Background: Heat-induced morphogenetic switch is Hsp90- and PKA-dependent.
Results: The G protein-coupled receptor Gpr1, upstream of PKA, regulates trehalose levels in glucose-grown cells.
Conclusion: The link between the PKA pathway and Hsp90-mediated regulation of heat-induced morphogenesis is trehalose.
Significance: Tight control of trehalose levels is required for morphogenesis in Candida albicans.
Keywords: Candida albicans, G Protein-coupled Receptor (GPCR), Hsp90, Morphogenesis, Protein Kinase A (PKA), Trehalose
Abstract
The ability to form hyphae in the human pathogenic fungus Candida albicans is a prerequisite for virulence. It contributes to tissue infection, biofilm formation, as well as escape from phagocytes. Cell elongation triggered by human body temperature involves the essential heat shock protein Hsp90, which negatively governs a filamentation program dependent upon the Ras-protein kinase A (PKA) pathway. Tight regulation of Hsp90 function is required to ensure fast appropriate response and maintenance of a wide range of regulatory and signaling proteins. Client protein activation by Hsp90 relies on a conformational change of the chaperone, whose ATPase activity is competitively inhibited by geldanamycin. We demonstrate a novel regulatory mechanism of heat- and Hsp90-dependent induced morphogenesis, whereby the nonreducing disaccharide trehalose acts as a negative regulator of Hsp90 release. By means of a mutant strain deleted for Gpr1, the G protein-coupled receptor upstream of PKA, we demonstrate that elevated trehalose content in that strain, resulting from misregulation of enzymatic activities involved in trehalose metabolism, disrupts the filamentation program in response to heat. Addition of geldanamycin does not result in hyphal extensions at 30 °C in the gpr1Δ/gpr1Δ mutant as it does in wild type cells. In addition, validamycin, a specific inhibitor of trehalase, the trehalose-degrading enzyme, inhibits cell elongation in response to heat and geldanamycin. These results place Gpr1 as a regulator of trehalose metabolism in C. albicans and illustrate that trehalose modulates Hsp90-dependent activation of client proteins and signaling pathways leading to filamentation in the human fungal pathogen.
Introduction
Candida albicans is an opportunistic human fungal pathogen that can cause superficial and severe invasive infections. Virulence of the fungus is in part dependent on the morphogenetic plasticity of C. albicans, as strains that are trapped in a particular growth form are avirulent (1–4). The complex trait of filamentous regulation is under the control of multiple signaling pathways, including the Ras-protein kinase A (PKA) pathway (5). This pathway regulates several biological processes in response to nutrient availability, such as stress resistance, growth, cell differentiation, and trehalose metabolism (6–8). In Saccharomyces cerevisiae, PKA activity is low, and trehalose levels increase upon nutrient starvation, whereas activation of the pathway by glucose or sucrose, via the G protein-coupled receptor Gpr1, results in low trehalose levels (9, 10). In C. albicans, the nature of the ligand of the homologous receptor Gpr1 remains controversial as the receptor is not required for sugar-induced cAMP signaling (7).
Trehalose is a nonreducing disaccharide that can be found in bacteria, fungi, plants, and insects. It is synthesized by two consecutive enzymatic reactions. The enzyme trehalose-6-phosphate synthase (TPS),3 encoded by TPS1, produces trehalose-6-phosphate, which is then converted into trehalose by the trehalose-6-phosphate phosphatase (TPP) enzyme encoded by TPS2 (11, 12). Trehalose biosynthesis plays a role in the virulence of different human and plant pathogenic fungi, as typically, deletion of the Tps1 and Tps2 orthologs leads to absence of trehalose formation, reduction or absence of virulence, and sensitivity to stress (13–21). However, molecular links among trehalose, the filamentous programs, and virulence have never been identified.
Human body temperatures induce filamentous growth in C. albicans, and this process is evoked by the release of repression by the essential molecular chaperone Hsp90 on its target proteins (22). The effect of Hsp90 on filamentation was originally suggested to be mediated via the Ras-PKA pathway, as strains deleted for positive regulators of the pathway are unable to form filaments upon compromise of Hsp90 function by its inhibitor geldanamycin, whereas strains deleted for negative regulators easily show the morphogenetic response (22). However, a clear point of interaction between the Ras-PKA pathway and Hsp90 could not be identified (23, 24).
In the current study, we provide evidence that supports a model of regulation of heat-induced filamentation by the chemical chaperone trehalose. In this model, the Ras-PKA pathway participates in the release of function of Hsp90 by regulating trehalose content, hence providing clues on the role of the signaling pathway in the Hsp90-dependent filamentation program. Heat-induced filamentation is severely reduced by the absence of Gpr1. We show that this defect is caused by increased intracellular trehalose content. In addition, validamycin, a trehalose analog, promotes trehalose accumulation and blocks filamentation in response to heat and geldanymycin. Our results clearly establish trehalose as a negative regulator of the heat-induced cell elongation program.
EXPERIMENTAL PROCEDURES
Strains and Growth Conditions
All C. albicans and S. cerevisiae strains used in this study are listed in Table 1. Under continuous shaking, C. albicans strains were grown at 37 °C, S. cerevisiae strains were grown at 30 °C. Strains were grown on rich medium (YP: 1% yeast extract, 2% bactopeptone) supplemented with 2% glucose (YPD) or 3% glycerol (YPG), or on synthetic complete medium (SC: 0.17% yeast nitrogen base without amino acids and without ammonium, supplemented with synthetic drop-out amino acid and nucleotide mixture as required, 0.5% ammonium sulfate) and supplemented with 2% glucose (SCD). Solid media contained 2% (w/v) Difco-agar in addition. When applicable, geldanamycin or validamycin was added to a concentration of 10 μm, unless stated otherwise.
TABLE 1.
C. albicans and S. cerevisiae strains and oligonucleotides used in this study
| Strain | Genotype | Source or reference |
|---|---|---|
| S. cerevisiae strains | ||
| W303-1A | MATa leu2–3, 112 ura3–1, trp1–92, his3–11, 15 ade2–1, can1–100 GAL, SUC2, MAL | 49 |
| LK41 | W303-1A gpr1Δ::URA3 | 9 |
| JS4 | W303-1A gpr1Δ::URA3 + CaGPR1int | This study |
| JS16 | W303-1A gpr1Δ::URA3 + ScGPR1int | This study |
| C. albicans strains | ||
| CAI4+URA | URA3/ura3Δ::λimm434 | 50 |
| HT10 | URA3/ura3Δ::λimm434 gpr1Δ::hisG/gpr1Δ::hisG | This study |
| LR2 | ura3Δ::λimm434/ura3Δ::λimm434 GPR1-URA3 /gpr1Δ::hisG | 7 |
| BSC7 | URA3/ura3Δ::λimm434 cdc25Δ::cmLEU2/cdc25Δ::cdHIS1 | This study |
| CR216 | ura3Δ::λimm434/ura3Δ::λimm434 | 8 |
| cdc35Δ::hisG/cdc35Δ::hisG-URA3-hisG | ||
| BSI3 | URA3/ura3Δ::λimm434 | This study |
| ira2Δ::FRT/ira2Δ::FRT | ||
| Oligonucleotides | ||
| pCaGPR1tF | GCAGTAAGAGTCACCAAAAAAAAAAAACGACAAACAAGTGATCCGAAGTGTGACGAATAAAGCAAACTCTCCAACTCAAAATGCCGGACCTAATATCAATAGC | |
| pCaGPR1tR | GAGCGRCATTCATGTCGGCTACTTGTCAATTTGTATTACGTTCCTTACTTTCCATTTTCAAACATCGCGATACAAAAACTTTACATTGGGGGTCTTTTTGAG | |
Construction of Plasmids and Strains
The C. albicans GPR1 gene was amplified with primers pCaGPR1tF and pCaGPR1tR (Table 1). The resulting pCaGPR1t PCR fragment was co-transformed with a BglII/MluI linearized YEplac112-pScGPR1t fragment to S. cerevisiae. pCaGPR1t and pScGPR1t were cloned in pYX012KanMX for genomic integration by digestion with EcoRI/SphI and SacI/SphI, respectively, and ligation. S. cerevisiae strains JS4 and JS16 were obtained by integrating pYX012KanMX-pCaGPR1t or pYX012KanMX-pScGPR1t, respectively, in the genomic locus of GPR1 in LK41. C. albicans strain HT10 was constructed by reintegration of URA3 in the genomic locus of LDR8-5 (7).
Glucose Transport
For determination of glucose uptake, cells were harvested and washed with 25 mm MES buffer (pH 6) and resuspended in buffer at 80 mg of cells, wet weight, per ml. 40 μl of cell suspension was preincubated at 30 °C. 10 μl of [14C]glucose was added to the appropriate final concentration at a specific activity of 500 cpm/nmol of glucose. After 1 min, 5 ml of ice-cold water was added, and the cells were filtered through a glass microfiber filter (Whatman GF/C) prewet with the unlabeled glucose solution at the same concentration and immediately washed twice with 5 ml of ice-cold water. For each determination, three samples and two blank samples were taken. 10 μl of the labeled glucose solution was used to determine the specific activity. The radioactivity was determined in a liquid scintillation counter (Beckman Coulter LS6500). Transport activity is expressed as nmol of glucose min−1 (g dry weight)−1.
Biochemical Determinations
Intracellular levels of cAMP and trehalose were determined as described previously (25). Intracellular levels of glucose 6-phosphate and ATP were determined as described previously (26).
For determination of extracellular trehalose content, the Waters Breeze HPLC (Waters Corporation) was used. Samples were taken over time, and cells were centrifuged and discarded. Extracellular medium was used in the analysis. Samples were analyzed at a flow rate of 1 ml/min, using 5 mm H2SO4 as eluant. Results were processed with the Breeze software. Trehalose content was determined as mm trehalose.
Enzymatic Determinations
Trehalase activity was determined as described previously (27). Hexokinase activity was determined as described previously (28).
For TPS and TPP activity determination, cells were harvested at indicated time points and washed with and extracted in 50 mm imidazole (pH 6.3) supplemented with 1 mm EDTA, 2 mm MgCl2, and protease inhibitor and desalted on Sephadex columns. For TPS activity determinations, samples were added to an assay mixture solution, containing (at final concentration) 40 mm Tricine (pH 7), 10 mm MgCl2, 4 mm uridine diphosphoglucose, and 8 mm glucose 6-phosphate. In the control samples, glucose 6-phosphate was omitted. Formed trehalose 6-phosphate was determined by the addition of phosphoenolpyruvate, NADH, and lactate dehydrogenase and measured as a difference in A340 nm before and after addition of pyruvate kinase. The amount of protein in the samples was determined as described by Lowry et al. (29). TPS activity was expressed as nanokatals of TPS (g of protein)−1. For TPP activity determinations, samples were added to an assay mixture solution, containing (at final concentration) 40 mm Tricine (pH 7), 10 mm MgCl2, and 2.5 mm trehalose 6-phosphate. In the control samples, trehalose 6-phosphate was omitted. Formed trehalose was determined by detection of glucose formed after hydrolysis by trehalase. The amount of protein in the samples was determined as described by Lowry et al. (29). TPP activity was expressed as nanokatals of TPP (g protein)−1.
Microscopy
Cells were grown in synthetic complete medium containing glucose at 37 °C. Experiments with geldanamycin were conducted as described previously (22). Cells were observed with a Zeiss Axioplan 2 fluorescence microscope, and images were captured with an Axio-Cam HRm camera by using Axiovision 3.0 software (Carl Zeiss, Thornwood, NY).
Gene Expression Analysis
Total RNA was extracted using the TRIzol reagent method. Complementary DNA was prepared from DNase-treated RNA samples with a Reverse Transcription kit (Promega). Quantitative PCR was performed using a StepOnePlus real-time PCR system (Applied Biosystems). Reactions were prepared with the Kapa SYBR Fast kit (Kapabiosystem) according to the manufacturer's instructions. The -fold regulation of the target gene was calculated using the comparative Ct method, using TEF1 Ct to normalize the data.
RESULTS
Gpr1 Is Required for Lowering Trehalose Content during Active Growth in C. albicans
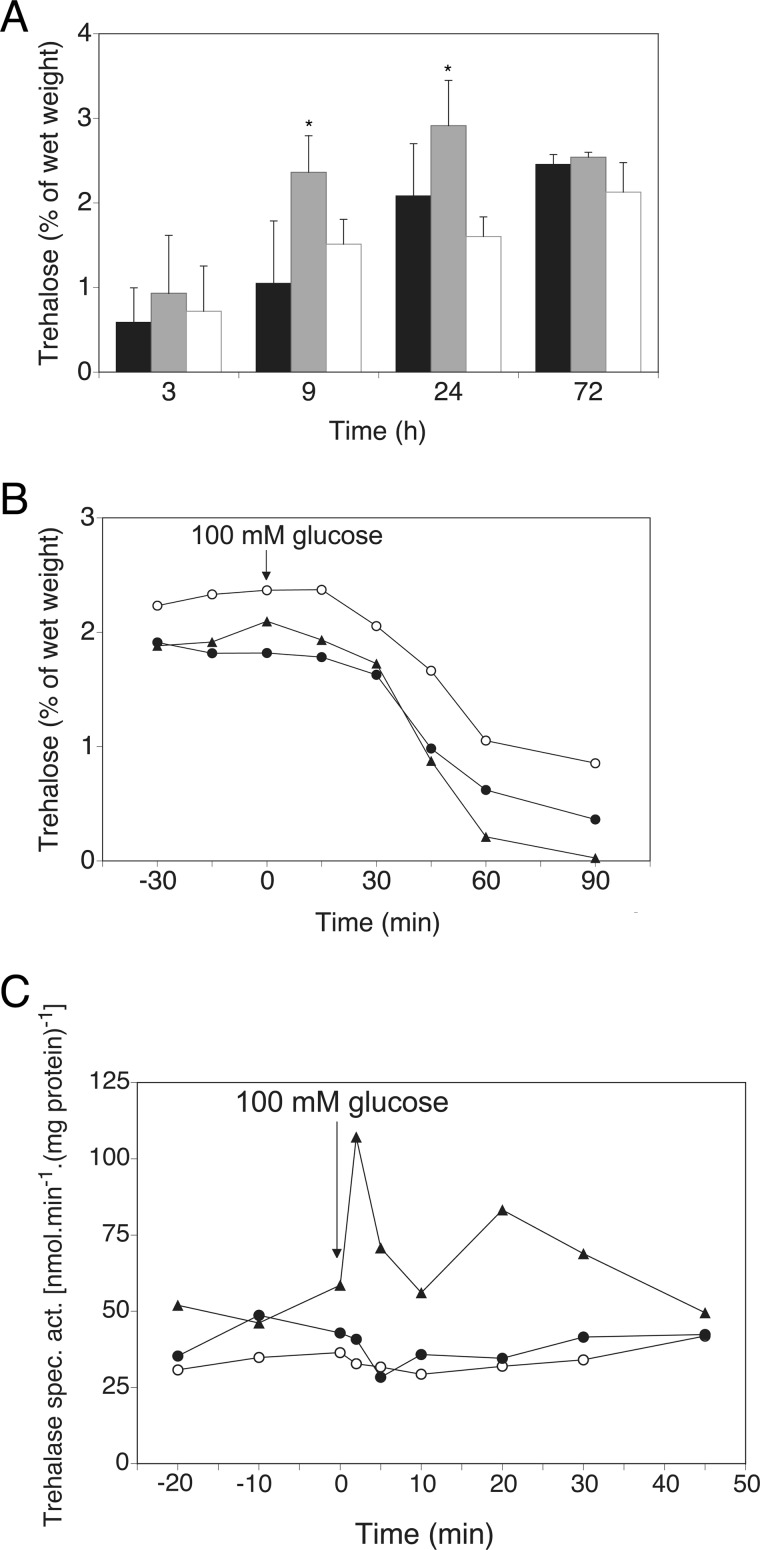
Previous findings have shown that absence of GPR1 in C. albicans leads to increased trehalose content as well as activity of the Tps1 enzyme, especially in heat shock conditions (30). We investigated the role of Gpr1 on trehalose content in C. albicans in conditions of 37 °C, the physiological temperature that is not experienced as a stress in the pathogenic fungus. Deletion of GPR1 resulted in increased levels of trehalose in exponentially growing cells (Fig. 1A), a growth phase during which trehalose does not normally accumulate. Significantly higher amounts of trehalose were measured in the deleted strain compared with wild type and GPR1-reintegrant strains. This peculiar accumulation occurred in glucose-containing growth conditions, however not in glycerol-containing media (data not shown). Defective export of trehalose in the surrounding medium was ruled out by HPLC analysis, which showed equal amounts of trehalose accumulating in the medium for all strains (data not shown). Higher trehalose content in the gpr1Δ/gpr1Δ strain did not impair the carbohydrate mobilization upon addition of glucose to sugar-deprived cells (Fig. 1B). We speculated that activation of trehalase, the enzyme that breaks down molecules of trehalose into glucose, would therefore occur prior to mobilization. Reports on neutral trehalase activity in C. albicans exist (31), but activation by resupplementation of missing nutrients has never been documented. In wild type S. cerevisiae, activation of neutral trehalase could be observed 2–5 min after glucose addition (Fig. 1C). However, only basal trehalase activity and no activation could be detected in C. albicans strains.
FIGURE 1.
Deletion of GPR1 leads to higher trehalose accumulation and normal trehalose mobilization. Trehalase activation is absent in C. albicans. A, trehalose levels are monitored over time at 37 °C, with a starting absorbance of 0.2 (A600 nm). Standard deviations (error bars) are calculated from three independent biological repeats. An asterisk (*) indicates significant difference compared with wild type (p < 0.05). The shaded bars represent, from black to white: wild type, gpr1Δ/gpr1Δ, gpr1Δ/gpr1Δ + riGPR1. B, trehalose levels are determined after addition of 100 mm glucose (time point 0) to glucose-deprived cells in fresh YP medium at 37 °C. ●, WT; ○, gpr1Δ/gpr1Δ; ▴, gpr1Δ/gpr1Δ + riGPR1. C, trehalase activation is determined after addition of 100 mm glucose (time point 0) to glucose-deprived cells in fresh YP medium at 37 °C. ●, WT C. albicans; ○, gpr1Δ/gpr1Δ C. albicans; ▴, WT S. cerevisiae.
Gpr1 in C. albicans is required to control basal trehalose levels in particular when cells are metabolically active and growing in favorable conditions. Absence of the receptor seems to mimic a nutrient-poor environment for the cells, leading to trehalose accumulation. However, Gpr1 does not play a role in the induction of trehalose breakdown, typically observed upon sugar addition, which differs from the situation in S. cerevisiae.
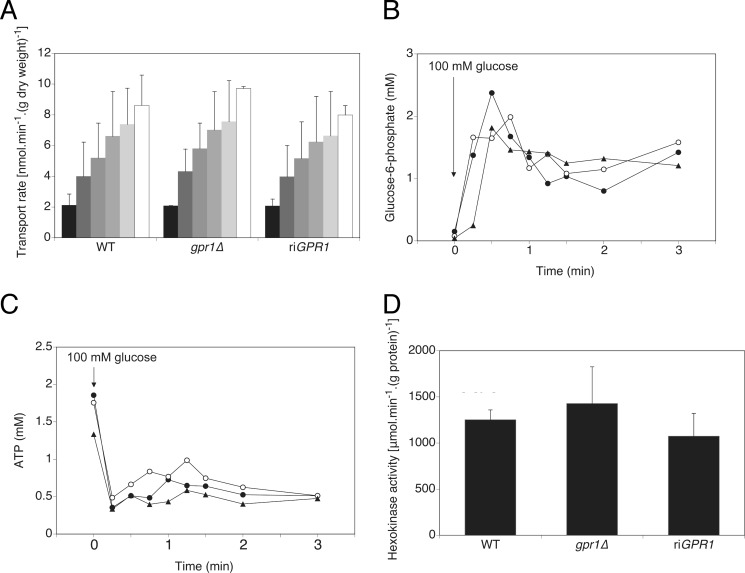
The substrates of trehalose are uridine diphosphoglucose and glucose 6-phosphate. They are converted into trehalose 6-phosphate in the first step of trehalose accumulation by the Tps1 enzyme (11). We aimed at determining whether increased trehalose content in gpr1Δ/gpr1Δ cells correlated with increased levels of glucose or glucose 6-phosphate. In none of the assays we performed we could detect a lower or higher level of these substrates in cells lacking GPR1, either before or after induction with glucose (Fig. 2). Specifically, Gpr1 does not play a role in the transport of glucose (Fig. 2A), and it does not control the rise of glucose phosphorylation following glucose addition (Fig. 2B). The transient increase of glucose 6-phosphate coincides with a drop of ATP levels, which was observed in both wild type and GPR1-deleted strains (Fig. 2C). The up-regulated conversion to trehalose could mask any increased glucose 6-phosphate production, possibly resulting from elevated hexokinase activity. However, the total hexo- and glucokinase activities were not significantly different between the two strains (Fig. 2D). Although these data were required to determine possible routes of regulation of trehalose metabolism via Gpr1, they evidently suggest other level(s) of regulation, one of them being the regulation of the trehalose synthesis enzymes, Tps1 and Tps2.
FIGURE 2.
Increased trehalose levels in gpr1Δ/gpr1Δ are not associated with altered glucose transport, glucose 6-phosphate, and ATP levels, and hexokinase activity. A, glucose transport is measured by adding increasing concentrations of radioactively labeled glucose. Progressive shading from black to white represents increasing glucose concentrations (1, 5, 10, 20, 50, and 100 mm). Standard deviations (error bars) are calculated from three independent biological repeats. B and C, glucose 6-phosphate (B) and ATP (C) levels were determined after addition of 100 mm glucose. ●, WT; ○, gpr1Δ/gpr1Δ; ▴, gpr1Δ/gpr1Δ + riGPR1. D, basal hexokinase activity is determined. Standard deviations are calculated from three independent biological repeats.
Gpr1 Strongly Regulates TPS Enzymatic Activity
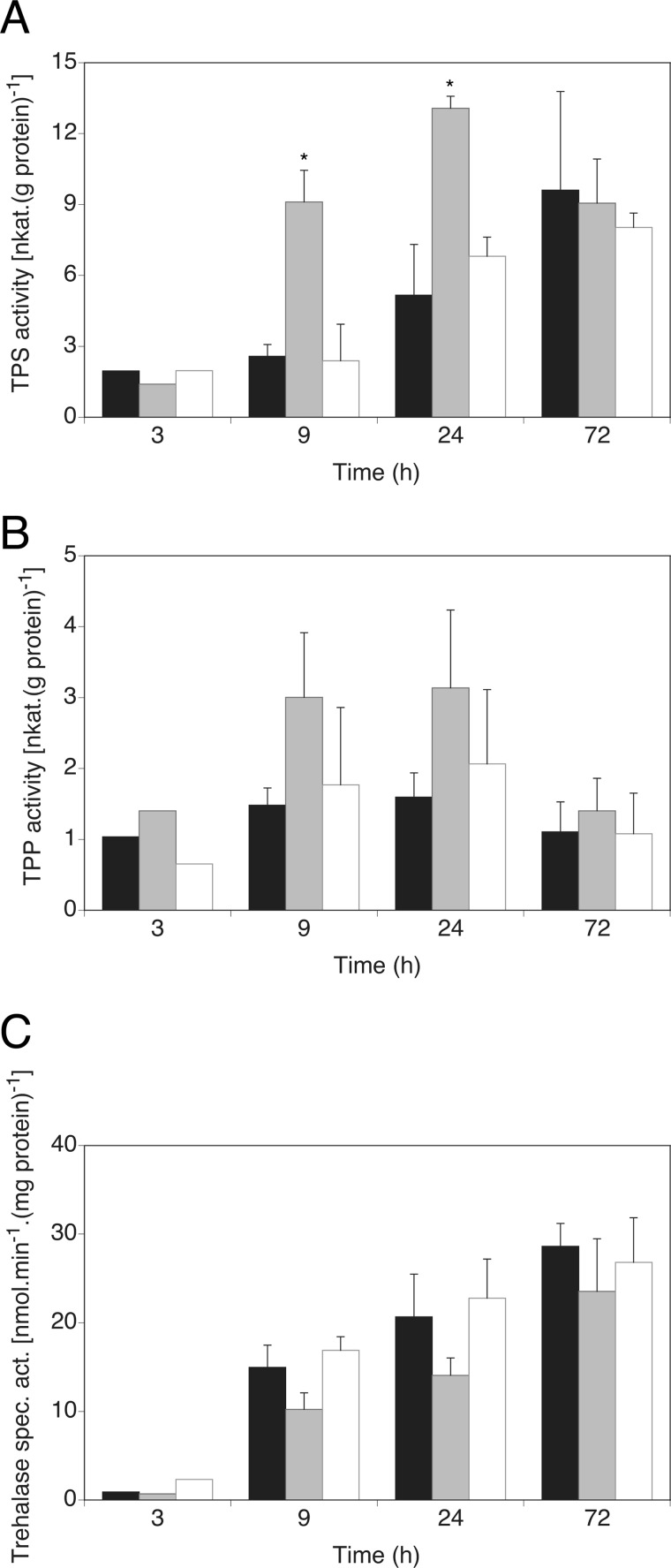
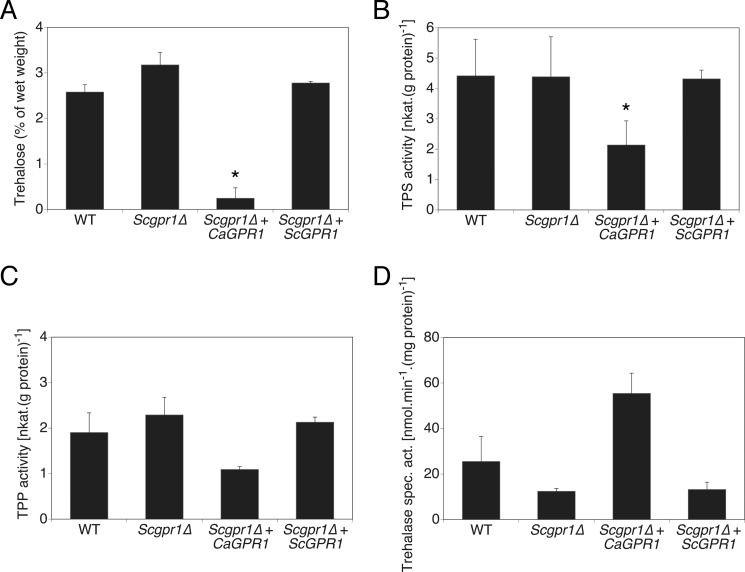
We measured TPS and TPP activities, the two main enzymatic activities responsible for trehalose synthesis, in C. albicans cells growing in glucose-containing medium for 3–72 h. A sharp rise in TPS activity was observed in gpr1Δ/gpr1Δ cells at 9 and 24 h of growth, coinciding with increased trehalose content at the same time of growth (Fig. 3A). The dephosphorylation step of trehalose 6-phosphate into trehalose by TPP was somewhat increased as well, but statistical significance could never be obtained (Fig. 3B). The steadily increased TPP activity is likely necessary to ensure trehalose 6-phosphate to be continuously converted into trehalose, as accumulation of this intermediate molecule is toxic to cells (19, 32). Transcriptional regulation of the TPS1 gene was not modulated differentially in wild type and gpr1Δ/gpr1Δ cells (data not shown), suggesting a post-translational regulatory mechanism. An increase of trehalose content in C. albicans is mostly accompanied by a decrease in neutral trehalase activity, at least as a response to heat or oxidative stress (33, 34). In nonstress conditions, neutral trehalase activity increased over time during growth of wild type cells (Fig. 3C). In absence of GPR1, trehalase activity could never be demonstrated as significantly different from wild type levels. Nevertheless, partial impairment of this activity was always observed in the mutant strain. To corroborate these findings, a strong and opposite direct effect on trehalose metabolism was apparent upon expression of C. albicans GPR1 in a strain of S. cerevisiae deleted for the homologous receptor (Fig. 4). Trehalose content was significantly reduced as well as TPS activity in this strain. Similar to the assays performed in C. albicans cells, trehalase and TPP activities seemed to be differentially regulated in the presence of C. albicans Gpr1, but the difference could not be determined as significant. Hence, the aberrant trehalose accumulation observed in C. albicans gpr1Δ/gpr1Δ cells seems to result from a higher rate of synthesis, mainly governed by post-transcriptional regulation of Tps1 and a lower catabolism.
FIGURE 3.
Increased trehalose levels in gpr1Δ/Δ correlate with aberrant activities of the trehalose metabolism and catabolism enzymes. TPS (A) TPP (B), and trehalase (C) activities are monitored over time. Standard deviations (error bars) are calculated from three independent biological repeats. An asterisk (*) indicates significant difference compared with wild type (p < 0.05). The shaded bars represent, from black to white: wild type, gpr1Δ/gpr1Δ, gpr1Δ/gpr1Δ + riGPR1.
FIGURE 4.
Expression of C. albicans GPR1 in a S. cerevisiae gpr1Δ strain mainly results in absence of trehalose accumulation and a reduced TPS activity. Trehalose levels (A), TPS (B), TPP (C), and trehalase activity (D) are measured in cells grown for 24 h in rich medium. Standard deviations (error bars) are calculated from three independent biological repeats. An asterisk (*) indicates significant difference compared with wild type (p < 0.05).
Heat-induced Morphogenesis Is Impaired by Increased Trehalose Levels
C. albicans wild type cells typically undergo a morphogenetic switch from yeast to filamentous forms at elevated temperature (>35 °C) in growth conditions with neutral pH (35). We had previously shown that deletion of GPR1 impaired invasive growth on solid media but not in liquid cultures in presence of serum (7). However, we show here that the gpr1Δ/gpr1Δ mutant was found to be impaired in the morphogenetic switch induced by elevated temperature (Fig. 5A, panels 1 and 2). In the conditions tested, the mutant did not display any reduced growth rate.
FIGURE 5.
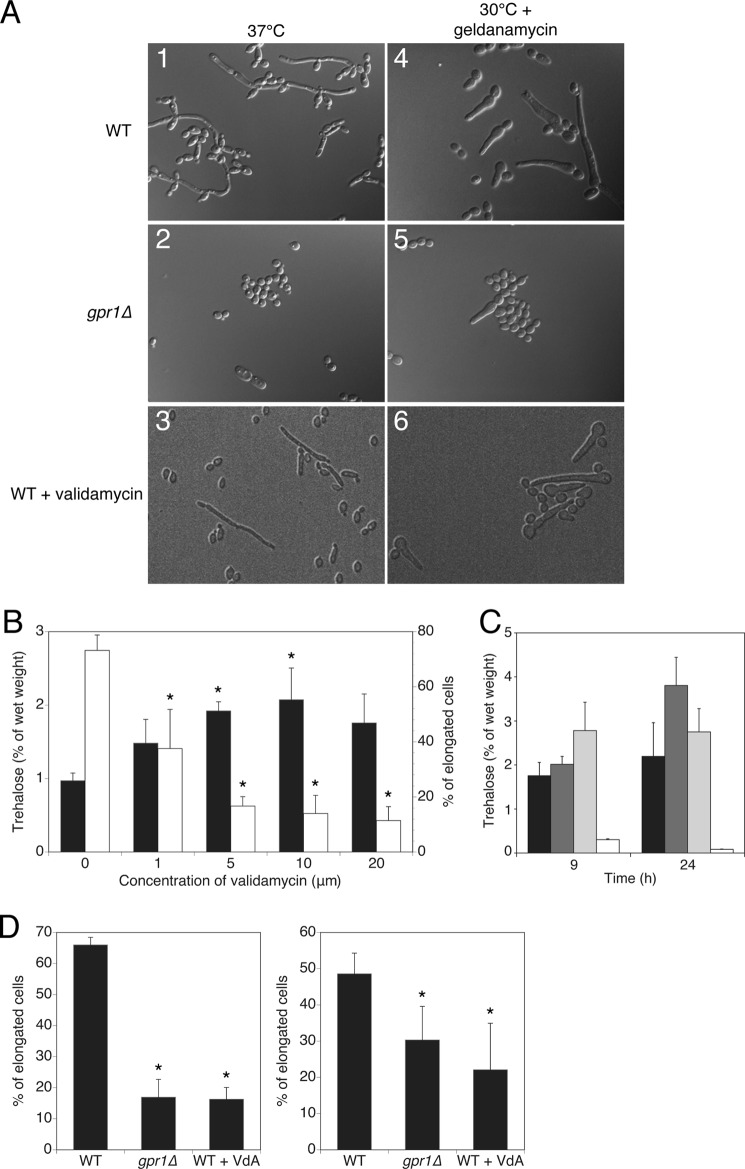
Increased trehalose levels counteract heat-induced filamentation. A, C. albicans cell morphology after 9 h at 37 °C (left column) and at 30 °C with 10 μm geldanamycin (right column) in wild type cells (top panels 1 and 4), in gpr1Δ/gpr1Δ cells (middle panels 2 and 5) and in wild type cells in the presence of 10 μm validamycin (bottom panels 3 and 6). B, trehalose content (white bars) and percentage of elongated cell phenotypes (black bars) in cells growing in the presence of increasing amount of trehalase inhibitor. C, trehalose content in strains deleted for three major regulators of the PKA pathway in C. albicans, namely CDC25 (dark gray bars), CDC35 (light gray bars), and IRA2 (white bars). Wild type levels are shown as reference levels (black bars). D, addition of validamycin mimicking the deletion of GPR1 in inhibiting cell elongation in response to heat (left graph) and in response to the Hsp90 inhibitor geldanamycin (right graph). Basal trehalose levels and percentage of elongated cells were determined after 9 h. An asterisk (*) indicates significant difference compared with wild type (p < 0.05).
We hypothesized that presence of elevated trehalose content in gpr1Δ/gpr1Δ cells at 37 °C may alter the heat-induced filamentation phenotype in the mutant strain. To further assess the role of trehalose in C. albicans cell elongation, we made use of validamycin, a specific inhibitor of trehalase, the enzyme breaking down trehalose. Validamycin has in vitro activity against several fungal trehalases, including the one of S. cerevisiae (36) and C. albicans (31). In vivo activity has been demonstrated against the trehalase enzyme of Rhizoctonia solani, the pathogen responsible for sheath light of rice plants (37). We demonstrate here that addition of increasing amounts of the trehalose analog led to increasing amounts of trehalose being accumulated in vivo (Fig. 5B), without altering cell growth rate (data not shown). As little as 5 μm compound ensured a 100% increase in intracellular trehalose levels. The increase in trehalose content coincided with a reduction up to 75% of elongated cell phenotypes observed at 37 °C, as the concentration of the inhibitor increased. Wild type cells treated with validamycin had a clear reduction in cell elongation (Fig. 5A, panel 3). These data demonstrate for the first time that trehalose is a potent inhibitor of morphogenesis in C. albicans.
Trehalose Restrains Cell Elongation by Release of Hsp90 Inhibition
The specific condition of cellular transition caused by elevated temperature is orchestrated by the molecular chaperone Hsp90 in C. albicans (22). Compromising Hsp90 function, by elevated temperature or chemical inhibitors, such as geldanamycin, leads to release of inhibition imposed on the morphogenetic program. We speculated that elevated trehalose levels could lead to a defect in the filamentation program governed by Hsp90, as it impaired cell elongation at 37 °C. We therefore examined whether the induction of filamentation resulting from Hsp90 inhibition by means other than temperature was also impaired in gpr1Δ/gpr1Δ cells. As gpr1Δ/gpr1Δ cells accumulate trehalose even at 30 °C while actively growing (data not shown and Ref. 30), we investigated whether geldanamycin was able to induce the filamentation phenotype in the mutant strain. Whereas extrusions of the cells were readily observed in wild type upon addition of geldanamycin at the noninducing temperature of 30 °C, the gpr1Δ/gpr1Δ mutant was partially defective in this response (Fig. 5A, panels 4 and 5). In addition, the observed filamentation in response to the Hsp90 inhibitor in an ira2Δ/ira2Δ mutant and absence thereof in a cdc25Δ/cdc25Δ or cdc35Δ/cdc35Δ mutant (22) equally correlates with low and high trehalose levels, respectively (Fig. 5C). Reinforcing the finding that trehalose can prohibit the cell elongation program centered around Hsp90 release, validamycin was able to counteract the effect of geldanamycin (Fig. 5A, panel 6).
As deletion of GPR1 or addition of validamycin to wild type cells increases trehalose content 2-fold, both conditions led to a significant decrease of cell elongation as well. In heat-inducing conditions at 37 °C, cell elongation was reduced by 75% in both conditions, whereas at the noninducing temperature of 30 °C but in the presence of the Hsp90 inhibitor geldanamycin a reduction of 25% was recorded in both cases (Fig. 5D). All of these data firmly establish trehalose as a negative regulator of filamentous development in C. albicans.
DISCUSSION
C. albicans Gpr1 Participates in the Maintenance of Trehalose Homeostasis at the Human Body Temperature
The G protein-coupled receptor Gpr1 in C. albicans was identified based on its high level of amino acid sequence identity with the ortholog of the model yeast S. cerevisiae. Despite the high degree of sequence homology between the two, their functions have diverged substantially, the most striking difference being the apparent loss of its role in fast glucose signaling to adenylate cyclase for cAMP production (7). A mild induction of TPS activity at 43 °C in the gpr1Δ/gpr1Δ strain and avirulence of a gpr1Δ/gpr1Δ tps2Δ/tps2Δ strain suggested a connection between the G protein-coupled receptor and trehalose metabolism, a feature suggested to be downstream of PKA (30). It is now clear from the present findings that these phenotypes were caused by the role of Gpr1 in negatively regulating the trehalose biosynthesis activity when cells are actively growing under optimal conditions and do not require trehalose as a compatible solute. Typically, trehalose is synthesized at the onset of nutrient depletion. Hydrolysis of trehalose upon readdition of nutrients is equally important, as it is an essential event in many cellular processes such as fungal spore germination, insect flight, and the resumption of growth in resting cells (38), as well as germ tube formation in C. albicans (39). This tight regulation of trehalose content is perturbed in a gpr1Δ/gpr1Δ strain, due to an increased activity of the trehalose synthesis enzymes Tps1 and Tps2 and a concomitant inactivation of neutral trehalase. One hypothesis is that in absence of Gpr1, the activity of the Ras-PKA pathway of C. albicans is lowered during exponential growth, which in turn reduces the repression of the trehalose biosynthesis enzymes. To gain support for this hypothesis is the finding that several mutants of components of the pathway display an aberrant trehalose content too. Mutants in genes that activate the pathway, such as CDC25 and CDC35, display a higher trehalose content than wild type, whereas mutants of inhibitors of the pathway, such as IRA2, present lower levels of trehalose. Contrary to previous reports (20), we could identify four and six potential PKA phosphorylation sites of the consensus sequence (R/K)X1–2(S/T) in Tps1 and Tps2, respectively, with two in each protein having the second most abundant consensus sequence KKX(S/T) (40). The pathway may therefore regulate enzymatic activities via phosphorylation by PKA rather than gene expression in C. albicans.
It is noteworthy to state that this phenomenon of trehalose accumulation in gpr1Δ/gpr1Δ cells is a glucose-dependent process, in contrast to the well characterized accumulation of the carbohydrate when glucose gets depleted. Gpr1 therefore seems to behave as a glucose sensor, yet not involved in fast signaling of glucose, but rather involved in the long term maintenance of glucose signaling leading to trehalose metabolism regulation.
Tight Control of Trehalose Levels Is Required for Filamentation
We used the trehalase inhibitor validamycin to validate the finding that trehalose is the causative agent that diminishes the potency of gpr1Δ/gpr1Δ cells to elongate. We establish the potency of validamycin to increase trehalose content in vivo in C. albicans. Addition of validamycin results in 100% increase in trehalose levels and simultaneous reduction of 75% in cell elongation. This links morphogenetic differences between wild type and gpr1Δ/gpr1Δ cells to trehalose content. Indeed, the observed reduction of cell elongation as well as trehalose accumulation of the gpr1Δ/gpr1Δ strain at 37 °C was comparable with that of the wild type strain treated with validamycin. The data here demonstrate that although high levels of trehalose in the gpr1Δ/gpr1Δ strain are not detrimental to cell growth, they diminish the ability of the mutant cells to undergo the morphogenetic switch. Hence, increased trehalose content correlates with inefficient cell elongation at 37 °C in C. albicans. Coincidently, other mutants of the Ras-PKA pathway also display altered phenotypes at 37 °C, which remarkably correlates with their trehalose content (22).4 These findings emphasize the biological connection among the Ras-PKA pathway, filamentation, and trehalose content.
A Biological Link among the Storage Carbohydrate, Filamentation, and Virulence Originates from the Membrane Receptor Gpr1 in C. albicans
Heat-induced filamentation in C. albicans is centered on the relief of the heat shock chaperone Hsp90 from the morphological signaling machinery (22). It can be mimicked efficiently at 30 °C by addition of geldanamycin, an Hsp90 inhibitor. Here, we propose that trehalose serves as a novel regulatory factor of the Hsp90-mediated cell elongation program. Down-regulation of PKA signaling leads to trehalose accumulation, which in turn accentuates Hsp90 function. As an important part of the heat shock response, trehalose binds to partially folded intermediate conformations of proteins (41). It has been reported that trehalose binds to heat shock proteins and can chaperone their activity (41, 42). However, whether trehalose aids the function of the chaperone, or vice versa, remains unclear. In the context of heat-induced filamentation, it becomes apparent from our findings that trehalose reinforces the chaperone function of Hsp90 on some of its client proteins belonging to the cell elongation pathway. The possibility that trehalose directly inhibits components of the Ras-PKA pathway cannot be excluded. However, serum, which activates PKA as well induces the morphogenetic program, is a potent activator of cell elongation in the gpr1Δ/gpr1Δ strain (7). This tends to indicate that the effect of trehalose is specific on Hsp90, which governs filamentation in response to heat.
Studies on trehalose relate to its physical and chemical properties or to the regulation of its biosynthesis and degradation, without directly addressing the interaction point between the carbohydrate and the targeted molecular process. In other words, we often know that trehalose is involved, but rarely do we know how and at which molecular level it plays its function. In the present study, we have identified a clear biological link among the Ras-PKA pathway, the increased trehalose synthesis, and the filamentation process governed in response to the human body temperature. We can also extrapolate from our findings on filamentation that this molecular link is likely to be the chaperone protein Hsp90.
How May Trehalose Inhibit the Morphogenetic Switch?
The present findings raise the interesting question of how a sugar molecule can modulate the signaling process leading to cell differentiation. At high temperature, trehalose can serve the role of a chaperone, which protects proteins from heat-induced denaturation and aggregation. Trehalose is a very potent protein stabilizer, in that it keeps proteins in native states in unfavorable conditions (43). Hence, overproduction of trehalose may overwhelm normal protein functioning (44). The obvious stabilized target in this study is the chaperone Hsp90, the central player in heat-induced filamentation in C. albicans (22). In that context, the presence of high trehalose content at inappropriate timing in a gpr1Δ/gpr1Δ strain or other positive regulators of the PKA pathway may maintain Hsp90 in a stable conformation, ensuring effective inhibition of the filamentation program even at the permissive temperature. Stable Hsp90 binds to its client proteins, disabling their function (45).
Second, the influence of trehalose on Hsp90 may be mediated through Hsf1, rather than Hsp90 itself. The hyperphosphorylation of Hsf1 leading to HSP expression, generally seen after heat shock, has been shown to be dependent upon mild increase of trehalose levels in S. cerevisiae (46). In C. albicans, 86% of up-regulated genes after heat shock, especially the HSP family genes, depend on Hsf1 for their up-regulation (47). In C. albicans, the increase in trehalose levels may lead to the repression of Hsp90 relief through the phosphorylation status of Hsf1. As Hsf1 participates in the basal expression of 75 genes even in the absence of heat shock, the essential transcription factor thus plays a key role in the modulation of protein folding-related functions in C. albicans even in the absence of stress. Interestingly, disaccharide metabolism was down-regulated by heat shock following Hsf1 depletion (47). A feedback loop to avoid overproduction of trehalose may then occur via the Hsf1 transcription factor.
Third, the accumulation of trehalose may initiate a redistribution of Hsp90 in the cells. In S. cerevisiae, Hsp90 translocates from the cytosol to the nucleus upon gradual depletion of glucose in the medium, whereas acute withdrawal of sugar does not elicit the same response (48). As depletion of glucose toward stationary phase coincides with formation of trehalose (12), one could therefore envisage that trehalose may disturb Hsp90 cellular distribution.
In conclusion, these findings preclude a direct correlation between the bioprotecting and stabilizing properties of trehalose and a biological activity in the filamentation process in C. albicans. Maintenance of low trehalose levels is required for the cell elongation pathway governed by the chaperone Hsp90 to be activated in the appropriate growth conditions. Trehalose may also perturb other molecular processes regulated by Hsp90. In that context, the effect of trehalose on Hsp90-dependent antifungal drug resistance mechanism remains to be established.
Acknowledgments
We thank Cindy Colombo, Deborah Seys, and Willy Verheyden for excellent technical assistance and Nico Vangoethem for graphical support.
This work was funded by Flemish Science Foundation, FWO Grants G.0804.11 and WO.026.11N and by research funds of KU Leuven Grants GOA/2007/08 and CREA/11/012.
J. Serneels, H. Tournu, and P. Van Dijck, unpublished results.
- TPS
- trehalose-6-phosphate synthase
- TPP
- trehalose-6-phosphate phosphatase
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine.
REFERENCES
- 1. Bahn Y. S., Staab J., Sundstrom P. (2003) Increased high-affinity phosphodiesterase PDE2 gene expression in germ tubes counteracts CAP1-dependent synthesis of cyclic AMP, limits hypha production and promotes virulence of Candida albicans. Mol. Microbiol. 50, 391–409 [DOI] [PubMed] [Google Scholar]
- 2. Cao F., Lane S., Raniga P. P., Lu Y., Zhou Z., Ramon K., Chen J., Liu H. (2006) The Flo8 transcription factor is essential for hyphal development and virulence in Candida albicans. Mol. Biol. Cell 17, 295–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lo H. J., Köhler J. R., DiDomenico B., Loebenberg D., Cacciapuoti A., Fink G. R. (1997) Nonfilamentous C. albicans mutants are avirulent. Cell 90, 939–949 [DOI] [PubMed] [Google Scholar]
- 4. Whiteway M., Bachewich C. (2007) Morphogenesis in Candida albicans. Annu. Rev. Microbiol. 61, 529–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Biswas S., Van Dijck P., Datta A. (2007) Environmental sensing and signal transduction pathways regulating morphopathogenic determinants of Candida albicans. Microbiol. Mol. Biol. Rev. 71, 348–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bockmühl D. P., Krishnamurthy S., Gerads M., Sonneborn A., Ernst J. F. (2001) Distinct and redundant roles of the two protein kinase A isoforms Tpk1p and Tpk2p in morphogenesis and growth of Candida albicans. Mol. Microbiol. 42, 1243–1257 [DOI] [PubMed] [Google Scholar]
- 7. Maidan M. M., De Rop L., Serneels J., Exler S., Rupp S., Tournu H., Thevelein J. M., Van Dijck P. (2005) The G protein-coupled receptor Gpr1 and the Gα protein Gpa2 act through the cAMP-protein kinase A pathway to induce morphogenesis in Candida albicans. Mol. Biol. Cell 16, 1971–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rocha C. R., Schröppel K., Harcus D., Marcil A., Dignard D., Taylor B. N., Thomas D. Y., Whiteway M., Leberer E. (2001) Signaling through adenylyl cyclase is essential for hyphal growth and virulence in the pathogenic fungus Candida albicans. Mol. Biol. Cell 12, 3631–3643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kraakman L., Lemaire K., Ma P., Teunissen A. W., Donaton M. C., Van Dijck P., Winderickx J., de Winde J. H., Thevelein J. M. (1999) A Saccharomyces cerevisiae G protein-coupled receptor, Gpr1, is specifically required for glucose activation of the cAMP pathway during the transition to growth on glucose. Mol. Microbiol. 32, 1002–1012 [DOI] [PubMed] [Google Scholar]
- 10. Lemaire K., Van de Velde S., Van Dijck P., Thevelein J. M. (2004) Glucose and sucrose act as agonist and mannose as antagonist ligands of the G protein-coupled receptor Gpr1 in the yeast Saccharomyces cerevisiae. Mol. Cell 16, 293–299 [DOI] [PubMed] [Google Scholar]
- 11. Reinders A., Bürckert N., Hohmann S., Thevelein J. M., Boller T., Wiemken A., De Virgilio C. (1997) Structural analysis of the subunits of the trehalose-6-phosphate synthase/phosphatase complex in Saccharomyces cerevisiae and their function during heat shock. Mol. Microbiol 24, 687–695 [DOI] [PubMed] [Google Scholar]
- 12. Thevelein J. M. (1984) Regulation of trehalose mobilization in fungi. Microbiol. Rev. 48, 42–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Al-Bader N., Vanier G., Liu H., Gravelat F. N., Urb M., Hoareau C. M., Campoli P., Chabot J., Filler S. G., Sheppard D. C. (2010) Role of trehalose biosynthesis in Aspergillus fumigatus development, stress response, and virulence. Infect. Immun. 78, 3007–3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fillinger S., Chaveroche M. K., van Dijck P., de Vries R., Ruijter G., Thevelein J., d'Enfert C. (2001) Trehalose is required for the acquisition of tolerance to a variety of stresses in the filamentous fungus Aspergillus nidulans. Microbiology 147, 1851–1862 [DOI] [PubMed] [Google Scholar]
- 15. Foster A. J., Jenkinson J. M., Talbot N. J. (2003) Trehalose synthesis and metabolism are required at different stages of plant infection by Magnaporthe grisea. EMBO J. 22, 225–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ngamskulrungroj P., Himmelreich U., Breger J. A., Wilson C., Chayakulkeeree M., Krockenberger M. B., Malik R., Daniel H. M., Toffaletti D., Djordjevic J. T., Mylonakis E., Meyer W., Perfect J. R. (2009) The trehalose synthesis pathway is an integral part of the virulence composite for Cryptococcus gattii. Infect. Immun. 77, 4584–4596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Petzold E. W., Himmelreich U., Mylonakis E., Rude T., Toffaletti D., Cox G. M., Miller J. L., Perfect J. R. (2006) Characterization and regulation of the trehalose synthesis pathway and its importance in the pathogenicity of Cryptococcus neoformans. Infect. Immun. 74, 5877–5887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Puttikamonkul S., Willger S. D., Grahl N., Perfect J. R., Movahed N., Bothner B., Park S., Paderu P., Perlin D. S., Cramer R. A., Jr. (2010) Trehalose-6-phosphate phosphatase is required for cell wall integrity and fungal virulence but not trehalose biosynthesis in the human fungal pathogen Aspergillus fumigatus. Mol. Microbiol. 77, 891–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van Dijck P., De Rop L., Szlufcik K., Van Ael E., Thevelein J. M. (2002) Disruption of the Candida albicans TPS2 gene encoding trehalose-6-phosphate phosphatase decreases infectivity without affecting hypha formation. Infect. Immun. 70, 1772–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zaragoza O., Blazquez M. A., Gancedo C. (1998) Disruption of the Candida albicans TPS1 gene encoding trehalose-6-phosphate synthase impairs formation of hyphae and decreases infectivity. J. Bacteriol. 180, 3809–3815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zaragoza O., de Virgilio C., Pontón J., Gancedo C. (2002) Disruption in Candida albicans of the TPS2 gene encoding trehalose-6-phosphate phosphatase affects cell integrity and decreases infectivity. Microbiology 148, 1281–1290 [DOI] [PubMed] [Google Scholar]
- 22. Shapiro R. S., Uppuluri P., Zaas A. K., Collins C., Senn H., Perfect J. R., Heitman J., Cowen L. E. (2009) Hsp90 orchestrates temperature-dependent Candida albicans morphogenesis via Ras1-PKA signaling. Curr. Biol. 19, 621–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shapiro R. S., Sellam A., Tebbji F., Whiteway M., Nantel A., Cowen L. E. (2012) Pho85, Pcl1, and Hms1 signaling governs Candida albicans morphogenesis induced by high temperature or Hsp90 compromise. Curr. Biol. 22, 461–470 [DOI] [PubMed] [Google Scholar]
- 24. Xu W., Mitchell A. P. (2012) Fungal Morphogenesis: in hot pursuit. Curr. Biol. 22, R225–227 [DOI] [PubMed] [Google Scholar]
- 25. Colombo S., Ma P., Cauwenberg L., Winderickx J., Crauwels M., Teunissen A., Nauwelaers D., de Winde J. H., Gorwa M. F., Colavizza D., Thevelein J. M. (1998) Involvement of distinct G-proteins, Gpa2 and Ras, in glucose- and intracellular acidification-induced cAMP signalling in the yeast Saccharomyces cerevisiae. EMBO J. 17, 3326–3341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rolland F., Wanke V., Cauwenberg L., Ma P., Boles E., Vanoni M., de Winde J. H., Thevelein J. M., Winderickx J. (2001) The role of hexose transport and phosphorylation in cAMP signalling in the yeast Saccharomyces cerevisiae. FEMS Yeast Res. 1, 33–45 [DOI] [PubMed] [Google Scholar]
- 27. Pernambuco M. B., Winderickx J., Crauwels M., Griffioen G., Mager W. H., Thevelein J. M. (1996) Glucose-triggered signalling in Saccharomyces cerevisiae: different requirements for sugar phosphorylation between cells grown on glucose and those grown on nonfermentable carbon sources. Microbiology 142, 1775–1782 [DOI] [PubMed] [Google Scholar]
- 28. Blázquez M. A., Lagunas R., Gancedo C., Gancedo J. M. (1993) Trehalose-6-phosphate, a new regulator of yeast glycolysis that inhibits hexokinases. FEBS Lett. 329, 51–54 [DOI] [PubMed] [Google Scholar]
- 29. Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275 [PubMed] [Google Scholar]
- 30. Maidan M. M., De Rop L., Relloso M., Diez-Orejas R., Thevelein J. M., Van Dijck P. (2008) Combined inactivation of the Candida albicans GPR1 and TPS2 genes results in avirulence in a mouse model for systemic infection. Infect. Immun. 76, 1686–1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Eck R., Bergmann C., Ziegelbauer K., Schönfeld W., Künkel W. (1997) A neutral trehalase gene from Candida albicans: molecular cloning, characterization and disruption. Microbiology 143, 3747–3756 [DOI] [PubMed] [Google Scholar]
- 32. Piper P. W., Lockheart A. (1988) A temperature-sensitive mutant of Saccharomyces cerevisiae defective in the specific phosphatase of trehalose biosynthesis. FEMS Microbiol. Lett. 49, 245–250 [Google Scholar]
- 33. Pedreño Y., González-Párraga P., Martínez-Esparza M., Sentandreu R., Valentín E., Argüelles J. C. (2007) Disruption of the Candida albicans ATC1 gene encoding a cell-linked acid trehalase decreases hypha formation and infectivity without affecting resistance to oxidative stress. Microbiology 153, 1372–1381 [DOI] [PubMed] [Google Scholar]
- 34. Zaragoza O., González-Párraga P., Pedreño Y., Alvarez-Peral F. J., Argüelles J. C. (2003) Trehalose accumulation induced during the oxidative stress response is independent of TPS1 mRNA levels in Candida albicans. Int. Microbiol. 6, 121–125 [DOI] [PubMed] [Google Scholar]
- 35. Sudbery P., Gow N., Berman J. (2004) The distinct morphogenic states of Candida albicans. Trends Microbiol. 12, 317–324 [DOI] [PubMed] [Google Scholar]
- 36. Kameda Y., Asano N., Yamaguchi T., Matsui K., Horii S., Fukase H. (1986) Validamycin G and validoxylamine G, new members of the validamycins. J. Antibiot. 39, 1491–1494 [DOI] [PubMed] [Google Scholar]
- 37. Asano N., Yamaguchi T., Kameda Y., Matsui K. (1987) Effect of validamycins on glycohydrolases of Rhizoctonia solani. J. Antibiot. 40, 526–532 [DOI] [PubMed] [Google Scholar]
- 38. Nwaka S., Holzer H. (1998) Molecular biology of trehalose and the trehalases in the yeast Saccharomyces cerevisiae. Prog. Nucleic Acids Res. Mol. Biol. 58, 197–237 [DOI] [PubMed] [Google Scholar]
- 39. Alvarez-Peral F. J., Argüelles J. C. (2000) Changes in external trehalase activity during human serum-induced dimorphic transition in Candida albicans. Res. Microbiol. 151, 837–843 [DOI] [PubMed] [Google Scholar]
- 40. Shabb J. B. (2001) Physiological substrates of cAMP-dependent protein kinase. Chem. Rev. 101, 2381–2411 [DOI] [PubMed] [Google Scholar]
- 41. Singer M. A., Lindquist S. (1998) Multiple effects of trehalose on protein folding in vitro and in vivo. Mol. Cell 1, 639–648 [DOI] [PubMed] [Google Scholar]
- 42. Elliott B., Haltiwanger R. S., Futcher B. (1996) Synergy between trehalose and Hsp104 for thermotolerance in Saccharomyces cerevisiae. Genetics 144, 923–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kaushik J. K., Bhat R. (2003) Why is trehalose an exceptional protein stabilizer? An analysis of the thermal stability of proteins in the presence of the compatible osmolyte trehalose. J. Biol. Chem. 278, 26458–26465 [DOI] [PubMed] [Google Scholar]
- 44. Sebollela A., Louzada P. R., Sola-Penna M., Sarone-Williams V., Coelho-Sampaio T., Ferreira S. T. (2004) Inhibition of yeast glutathione reductase by trehalose: possible implications in yeast survival and recovery from stress. Int. J. Biochem. Cell Biol. 36, 900–908 [DOI] [PubMed] [Google Scholar]
- 45. Taipale M., Jarosz D. F., Lindquist S. (2010) HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat. Rev. Mol. Cell Biol. 11, 515–528 [DOI] [PubMed] [Google Scholar]
- 46. Conlin L. K., Nelson H. C. (2007) The natural osmolyte trehalose is a positive regulator of the heat-induced activity of yeast heat shock transcription factor. Mol. Cell. Biol. 27, 1505–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nicholls S., Leach M. D., Priest C. L., Brown A. J. (2009) Role of the heat shock transcription factor, Hsf1, in a major fungal pathogen that is obligately associated with warm-blooded animals. Mol, Microbiol, 74, 844–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tapia H., Morano K. A. (2010) Hsp90 nuclear accumulation in quiescence is linked to chaperone function and spore development in yeast. Mol. Biol. Cell 21, 63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thomas B. J., Rothstein R. (1989) Elevated recombination rates in transcriptionally active DNA. Cell 56, 619–630 [DOI] [PubMed] [Google Scholar]
- 50. Fonzi W. A., Irwin M. Y. (1993) Isogenic strain construction and gene mapping in Candida albicans. Genetics 134, 717–728 [DOI] [PMC free article] [PubMed] [Google Scholar]