Abstract
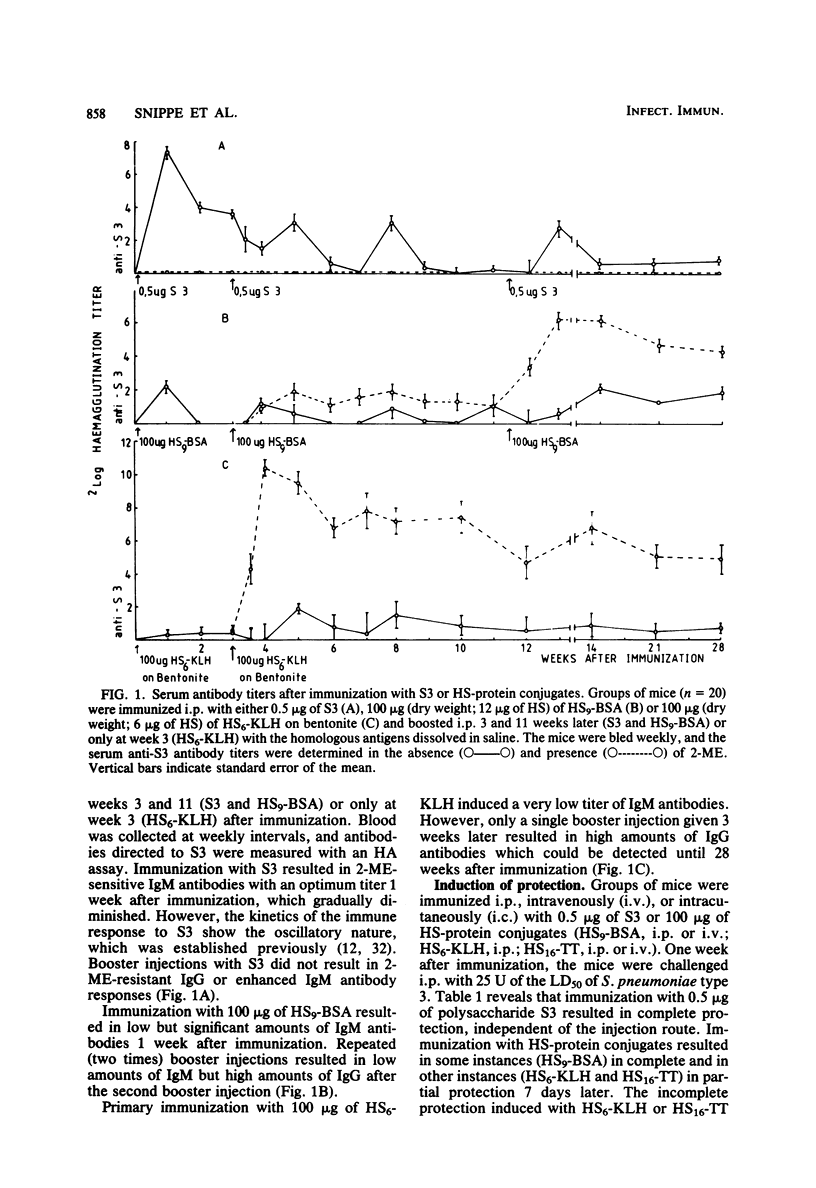
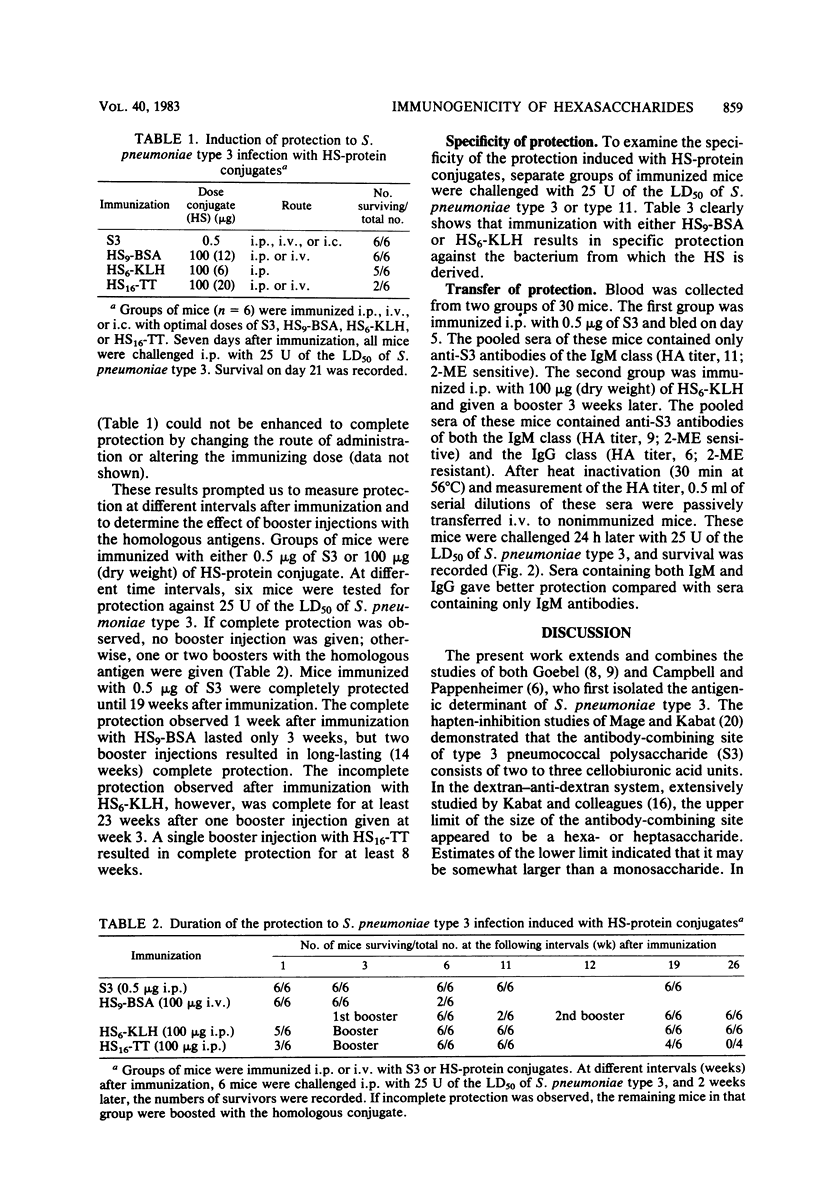
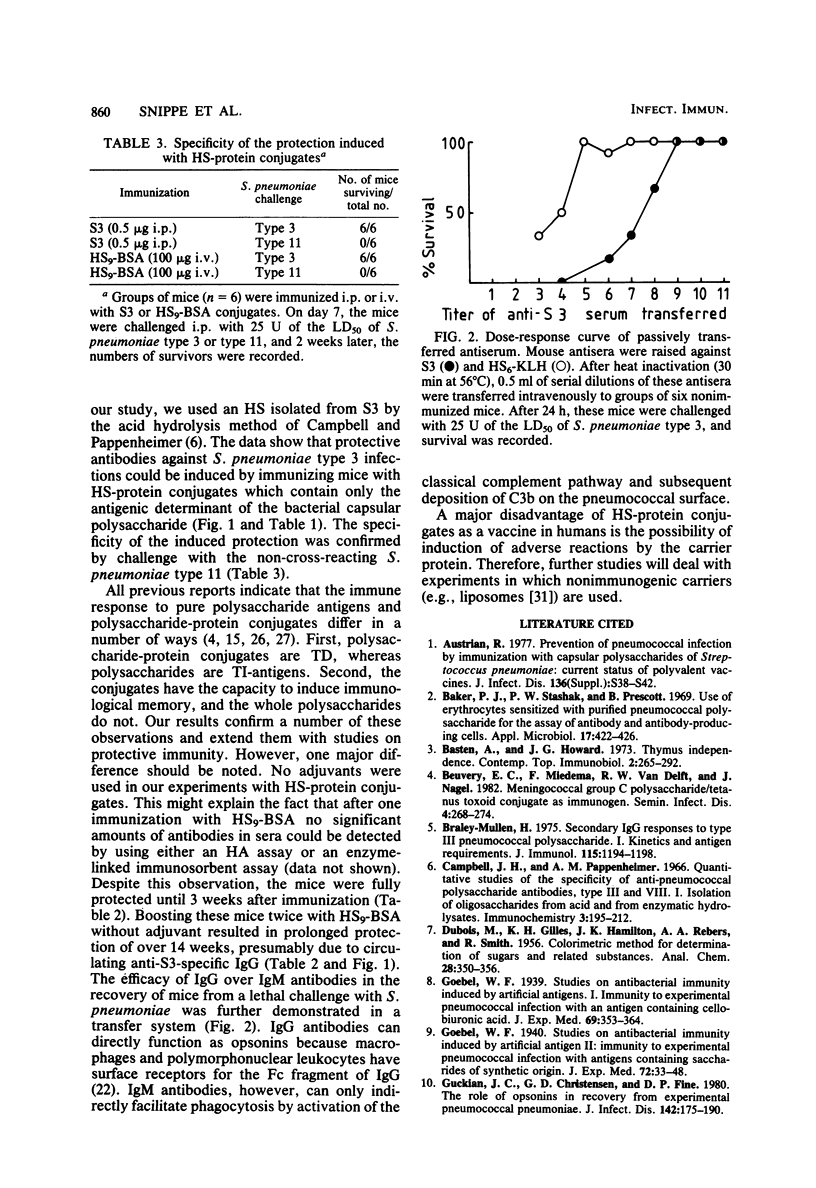
Hexasaccharide (HS) containing 3 U of cellobiuronic acid was isolated from Streptococcus pneumoniae type 3 capsular polysaccharide S3 and coupled to bovine serum albumin (BSA), keyhole limpet hemocyanin (KLH), or tetanus toxoid (TT). The immunogenicity of these HS-protein conjugates in BALB/c mice was studied by measuring the production of circulating antibodies and the induction of protective immunity to viable S. pneumoniae type 3. Immunization of BALB/c mice with 0.5 micrograms of S3 resulted in the induction of immunoglobulin M (IgM) antibodies and complete protection against 25 U of a mean lethal dose of S. pneumoniae type 3 for 19 weeks after immunization. BALB/c mice immunized with 100 micrograms of HS9-BSA (containing 12 micrograms of HS) were also protected due to circulating IgM antibodies. Repeated injections with either 100 micrograms of HS9-BSA (three immunizations) or 100 micrograms of HS6-KLH (two immunizations) resulted in high levels of circulating IgG antibodies. These HS-protein conjugates induced complete protection which lasted at least 14 (HS9-BSA), 23 (HS6-KLH), or 8 (HS16-TT) weeks after the last immunization. Protection against viable S. pneumoniae type 3 could be passively transferred to nonimmunized mice by antisera containing IgM or IgG antibodies or both. Sera containing both IgM and IgG antibodies gave better protection than sera containing only IgM antibodies. The specificity of the induced protection was confirmed by challenge with the non-cross-reacting S. pneumoniae type 11.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austrian R. Prevention of pneumococcal infection by immunization with capsular polysaccharides of Streptococcus pneumoniae: current status of polyvalent vaccines. J Infect Dis. 1977 Aug;136 (Suppl):S38–S42. doi: 10.1093/infdis/136.supplement.s38. [DOI] [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Prescott B. Use of erythrocytes sensitized with purified pneumococcal polysaccharides for the assay of antibody and antibody-producing cells. Appl Microbiol. 1969 Mar;17(3):422–426. doi: 10.1128/am.17.3.422-426.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braley-Mullen H. Secondary IgG responses to type III pneumococcal polysaccharide. I. Kinetics and antigen requirements. J Immunol. 1975 Nov;115(5):1194–1198. [PubMed] [Google Scholar]
- Campbell J. H., Pappenheimer A. M., Jr Quantitative studies of the specificity of anti-pneumococcal polysaccharide antibodies, types 3 and 8. I. Isolation of oligosaccharides from acid and from enzymatic hydrolysates of S3 and S8. Immunochemistry. 1966 May;3(3):195–212. doi: 10.1016/0019-2791(66)90184-4. [DOI] [PubMed] [Google Scholar]
- Goebel W. F. STUDIES ON ANTIBACTERIAL IMMUNITY INDUCED BY ARTIFICIAL ANTIGENS : I. IMMUNITY TO EXPERIMENTAL PNEUMOCOCCAL INFECTION WITH AN ANTIGEN CONTAINING CELLOBIURONIC ACID. J Exp Med. 1939 Feb 28;69(3):353–364. doi: 10.1084/jem.69.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel W. F. STUDIES ON ANTIBACTERIAL IMMUNITY INDUCED BY ARTIFICIAL ANTIGENS : II. IMMUNITY TO EXPERIMENTAL PNEUMOCOCCAL INFECTION WITH ANTIGENS CONTAINING SACCHARIDES OF SYNTHETIC ORIGIN. J Exp Med. 1940 Jun 30;72(1):33–48. doi: 10.1084/jem.72.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guckian J. C., Christensen G. D., Fine D. P. The role of opsonins in recovery from experimental pneumococcal pneumonia. J Infect Dis. 1980 Aug;142(2):175–190. doi: 10.1093/infdis/142.2.175. [DOI] [PubMed] [Google Scholar]
- Heidelberger M., Roy N., Glaudemans C. P. Inhibition of aldobiouronates in the precipitation of pneumococcal type II and III systems. Biochemistry. 1969 Dec;8(12):4822–4824. doi: 10.1021/bi00840a025. [DOI] [PubMed] [Google Scholar]
- Hiernaux J. R., Baker P. J., Delisi C., Rudbach J. A. Modulation of the immune response to lipopolysaccharide. J Immunol. 1982 Mar;128(3):1054–1058. [PubMed] [Google Scholar]
- Inman J. K., Merchant B., Claflin L., Tacey S. E. Coupling of large haptens to proteins and cell surfaces: preparation of stable, optimally sensitized erythrocytes for hapten-specific, hemolytic plaque assays. Immunochemistry. 1973 Mar;10(3):165–174. doi: 10.1016/0019-2791(73)90005-0. [DOI] [PubMed] [Google Scholar]
- Jennings H. J., Lugowski C. Immunochemistry of groups A, B, and C meningococcal polysaccharide-tetanus toxoid conjugates. J Immunol. 1981 Sep;127(3):1011–1018. [PubMed] [Google Scholar]
- Kamerling J. P., Gerwig G. J., Vliegenthart J. F., Clamp J. R. Characterization by gas-liquid chromatography-mass spectrometry and proton-magnetic-resonance spectroscopy of pertrimethylsilyl methyl glycosides obtained in the methanolysis of glycoproteins and glycopeptides. Biochem J. 1975 Dec;151(3):491–495. doi: 10.1042/bj1510491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz D. H., Benacerraf B. The regulatory influence of activated T cells on B cell responses to antigen. Adv Immunol. 1972;15:1–94. doi: 10.1016/s0065-2776(08)60683-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAGE R. G., KABAT E. A. THE COMBINING REGIONS OF THE TYPE III PNEUMOCOCCUS POLYSACCHARIDE AND HOMOLOGOUS ANTIBODY. Biochemistry. 1963 Nov-Dec;2:1278–1288. doi: 10.1021/bi00906a019. [DOI] [PubMed] [Google Scholar]
- Messner R. P., Jelinek J. Receptors for human gamma G globulin on human neutrophils. J Clin Invest. 1970 Dec;49(12):2165–2171. doi: 10.1172/JCI106435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneerson R., Barrera O., Sutton A., Robbins J. B. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J Exp Med. 1980 Aug 1;152(2):361–376. doi: 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein K. E., Zopf D. A., Johnson B. M., Miller C. B., Paul W. E. The immune response to an isomaltohexosyl-protein conjugate, a thymus-dependent analogue of alpha(1 replaced by 6) dextran. J Immunol. 1982 Mar;128(3):1350–1354. [PubMed] [Google Scholar]
- Svenson S. B., Lindberg A. A. Coupling of acid labile Salmonella specific oligosaccharides to macromolecular carriers. J Immunol Methods. 1979;25(4):323–335. doi: 10.1016/0022-1759(79)90025-5. [DOI] [PubMed] [Google Scholar]
- Tittle T. V., Rittenberg M. B. Expression of IgG memory response in vitro to thymus-dependent and thymus-independent antigens. Cell Immunol. 1978 Jan;35(1):180–190. doi: 10.1016/0008-8749(78)90138-7. [DOI] [PubMed] [Google Scholar]
- Tsai C. S. Determination of degree of polymerization of N-acetyl chitooligoses by chromatographic methods. Anal Biochem. 1970 Jul;36(1):114–122. doi: 10.1016/0003-2697(70)90338-6. [DOI] [PubMed] [Google Scholar]
- Weigle W. O. Cyclical production of antibody as a regulatory mechanism in the immune response. Adv Immunol. 1975;21:87–111. doi: 10.1016/s0065-2776(08)60219-9. [DOI] [PubMed] [Google Scholar]
- van Houte A. J., Snippe H., Willers J. M. Characterization of immunogenic properties of haptenated liposomal model membranes in mice. I. Thymus independence of the antigen. Immunology. 1979 Jun;37(2):505–514. [PMC free article] [PubMed] [Google Scholar]


