Background: The period length of the Neurospora circadian clock is dependent on expression levels of its core transcription factor WCC.
Results: Glycogen synthase kinase (GSK) forms a complex with the WCC and regulates its abundance.
Conclusion: GSK modulates the period length of the circadian clock.
Significance: GSK is a novel clock component of Neurospora transducing signals to the core oscillator.
Keywords: Circadian Rhythms, Glycogen Synthase Kinase 3, Phosphorylation, Protein Expression, Transcription Factors, GSK, WCC, Circadian Clock
Abstract
Timekeeping by circadian clocks relies upon precise adjustment of expression levels of clock proteins. Here we identify glycogen synthase kinase (GSK) as a novel and critical component of the circadian clock of Neurospora crassa that regulates the abundance of its core transcription factor white collar complex (WCC) on a post-transcriptional level. We show that GSK specifically binds and phosphorylates both subunits of the WCC. Reduced expression of GSK promotes an increased accumulation of WC-1, the limiting factor of the WCC, causing an acceleration of the circadian clock and a shorter free-running period.
Introduction
Circadian clocks are biological oscillators that are found in organisms ranging from cyanobacteria to mammals where they allow anticipation of periodic changes in the environment associated with the rotation of the earth. Eukaryotic circadian clocks based on transcriptional/translational feedback loops (TTFL),3 in which negatively acting clock proteins rhythmically repress their own synthesis by inhibiting the activity of their transcription factors, thereby generating self-sustained oscillations with a period length of about 24 h. Function, activity, turnover as well as subcellular localization of clock proteins are tightly regulated post-transcriptionally contributing to robust circadian oscillations on a transcriptional level. In particular, phosphorylation of core clock proteins is crucial for clock function (1–3). Various kinases and phosphatases mediate phosphorylation and dephosphorylation of clock components and their complex interplay determines fate and function of the clock proteins they modify (1).
In the filamentous fungus Neurospora crassa the TTFL of the circadian clock consists of the transcription factors white collar-1 AND white collar-2 (WC-1 and WC-2), which form the white collar complex (WCC), and its negative regulator frequency (FRQ) (4–6). WC-1 is also a blue light receptor enabling Neurospora to synchronize its endogenous clock with the exogenous 24 h day/night cycle (7). Active WCC directly and indirectly drives the expression of several hundred clock controlled genes (ccgs) (8, 9). FRQ, which is expressed under the direct control of WCC, inactivates the transcription factor by mediating its phosphorylation by casein kinase 1a (CK1a) (10). Over the course of a circadian cycle, FRQ is progressively phosphorylated by CK1a and CK2 and then degraded via the ubiquitin proteasome pathway (11–13). As the levels of FRQ decrease, dephosphorylation of WCC by protein phosphatase 2A (PP2A) reactivates the transcription factor, thereby initiating a new circadian cycle (10). Active WCC is unstable and DNA binding triggers its rapid degradation. Inactivation of the WCC by FRQ-dependent phosphorylation stabilizes the WCC and facilitates its accumulation in an inactive form (14).
In all eukaryotic clocks casein kinases 1 are essential to generate rhythmic oscillations (1, 15, 16). Other kinases are involved in modulating period length and phase of the clocks. Although they are not essential for circadian rhythmicity per se (1), they might be important under entrained conditions (17). Among these, glycogen synthase kinase 3 (GSK3) fulfills functions in the Drosophila and mammalian clocks. The Drosophila GSK3 ortholog SHAGGY (SGG) phosphorylates the negative regulators timeless (TIM) and period (dPER), which are in a cytoplasmic complex. Phosphorylation of TIM by SGG fosters its degradation in response to light and allows nuclear entry of dPER, while phosphorylation of dPER by SGG delays its nuclear entry (18, 19). Mammalian GSK3β phosphorylates the clock transcription factors CLOCK and BMAL-1, targeting both for subsequent degradation (20, 21). The negative circadian regulators PER2 and CRY2 are also phosphorylated by GSK3β, regulating their nuclear entry and proteasomal degradation, respectively (22, 23). Inhibition or down-regulation of GSK has opposing effects on the mammalian and Drosophila clock. In mammals a shortening of the period length is observed (24), while the period length increases in Drosophila (18).
Here we show that GSK also plays a role in setting the pace of the circadian clock of Neurospora. GSK is not part of the core feedback loop of the clock. It binds the WCC, presumably promoting its degradation via phosphorylation, thereby lengthening the circadian period by regulating the core clock transcription factor levels.
EXPERIMENTAL PROCEDURES
Neurospora Strains and Culture Conditions
The Neurospora glycogen synthase kinase (GSK, NCU04185.2) heterokaryon knock-out strain gskhet was obtained from the Fungal Genetics Stock Center (FGSC #11500) along with the corresponding background control strain wt9718. Conidial suspensions in 1 m sorbitol were prepared from samples grown on standard solid growth medium (2.2% agar, 0.3% d+ glucose monohydrate, 0.17% l-arginine, 1× Vogel's medium, and 0.1% biotin). 200 μg/ml hygromycin B (Applichem) was added to the solid growth medium to enrich for gsk knock-out conidia. Race tubes contained 2% agar, 0.1% d+ glucose monohydrate, 0.17% l-arginine, 1× Vogel's medium, and 0.1% biotin. Where applicable, QA and H2O2 were added in concentrations as indicated in the figures. Liquid culture medium contained 2% glucose, 0.5% arginine, and 1× Vogel's medium. Cycloheximide (CHX) was added at a concentration of 10 μg/ml where indicated.
The qa-gsk knock-in strain was generated by replacing the endogenous promoter of gsk by the inducible qa2-promoter along with N-terminal tagging of GSK with a FLAG epitope using the protocol of Ref. 25. The construct was knocked into a background strain expressing luciferase under the control of the frq promoter (ras-1bd, pfrq-luc, Ref. 9).
The strains Δwc-1, Δwc-2, frq10/qa-frq, and frq10 and the control strain wt74 contain the ras-1bd mutation (26). Analysis of race tubes was performed using the Chrono program (T. Roenneberg, LMU Munich).
For real-time luciferase measurements, 96-well plates were prepared with medium as described elsewhere (9) and supplied with QA in concentrations as indicated. Experiments were performed in an EnVision Xcite Multiplate Reader (Perkin Elmer), and the luciferase readout was evaluated with MultiCycle software (Actimetrics).
Protein Extraction and Analysis
Extraction of Neurospora protein was performed as described (27). Protein concentrations were measured with NanoDrop® (PeqLab), and 400 μg of protein were loaded unless stated otherwise. Western blotting was performed as described (10, 28). Enhanced chemiluminescence signals were detected with x-ray films. Quantification of Western blots was performed using ImageJ software (Rasband, W.S., ImageJ, NIH). Subcellular fractionations were performed as recently described (29). Antibodies used against FRQ, WC-1, and WC-2 were previously described (28, 30). For GSK, a peptide antibody was raised in rabbit against the extreme C terminus of the protein (NH2-DNFTPMNKSEMMAKLD-COOH) (Pineda, Berlin). When used in a 1:500 dilution, Western blot analysis with the affinity-purified GSK antibody led to a strong signal at the correct MW (∼45 kDa) with low or no background. In the qa-gsk knock-in strain, the intensity of the band corresponding to GSK was strongly reduced when cultures were grown without QA in the medium (see Fig. 1A).
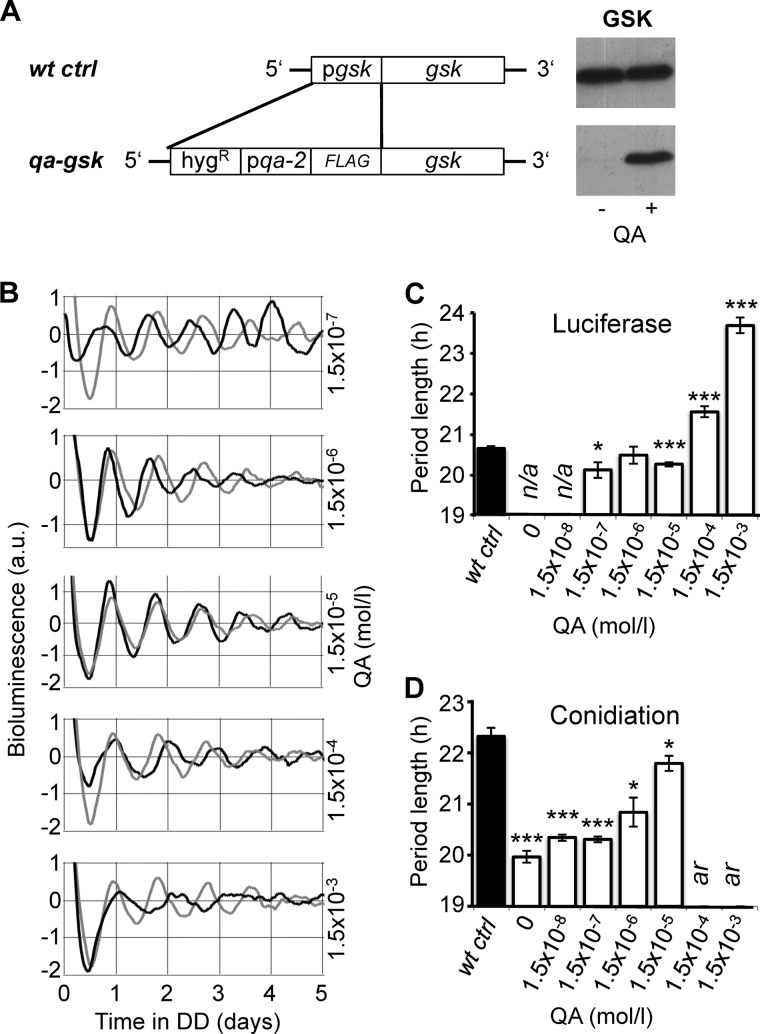
FIGURE 1.
Dose-dependent expression of GSK modulates the period length of the circadian clock. A, schematic representation of the knock-in cassette used to replace the gsk promoter with the QA inducible qa-2 promoter. The cassette was transformed into the ras-1bd, pfrq-luc strain, which expresses luciferase under the control of the frq promoter. Representative Western blots show levels of GSK in the qa-gsk strain compared with its corresponding background control strain wt ctrl. Cultures were grown in light at 25 °C in the presence or absence of 1.5 × 10−3 m QA. B, rhythmic expression of the frq-luc promoter in qa-gsk (black traces) and wt ctrl (gray traces) in the presence of the indicated QA concentrations. Luciferase activity of the strains was continuously monitored in DD after a 1 h light pulse. Representative bioluminescence records of individual cultures are shown. C, summary of the average period lengths of qa-gsk (white bars) and wt ctrl (black bar) as determined by luciferase measurements at the indicated QA concentrations. Error bars show ± S.E. For wt ctrl the average period length at all QA concentrations is shown. n/a, experiment was not performed under these conditions. See also supplemental Table S1. D, summary of the average period lengths of qa-gsk (white bars) and wt ctrl (black bar) grown on racetubes with the indicated QA concentrations (± S.E.). For wt ctrl the average period length at 0 and 1.5 × 10−3 m QA is shown. See also supplemental Table S2. ar, arrhythmic. * and *** indicate p values of ≤0.05 and ≤0.0005, respectively using two-tailed Student's t-test.
In Vitro Phosphorylation Assay
Recombinant His-tagged GSK and CK1a were purified from Escherichia coli. GSK and CK1a cDNA fragments were cloned into pQE30 (Qiagen) plasmids using PstI and SphI restriction sites. Kinase-encoding vectors and an empty plasmid (mock control) were transformed into the Escherichia coli strain M15 Prep4 (Qiagen). The cells were induced overnight with 0.1 mm IPTG at 22 °C and His-tagged proteins were purified at 4 °C using the standard His tag purification protocol (QIAexpressionist, Qiagen). GSK, CK1a, and mock control elutions were re-buffered into stock buffer (50 mm HEPES, 300 mm NaCl, 5 mm MgCl2, and 1 mm DTT, 10% glycerol, pH 7.4) and stored at −20 °C. The amount of enzyme required for saturating phosphorylation was determined by a series of dilutions, and 18.24 μg of CK1a, 38.88 μg of GSK, and 24 μg of mock control proteins were added to 200 μg of total protein in a final reaction volume of 30 μl. Samples were incubated for 1 h at 25 °C. Final reaction mixture contained 50 mm HEPES/KOH, pH 7.4, 150 mm NaCl, 10 mm MgCl2, 10 mm ATP, leupeptin (2 μg/ml), pepstatin A (2 μg/ml), PMSF (1 mm), and PhosStop® phosphatase inhibitor mixture (Roche). Samples were analyzed by SDS-PAGE and immunoblotting. GSK inhibitors indirubin-3′-monoxime and kenpaullone (Merck) were used at a final concentration of 3 μm.
GSK Co-immunoprecipitation
Purified GSK antibody was bound to 100 μl of protein A-Sepharose CL-4B beads (Amersham Biosciences) and blocked with 5% milk in TBS. After washing with standard protein extraction buffer, 5 mg of total protein and fresh protease inhibitors were added, and the mixture was incubated overnight at 4 °C. After two washing steps, bound proteins were eluted by incubating the beads with 1× Laemmli buffer at 95 °C for 5 min.
Quantitative Real-time PCR
Total mRNA from ground frozen mycelia was prepared using peqGOLD TriFASTTM (peqLab, Germany) and reverse transcribed using the QuantiTect Reverse Transcription Kit (Qiagen, Germany) following the manufacturer's instructions. Transcript levels were analyzed by quantitative real-time PCR as described previously (29). Results shown are from at least three independent experiments. Sequences of primers and probes used for RT-PCR were: actin: F: gat gac aca gat cgt ttt cga gac t, R: cgg agg cgt aga gag aaa gga, Probe: 6-FAM-ccg cct tct acg tct cca tcc a-TAMRA; wc-1: F: acc tcg ctg tcc tcg att tg, R: tgc tgg gcc tct ttc aac tc, Probe: 6-FAM-ccg tcc gac atc gtg ccg g-TAMRA; frq: F: ttg taa tga aag gtg tcc gaa ggt, R: gga gga aga agc gga aaa cg, Probe: 6-FAM-acc tcc caa tct ccg aac tcg cct g-TAMRA; wc-2: F: agt ttg cac cca atc cac aga, R: agg gtc gag cca tca tga ac, Probe: 6-FAM-agt cgc ctt tct gcc agg ccg-TAMRA; gsk: F: ccc gac tcg agg cac aac t, R: tgg ata gct cat gac ggg taa agt, Probe: 6-FAM-acc gtt agg gat ctg cca ccg ctc tt-TAMRA.
RESULTS
Down-regulation of GSK Results in a Short Period Phenotype
Because GSK is an essential protein, we generated a knock-in strain expressing GSK under the control of the qa-2 promoter, which is inducible in proportion to the concentration of quinic acid (QA) in the medium (Fig. 1A). A rasbd strain with a luciferase reporter under the control of the frq promoter was used as the background strain (rasbd, pfrq-luc (9); hereafter referred as to wt ctrl). The resulting strain, rasbd, pfrq-luc, qa-flag-gsk (hereafter referred to as qa-gsk) expressed only trace amounts of GSK without QA, while near-wild type GSK protein levels were produced with the addition of 1.5 mm QA into the growth medium (Fig. 1A).
We performed real-time luciferase measurements to study the effects of GSK on the circadian clock. For this we supplied the growth medium with of QA ranging from 1.5 × 10−7 m to 1.5 × 10−3 m. The results show that QA affects the period length in the qa-gsk strain but not in the wt ctrl in a concentration-dependent manner (Fig. 1B). At 1.5 × 10−7 m QA qa-gsk exhibits a slightly but significantly shorter period than wt ctrl. The period length of qa-gsk lengthened with increasing QA concentrations and was >3 h longer than wt ctrl at 1.5 × 10−3 m (Fig. 1C and supplemental Table S1). In addition, the robustness of the bioluminescence rhythm of qa-gsk decreased at high QA concentrations. The data indicate that expression levels of GSK correlate with period length.
Next we assessed period length by analyzing the conidiation rhythm of Neurospora on racetubes. Also under these conditions the period length of qa-gsk was dependent on QA concentration. In the absence of QA the period of qa-gsk was >2 h shorter than the period of the wt ctrl, while the period length of both strains was similar at 1.5 × 10−5 m. At QA concentrations of 1.5 × 10−4 m and 1.5 × 10−3 m conidiation rhythms of the qa-gsk strain was lost, which could be due to the decreased robustness of the clock on a molecular level (Fig. 1B). Moreover, the strain failed to entrain to light/dark (12 h/12 h) cycles at these QA levels (not shown). As in the real-time luciferase measurements, rhythmic conidiation was also unaffected by QA in the wt ctrl strain (supplemental Table S2).
In summary, GSK lengthens the circadian period in a dose-dependent manner. GSK levels substantially lower than wild type significantly shorten but do not abolish circadian rhythm, suggesting a modulating role of GSK in the Neurospora clock.
GSK Activity Affects Levels of WC-1
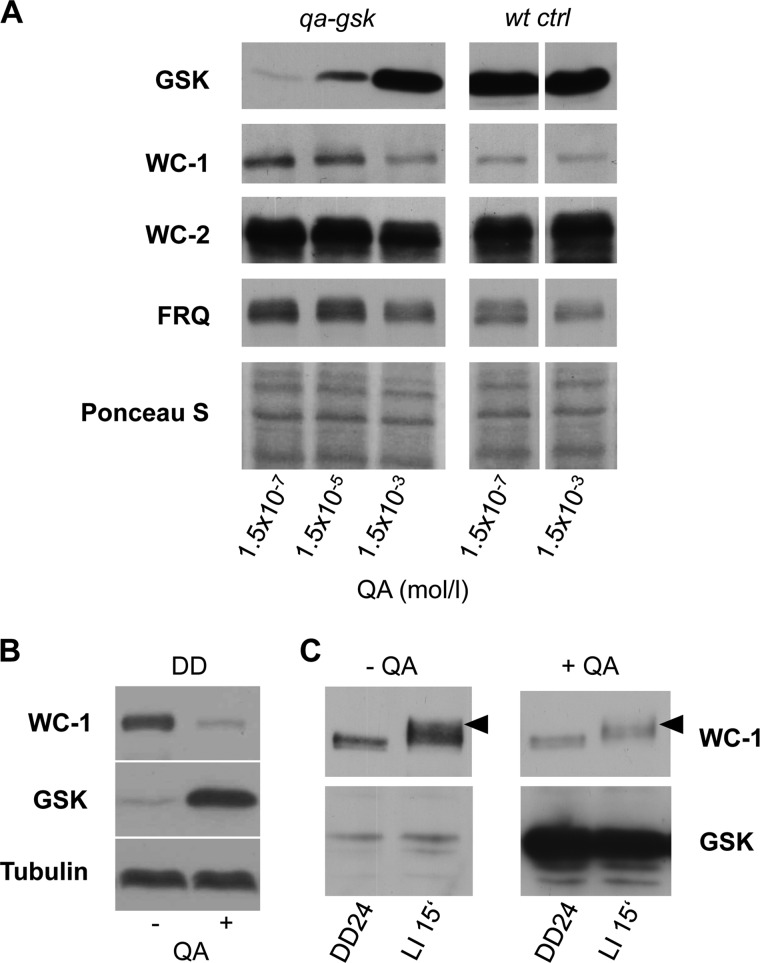
We next investigated the impact of GSK expression of core clock components. To be able to reliably compare protein levels in cultures supplied with different QA concentrations we grew Neurospora under constant light conditions, where the clock components are constitutively expressed and clock driven fluctuations due to differences in period lengths do not occur. As expected, GSK levels in qa-gsk increased in a QA-dependent manner but were unaffected by QA in the wt ctrl strain (Fig. 2A). Interestingly, WC-1 levels were elevated when GSK expression was low (1.5 × 10−7 m QA), and similar to those in the wt ctrl strain when GSK was expressed at wild type levels (1.5 × 10−3 m QA). FRQ levels were also slightly elevated at low GSK, while no difference in WC-2 protein could be observed (Fig. 2A).
FIGURE 2.
Down-regulation of GSK leads to increased WC-1 levels. A, QA titration: Western blots showing levels of GSK, WC-1, WC-2, and FRQ in the qa-gsk and wt ctrl strains from cultures grown at 25 °C in light at the indicated QA concentrations. A Ponceau stain of the blot is shown as a loading control. B, down-regulation of GSK affects WC-1 independent of light. Representative Western blots showing levels of WC-1 and GSK in qa-gsk grown in darkness for 30 h (DD30) in the presence or absence of 1.5 × 10−3 m QA. As a loading control the blot was additionally decorated for tubulin. C, Western blot showing that light induced hyperphosphorylation of WC-1 is not affected when GSK levels are low. Cultures grown in the presence or absence of QA in constant darkness for 24 h (DD24) were transferred to light and harvested after 15 min (LI 15′). Black arrowheads indicate hyperphosphorylated WC-1 species.
GSK down-regulation affects the free running period in constant darkness indicating that the main function of GSK in the clock is independent of light. WC-1 is a blue light photoreceptor that is phosphorylated and degraded in a light dependent manner. Accumulation of WC-1 upon down-regulation of GSK was independent of light and was not affected by temperature (Fig. 2B and supplemental Fig. S1). Rapid hyperphosphorylation of WC-1, which is a hallmark of the light activation of the WCC (31), was also independent of the expression levels of GSK (Fig. 2C), indicating that GSK activity is not essential in light dependent phosphorylation of the WCC. Thus, the data suggest a light and temperature independent effect of GSK on WC-1 abundance.
Period Length Is Short in a Heterokaryon gsk Knock-out Strain
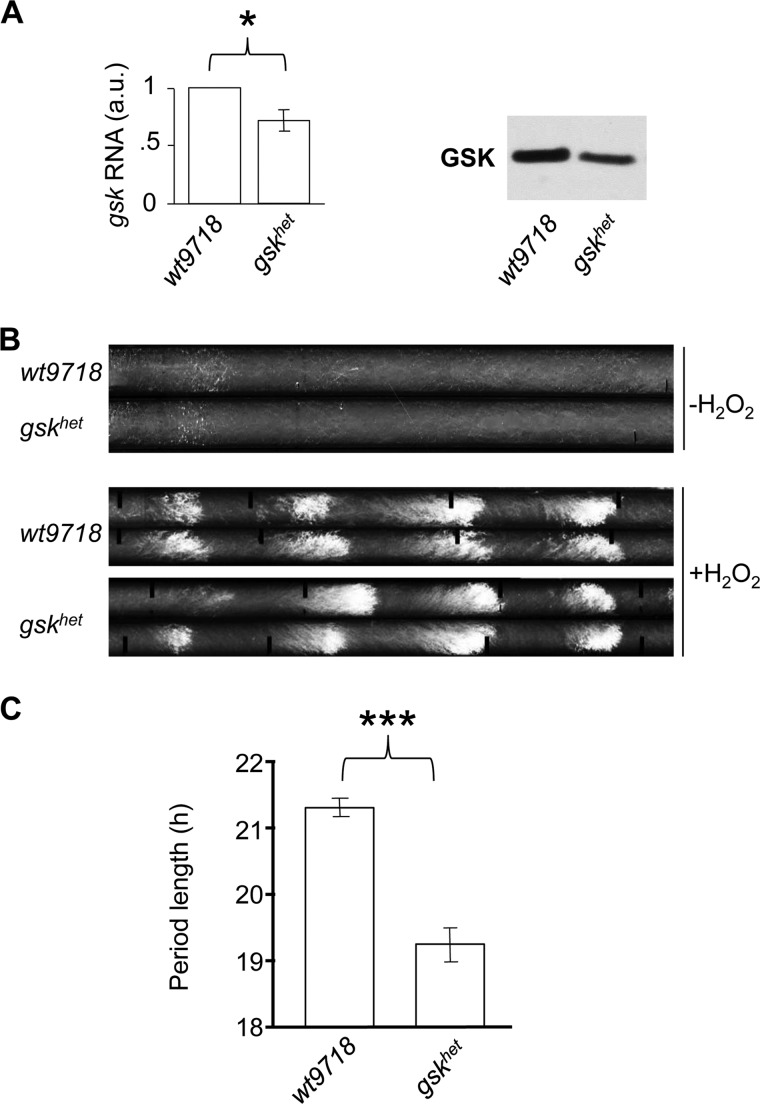
To confirm the effects of altered GSK activity on the Neurospora circadian clock, we performed additional experiments using a gsk heterokaryotic knockdown strain (gskhet, FGSC#11500). gskhet expressed reduced levels of GSK protein and mRNA compared with its corresponding wild-type control strain wt9718 independent of temperature (Fig. 3A and supplemental Fig. S2). Neurospora strains carrying the mutant rasbd allele produce a circadian banding pattern in race tubes. In non-bd strains banding on racetubes is suppressed due to accumulating CO2 but can be induced by oxidative stress (26). To assess the circadian conidiation rhythm of gskhet and the wt9718 racetube medium was supplied with 10 mm H2O2, which resulted in a visible circadian banding pattern (Fig. 3B). When grown at 30 °C, the period length of gskhet was 2 h shorter than that of wt9718 (Fig. 3C and supplemental Table S2) essentially phenocopying the effect of the dose-dependent down-regulation of GSK in the qa-gsk strain. No significant difference in period length between wt9718 and gskhet was observed at 25 °C (supplemental Table S2). This might be due to the still rather high levels of GSK in the gskhet strain, which may not be limiting at 25 °C.
FIGURE 3.
Period length is affected in the gsk knockdown strain gskhet. A, total levels of GSK mRNA and protein are lower in the gskhet strain compared with its background control strain wt9718 in constant light (LL) at 30 °C. * indicates a p value of ≤0.05 using two-tailed Student's t-test. B, representative banding patterns of the circadian conidiation rhythm in strains that are wild type for ras-1, with or without 10 mm H2O2 at 30 °C. C, summary of the average period lengths from racetubes grown at 30 °C (±S.E.). *** indicates a p value of ≤0.0005 using two-tailed Student's t-test. See also supplemental Table S2.
WC-1 Levels Are Elevated Post-transcriptionally in gskhet
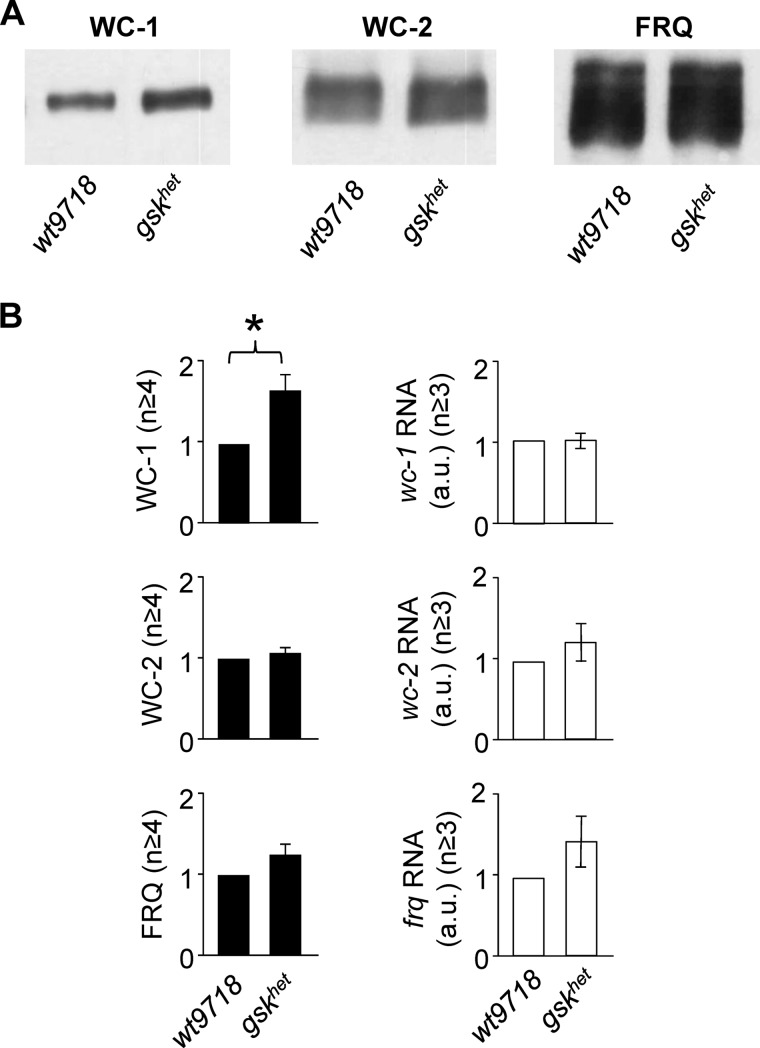
We next assessed the expression levels of clock proteins in gskhet and wt9718 (Fig. 4A). In cultures grown at 30 °C, levels of WC-1 were 1.7-fold higher in gskhet than in the wt9718 (p = 0.0071). WC-2 expression was similar in both strains and levels of FRQ were slightly higher in gskhet than in wt9718. Clock protein and mRNA levels were not significantly affected in gskhet when the strain was grown at 25 °C (supplemental Fig. S3, A and B). We then quantified the levels of clock gene mRNAs (Fig. 4B, right panels). Levels of wc-1 RNA were similar in gskhet and wt9718 indicating that the accumulation of WC-1 in gskhet is due to a post-transcriptional effect of GSK. Levels wc-2 RNA levels were not affected by GSK in accordance with WC-2 protein expression. frq RNA was slightly elevated in gskhet, suggesting that the observed increase in FRQ is due to increased WCC activity, resulting from the higher WC-1 levels.
FIGURE 4.
WC-1 levels are elevated in gskhet in a post-transcriptional manner. A, representative Western blots showing levels of clock proteins in the gskhet strain compared with its background control strain wt9718 at steady state conditions (LL, 30 °C). B, quantification of WC-1, WC-2, and FRQ protein (left panels) and mRNA (right panels) from at least three independent experiments are shown (± S.E.). wt9718 RNA and protein levels were used as reference. Differences between strains were analyzed using two-tailed Student's t-test, and p values ≤0.05 are indicated with *.
The results suggest a destabilizing role of GSK in the turnover of WC-1 in constant conditions (DD). However, WC-1 is stable for many hours in DD (32, 33). Even in a strain that does neither express functional FRQ nor VVD, which both stabilize WCC in positive feedback loops (7, 29), WC-1 was stable (supplemental Fig. S4A). Accordingly, a cycloheximide (CHX) assay is not suited to resolve differences in the half-life of WC-1 on this timescale. Rapid degradation of WC-1 is induced by light (10, 31). Light induced turnover of WC-1 was not affected by GSK (supplemental Fig. S4, B and C).
GSK Associates with WCC in Vivo
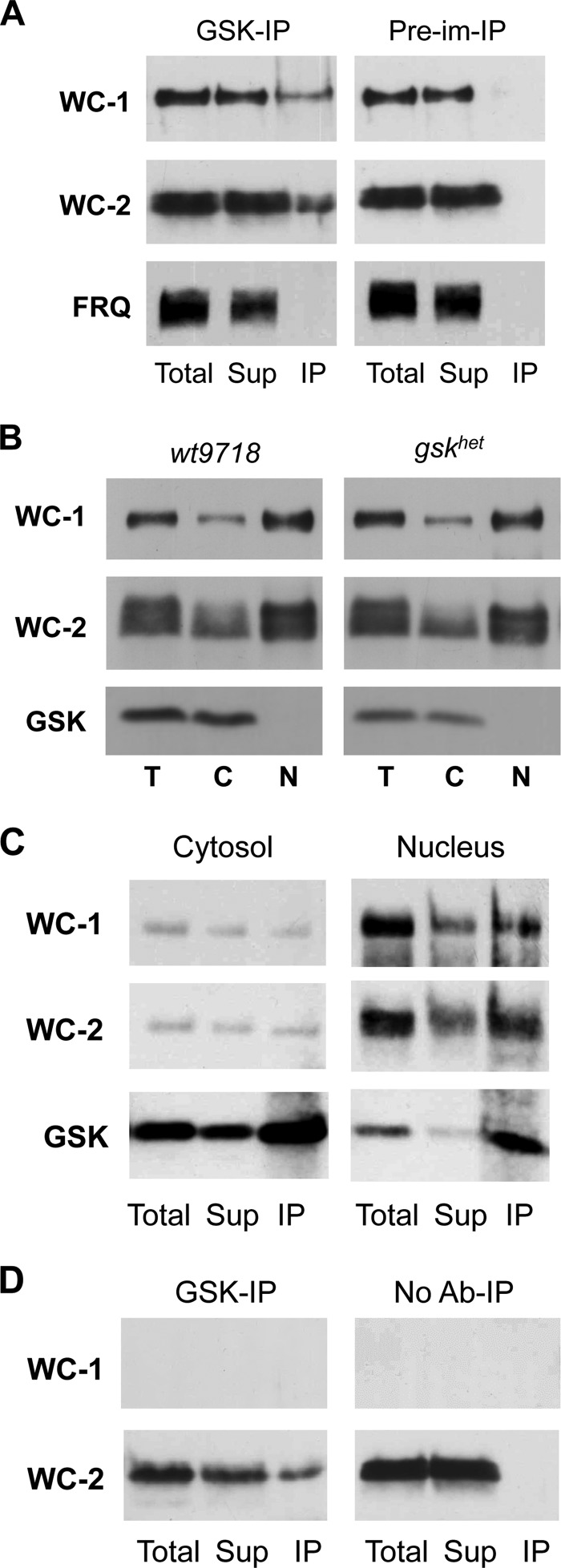
To elucidate whether GSK interacts directly with one of the core clock proteins we performed co-immunoprecipitation assays (Co-IP). We found that WC-1 and WC-2 were co-immunoprecipitated with GSK, while FRQ was not detected in a complex with GSK (Fig. 5A). Only a small fraction of the WCC (∼10%) co-precipitated with GSK under the conditions used.
FIGURE 5.
GSK binds to WCC in vivo. A, GSK is in a complex with the WCC. Co-immunoprecipitation (Co-IP) using GSK antibody and pre-immune serum from total cell extracts of wt9718. B, GSK is predominantly localized in the cytosol. Light grown cultures from the indicated strains were incubated at 30 °C and mycelia was subjected to subcellular fractionation. Western blots were immunoprobed for WC-1, WC-2, and GSK. C, GSK is bound to WCC in both cytosol and nucleus. GSK co-IP assays from nuclear and cytosolic fractions of wt9718 (LL) are shown. D, co-IP using GSK antibody and no antibody control from total cell extracts of Δwc-1. For the Co-IPs 200 μg total and an equivalent of 200 μg supernatant and 800 μg (A and D) or 1600 μg (C) IP protein were loaded.
Subcellular fractionation revealed that the majority of GSK was localized in the cytosol, while the WCC, which rapidly shuttles between compartments (14), was predominantly nuclear (Fig. 5B). Co-IPs from cytosolic and nuclear fractions showed that the WCC interacts with GSK in both compartments (Fig. 5C).
WC-2 was co-precipitated with GSK from a cell extract of a Δwc-1 strain, demonstrating that GSK associates with WC-2 independent of WC-1 (Fig. 5D). Since WC-1 is unstable and does not accumulate in the absence of WC-2, we could not analyze whether GSK also directly associates with WC-1.
GSK Phosphorylates the WCC in Vitro
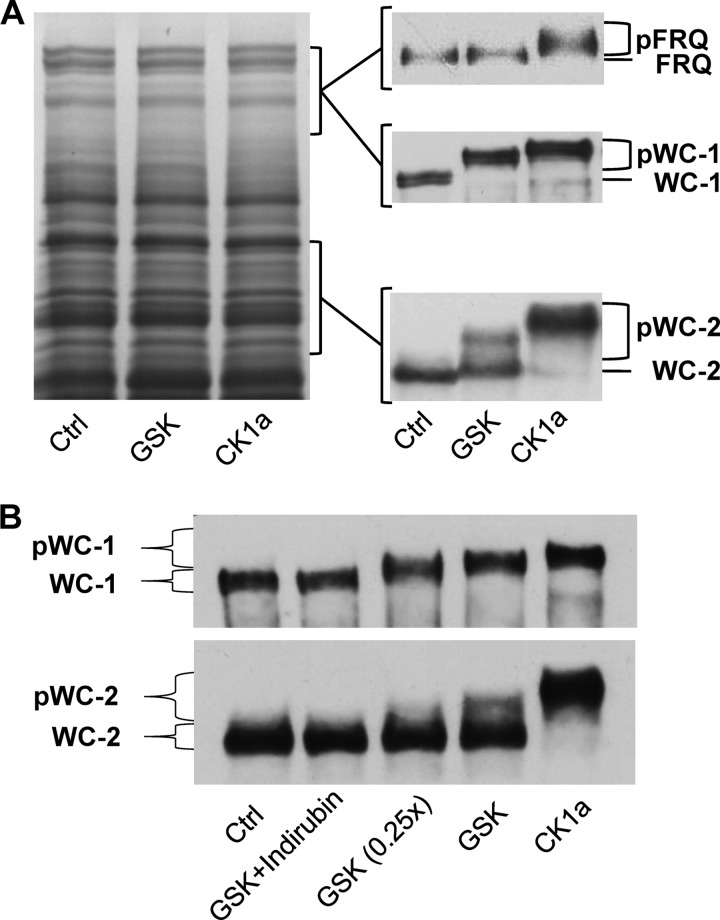
Given the observation that GSK binds to the WCC in vivo, we asked whether the transcription factor is also a substrate of GSK. Therefore, we tested whether recombinant Neurospora GSK is capable of phosphorylating the WCC in vitro. CK1a, which is known to phosphorylate the WCC, was used as a positive control. However, when a purified kinase is incubated with a purified protein in vitro, the enzyme will generally phosphorylate the protein to some extent. Specificity and physiological relevance of such phosphorylations are difficult to estimate. To test whether the WCC is a specific substrate of GSK, whole cell protein extract was incubated with purified recombinant GSK and ATP. SDS-PAGE and Coomassie Blue staining revealed that the overall electrophoretic mobility of Neurospora proteins was not affected by recombinant GSK, demonstrating that the majority of proteins were not efficiently phosphorylated (Fig. 6A, left). In contrast, the entire pool of WC-1 and a substantial portion of WC-2 were shifted to a higher electrophoretic mobility (Fig. 6A, right), indicating that they were efficiently and specifically phosphorylated by GSK despite the presence of a huge excess of other proteins. The electrophoretic mobility shift was dose-dependent on GSK (Fig. 6B) and efficiently prevented by the GSK inhibitor indirubin (Fig. 6B). The data demonstrate that the WCC is a specific substrate of GSK. Recombinant CK1a phosphorylated WC-1 and WC-2 in a total protein extract but not Neurospora proteins in general, confirming that both clock proteins are specific substrates of CK1a (6).
FIGURE 6.
GSK specifically phosphorylates the WCC in vitro. Neurospora GSK, CK1a, and control (mock purification) proteins were added to Neurospora total cell extracts. A, left: representative Coomassie Blue staining of an in vitro phosphorylation assay gel. Note that there is no overall phosphorylation of proteins. Approximate locations of WC-1, WC-2, and FRQ proteins on the gel are indicated. Right: representative Western blots of WC-1, WC-2, and FRQ from the in vitro phosphorylation assay are shown. Hyperphosphorylated species are indicated as pWC-1, pWC-2, and pFRQ. For in vitro phosphorylation of WC-1 and WC-2 total protein extracts was obtained from wt9718 cultures grown in light at 25 °C. Hypophosphorylated FRQ protein was obtained by inducing FRQ from a quinic acid-inducible promoter in a frq-null background (frq10 qa-frq) for 4 h, and extracts from this strain were phosphorylated in vitro. B, Western blot showing that phosphorylation of WC-1 and WC-2 by GSK is dependent on the amount of GSK added and can be selectively inhibited by GSK inhibitor indirubin.
We also asked whether FRQ is a substrate of GSK. Under steady-state conditions, FRQ is heterogeneously phosphorylated at numerous sites (11, 34, 35). Hence, additional phosphorylation by GSK may be difficult to detect. To generate a pool of hypo-phosphorylated FRQ, we expressed for 4 h a pulse of FRQ from a qa-inducible promoter in a frq-null background (frq10 qa-frq). Subsequently a protein extract was prepared and incubated with recombinant GSK. Although FRQ harbors many putative GSK phosphorylation sites, it was not visibly modified by the kinase in the in vitro assays (Fig. 6A), suggesting that it is not an efficient substrate of GSK. In contrast, incubation of the extract with CK1a resulted in efficient hyperphosphorylation of FRQ.
DISCUSSION
Here we identified GSK as a new component of the circadian clock of Neurospora crassa. GSK binds the circadian transcription factor WCC in vivo and phosphorylates both subunits with high specificity in a complex protein environment in vitro. Reduced expression of GSK in vivo resulted in elevated accumulation of WC-1, the limiting subunit of the WCC, and a shorter free-running period of the circadian clock. Wild type period length could be restored by induced expression of GSK, which in turn led to a reduction of total WC-1 levels. Induced expression of WCC is sufficient to shorten period length in a dose-dependent manner (36).
GSK is involved in the molecular mechanisms of circadian clocks in mammals and flies. Similar to our observations in Neurospora, GSK-mediated phosphorylation of clock proteins in mammals and Drosophila is also not essential for the clock but rather impacts on the circadian time-keeping process in a subtle manner. Specific inhibition of GSK or down-regulation by siRNA results in period shortening of the mouse circadian clock (24). In contrast, lithium, which is known to inhibit GSK activity, was reported to have a period lengthening effect in mammals (24, 37). These observations are comparable to the ones made in Neurospora, where lithium causes severe period lengthening (38), while down-regulation of GSK led to significant period shortening. Inhibition of GSK could have a different effect than down-regulation of the kinase. Since it is known to have profound effects on many cellular processes unrelated to GSK (reviewed in Ref. 39) it cannot be excluded that other targets of lithium impact on the circadian clock.
The findings suggest that the functions of GSK might be similar in the clocks of Neurospora and mammals. In Neurospora GSK affects expression levels of the WCC. In mammals, GSK3β phosphorylates and destabilizes CLOCK and BMAL1, the positive elements of the mammalian clock (20, 21). However, PER2 and CRY2 are also phosphorylated by GSK3β, which impacts on nuclear entry and proteasomal degradation of the proteins, respectively (22, 23). We could not find evidence that the negative clock regulator FRQ in Neurospora is a target of GSK.
Contrary to Neurospora, the Drosophila GSK homolog SHAGGY (SGG) phosphorylates the negative elements of the clock, timeless (TIM) and period (dPER). Accordingly, a reduction in SGG activity causes an increase in period length (18). It was recently shown that, as part of a multi-kinase hierarchical phosphorylation cascade, SGG phosphorylates and delays nuclear entry of dPER (19). Similarly, nuclear translocation of PER2 in mammaIs is also influenced by GSK3β phosphorylation (23). The subcellular localization of the WCC was not affected by reduced GSK levels (gskhet strain, Fig. 5B).
GSK appears to regulate accumulation of WCC on a post-transcriptional level, as wc-1 RNA levels were not affected by down-regulation of the kinase. Since GSK affects the free-running period of Neurospora the kinase must act on the dark form of the WCC. While the transient hyperphosphorylation and destabilization of the WCC upon light exposure has been analyzed in considerable detail (29, 32, 40), little is known about the turnover of WCC in constant conditions. It has been shown that the WCC is extremely stable in DD (32, 41). We show that GSK hyperphosphorylates the WCC in vitro. In vivo, GSK is bound to only a minor fraction of WCC, suggesting that GSK is limiting and may act in a processive manner. Since hyperphosphorylated forms of WC-1 and WC-2 do not accumulate, the fraction of the WCC phosphorylated by GSK may be rapidly degraded, while the majority of WCC, which is not in complex with GSK, is stable in constant conditions. Thus, we hypothesize that by promoting phosphorylation and turnover of a small fraction of the WCC, GSK fine-tunes the steady state levels of the WCC. The signals and metabolic cues regulating the interaction of GSK with the WCC and its subsequent phosphorylation, as well as the sites in WCC phosphorylated by GSK are not known and remain to be investigated.
Acknowledgment
We thank Sabine Schultz for excellent technical assistance.
The work was supported by a PhD fellowship of CellNetworks (to Ö. T.) and by grants of the Deutsche Forschungsgemeinschaft.

This article contains supplemental Tables S1 and S2 and Figs. S1–S4.
- TTFL
- transcriptional/translational feedback loops
- WCC
- white collar complex
- CK
- casein kinase
- GSK
- glycogen synthase kinase
- CHX
- cycloheximide
- QA
- quinic acid.
REFERENCES
- 1. Reischl S., Kramer A. (2011) Kinases and phosphatases in the mammalian circadian clock. FEBS Letters 585, 1393–1399 [DOI] [PubMed] [Google Scholar]
- 2. Diernfellner A. C., Schafmeier T. (2011) Phosphorylations: Making the Neurospora crassa circadian clock tick. FEBS Letters 585, 1461–1466 [DOI] [PubMed] [Google Scholar]
- 3. Mehra A., Baker C. L., Loros J. J., Dunlap J. C. (2009) Post-translational modifications in circadian rhythms. Trends Biochem. Sci. 34, 483–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guo J., Cheng P., Yuan H., Liu Y. (2009) The exosome regulates circadian gene expression in a post-transcriptional negative feedback loop. Cell 138, 1236–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garceau N. Y., Liu Y., Loros J. J., Dunlap J. C. (1997) Alternative initiation of translation and time-specific phosphorylation yield multiple forms of the essential clock protein FREQUENCY. Cell 89, 469–476 [DOI] [PubMed] [Google Scholar]
- 6. He Q., Cha J., Lee H. C., Yang Y., Liu Y. (2006) CKI and CKII mediate the FREQUENCY-dependent phosphorylation of the WHITE COLLAR complex to close the Neurospora circadian negative feedback loop. Genes Dev. 20, 2552–2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schafmeier T., Diernfellner A. C. (2011) Light input and processing in the circadian clock of Neurospora. FEBS Letters 585, 1467–1473 [DOI] [PubMed] [Google Scholar]
- 8. Smith K. M., Sancar G., Dekhang R., Sullivan C. M., Li S., Tag A. G., Sancar C., Bredeweg E. L., Priest H. D., McCormick R. F., Thomas T. L., Carrington J. C., Stajich J. E., Bell-Pedersen D., Brunner M., Freitag M. (2010) Transcription factors in light and circadian clock signaling networks revealed by genomewide mapping of direct targets for Neurospora white collar complex. Eukaryot Cell 9, 1549–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sancar G., Sancar C., Brügger B., Ha N., Sachsenheimer T., Gin E., Wdowik S., Lohmann I., Wieland F., Höfer T., Diernfellner A., Brunner M. (2011) A global circadian repressor controls antiphasic expression of metabolic genes in Neurospora. Mol. Cell 44, 687–697 [DOI] [PubMed] [Google Scholar]
- 10. Schafmeier T., Haase A., Káldi K., Scholz J., Fuchs M., Brunner M. (2005) Transcriptional feedback of Neurospora circadian clock gene by phosphorylation-dependent inactivation of its transcription factor. Cell 122, 235–246 [DOI] [PubMed] [Google Scholar]
- 11. Tang C. T., Li S., Long C., Cha J., Huang G., Li L., Chen S., Liu Y. (2009) Setting the pace of the Neurospora circadian clock by multiple independent FRQ phosphorylation events. Proc. Natl. Acad. Sci. U.S.A. 106, 10722–10727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Querfurth C., Diernfellner A. C., Gin E., Malzahn E., Höfer T., Brunner M. (2011) Circadian conformational change of the Neurospora clock protein FREQUENCY triggered by clustered hyperphosphorylation of a basic domain. Mol. Cell 43, 713–722 [DOI] [PubMed] [Google Scholar]
- 13. He Q., Cheng P., Yang Y., He Q., Yu H., Liu Y. (2003) FWD1-mediated degradation of FREQUENCY in Neurospora establishes a conserved mechanism for circadian clock regulation. EMBO J. 22, 4421–4430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schafmeier T., Diernfellner A., Schäfer A., Dintsis O., Neiss A., Brunner M. (2008) Circadian activity and abundance rhythms of the Neurospora clock transcription factor WCC associated with rapid nucleo-cytoplasmic shuttling. Genes Dev. 22, 3397–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Peschel N., Helfrich-Förster C. (2011) Setting the clock–by nature: circadian rhythm in the fruitfly Drosophila melanogaster. FEBS letters 585, 1435–1442 [DOI] [PubMed] [Google Scholar]
- 16. Tataroğlu O., Schafmeier T. (2010) Of switches and hourglasses: regulation of subcellular traffic in circadian clocks by phosphorylation. EMBO Reports 11, 927–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Merrow M., Roenneberg T. (2007) Circadian entrainment of Neurospora crassa. Cold Spring Harb. Symp. Quant. Biol. 72, 279–285 [DOI] [PubMed] [Google Scholar]
- 18. Martinek S., Inonog S., Manoukian A. S., Young M. W. (2001) A role for the segment polarity gene shaggy/GSK-3 in the Drosophila circadian clock. Cell 105, 769–779 [DOI] [PubMed] [Google Scholar]
- 19. Ko H. W., Kim E. Y., Chiu J., Vanselow J. T., Kramer A., Edery I. (2010) A hierarchical phosphorylation cascade that regulates the timing of PERIOD nuclear entry reveals novel roles for proline-directed kinases and GSK-3β/SGG in circadian clocks. J. Neurosci. 30, 12664–12675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spengler M. L., Kuropatwinski K. K., Schumer M., Antoch M. P. (2009) A serine cluster mediates BMAL1-dependent CLOCK phosphorylation and degradation. Cell Cycle 8, 4138–4146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sahar S., Zocchi L., Kinoshita C., Borrelli E., Sassone-Corsi P. (2010) Regulation of BMAL1 protein stability and circadian function by GSK3β-mediated phosphorylation. PLoS One 5, e8561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kurabayashi N., Hirota T., Sakai M., Sanada K., Fukada Y. (2010) DYRK1A and glycogen synthase kinase 3β, a dual-kinase mechanism directing proteasomal degradation of CRY2 for circadian timekeeping. Mol. Cell. Biol. 30, 1757–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Iitaka C., Miyazaki K., Akaike T., Ishida N. (2005) A role for glycogen synthase kinase-3β in the mammalian circadian clock. J. Biol. Chem. 280, 29397–29402 [DOI] [PubMed] [Google Scholar]
- 24. Hirota T., Lewis W. G., Liu A. C., Lee J. W., Schultz P. G., Kay S. A. (2008) A chemical biology approach reveals period shortening of the mammalian circadian clock by specific inhibition of GSK-3β. Proc. Natl. Acad. Sci. U.S.A. 105, 20746–20751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Colot H. V., Park G., Turner G. E., Ringelberg C., Crew C. M., Litvinkova L., Weiss R. L., Borkovich K. A., Dunlap J. C. (2006) A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc. Natl. Acad. Sci. U.S.A. 103, 10352–10357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Belden W. J., Larrondo L. F., Froehlich A. C., Shi M., Chen C. H., Loros J. J., Dunlap J. C. (2007) The band mutation in Neurospora crassa is a dominant allele of ras-1 implicating RAS signaling in circadian output. Genes Dev. 21, 1494–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Diernfellner A. C., Schafmeier T., Merrow M. W., Brunner M. (2005) Molecular mechanism of temperature sensing by the circadian clock of Neurospora crassa. Genes Dev. 19, 1968–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Görl M., Merrow M., Huttner B., Johnson J., Roenneberg T., Brunner M. (2001) A PEST-like element in FREQUENCY determines the length of the circadian period in Neurospora crassa. EMBO J. 20, 7074–7084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Malzahn E., Ciprianidis S., Káldi K., Schafmeier T., Brunner M. (2010) Photoadaptation in Neurospora by competitive interaction of activating and inhibitory LOV domains. Cell 142, 762–772 [DOI] [PubMed] [Google Scholar]
- 30. Sancar G., Sancar C., Brunner M., Schafmeier T. (2009) Activity of the circadian transcription factor White Collar Complex is modulated by phosphorylation of SP-motifs. FEBS Lett. 583, 1833–1840 [DOI] [PubMed] [Google Scholar]
- 31. He Q., Shu H., Cheng P., Chen S., Wang L., Liu Y. (2005) Light-independent phosphorylation of WHITE COLLAR-1 regulates its function in the Neurospora circadian negative feedback loop. J. Biol. Chem. 280, 17526–17532 [DOI] [PubMed] [Google Scholar]
- 32. Lee K., Loros J. J., Dunlap J. C. (2000) Interconnected feedback loops in the Neurospora circadian system. Science 289, 107–110 [DOI] [PubMed] [Google Scholar]
- 33. Schafmeier T., Káldi K., Diernfellner A., Mohr C., Brunner M. (2006) Phosphorylation-dependent maturation of Neurospora circadian clock protein from a nuclear repressor toward a cytoplasmic activator. Genes Dev. 20, 297–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu Y., Loros J., Dunlap J. C. (2000) Phosphorylation of the Neurospora clock protein FREQUENCY determines its degradation rate and strongly influences the period length of the circadian clock. Proc. Natl. Acad. Sci. U.S.A. 97, 234–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baker C. L., Kettenbach A. N., Loros J. J., Gerber S. A., Dunlap J. C. (2009) Quantitative proteomics reveals a dynamic interactome and phase-specific phosphorylation in the Neurospora circadian clock. Mol. Cell 34, 354–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cheng P., Yang Y., Liu Y. (2001) Interlocked feedback loops contribute to the robustness of the Neurospora circadian clock. Proc. Natl. Acad. Sci. U.S.A. 98, 7408–7413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Johnsson A., Pflug B., Engelmann W., Klemke W. (1979) Effect of lithium carbonate on circadian periodicity in humans. Pharmakopsychiatr Neuropsychopharmakol 12, 423–425 [DOI] [PubMed] [Google Scholar]
- 38. Jolma I. W., Falkeid G., Bamerni M., Ruoff P. (2006) Lithium leads to an increased FRQ protein stability and to a partial loss of temperature compensation in the Neurospora circadian clock. J. Biol. Rhythms 21, 327–334 [DOI] [PubMed] [Google Scholar]
- 39. O'Brien W. T., Klein P. S. (2009) Validating GSK3 as an in vivo target of lithium action. Biochem. Soc. Trans. 37, 1133–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Talora C., Franchi L., Linden H., Ballario P., Macino G. (1999) Role of a white collar-1-white collar-2 complex in blue-light signal transduction. EMBO J. 18, 4961–4968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. He Q., Cheng P., Yang Y., Wang L., Gardner K. H., Liu Y. (2002) White collar-1, a DNA binding transcription factor and a light sensor. Science 297, 840–843 [DOI] [PubMed] [Google Scholar]