity.
DISCUSSION
Both the binding to GDP-loaded Gα subunits and the GDI activity of GL require residues beyond the 19-residue conserved core sequence (14, 41). Because the C-terminal flanking sequences of GLs are highly diverse among GoLoco proteins (7), it has been hypothesized that the variable C-terminal tail sequences of GLs are the specificity determinants governing GL/Gα interactions. In the present study, we demonstrate that only a few residues (3–4 aa) C-terminal to the conserved GL core are required for LGN GLs to bind to and to inhibit GDP dissociation of Gαi·GDP, a finding that is in sharp contrast to that of RGS14 GL. Sequence alignment analysis suggests that the conformation of the GL peptide in the Gαi1·RGS14 structure is likely a unique example of GL/Gα interaction. The LGN GL/Gαi interaction described in the current study instead is likely a general binding mode between GLs and Gα. The structures of LGN GLs in complex with Gαi·GDP also suggest that the short variable C-terminal sequences of LGN GLs are unlikely to determine their binding specificity to Gα subunits. Consistently, previous studies have shown that LGN GLs bind to all three forms of GDP-loaded Gαi (i1, i2, and i3). As for the Gαo·GDP binding, discrepancies exist in the literature. An early study by McCudden et al. (40) reported that LGN GLs selectively bind to Gαi·GDP, but not to Gαo·GDP or Gαs·GDP. Recently, Kopein et al. (42) found that LGN, as well as its Drosophila homolog Pins, can bind robustly to both GDP-loaded Gαo and Gαi.
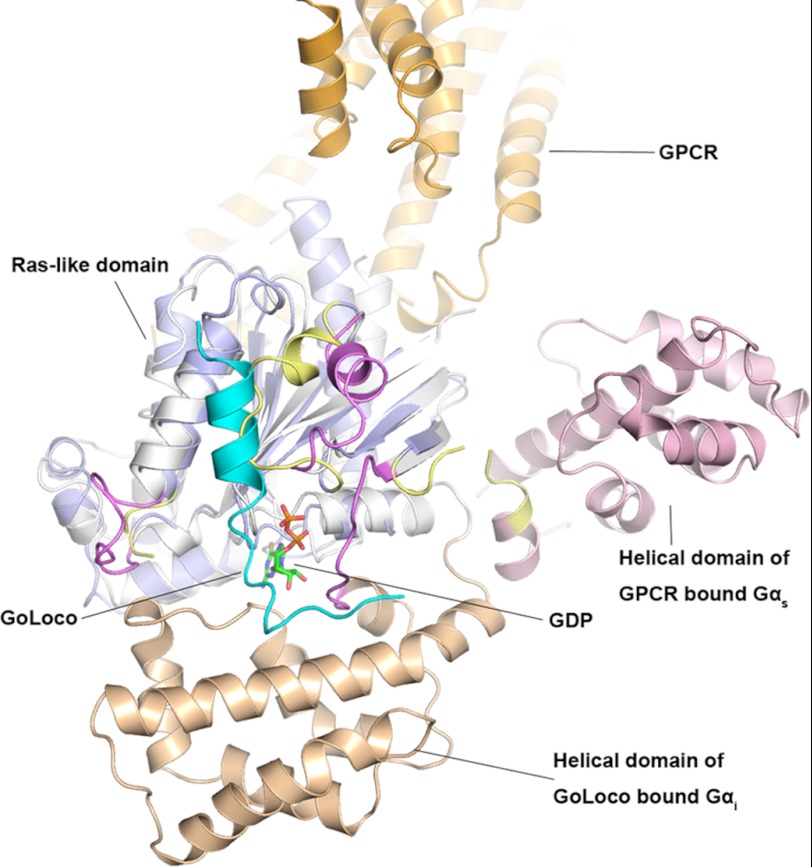
We have demonstrated in this study that every one of the four LGN GLs can bind to Gαi·GDP with high affinity. Additionally, although the LGN GL peptides are much shorter than their counterpart from RGS14, LGN GLs also act as potent GDIs. The structures of the LGN GL3 and GL4 in complex with Gαi suggest that both the double-arginine finger and the short variable tail of the GL peptides are important for their GDI activities. The di-arginine finger makes extensive salt bridges with the phosphates of GDP, and the GDP in return makes contacts with both the Ras-like and all-helical domains of Gαi. The variable C-terminal tail of the GL peptides further interacts with the all-helical domain of Gαi. Thus, in addition to stabilizing GDP bound to Gαi, the binding of GL peptide further promotes the closed conformation of Gαi (i.e., by restricting the opening of the all-helical domain and subsequent dissociation of GDP from Gαi; Fig. 7).
FIGURE 7.
Comparison of Gαi1·GL4 structure with the structure of the fully activated Gα conformation derived from the β2-AR-Gαβγ structure. The all helical domain, the Ras-like domain, and three switches of GPCR bound Gαs are shown in light blue, pink, and yellow, respectively. Part of the GPCR (β2-AR) is shown in orange. The coloring of the Gαi1·GL4 complex is the same as in Fig. 4A.
The characteristic multiple GLs in LGN and its Drosophila homolog Pins have been implicated to play a role in regulating their intramolecular interactions between TPR repeats and GLs in response to the binding of Gαi·GDP and NuMA/Mud (34, 43). In addition to this, the multiple GLs in LGN (Pins) also function as a scaffold in regulating the localization of related protein complexes and organizing signaling pathways mediating spindle orientations. The detailed characterizations of interactions between LGN-GLs and Gαi·GDP in this work demonstrate that in its open state the four LGN GLs have equal capacity to bind to Gαi·GDP (Fig. 6E). In another word, the stoichiometry of LGN/Gαi·GDP complex in vivo likely depends on the concentration of Gαi·GDP, which in turn regulates the cortical localization of LGN-bound proteins, such as NuMA. Recently, it was found that the extrinsic GPCR Tre1 signaling determines the orientation of cortical polarity in the asymmetric cell division of Drosophila neuroblast (44). Tre1 was shown to activate Gαo, and the GTP form Gαo can specifically associate with the first GL of Pins (44). Thus, the presence of multiple GLs allows Pins to function as a scaffold to simultaneously engage Gαo- and Gαi-mediated signaling events during asymmetric cell division.
Acknowledgment
We thank the Shanghai Synchrotron Radiation Facility BL17U for x-ray beam time.
This work was supported by National Major Basic Research Program of China Grants 2009CB918600 and 2011CB808505, National Science Foundation of China Grants 20973040 and 31070642, Science & Technology Commission of Shanghai Municipality Grant 08DZ2270500), and by Grants 663808, 664009, 660709, 663610, 663811, HKUST6/CRF/10, and SEG_HKUST06 from the Research Grants Council of Hong Kong to MZ.
The atomic coordinates and structure factors (codes 4G5O, 4G5Q, 4G5R, and 4G5S) have been deposited in the Protein Data Bank (http://wwpdb.org/).
- GPCR
- G protein-coupled receptor
- GL
- GoLoco
- GDI
- guanine nucleotide dissociation inhibitor
- TPR
- tetratricopeptide
- aa
- amino acids
- ITC
- isothermal titration calorimetry.
REFERENCES
- 1. Malbon C. C. (2005) G proteins in development. Nat. Rev. Mol. Cell Biol. 6, 689–701 [DOI] [PubMed] [Google Scholar]
- 2. Sprang S. R. (1997) G protein mechanisms. Insights from structural analysis. Annu. Rev. Biochem. 66, 639–678 [DOI] [PubMed] [Google Scholar]
- 3. Gilman A. G. (1987) G proteins. Transducers of receptor-generated signals. Annu. Rev. Biochem. 56, 615–649 [DOI] [PubMed] [Google Scholar]
- 4. Sunahara R. K., Dessauer C. W., Gilman A. G. (1996) Complexity and diversity of mammalian adenylyl cyclases. Annu. Rev. Pharmacol. Toxicol. 36, 461–480 [DOI] [PubMed] [Google Scholar]
- 5. Singer W. D., Brown H. A., Sternweis P. C. (1997) Regulation of eukaryotic phosphatidylinositol-specific phospholipase C and phospholipase D. Annu. Rev. Biochem. 66, 475–509 [DOI] [PubMed] [Google Scholar]
- 6. Kozasa T., Jiang X., Hart M. J., Sternweis P. M., Singer W. D., Gilman A. G., Bollag G., Sternweis P. C. (1998) p115 RhoGEF, a GTPase activating protein for Gα12 and Gα13. Science 280, 2109–2111 [DOI] [PubMed] [Google Scholar]
- 7. Willard F. S., Kimple R. J., Siderovski D. P. (2004) Return of the GDI. The GoLoco motif in cell division. Annu. Rev. Biochem. 73, 925–951 [DOI] [PubMed] [Google Scholar]
- 8. Siderovski D. P., Diversé-Pierluissi M., De Vries L. (1999) The GoLoco motif. A Gαi/o binding motif and potential guanine-nucleotide exchange factor. Trends Biochem. Sci. 24, 340–341 [DOI] [PubMed] [Google Scholar]
- 9. Bernard M. L., Peterson Y. K., Chung P., Jourdan J., Lanier S. M. (2001) Selective interaction of AGS3 with G-proteins and the influence of AGS3 on the activation state of G-proteins. J. Biol. Chem. 276, 1585–1593 [DOI] [PubMed] [Google Scholar]
- 10. Kimple R. J., De Vries L., Tronchère H., Behe C. I., Morris R. A., Gist Farquhar M., Siderovski D. P. (2001) RGS12 and RGS14 GoLoco motifs are Gαi interaction sites with guanine nucleotide dissociation inhibitor activity. J. Biol. Chem. 276, 29275–29281 [DOI] [PubMed] [Google Scholar]
- 11. Natochin M., Gasimov K. G., Artemyev N. O. (2001) Inhibition of GDP/GTP exchange on Gα subunits by proteins containing G-protein regulatory motifs. Biochemistry 40, 5322–5328 [DOI] [PubMed] [Google Scholar]
- 12. Granderath S., Stollewerk A., Greig S., Goodman C. S., O'Kane C. J., Klämbt C. (1999) loco encodes an RGS protein required for Drosophila glial differentiation. Development 126, 1781–1791 [DOI] [PubMed] [Google Scholar]
- 13. Ponting C. P. (1999) Raf-like Ras/Rap-binding domains in RGS12- and still-life-like signalling proteins. J. Mol. Med. 77, 695–698 [DOI] [PubMed] [Google Scholar]
- 14. Kimple R. J., Kimple M. E., Betts L., Sondek J., Siderovski D. P. (2002) Structural determinants for GoLoco-induced inhibition of nucleotide release by Gα subunits. Nature 416, 878–881 [DOI] [PubMed] [Google Scholar]
- 15. Bosch D. E., Kimple A. J., Sammond D. W., Muller R. E., Miley M. J., Machius M., Kuhlman B., Willard F. S., Siderovski D. P. (2011) Structural determinants of affinity enhancement between GoLoco motifs and G-protein α subunit mutants. J. Biol. Chem. 286, 3351–3358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sammond D. W., Eletr Z. M., Purbeck C., Kimple R. J., Siderovski D. P., Kuhlman B. (2007) Structure-based protocol for identifying mutations that enhance protein-protein binding affinities. J. Mol. Biol. 371, 1392–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sammond D. W., Bosch D. E., Butterfoss G. L., Purbeck C., Machius M., Siderovski D. P., Kuhlman B. (2011) Computational design of the sequence and structure of a protein-binding peptide. J. Am. Chem. Soc. 133, 4190–4192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bosch D. E., Willard F. S., Ramanujam R., Kimple A. J., Willard M. D., Naqvi N. I., Siderovski D. P. (2012) A P-loop mutation in Gα subunits prevents transition to the active state. Implications for G-protein signaling in fungal pathogenesis. PLoS Pathog. 8, e1002553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Du Q., Stukenberg P. T., Macara I. G. (2001) A mammalian partner of inscuteable binds NuMA and regulates mitotic spindle organization. Nat. Cell Biol. 3, 1069–1075 [DOI] [PubMed] [Google Scholar]
- 20. Zhu J., Wen W., Zheng Z., Shang Y., Wei Z., Xiao Z., Pan Z., Du Q., Wang W., Zhang M. (2011) LGN/mInsc and LGN/NuMA complex structures suggest distinct functions in asymmetric cell division for the Par3/mInsc/LGN and Gαi/LGN/NuMA pathways. Mol. Cell 43, 418–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Du Q., Taylor L., Compton D. A., Macara I. G. (2002) LGN blocks the ability of NuMA to bind and stabilize microtubules. A mechanism for mitotic spindle assembly regulation. Curr. Biol. 12, 1928–1933 [DOI] [PubMed] [Google Scholar]
- 22. Du Q., Macara I. G. (2004) Mammalian Pins is a conformational switch that links NuMA to heterotrimeric G proteins. Cell 119, 503–516 [DOI] [PubMed] [Google Scholar]
- 23. Siller K. H., Cabernard C., Doe C. Q. (2006) The NuMA-related Mud protein binds Pins and regulates spindle orientation in Drosophila neuroblasts. Nat. Cell Biol. 8, 594–600 [DOI] [PubMed] [Google Scholar]
- 24. Izumi Y., Ohta N., Hisata K., Raabe T., Matsuzaki F. (2006) Drosophila Pins-binding protein Mud regulates spindle-polarity coupling and centrosome organization. Nat. Cell Biol. 8, 586–593 [DOI] [PubMed] [Google Scholar]
- 25. Bowman S. K., Neumüller R. A., Novatchkova M., Du Q., Knoblich J. A. (2006) The Drosophila NuMA homolog Mud regulates spindle orientation in asymmetric cell division. Dev. Cell 10, 731–742 [DOI] [PubMed] [Google Scholar]
- 26. Colombo K., Grill S. W., Kimple R. J., Willard F. S., Siderovski D. P., Gönczy P. (2003) Translation of polarity cues into asymmetric spindle positioning in Caenorhabditis elegans embryos. Science 300, 1957–1961 [DOI] [PubMed] [Google Scholar]
- 27. Gotta M., Dong Y., Peterson Y. K., Lanier S. M., Ahringer J. (2003) Asymmetrically distributed C. elegans homologs of AGS3/PINS control spindle position in the early embryo. Curr. Biol. 13, 1029–1037 [DOI] [PubMed] [Google Scholar]
- 28. Srinivasan D. G., Fisk R. M., Xu H., van den Heuvel S. (2003) A complex of LIN-5 and GPR proteins regulates G protein signaling and spindle function in C. elegans. Genes Dev. 17, 1225–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bellaïche Y., Radovic A., Woods D. F., Hough C. D., Parmentier M. L., O'Kane C. J., Bryant P. J., Schweisguth F. (2001) The partner of Inscuteable/Discs-large complex is required to establish planar polarity during asymmetric cell division in Drosophila. Cell 106, 355–366 [DOI] [PubMed] [Google Scholar]
- 30. Sans N., Wang P. Y., Du Q., Petralia R. S., Wang Y. X., Nakka S., Blumer J. B., Macara I. G., Wenthold R. J. (2005) mPins modulates PSD-95 and SAP102 trafficking and influences NMDA receptor surface expression. Nat. Cell Biol. 7, 1179–1190 [DOI] [PubMed] [Google Scholar]
- 31. Zhu J., Shang Y., Xia C., Wang W., Wen W., Zhang M. (2011) Guanylate kinase domains of the MAGUK family scaffold proteins as specific phospho-protein-binding modules. EMBO J. 30, 4986–4997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yu F., Kuo C. T., Jan Y. N. (2006) Drosophila neuroblast asymmetric cell division. Recent advances and implications for stem cell biology. Neuron 51, 13–20 [DOI] [PubMed] [Google Scholar]
- 33. Kaushik R., Yu F., Chia W., Yang X., Bahri S. (2003) Subcellular localization of LGN during mitosis. Evidence for its cortical localization in mitotic cell culture systems and its requirement for normal cell cycle progression. Mol. Biol. Cell 14, 3144–3155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nipper R. W., Siller K. H., Smith N. R., Doe C. Q., Prehoda K. E. (2007) Gαi generates multiple Pins activation states to link cortical polarity and spindle orientation in Drosophila neuroblasts. Proc. Natl. Acad. Sci. U.S.A. 104, 14306–14311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Otwinowski Z., Minor W. (1997) Methods in Enzymology, Vol. 276, pp. 307–326, Elsevier, New York: [DOI] [PubMed] [Google Scholar]
- 36. Vagin A., Teplyakov A. (1997) MOLREP: an automated program for molecular replacement. J. Appl. Crystallogr. 30, 1022–1025 [Google Scholar]
- 37. Murshudov G. N., Vagin A. A., Dodson E. J. (1997) Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D 53, 240–255 [DOI] [PubMed] [Google Scholar]
- 38. Adams P. D., Afonine P. V., Bunkóczi G., Chen V. B., Davis I. W., Echols N., Headd J. J., Hung L. W., Kapral G. J., Grosse-Kunstleve R. W., McCoy A. J., Moriarty N. W., Oeffner R., Read R. J., Richardson D. C., Richardson J. S., Terwilliger T. C., Zwart P. H. (2010) PHENIX. A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Emsley P., Cowtan K. (2004) Coot. Model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 40. McCudden C. R., Willard F. S., Kimple R. J., Johnston C. A., Hains M. D., Jones M. B., Siderovski D. P. (2005) Gα selectivity and inhibitor function of the multiple GoLoco motif protein GPSM2/LGN. Biochim. Biophys. Acta 1745, 254–264 [DOI] [PubMed] [Google Scholar]
- 41. Adhikari A., Sprang S. R. (2003) Thermodynamic characterization of the binding of activator of G protein signaling 3 (AGS3) and peptides derived from AGS3 with Gαi1. J. Biol. Chem. 278, 51825–51832 [DOI] [PubMed] [Google Scholar]
- 42. Kopein D., Katanaev V. L. (2009) Drosophila GoLoco-protein Pins is a target of Gαo-mediated G protein-coupled receptor signaling. Mol. Biol. Cell 20, 3865–3877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Smith N. R., Prehoda K. E. (2011) Robust spindle alignment in Drosophila neuroblasts by ultrasensitive activation of pins. Mol. Cell 43, 540–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yoshiura S., Ohta N., Matsuzaki F. (2012) Tre1 GPCR signaling orients stem cell divisions in the Drosophila central nervous system. Dev. Cell 22, 79–91 [DOI] [PubMed] [Google Scholar]
- 45. Chen V. B., Arendall W. B., 3rd, Headd J. J., Keedy D. A., Immormino R. M., Kapral G. J., Murray L. W., Richardson J. S., Richardson D. C. (2010) MolProbity. All-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 [DOI] [PMC free article] [PubMed] [Google Scholar]