Background: α-Amylase was thought to be the sole enzyme that determines starch digestion rate. Mucosal α-glucosidases were considered to simply convert post-α-amylase dextrins to glucose.
Results: The mucosal α-glucosidases can digest a wide range of α-limit dextrin molecules and digest starches from various sources differently.
Conclusion: Starch chemical structure drives the digestion difference at the brush-border area.
Significance: The findings provide new insight into controlling the glycemic response.
Keywords: Carbohydrate, Carbohydrate Chemistry, Carbohydrate Metabolism, Carbohydrate Structure, Glucose, α-Glucosidase, Glucogenesis, Maltase-glucoamylase, Starch Digestibility, Sucrase-isomaltase
Abstract
The quality of starch digestion, related to the rate and extent of release of dietary glucose, is associated with glycemia-related problems such as diabetes and other metabolic syndrome conditions. Here, we found that the rate of glucose generation from starch is unexpectedly associated with mucosal α-glucosidases and not just α-amylase. This understanding could lead to a new approach to regulate the glycemic response and glucose-related physiologic responses in the human body. There are six digestive enzymes for starch: salivary and pancreatic α-amylases and four mucosal α-glucosidases, including N- and C-terminal subunits of both maltase-glucoamylase and sucrase-isomaltase. Only the mucosal α-glucosidases provide the final hydrolytic activities to produce substantial free glucose. We report here the unique and shared roles of the individual α-glucosidases for α-glucans persisting after starch is extensively hydrolyzed by α-amylase (to produce α-limit dextrins (α-LDx)). All four α-glucosidases share digestion of linear regions of α-LDx, and three can hydrolyze branched fractions. The α-LDx, which were derived from different maize cultivars, were not all equally digested, revealing that the starch source influences glucose generation at the mucosal α-glucosidase level. We further discovered a fraction of α-LDx that was resistant to the extensive digestion by the mucosal α-glucosidases. Our study further challenges the conventional view that α-amylase is the only rate-determining enzyme involved in starch digestion and better defines the roles of individual and collective mucosal α-glucosidases. Strategies to control the rate of glucogenesis at the mucosal level could lead to regulation of the glycemic response and improved glucose management in the human body.
Introduction
Glucose, the singularly most highly regulated nutrient in the body, has three originating sources: glycogen, the storage molecule that is mobilized by glucagon; endogenous de novo glucose (gluconeogenesis); and dietary glucose-containing carbohydrates. Starch, the main dietary glucose source, consists of two α-glucans: amylose and amylopectin. Both are composed of linear chains of d-glucose units joined by α-1,4-glycosidic linkages and are branched through α-1,6-linkages. Amylose has long linear chains with few branches (0.3–0.5% of total linkages); amylopectin is a much larger molecule in the 108-Da range with shorter linear chains and is highly branched (4–5% of total linkages) (1). It is noteworthy that dietary glucose is of such critical importance to the body that it has a complex enzyme system to maximize its extraction from the diet. To generate dietary glucose from starch or the range of maltodextrins commonly added to processed foods, two α-amylases and four intestinal mucosal α-glucosidase subunits join forces (2). α-Amylase, found in mature salivary and pancreatic secretions, hydrolyzes the internal α-1,4-linkages but bypasses the α-1,6-linkages and thus produces α-limit dextrins (α-LDx),4 a mixture of short linear α-glucans and branched malto-oligosaccharides (3), after extensive hydrolysis. The four intestinal mucosal α-glucosidases comprise two brush border-anchored complexes of N-terminal (Nt) and C-terminal (Ct) subunits of maltase-glucoamylase (MGAM) and sucrase-isomaltase (SI) to carry out hydrolysis of the α-amylase degradation products to free absorbable glucose. The four α-glucosidases have maltase-type activity to cleave α-1,4-linkages from the end of molecules, and Nt-SI is known to have additional activity to break α-1,6-linkages of isomaltose and isomaltulose (4).
To control the dietary glucose delivery from starchy foods to the body, pancreatic α-amylase has been the focus of attention because it quickly breaks down starch molecules. Thus, based on the susceptibility to α-amylase hydrolysis alone, starch is nutritionally classified into rapidly digestible, slowly digestible, and resistant starches (5). The marketed slowly digestible carbohydrate and resistant starches that are developed for weight control or other low glycemic response purposes are based on this classification. For those in vitro tests, the role of the intestinal mucosal α-glucosidases was assumed to simply covert α-amylase hydrolysis products to glucose, and fungal amyloglucosidase was used. Perhaps because of this, the physiology role of mucosal α-glucosidases in generating starch digestion products has been neglected. In this study, we investigated the complexity of the downstream digestion process of starch and the unique and shared roles of the individual α-glucosidases in digesting α-LDx.
EXPERIMENTAL PROCEDURES
Gelatinized starch molecules (10 mg/ml in phosphate-buffered saline) were incubated with human recombinant pancreatic α-amylase (10 units/5 mg of starch) at 37 °C until the amount of reducing sugar and did not change significantly (6). After inactivation of α-amylase by heating in a boiling water bath, aliquot-diluted α-amylase hydrolysate (10 μl of 2.5 mg/ml PBS) was further incubated with four recombinant mucosal glucosidase subunits at 37 °C for 1 h. The two N termini were from cloned human genes (7, 8), and the two C termini were from cloned mouse genes (9). SI was applied at 10 times (2 μg for 25 μg of starch) the amount of MGAM (0.2 μg) for the glucogenesis study (Table 1) because SI is more abundant than MGAM in the small intestine (10). Glucogenesis was determined by the glucose oxidase/peroxidase assay (11), and the residue structure was studied by high performance anion exchange chromatography (Ref. 12 and data not shown). wx α-LDx (25 μg) was incubated with four subunits and at three amounts (0.05, 0.1, and 0.2 μg of Nt- and Ct-MGAM; 0.25, 0.5, and 1 μg of Nt- and Ct-SI), and real-time (up to 6 h) residue structure change was examined by high performance size exclusion chromatography (HP-1090, Agilent Technologies) equipped with a refractive index detector (RID-10A, Shimadzu Corp., Kyoto, Japan) using two Zorbax PSM 60S columns (50 °C; Agilent Technologies) with a mobile phase of dimethyl sulfoxide containing 50 mm lithium chloride at a flow rate of 0.5 ml/min. Standards of glucose, maltose, maltotriose, maltopentaose, maltoheptaose, and pullulan (5900 Da) were applied to calibrate the elution time and molecular size.
TABLE 1.
Glucogenesis of maize α-LDx by the combination of four mucosal α-glucosidase subunits and individual subunits
Values are means ± S.E., and different letters express statistical difference among means within rows (one-way analysis of variance and Tukey's tests; α = 0.05). Letters indicate the glucogenesis differences due to the effect of enzymes (combined and individual α-glucosidase subunits) on the same substrate. The assays were done in triplicates.
| Normal maize | wx | aewx | duwx | |
|---|---|---|---|---|
| Combination | 86.5 ± 1.2a | 75.6 ± 1.8a | 90.6 ± 4.0a | 86.2 ± 3.7a |
| Ct-MGAM | 79.4 ± 0.3a,b | 70.6 ± 1.6a,b | 80.5 ± 1.7b | 71.4 ± 10.2a |
| Ct-SI | 77.6 ± 1.2a,b | 64.6 ± 1.6b,c | 73.7 ± 1.8b | 70.1 ± 1.5a |
| Nt-SI | 61.8 ± 10.2b,c | 57.4 ± 3.4c | 56.1 ± 1.1c | 65.8 ± 2.3a |
| Nt-MGAM | 45.1 ± 2.4c | 38.2 ± 1.5d | 34.0 ± 0.5d | 35.3 ± 3.6b |
Maltose and three branched sugars (isomaltose (6-O-α-d-glucopyranosyl-d-glucose), panose (6-O-α-d-glucopyranosyl-d-maltose), and 63-glucosylmaltotriose (6-O-α-d-glucopyranosyl-d-maltotriose) (1–200 mm)) were applied to determine the kinetic constants of individual α-glucosidase subunits (5 μg/ml) at 37 °C in 10 mm PBS (pH 6.8). The released glucose per min was determined by the glucose oxidase/peroxidase assay. Kinetic values (Km and Vmax) were calculated using SigmaPlot 12 (Systat Software Inc., San Jose, CA).
RESULTS
Four recombinant mucosal α-glucosidases were used individually and combined to digest hydrolyzed gelatinized starches from normal maize and three waxy maize mutants (wx, aewx, and duwx) with varying amylopectin structures. In our in vitro system, which was maximized for extensive digestion, individual enzymes did not equally digest the components found in α-LDx, with the digestive capacity ranging from 34 to 81% (based on starch dry mass) (Table 1). The digestive capacity of the α-glucosidase subunits using the α-LDx generated from the four starches was consistently on the order of Ct-MGAM > Ct-SI > Nt-SI > Nt-MGAM.
α-LDx are mixtures of molecules that include short linear oligomers (mainly DP 2, 3, and 4 in Fig. 1a) and densely branched structures (Fr 1 in Fig. 1a). The molecular weight distribution of wx α-LDx changed during individual α-glucosidase digestions; Ct-MGAM, Ct-SI, and Nt-SI digested the linear oligomers and large branched molecules equally well (Fig. 1a).
FIGURE 1.
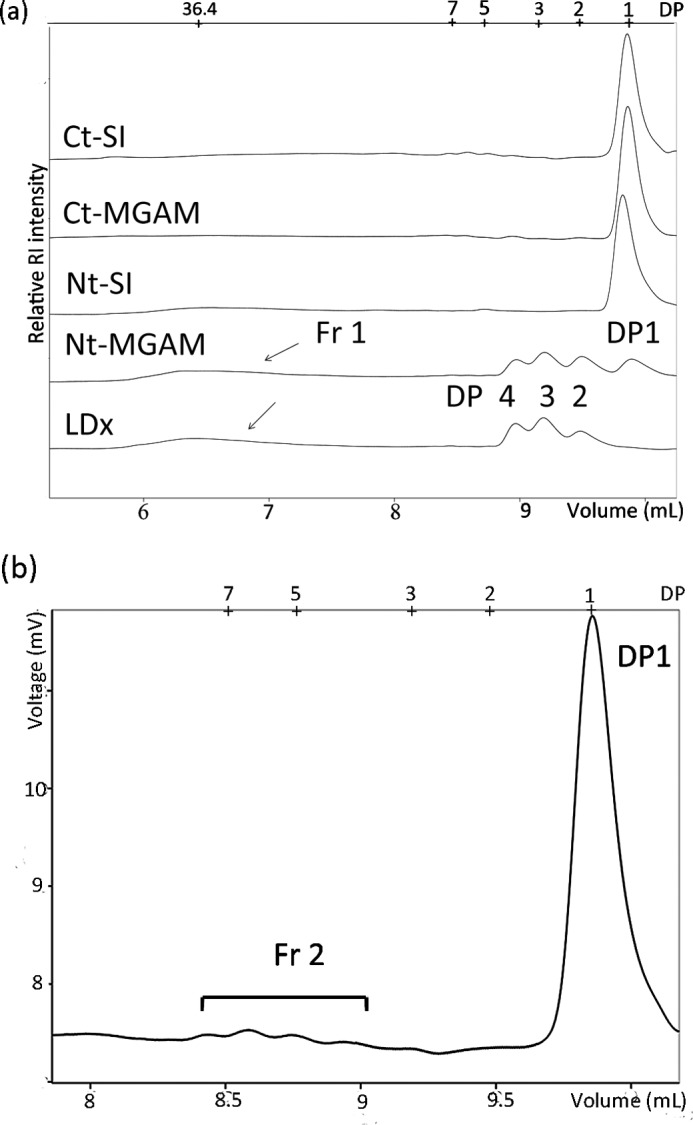
Chromatographs (obtained by high performance size exclusion chromatography with a refractive index detector) of wx α-LDx before and after Ct-MGAM, Ct-MGAM, Nt-SI, and Nt-MGAM extreme digestion. a, part of the chromatograph of Ct-SI is examined more closely in b. The large glucans (fraction 1 (Fr 1)) were digested by Ct-SI, Ct-MGAM, and Nt-SI, but not by Nt-MGAM. b, fraction 2 (Fr 2) is proposed as the slowly digestible or hard-to-digest fraction. DP, degree of polymerization; RI, refraction index detector.
Only two of the α-glucosidase subunits, Nt-SI and Nt-MGAM, had significant debranching activity on isomaltose, panose, and 63-glucosylmaltotriose, with the activity of Nt-SI being ∼9–40 times higher than that of Nt-MGAM depending on the branch pattern (Table 2). Both Ct-MGAM and Ct-SI released little glucose from the three branched sugars after long incubation times. Ct-MGAM showed the highest maltase activity, followed by Nt-MGAM, Ct-SI, and Nt-SI.
TABLE 2.
Kinetic constants of individual α-glucosidase subunits
Ct-MGAM and Ct-SI did not produce significant amounts of glucose from three branched sugars to determine the kinetic constants.
| Substrates and constants | Enzymes |
|||
|---|---|---|---|---|
| Ct-MGAM | Nt-MGAM | Ct-SI | Nt-SI | |
| Maltose | ||||
| Km (mm) | 2.29 ± 0.15 | 5.35 ± 0.62 | 2.54 ± 0.39 | 8.68 ± 0.99 |
| Kcat (s−1) | 156.31 ± 1.98 | 87.67 ± 2.27 | 24.41 ± 0.72 | 37.13 ± 1.04 |
| Kcat/Km | 68.37 ± 12.96 | 16.38 ± 3.64 | 9.62 ± 1.83 | 4.28 ± 1.06 |
| Isomaltose | ||||
| Km (mm) | 155.79 ± 19.88 | 10.55 ± 1.98 | ||
| Kcat (s−1) | 3.29 ± 0.23 | 9.05 ± 0.43 | ||
| Kcat/Km | 0.02 ± 0.01 | 0.86 ± 0.22 | ||
| Panose | ||||
| Km (mm) | 73.69 ± 11.45 | 12.37 ± 2.18 | ||
| Kcat (s−1) | 3.49 ± 0.23 | 5.09 ± 0.23 | ||
| Kcat/Km | 0.05 ± 0.02 | 0.41 ± 0.11 | ||
| 63-Glucosylmaltotriose | ||||
| Km (mm) | 103.62 ± 13.73 | 12.22 ± 1.08 | ||
| Kcat (s−1) | 4.77 ± 0.31 | 5.43 ± 0.13 | ||
| Kcat/Km | 0.05 ± 0.02 | 0.44 ± 0.12 | ||
A fraction of α-LDx was resistant under the conditions of the study to both α-amylase and α-glucosidase. The four α-glucosidases combined did not completely digest the α-LDx, reaching only 86, 76, 91, and 86% total hydrolysis for normal maize, wx, aewx, and duwx, respectively (Table 1). Fig. 1b shows the molecular weight distribution of the remaining fraction of wx α-LDx after Ct-SI digestion.
The four mucosal α-glucosidases have complementary roles. The four α-glucosidases combined had the highest glucogenesis (Table 1). The activity of the Ct-MGAM subunit was almost equal to that of the whole SI enzyme complex (Fig. 2a), and the activity of the Ct-SI subunit was almost equal to that of the whole MGAM enzyme complex (Fig. 2b).
FIGURE 2.
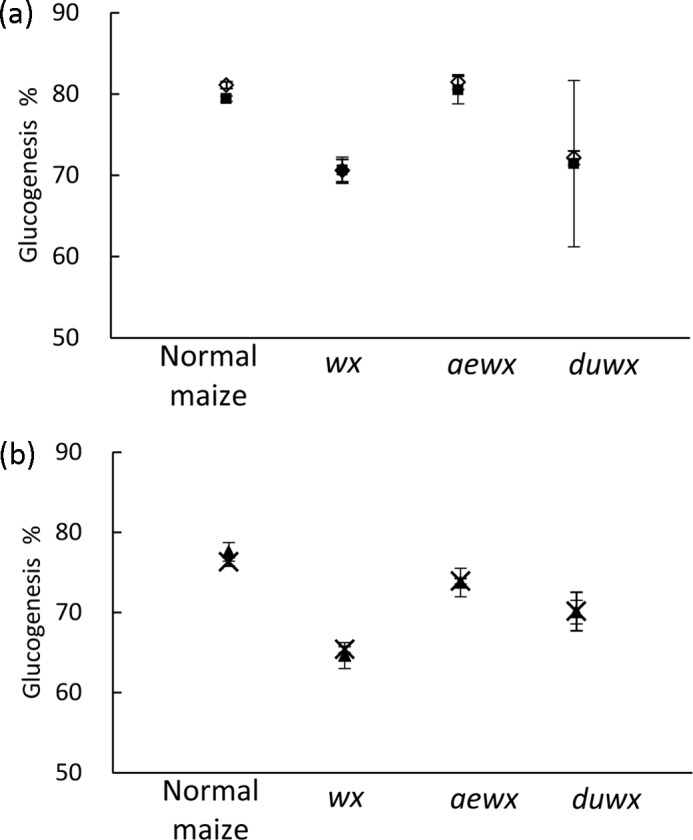
Glucogenesis from four maize α-LDx by a C-terminal subunit and the non-corresponding complex. a, a combination of Nt-SI and Ct-SI was applied to predict SI complex (♢) digestion and for comparison with Ct-MGAM (■). b, a combination of Nt-MGAM and Ct-MGAM was applied to predict MGAM complex (×) digestion and for comparison with Ct-SI (▴). Error bars present S.D. among triplicates.
DISCUSSION
Each individual α-mucosal glucosidase subunit digested α-LDx in a different manner. Ct-MGAM had the highest digestive capacity, followed by Ct-SI, Nt-SI, and Nt-MGAM. The digestion order was the same for all four α-LDx obtained from various maize starches, thus demonstrating that the four α-glucosidases have different roles in the downstream digestion of starch.
The amount of glucose released by Nt-MGAM was near the total anhydroglucose amount of the linear glucans, which was 47% in the α-LDx from normal maize (calculated from the chromatographic peak areas). The other three subunits additionally digested branched fractions and resulted in higher glucose production compared with Nt-MGAM. The high capacity of Ct-MGAM and Ct-SI to digest the branched fraction of α-LDx was unexpected because they have only maltase and glucoamylase activities and no significant debranching ability or endohydrolytic activity (data not shown). The molecular weight distribution changed during individual α-glucosidase digestions, further confirming that Ct-MGAM, Ct-SI, and Nt-SI independently digest many of the large branched fractions (Fr 1 in Fig. 1a) and linear oligomers as well. Perhaps the high digestive activity of Ct-MGAM and Ct-SI on α-LDx is due to their ability to effectively cleave α-1,4-linkages close to the branch points, which is different from α-amylase. Related to the high hydrolytic activity of the C-terminal subunits, we recently showed that their selective inhibition with acarbose is an approach to slow glucogenesis of α-LDx (13).
A new slowly digestible or hard-to-digest dextrin was identified as the undigested residual fraction after exhaustive digestion of the α-LDx with the combination of α-glucosidases. We hypothesize that the slowly digestible dextrin has an overall degree of polymerization in the range of 4–10 glucose units and contains short internal chain lengths with short branch(es), as indicated in Fig. 1b (Fr 2). Further structural study is required to elucidate the exact nature of this slowly digestible dextrin fraction.
Our study presents a view of the complementary roles of four mucosal α-glucosidases. Their combination had the highest glucogenesis (Table 1) and covered a broader spectrum of activities than any individual enzyme. The two C-terminal subunits (Ct-MGAM and Ct-SI) were almost equivalent in digestive capacity in this system, which was maximized for digestion (Table 1). Reflecting the relative abundance of SI in the body, SI subunits were applied at 10 times the amount of MGAM subunits to digest α-LDx. We found that the activity of the Ct-MGAM subunit was almost equal to that of the entire SI enzyme complex, which contains both N- and C-terminal subunits (Fig. 2a). Likewise, the digestive capacity of Ct-SI alone was equivalent to that of the MGAM complex, with both N- and C-terminal subunits (Fig. 2b). This finding was consistent for the digestion of the four maize α-LDx. Thus, our results show that the C-terminal subunits are the more powerful digestive enzymes, with both maltase and glucoamylase activities (in addition to the sucrase activity of Ct-SI), and may compensate to maintain the function of dietary glucose generation when one of the enzyme complexes is missing (such as in congenital sucrase-isomaltase deficiency).
The extent of mucosal α-glucosidase digestion was influenced by the structure of the α-LDx. This infers that the degree and perhaps the rate of glucose generation may be manipulated by choosing the appropriate starchy food. Digestion at the mucosal α-glucosidase level was previously thought to simply convert α-amylase-degraded products to glucose without differentiation due to starch source. In this study, starches differing in amylose content and amylopectin structures were chosen to test this conventional view. Normal maize consisting of both amylose and amylopectin and three waxy amylose-free maize cultivars were chosen because their amylopectin structures differ in chain length and branch density (14, 15), which are factors known to be associated with differing susceptibility to α-amylase. aewx amylopectin has a loosely branched structure with a high proportion of long chains (B2 chains) and an overall longer average chain length. duwx has a densely branched structure with a high proportion of short chains (A and B1 chains) and a shorter average chain length. wx amylopectin has chain properties between those of aewx and duwx. We found that α-LDx from these starches were digested unequally by both individual and combined α-glucosidases. The α-LDx of wx maize was less digestible by mucosal α-glucosidases than the α-LDx of normal maize, perhaps due to the rapid digestion of the linear amylose of gelatinized normal maize starch. This is the first study showing that α-LDx structural differences influence α-glucosidase digestion patterns, and our results suggest that starch sources may affect mucosal glucose release rates in the body.
The role of α-amylase in determining starch digestion rate has been further challenged in this study. α-Amylase quickly breaks down starch molecules but is a poor contributor to glucogenesis. Mucosal α-glucosidases are the enzymes that complete the digestion process. It is not easy to predict the ratio of two enzyme types in vivo due to the prandial secreting and dynamic activities along the small intestine. However, the branched glucans, examined in the body after consuming amylopectin, were found to be digested differently by individual mucosal α-glucosidases in this study. In addition to the previously reported finding that product inhibition of α-glucosidase can regulate digestion rate (10), we have shown that α-amylase degradation products are digested in different manners at the brush-border area.
Taken together, starch sources influence glucose generation at the small intestine mucosal α-glucosidase level, and it appears that the structures that make up α-LDx are digested at different rates, with one fraction appearing quite difficult to digest. This provides the basis for the next study using physiologically relevant conditions in which enzyme concentration, distribution, and activities are considered.
By developing starchy foods or starch-based ingredients with different susceptibilities to the mucosal α-glucosidases or through direct manipulation of mucosal α-glucosidase activities such as digestion product inhibition (10) or natural inhibitors (16, 17), one can envision some degree of control of glucose delivery. Regulation of the glycemic response as well as glucose-related physiologic responses may ultimately be achieved by the selection of α-glucogenic dietary components.
Acknowledgments
We thank Yuan Yao (Whistler Center for Carbohydrate Research, Purdue University) for assistance in providing aewx and duwx maize starch; Lyann Sim (University of Toronto, Ontario, Canada), Kyra Jones (University of Waterloo), and Stephen E. Avery (Baylor College of Medicine) for assistance in preparing recombinant enzymes; and Mario Pinto (Simon Fraser University, Burnaby, Canada) for discussion.
This work was supported by the Whistler Center for Carbohydrate Research and in part by United States Department of Agriculture/Agricultural Research Service (USDA/ARS) Grant 6250-51000-052, Heart and Stroke Foundation of Ontario Grant NA-6305, and Canadian Institutes of Health Research Grant MOP 111237. Part of this work was carried by at the Texas Medical Center Digestive Diseases Center (supported by National Institutes of Health Grant DK58338).
- α-LDx
- α-limit dextrin(s)
- Nt
- N-terminal subunit
- Ct
- C-terminal subunit
- MGAM
- maltase-glucoamylase
- SI
- sucrase-isomaltase.
REFERENCES
- 1. Hizukuri S., Abe J., Hanashiro I. (1996) in Carbohydrates in Food (Eliasson A.-C., ed) pp. 347–429, Marcel Dekker, Inc., New York [Google Scholar]
- 2. Quezada-Calvillo R., Sim L., Ao Z., Hamaker B. R., Quaroni A., Brayer G. D., Sterchi E. E., Robayo-Torres C. C., Rose D. R., Nichols B. L. (2008) Luminal starch substrate “brake” on maltase-glucoamylase activity is located within the glucoamylase subunit. J. Nutr. 138, 685–692 [DOI] [PubMed] [Google Scholar]
- 3. Robyt J., French D. (1963) Action pattern and specificity of an amylase from Bacillus subtilis. Arch. Biochem. Biophys. 100, 451–467 [DOI] [PubMed] [Google Scholar]
- 4. Siddons R. C. (1970) Heat inactivation and Sephadex chromatography of the small intestine. Biochem. J. 116, 71–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Englyst H. N., Kingman S. M., Cummings J. H. (1992) Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 46, S33–S50 [PubMed] [Google Scholar]
- 6. Nelson N. (1944) A photometric adaptation of the Somogyi method for the determination of glucose. J. Biol. Chem. 153, 375–380 [Google Scholar]
- 7. Sim L., Quezada-Calvillo R., Sterchi E. E., Nichols B. L., Rose D. R. (2008) Human intestinal maltase-glucoamylase: crystal structure of the N-terminal catalytic subunit and basis of inhibition and substrate specificity. J. Mol. Biol. 375, 782–792 [DOI] [PubMed] [Google Scholar]
- 8. Sim L., Willemsma C., Mohan S., Naim H. Y., Pinto B. M., Rose D. R. (2010) Structural basis for substrate selectivity in human maltase-glucoamylase and sucrase-isomaltase N-terminal domains. J. Biol. Chem. 285, 17763–17770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lin A. H., Nichols B. L., Quezada-Calvillo R., Avery S. E., Sim L., Rose D. R., Naim H. Y., Hamaker B. R. (2012) Unexpected high digestion rate of cooked starch by the C-terminal maltase-glucoamylase small intestine mucosal α-glucosidase subunit. PLoS ONE 7, e35473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Quezada-Calvillo R., Robayo-Torres C. C., Ao Z., Hamaker B. R., Quaroni A., Brayer G. D., Sterchi E. E., Baker S. S., Nichols B. L. (2007) Luminal substrate “brake” on mucosal maltase-glucoamylase activity regulates total rate of starch digestion to glucose. J. Pediatr. Gastroenterol. Nutr. 45, 32–43 [DOI] [PubMed] [Google Scholar]
- 11. Trinder P. (1969) Determination of blood glucose using 4-aminophenazone as oxygen acceptor. J. Clin. Pathol. 22, 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ao Z., Simsek S., Zhang G., Venkatachalam M., Reuhs B. L., Hamaker B. R. (2007) Starch with a slow digestion property produced by altering its chain length, branch density, and crystalline structure. J. Agric. Food Chem. 55, 4540–4547 [DOI] [PubMed] [Google Scholar]
- 13. Lee B. H., Eskandari R., Jones K., Reddy K. R., Quezada-Calvillo R., Nichols B. L., Rose D. R., Hamaker B. R., Pinto B. M. (2012) Modulation of starch digestion for slow glucose release through toggling of activities of mucosal α-glucosidases. J. Biol. Chem. 287, 31929–31938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yuan R. C., Thompson D. B., Boyer C. D. (1993) Fine structure of amylopectin in relation to gelatinization and retrogradation behavior of maize starches from three wx-containing genotypes in two inbred lines. Cereal Chem. 70, 81–89 [Google Scholar]
- 15. Fuwa H., Glover D. V., Miyaura K., Inouchi N., Konishi Y., Sugimoto V. (1987) Chain length distribution of amylopectins of double and triple mutants containing the waxy gene in the inbred Oh43 maize background. Starch 39, 295–298 [Google Scholar]
- 16. Sim L., Jayakanthan K., Mohan S., Nasi R., Johnston B. D., Pinto B. M., Rose D. R. (2010) New glucosidase inhibitors from an ayurvedic herbal treatment for type 2 diabetes: structures and inhibition of human intestinal maltase-glucoamylase with compounds from Salacia reticulata. Biochemistry 49, 443–451 [DOI] [PubMed] [Google Scholar]
- 17. Eskandari R., Jones K., Reddy K. R., Jayakanthan K., Chaudet M., Rose D. R., Pinto B. M. (2011) Probing the intestinal α-glucosidase enzyme specificities of starch-digesting maltase-glucoamylase and sucrase-isomaltase: synthesis and inhibitory properties of 3′- and 5′-maltose-extended de-O-sulfonated ponkoranol. Chemistry 17, 14817–14825 [DOI] [PubMed] [Google Scholar]


