Background: Decidual glycodelin-A (GdA) is a regulator of immune cells.
Results: GdA binds to L-selectin and induces IL-6 expression in monocytes/macrophages, which suppress the Th-1 response of T-cells.
Conclusion: GdA has regulatory roles in monocytes/macrophages, which may contribute to the Th-1/Th-2 shift in early pregnancy.
Significance: The results increase our knowledge of the regulation of monocyte/macrophage functions and may provide insights into the pathology of complicated pregnancy.
Keywords: Cytokine, ERK, Glycoprotein, Macrophages, Monocytes, Pregnancy, Sialic Acid, T-cell, L-selectin, Glycodelin-A
Abstract
Macrophages represent the second major type of decidual leukocytes at the fetomaternal interface. Changes in macrophage number and activity are associated with fetal loss and pregnancy complications. Glycodelin-A (GdA) is an abundant glycoprotein in the first-trimester decidua. It is involved in fetomaternal defense and early placental development through its regulatory activities in various immune cells. The N-glycosylation of GdA mediates the binding and therefore the activities of the molecule. In this study, we studied the biological activities of GdA in the functions of human monocytes/macrophages. GdA was purified from amniotic fluid by affinity chromatography. GdA treatment did not affect the viability, cell death, or phagocytic activity of the monocytes/macrophages. GdA, but not recombinant glycodelin without glycosylation, induced IL-6 production as demonstrated by cytokine array, intracellular staining, and ELISA. GdA also induced phosphorylation of ERK in monocytes/macrophages. The involvement of ERKs in IL-6 induction was confirmed using pharmacological inhibitors. Co-immunoprecipitation showed that L-selectin on the monocytes/macrophages was the binding protein of GdA. Treatment with anti-L-selectin antibody reduced GdA binding and GdA-induced IL-6 production. GdA-treated macrophages suppressed IFN-γ expression by co-cultured T-helper cells in an IL-6-dependent manner. These results show that GdA interacts with L-selectin to induce IL-6 production in monocytes/macrophages by activating the ERK signaling pathway. In turn, the increased IL-6 production suppresses IFN-γ expression in T-helper cells, which may play an important role in inducing a Th-2-polarized cytokine environment that flavors the immunotolerance of the fetoplacental unit.
Introduction
Glycodelin-A (GdA)3 is a glycoprotein known for its role in fetomaternal defense and placental development during early pregnancy (1, 2), consistent with its peak expression in the decidua between 6 and 12 weeks of gestation (1). GdA suppresses proliferation and induces apoptosis of T-cells (2), regulates B-cell responses (3), and induces a tolerogenic phenotype in dendritic cells (4). Recently, GdA was found to modulate cytokine production in natural killer cells (5) and to induce Th-2 shift in cytokines (6, 7), which is crucial to maintenance of pregnancy. The importance of GdA in pregnancy is also indicated by its lower levels in serum and uterine flushings of women with infertility, first-trimester miscarriage, and recurrent miscarriage (1, 8).
CD14+ monocytes/macrophages comprise 20–30% of the leukocytes at the implantation site. These are the second major type of immune cells at the fetomaternal interface. During early pregnancy, they are actively recruited to the decidua from the blood circulation and produce cytokines and other soluble factors that support fetal tolerance and placental development (9, 10). Their number at the fetomaternal interface remains constant throughout pregnancy (9). Aberrant activities of monocytes/macrophages are associated with pregnancy complications or loss (9, 11). Despite the importance of these cells in early pregnancy, little is known about the regulation of their functions. The first objective of this study was to evaluate the effect of GdA on the biological activities of monocytes and macrophages.
The glycans of GdA, especially their terminal sialic acids, mediate the binding and biological activities of the molecule in several cell types (2, 12–14). The clinical importance of sialic acids in GdA has been demonstrated in patients with gestational diabetes mellitus; GdA from these patients shows both reduced α2–6 sialylation and immunomodulatory activities (15). Our second objective was to study the role of sialylation and to identify the receptor for the action of GdA on monocytes/macrophages.
EXPERIMENTAL PROCEDURES
Purification of GdA from Human Amniotic Fluid
The Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster approved the protocol of this study. GdA was purified from amniotic fluid by affinity chromatography using anti-glycodelin monoclonal antibody (clone F43-7F9) as described (16). The purified protein was confirmed to be glycodelin by Western blotting and mass spectrometry (supplemental Fig. S1). Desialylation of GdA was done by incubation with sialidase-coated agarose beads (Sigma) at 37 °C for 18 h (14, 15). Successful desialylation was confirmed by decreased binding to wheat germ agglutinin lectin (12). Recombinant glycodelin without glycosylation was produced and purified from Escherichia coli DH5α (Invitrogen) (17). Purified GdA and recombinant glycodelin were found to contain <0.1 endotoxin unit/ml as determined by limulus amebocyte lysate assay (GenScript, Piscataway, NJ).
Primary Monocyte and T-helper Cell Isolation, Macrophage Differentiation, and Cell Culture
Human female peripheral blood was obtained from the Hong Kong Red Cross. Primary CD14+ monocytes and CD3+CD4+ T-helper cells were isolated by Ficoll-Paque density gradient centrifugation (GE Healthcare), followed by negative immunomagnetic separation (Miltenyi Biotec Inc., Bergisch Gladbach, Germany). The purity of the isolated monocytes and T-helper cells was 90–95% as determined by flow cytometry (supplemental Fig. S2). The cells were cultured in 10% FBS-supplemented RPMI 1640 medium (Sigma). Macrophages were prepared by treating monocytes with 50 ng/ml GM-CSF (PeproTech, Rocky Hill, NJ) for 6 days. GM-CSF was used because it is the main differentiation factor for tissue macrophages in vivo (18–20). The monocytic acute leukemia cell line THP-1 (American Type Culture Collection, Manassas, VA) was cultured in RPMI 1640 medium supplemented with 10% FBS and 0.05 mm 2-mercaptoethanol.
GdA Binding Assay
GdA was fluorescently labeled using the Alexa Fluor 488 protein labeling kit (Molecular Probes, Carlsbad, CA) (15). Monocytes/macrophages (5 × 105) were fixed with intracellular fixation buffer (eBioscience, San Diego, CA) before incubation with 1 μg/ml labeled GdA for 2 h. The cells were analyzed using a BD FACSCanto II flow cytometer (BD Biosciences). The data were analyzed using FlowJo 7.6.3 software (Tree Star Inc., Ashland, OR). Cells incubated with an equimolar amount of an unrelated protein (Alexa Fluor 488-labeled goat IgG) were used as a negative control.
Determination of Cell Viability and Cell Death
Monocytes/macrophages (3 × 104) were incubated with 0.01, 0.1, 1m or 10 μg/ml GdA for 72 h. The viability of the cells was determined by the 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-(phenylamino)carbonyl)-2H-tetrazolium hydroxide (XTT) cell viability assay (Roche Diagnostic). Apoptotic and necrotic cell deaths were determined by flow cytometry using YO-PRO®-1 and propidium iodide (Invitrogen) as described (6, 12, 15) and analyzed by flow cytometer using 525- and 610-nm band pass filters.
Phagocytosis Assay
The effect of GdA on the phagocytic activity of the cells was analyzed by using a phagocytosis assay kit (Cayman Chemical) according to the manufacturer's protocol.
Cytokine Profiling of Monocytes
Monocytes (1 × 106) were treated with 10 μg/ml GdA for 48 h. The cytokine profile was determined semiquantitatively by human cytokine antibody array (RayBiotech, Inc., Norcross, GA) according to the manufacturer's protocol. The density of the cytokine spots was analyzed by densitometry using Quantity One software (Bio-Rad).
Determination of IL-6 Secretion in the Culture Medium by ELISA
Monocytes, THP-1 cells, and macrophages (5 × 105) were treated with 0.1, 1, and 10 μg/ml GdA or 10 μg/ml recombinant GdA for 48 h. As the basal secretion of IL-6 by the monocytes and THP-1 cells was low, the IL-6 production capacity of these cells were studied after exposure to LPS (1 μg/ml; Sigma), a commonly used activator of cytokine secretion by monocytes (21, 22), for 6 h. The duration of LPS activation was determined by studying the dynamic effect of LPS on IL-6 production in both cell types after GdA treatment, which showed a significant (p < 0.05) effect of GdA on IL-6 production after 6 h of LPS activation compared with the control without GdA treatment (supplemental Table S1). The level of IL-6 in the conditioned medium was determined by an ELISA-based assay (human IL-6 CytoSetTM, Invitrogen) (5).
Intracellular IL-6 Staining of Monocytes
Primary monocytes (1 × 106) were treated with 10 μg/ml GdA for 48 h. LPS (1 μg/ml) and brefeldin A (3 μg/ml) were added 6 h before the end of treatment. Cells were then fixed with intracellular fixation buffer for 10 min at room temperature and permeabilized with permeabilization buffer (eBioscience) for 5 min. The cells were washed and resuspended in 80 μl of permeabilization buffer containing 20 μl of FITC-labeled anti-IL-6 antibody (BD Biosciences) for 20 min at room temperature in the dark. The cells were resuspended in blocking buffer for flow cytometric analysis.
Effect of GdA on Activated ERKs in Monocytes/Macrophages
Monocytes, macrophages, and THP-1 cells (5 × 106) were incubated with 10 μg/ml GdA for different times (THP-1 cells, 0–24 h; and monocytes and macrophages, 0–6 h). The cells were lysed using CytoBuster protein extraction reagent (Merck). The protein lysates were resolved by 12% SDS-PAGE and transferred to a PVDF membrane for Western blot analysis using antibodies against ERKs (1:1000; Cell Signaling, Danvers, MA), phosphorylated ERKs (1:2000; Cell Signaling), and β-actin (Sigma). The protein bands were quantified by densitometry.
Effects of Inhibitors of ERK Kinase, p38, and NF-κB on the Stimulatory Effect of GdA on IL-6 Production in THP-1 Cells
THP-1 cells (5 × 105) were incubated with 10 μg/ml GdA in the presence or absence of ERK kinase inhibitors (PD98059, 10 μm; or U0126, 1 μm), NF-κB inhibitors (caffeic acid phenethyl ester and BAY-11708, 10 μm), or p38 inhibitors (SB202190, 5 μm; or SB203580, 10 μm) for 48 h. The cells were activated by LPS (1 μg/ml) for 6 h before the end of the experiment. The viabilities of the treated cells and the IL-6 level in the conditioned medium were then determined by XTT assay and ELISA, respectively, as described above.
Effects of Anti-L-selectin Antibodies on GdA Binding to and IL-6 Secretion by Monocytes
L-selectin expression in monocytes, macrophages, and THP-1 cells was determined by flow cytometry. In brief, 5 × 105 cells were incubated successively with mouse anti-human L-selectin antibody (Abcam, Cambridge, MA) and FITC-labeled anti-mouse antibody in PBS containing 1% BSA and 0.1% sodium azide. Cells treated with FITC-labeled anti-mouse antibody alone were used as controls. L-selectin expression in the cells was analyzed by flow cytometry.
The effects of anti-L-selectin antibodies on GdA binding and IL-6 secretion were investigated by incubating monocytes with fluorescently labeled GdA in the presence of anti-L-selectin antibody or control antibody at a molar ratio of 1:5 for 48 h. The fluorescent signal and the IL-6 level in the conditioned medium were then analyzed by flow cytometry and ELISA, respectively, as described above.
Interaction between GdA and L-selectin in Monocytes
Membrane proteins of 2 × 107 monocytes were extracted using a commercial membrane protein extraction kit (ProteoExtract transmembrane protein extraction kit, Novagen) according to the manufacturer's instructions. The extracted membrane protein fractions or IgG-fused recombinant human L-selectin chimeric proteins (R&D Systems, Minneapolis, MN) were incubated with native or desialylated GdA in PBS at 4 °C. After overnight incubation with gentle shaking, anti-glycodelin antibody (clone F43-7F9)-conjugated Sepharose beads (GE Healthcare) and protein G-Sepharose beads (GE Healthcare) were used to precipitate the complexes of GdA and GdA-interacting protein and of GdA and recombinant L-selectin, respectively. The captured complex was washed thoroughly, resolved by 12% SDS-PAGE, and analyzed by Western blotting using anti-L-selectin (Abcam) or anti-glycodelin (Abcam) antibody. To ensure equal loading of membrane protein extracts onto the Sepharose beads, the flow-through fractions of the extracts were collected after immunoprecipitation and analyzed by Western blotting using anti-CD14 antibody (Abcam).
Role of GdA-induced IL-6 Production in Macrophages in Th-1, Th-2, and Th-17 Cytokine Production in T-helper Cells
Macrophages (1 × 106) were treated with GdA (10 μg/ml) for 48 h and washed. GdA-treated macrophages and autologous T-helper cells (1 × 106) were then co-cultured in vitro without direct contact using a culture insert in the presence or absence of anti-IL-6 antibody (10 μg/ml) for 42 h. Phorbol myristate acetate (50 ng/ml), ionomycin (1 μg/ml), and brefeldin A (3 μg/ml) were then added to activate the T-helper cells after removal of the macrophages. Intracellular Th-1 (IFN-γ), Th-2 (IL-4 and IL-10), and Th-17 (IL-17) cytokine stainings were performed on the T-helper cells as described above using fluorescence-conjugated antibody against IFN-γ, IL-4, and IL-17 (Miltenyi Biotec Inc.). To confirm the sustained stimulatory effect of GdA on IL-6 production, washed macrophages after 48 h of GdA treatment were further cultured for 42 h. The IL-6 level in the conditioned medium was then determined.
Data Analysis
All values are expressed as the mean ± S.E. For all experiments, the non-parametric analysis of variance by rank test for comparisons was used to identify differences between groups. If the data were normally distributed, parametric Student's t test or the non-parametric Mann-Whitney U test was used where appropriate as the post-test. The data were analyzed using SigmaStat 2.03 (Jandel Scientific, San Rafael, CA). A p value of <0.05 was considered significant.
RESULTS
GdA Binds to Monocytes and Macrophages and Induces IL-6 Secretion
Flow cytometric analyses showed similar binding of fluorescently labeled GdA to all three cell types tested (monocytes, 81.1 ± 2.5%; THP-1 cells, 78.2 ± 7.8%; and macrophages, 84.2 ± 4.7%) (Fig. 1). No positive signal was observed in the negative control using fluorescently labeled IgG. GdA treatment significantly (p < 0.05) stimulated IL-6 production in monocytes as demonstrated by cytokine array and intracellular cytokine staining (Fig. 2). The results were confirmed by ELISA, which showed that the action of GdA was dose-dependent (Table 1). Similar observations were found in THP-1 cells and macrophages (Table 1). The treatment did not affect the viability (supplemental Fig. S3A), cell death (supplemental Fig. S3B), and phagocytic activity (supplemental Table S2) of the monocytes/macrophages.
FIGURE 1.
Binding of GdA to monocytes and macrophages. Monocytes, THP-1 cells, and macrophages were incubated with 1 μg/ml Alexa Fluor 488-labeled GdA for 2 h, followed by flow cytometric analysis. An unrelated protein (Alexa Fluor 488-labeled goat IgG) was used as a negative control. Data are the mean ± S.E. The results shown are representative of four replicate experiments.
FIGURE 2.
Effects of GdA on IL-6 production in monocytes. Monocytes were incubated with 10 μg/ml GdA for 48 h. A, cytokine secretion in the culture medium determined by cytokine array and analyzed by densitometry. The results are expressed as -fold change relative to the control without GdA treatment. Data are the mean ± S.E. The results shown are representative of four replicate experiments. B, intracellular IL-6 content determined by flow cytometry. LPS (1 μg/ml) and brefeldin A (3 μg/ml) were added 6 h before the end of the GdA treatment. Data are the mean ± S.E. (n = 6). *, p < 0.05 compared with the control.
TABLE 1.
GdA-induced IL-6 secretion by monocytes/macrophages
Monocytes, THP-1 cells, and macrophages (5 × 105) were incubated with 0.1, 1, or 10 μg/ml GdA for 48 h. IL-6 secretion into the culture medium was measured by ELISA (n = 8). IL-6 secretion into the culture medium of monocytes and THP-1 cells was measured after LPS activation. Data are the mean ± S.E.
| IL-6 level |
|||
|---|---|---|---|
| Monocytes | THP-1 cells | Macrophages | |
| pg/ml | |||
| Control | 654.6 ± 265.0 | 952.4 ± 370.4 | 4429.7 ± 752.6 |
| GdA (0.1 μg/ml) | 834.7 ± 315.9 | 1118.9 ± 459.4 | 5305.4 ± 909.8 |
| GdA (1 μg/ml) | 871.0 ± 344.2 | 3331.5 ± 507.9 | 6383.7 ± 1148.4 |
| GdA (10 μg/ml) | 1199.4 ± 391.4a | 4802.1 ± 1107.5a | 9213.6 ± 1277.9a |
a p < 0.05 compared with the corresponding control without GdA treatment.
In addition to IL-6, the cytokine array showed that GdA also significantly up-regulated IL-1β and IL-3 production in monocytes (Fig. 2). In contrast to monocytes, our preliminary data show that GdA could only stimulate IL-6 production in macrophages (data not shown). To study the signaling pathways that are shared by both monocytes and macrophages for the GdA activity, we focused on IL-6 production in the subsequent experiments.
GdA Induces IL-6 Production via the ERK Pathway
Treatment with GdA for 6 h significantly (p < 0.05) increased the levels of phosphorylated ERKs (ERK1, 44 kDa; and ERK2, 42 kDa) but not those of total ERK in THP-1 cells (Fig. 3A). Similar observations were obtained in monocytes and macrophages after 15 min or 6 h of GdA treatment (Fig. 3A). This action on ERK mediated the stimulatory effect of GdA on IL-6 secretion, as ERK kinase inhibitors (PD98059 and U0126) abolished such effects in THP-1 cells (Fig. 3B). The inhibitors alone did not affect IL-6 production (Fig. 3B) or viability (supplemental Fig. S4) of the treated cells. On the other hand, NF-κB and p38 inhibitors had no effect on the GdA-induced IL-6 production in THP-1 cells (Fig. 3B).
FIGURE 3.
Effects of GdA on ERK activation and IL-6 production. A, upper, effect of GdA treatment on ERK activation in THP-1 cells, monocytes, and macrophages at different time points. Representative Western blots for ERKs from six individual experiments are shown. Lower, phosphorylated ERK (pERK) protein bands (phospho-ERK1, 44 kDa; and phospho-ERK2, 42 kDa) were quantified by densitometry. The results are expressed as the band density post-treatment normalized to the base-line density at time 0. Total ERK and β-actin were used for normalization. Data are the mean ± S.E. *, p < 0.05 compared with the corresponding control at time zero. B, THP-1 cells were incubated with GdA in the presence or absence of ERK kinase inhibitors (PD98059, 10 μm; or U0126, 1 μm), NF-κB inhibitors (caffeic acid phenethyl ester (CAPE) and BAY-11708, 10 μm), or p38 inhibitors (SB202190, 5 μm; or SB203580, 10 μm) for 48 h (n = 12). IL-6 levels in the culture medium were measured by ELISA and expressed as a percentage relative to the control without treatment (494.0 ± 162.6 pg/ml). Data are the mean ± S.E. *, p < 0.05 compared with the THP-1 cells treated with GdA without inhibitor.
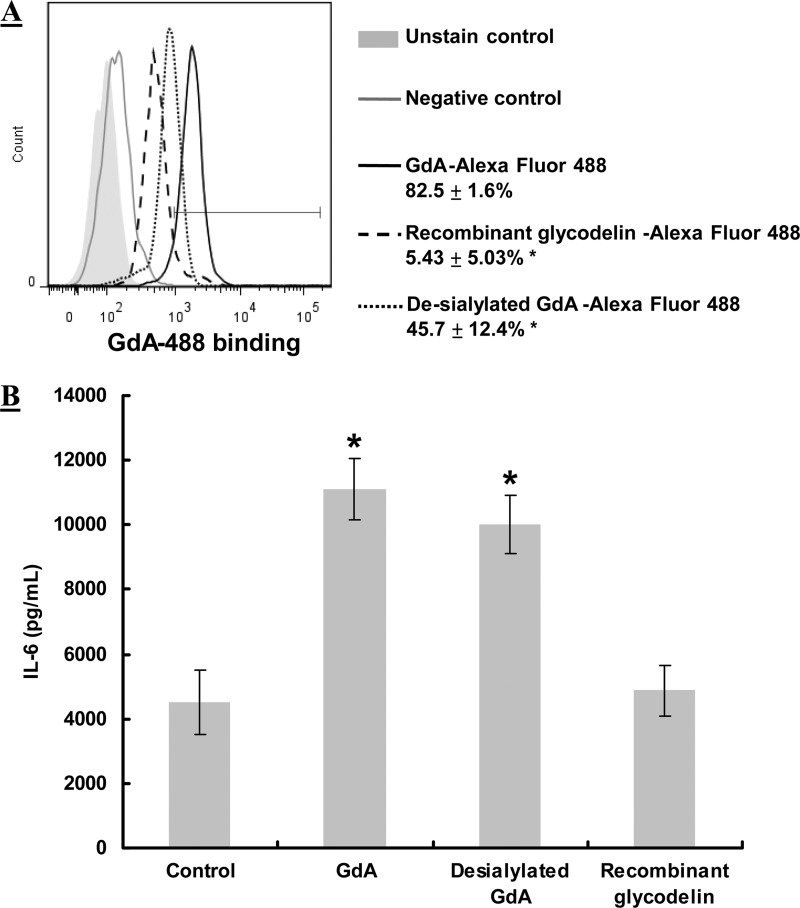
Sialylation of GdA Is Not Needed for GdA-induced IL-6 Secretion
Sialylation mediates the binding and a number of biological activities of GdA in various cell types (2, 12–14). Here, we investigated the role of sialylation in GdA-induced IL-6 production in monocytes. Although desialylation significantly (p < 0.05) reduced the binding of GdA to monocytes (Fig. 4A), desialylated GdA had a similar stimulatory effect on IL-6 secretion compared with native GdA (Fig. 4B). In contrast, non-glycosylated recombinant glycodelin from E. coli showed very low or no binding to monocytes and did not stimulate IL-6 secretion (p < 0.05).
FIGURE 4.
Effect of desialylation on GdA binding to and activities of monocytes. A, flow cytometric analysis of the binding of normal GdA, desialylated GdA, and recombinant glycodelin to monocytes (n = 5). Monocytes were incubated with Alexa Fluor 488-conjugated GdA or desialylated GdA for 2 h. An unrelated protein (Alexa Fluor 488-labeled goat IgG) was used as a negative control. *, p < 0.05 compared with normal GdA. B, IL-6 production in monocytes treated with GdA, desialylated GdA, and recombinant glycodelin (n = 6). Monocytes (5 × 105) were incubated with 10 μg/ml GdA and desialylated GdA for 48 h. IL-6 secretion into the culture medium was measured by ELISA. *, p < 0.05 compared with the control without treatment. Data are the mean ± S.E.
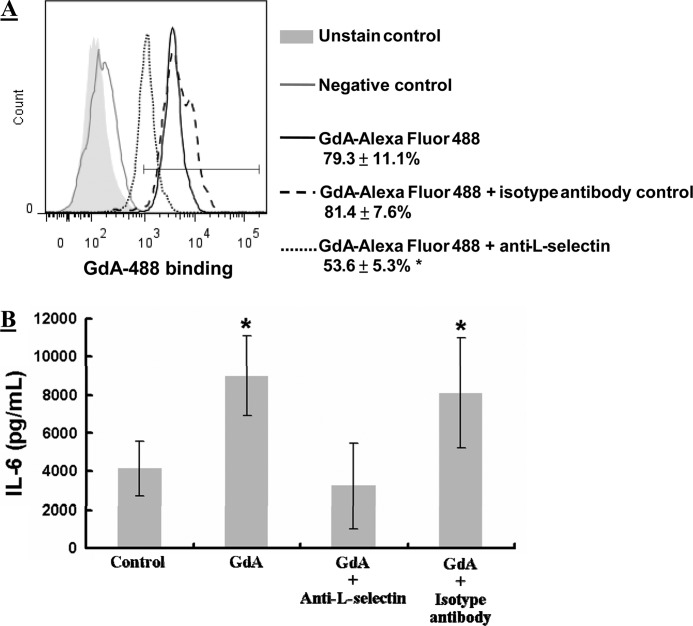
Anti-L-selectin Antibody Blocks GdA Binding and GdA-induced IL-6 Secretion
Flow cytometric analysis demonstrated surface expression of L-selectin in monocytes, THP-1 cells, and macrophages (supplemental Fig. S5), consistent with previous studies (23, 24). The inclusion of anti-L-selectin antibody significantly (p < 0.05) reduced the binding of fluorescently labeled GdA to monocytes (Fig. 5A) and abolished the GdA-induced IL-6 secretion (Fig. 5B). In contrast, no effect was observed when an isotype control antibody was used.
FIGURE 5.
Effect of anti-L-selectin antibody on GdA binding to and IL-6 production in monocytes. A, flow cytometric analysis of the binding of GdA to monocytes in the presence or absence of anti-L-selectin antibody (n = 4). Monocytes were incubated with Alexa Fluor 488-conjugated GdA in the presence of a 5-fold molar excess of anti-L-selectin antibody or isotype antibody for 2 h. An unrelated protein (Alexa Fluor 488-labeled goat IgG) was used as a negative control. *, p < 0.05 compared with GdA alone. B, IL-6 production in monocytes upon GdA treatment with or without anti-L-selectin antibody (n = 6). Monocytes were incubated with GdA in the presence of a 5-fold molar excess of anti-L-selectin antibody or isotype antibody for 48 h. IL-6 secretion into the culture medium was measured by ELISA. *, p < 0.05 compared with the control without treatment. Data are the mean ± S.E.
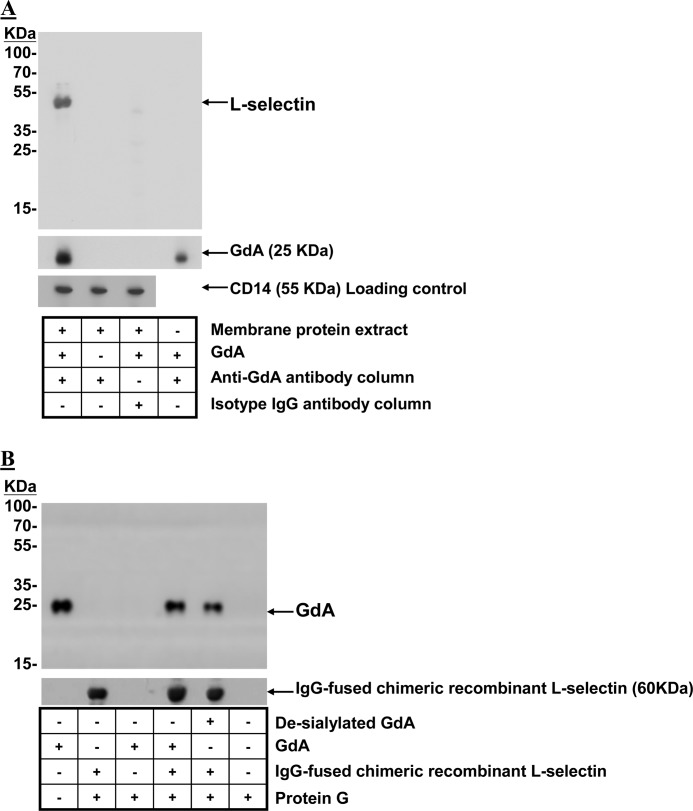
L-selectin Interacts with GdA
Co-immunoprecipitation confirmed the interaction between L-selectin and GdA. L-selectin immunoreactivity was detected in the immunoprecipitated complex from membrane protein extracts of monocytes (Fig. 6A). No L-selectin immunoreactivity was observed when GdA was omitted during co-immunoprecipitation or GdA-treated membrane protein extracts were immunoprecipitated by isotope antibody-conjugated beads. The results were further confirmed by repeating the experiment using recombinant L-selectin (Fig. 6B). The analysis showed that protein G did not interact with GdA and that anti-glycodelin antibody did not cross-react with L-selectin. Desialylated GdA was able to co-immunoprecipitate with L-selectin and to a similar extent as native GdA (Fig. 6B).
FIGURE 6.
Interaction between GdA and L-selectin. A, GdA-treated membrane protein extracts from monocytes were co-immunoprecipitated with anti-glycodelin antibody-conjugated Sepharose beads. Western blotting was performed on the eluted fractions with anti-L-selectin and anti-glycodelin antibodies. Membrane protein extracts without GdA treatment or co-immunoprecipitated with isotope antibody-conjugated Sepharose beads after GdA treatment served as controls. Equal loading of membrane protein extracts onto the Sepharose beads was confirmed by Western blotting of the flow-through fractions using anti-CD14 antibodies. Representative Western blots from four individual experiments are shown. B, IgG-fused recombinant human L-selectin chimeric proteins were incubated with native or desialylated GdA in PBS at 4 °C. Protein G-Sepharose beads were used to precipitate the GdA-recombinant L-selectin complex. The captured complex was resolved by SDS-PAGE and analyzed by Western blotting using anti-L-selectin or anti-glycodelin antibody. Representative Western blots from four individual experiments are shown.
GdA-treated Macrophages Suppress Intracellular Th-1 Cytokine Production in T-helper Cells
GdA-treated macrophages had significantly (p < 0.05, n = 4) higher IL-6 production (3252.4 ± 332.8 pg/ml) compared with the control (797.1 ± 222.5 pg/ml), even 42 h after the removal of GdA. Th-1 (IFN-γ), Th-2 (IL-4 and IL-10), and Th-17 (IL-17) cytokine production in T-helper cells was determined by intracellular staining (Fig. 7). Macrophages significantly (p < 0.05) stimulated IFN-γ, IL-4, IL-10, and IL-17 production in the co-cultured T-helper cells. This stimulatory effect of macrophages on Th-1 cytokine IFN-γ production was suppressed by GdA treatment. The inclusion of anti-IL-6 antibody abolished the suppressive activity of GdA (Fig. 7). In contrast, GdA pretreatment had no effect on the macrophage-induced Th-2 and Th-17 cytokine production in T-helper cells.
FIGURE 7.
Effects of GdA-induced IL-6 production in macrophages on IFN-γ, IL-4, IL-10, and IL-17 cytokine production in T-helper cells. Macrophages were treated with GdA (10 μg/ml) for 48 h and washed before co-culture with autologous T-helper cells in a co-culture insert with or without anti-IL-6 antibody for 42 h. Phorbol myristate acetate (50 ng/ml), ionomycin (1 μg/ml), and brefeldin A (3 μg/ml) were then added to activate the T-helper cells after removal of the macrophages. Intracellular IFN-γ, IL-4, IL-10, and IL-17 cytokine stainings were performed on the T-helper cells. Data are the mean ± S.E. (n = 4). *, p < 0.05 compared with the T-helper cell/macrophage co-culture group.
DISCUSSION
Our results show that native GdA binds to peripheral blood monocytes, in keeping with a previous observation on the binding of GdA from first-trimester decidual tissue to a monocytic cell line, U937 (25). We also provide the first demonstration of the binding of native GdA to the GM-CSF-differentiated macrophages. Little is known about the actions of GdA on macrophages. There is only one study showing a lack of effect of GdA on the apoptosis and phagocytic activity of phorbol myristate acetate-differentiated macrophages (26), consistent with our results. However, phorbol myristate acetate is not a physiological stimulator of monocyte differentiation (27). More importantly, phorbol myristate acetate-differentiated macrophages are different from blood macrophages in terms of expression of macrophage markers, functional characteristics (28), and plasticity to stimulus-directed polarization and tolerance to apoptosis (29).
The reported effects of glycodelin on monocytes are variable. Whereas Mukhopadhyay et al. (30) found that recombinant glycodelin did not affect apoptosis of U937 cells, another study using recombinant glycodelin from yeast reported induction of apoptotic changes in U937 cells and primary monocytes (31). Consistent with this latter study, native GdA has been shown to induce the apoptosis of primary monocytes (26). However, our results show that native GdA neither affects the viability of monocytes nor induces their cell death. These contradictory results from various investigators may be due to different sources of glycodelin used in their studies. In the most recent studies, recombinant glycodelin produced from yeast or a baculoviral system was used. These recombinant glycodelin isoforms have different glycosylation compared with native GdA (32). Another explanation may be the use of different cell preparation procedures. For example, whereas negative selection was used for the isolation of blood monocytes in our study, positive selection using anti-CD14 antibody was employed in another study (26). Some separation procedures may activate leukocytes (33), and selection of monocytes with anti-CD14 antibody has been reported to activate monocytes (34).
GdA up-regulates IL-6 production in monocytes and macrophages. IL-6 is expressed in the decidua and placenta (35, 36). Interestingly, GdA is abundant in the human decidua. Because decidual monocytes/macrophages produce an increased amount of IL-6 (37, 38), this may be accounted for by GdA stimulation. Apart from decidua, GdA also enhances IL-6 production in natural killer cells (5) and endometrial cells (39). IL-6 plays an important role in the development and growth of the fetoplacental unit (40–43). It also regulates trophoblast invasion by increasing the activity of matrix metalloproteinase-2 and -9 (41) and induces chemotaxis (44), cell migration (42), and chorionic gonadotropin production (43) of trophoblasts. On the other hand, IL-6 deficiency is associated with pregnancy disorders such as first-trimester spontaneous abortion (36) and preeclampsia (45). Thus, GdA-induced IL-6 up-regulation in monocytes/macrophages may be one of the mechanisms that enhance early placental development.
Proper balance among the activities of and cytokine secretion by Th-1, Th-2, and Th-17 cells is important for maintenance of pregnancy (46). We have previously reported that GdA modulates cytokine production in T-cells by preferential induction of the death of Th-1 cells (6), thereby inducing a Th-2-dominated cytokine environment that favors immunotolerance of the fetoplacental unit (47). The present data demonstrate that GdA can further suppress the production of IFN-γ, a Th-1-type cytokine, in T-helper cells indirectly via its stimulatory effect on macrophage-derived IL-6 production. IL-6 has been reported to promote the differentiation of Th-2-type cells, whereas it inhibits that of Th-1-type cells (48). IFN-γ is a proinflammatory cytokine that acts as the major contributor in the Th-1-type response. Excess maternal inflammatory response causes accumulation of neutrophils and macrophages in the decidua, up-regulation of inflammatory cytokines, and tissue damage by apoptosis (46, 49, 50). Increased IFN-γ has been linked with fetal loss, recurrent miscarriage, and gestational complications such as preeclampsia and premature delivery (46, 49, 51). Thus, GdA may improve fetal survival and contribute to the maintenance of pregnancy by promoting a Th-2 cytokine-dominant environment via its regulatory effect on various types of immune cells.
Three signaling pathways, namely p38 (52, 53), NF-κB (54), and ERK (53) signaling pathways, are associated with the regulation of IL-6 production in monocytes and macrophages. Two observations indicate that the ERK pathway mediates the GdA-induced IL-6 production in these cells. First, GdA activates ERK in monocytes and macrophages similar to its action on ERK activity in other cell types (6, 14, 55). Second, only inhibitors of the ERK pathway, but not of the other pathways studied, could abolish the GdA-induced IL-6 production. However, signaling pathway(s) other than ERK may also be involved in the regulation of GdA-induced IL-6 secretion. For example, our results showed that there is a trend of decreasing GdA-induced IL-6 production by NF-κB inhibitor, although it is not statistically significant (Fig. 3B). Further study is required to confirm the role of NF-κB in the activity of GdA.
Glycosylation of GdA is critical for the binding and biological activities of this glycoprotein in different cell types (2, 12–14). We demonstrated that non-glycosylated glycodelin has a minimal binding capacity and IL-6-inducing activity in monocytes/macrophages. Sialic acid is the terminal carbohydrate residue in many exposed N-glycans, and it often contributes to the binding of glycoproteins to their receptors (56). The sialic acid moieties are involved in the apoptosis-inducing action of GdA on T-cells (57). GdA, but not non-sialylated glycodelin-S, which differs from GdA only by glycosylation, possesses immunosuppressive activities on lymphocytes, and such activities are abolished after desialylation of GdA (12, 58). We recently demonstrated that GdA binds to siglec-6 in a sialic acid-dependent manner to suppress trophoblast invasion (14, 59). In this study, although desialylated GdA showed reduced binding to monocytes, it induced IL-6 production in monocytes similar to native GdA. These observations indicate that, in addition to sialic acid-binding molecule(s), another carbohydrate-binding protein(s) is involved in the GdA-monocyte/macrophage interaction (see below). This is further supported by the observation that desialylation cannot completely abolish the ability of GdA to bind to monocytes/macrophages. Multiple binding proteins/receptors of GdA have also been found in human spermatozoa and in the trophoblast (14, 60, 61). The sialic acid-binding protein responsible for GdA binding is still unknown. Siglec-6 (14) has been found to be a sialic acid-dependent receptor of GdA on the human trophoblast. Siglec family members appear in the monocytes/macrophages and may play an important role in their cellular functions (62). The possible interaction between monocyte/macrophage-derived siglec and GdA remains to be proven.
In this study, our aim was to find a sialic acid-independent binding partner of GdA, which mediates the IL-6-inducing activity on monocytes/macrophages. Selectins are a family of adhesion and signaling molecules expressed by most leukocytes (63–66). They bind to the carbohydrate moieties of target proteins. The physiological ligands of selectins include sialyl-Lewis X, Lewis X, and Gal-GlcNAc glycan epitopes (Consortium for Functional Glycomics). In this study, several observations support the presence of a sialic acid-independent interaction of GdA with L-selectin. (i) L-selectin was expressed on the surface of monocytes/macrophage. (ii) L-selectin was immunoprecipitated by GdA from monocytic membrane extract. (iii) Both native and desialylated GdA were immunoprecipitated with recombinant L-selectin. (iv) Anti-L-selectin antibody inhibited GdA binding to monocytes. (v) GdA possesses Lewis X and Gal-GlcNAc epitopes known to be recognized by L-selectin (12). (vi) GdA has no sialyl-Lewis X epitope (12).
Selectins were originally identified as cell adhesion molecules. Recently, selectin was shown to act as a signaling molecule (63–66). L-selectin activation induces calcium flux (67, 68), tyrosine phosphorylation (69), and ERK activation (69–71) in immune cells. Our results consistently showed that anti-L-selectin antibody abolished the GdA-induced IL-6 secretion.
To conclude, our data suggest that GdA binds to L-selectin and induces IL-6 expression through ERK activation in monocytes/macrophages. In turn, IL-6 suppresses IFN-γ secretion by T-helper cells, which may play an important role in the maintenance of pregnancy. Aberrant macrophage activity is associated with fetal loss and pregnancy complications (9). Further elucidation of the biological functions of GdA, particularly its mechanisms of action on monocytes and macrophages, will further the understanding of the pathophysiology and may provide the basis for the development of new treatment strategies.
This work was supported in part by University of Hong Kong Grant 201007176033, Hong Kong Research Grant Council Grant HKU774212, the Helsinki University Central Hospital Research Fund, and the Academy of Finland.

This article contains supplemental Figs. S1–S5 and Tables S1 and S2.
- GdA
- glycodelin-A.
REFERENCES
- 1. Seppälä M., Taylor R. N., Koistinen H., Koistinen R., Milgrom E. (2002) Glycodelin: a major lipocalin protein of the reproductive axis with diverse actions in cell recognition and differentiation. Endocr. Rev. 23, 401–430 [DOI] [PubMed] [Google Scholar]
- 2. Lee C. L., Lam K. K., Koistinen H., Seppälä M., Kurpisz M., Fernandez N., Pang R. T., Yeung W. S., Chiu P. C. (2011) Glycodelin-A as a paracrine regulator in early pregnancy. J. Reprod. Immunol. 90, 29–34 [DOI] [PubMed] [Google Scholar]
- 3. Yaniv E., Borovsky Z., Mishan-Eisenberg G., Rachmilewitz J. (2003) Placental protein 14 regulates selective B-cell responses. Cell. Immunol. 222, 156–163 [DOI] [PubMed] [Google Scholar]
- 4. Scholz C., Toth B., Brunnhuber R., Rampf E., Weissenbacher T., Santoso L., Friese K., Jeschke U. (2008) Glycodelin-A induces a tolerogenic phenotype in monocyte-derived dendritic cells in vitro. Am. J. Reprod. Immunol. 60, 501–512 [DOI] [PubMed] [Google Scholar]
- 5. Lee C. L., Chiu P. C., Lam K. K., Chan R. W., Chu I. K., Koistinen R., Koistinen H., Seppälä M., Lee K. F., Yeung W. S. (2010) Glycodelin-A modulates cytokine production of peripheral blood natural killer cells. Fertil. Steril. 94, 769–771 [DOI] [PubMed] [Google Scholar]
- 6. Lee C. L., Chiu P. C., Lam K. K., Siu S. O., Chu I. K., Koistinen R., Koistinen H., Seppälä M., Lee K. F., Yeung W. S. (2011) Differential actions of glycodelin-A on Th-1 and Th-2 cells: a paracrine mechanism that could produce the Th-2-dominant environment during pregnancy. Hum. Reprod. 26, 517–526 [DOI] [PubMed] [Google Scholar]
- 7. Mishan-Eisenberg G., Borovsky Z., Weber M. C., Gazit R., Tykocinski M. L., Rachmilewitz J. (2004) Differential regulation of Th-1/Th-2 cytokine responses by placental protein 14. J. Immunol. 173, 5524–5530 [DOI] [PubMed] [Google Scholar]
- 8. Mackenna A., Li T. C., Dalton C., Bolton A., Cooke I. (1993) Placental protein 14 levels in uterine flushing and plasma of women with unexplained infertility. Fertil. Steril. 59, 577–582 [DOI] [PubMed] [Google Scholar]
- 9. Mor G., Abrahams V. M. (2003) Potential role of macrophages as immunoregulators of pregnancy. Reprod. Biol. Endocrinol. 1, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Renaud S. J., Graham C. H. (2008) The role of macrophages in utero-placental interactions during normal and pathological pregnancy. Immunol. Invest. 37, 535–564 [DOI] [PubMed] [Google Scholar]
- 11. Duclos A. J., Haddad E. K., Baines M. G. (1995) Presence of activated macrophages in a murine model of early embryo loss. Am. J. Reprod. Immunol. 33, 354–366 [DOI] [PubMed] [Google Scholar]
- 12. Lee C. L., Pang P. C., Yeung W. S., Tissot B., Panico M., Lao T. T., Chu I. K., Lee K. F., Chung M. K., Lam K. K., Koistinen R., Koistinen H., Seppälä M., Morris H. R., Dell A., Chiu P. C. (2009) Effects of differential glycosylation of glycodelins on lymphocyte survival. J. Biol. Chem. 284, 15084–15096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chiu P. C., Chung M. K., Koistinen R., Koistinen H., Seppälä M., Ho P. C., Ng E. H., Lee K. F., Yeung W. S. (2007) Glycodelin-A interacts with fucosyltransferase on human sperm plasma membrane to inhibit spermatozoa-zona pellucida binding. J. Cell Sci. 120, 33–44 [DOI] [PubMed] [Google Scholar]
- 14. Lam K. K., Chiu P. C., Lee C. L., Pang R. T., Leung C. O., Koistinen H., Seppälä M., Ho P. C., Yeung W. S. (2011) Glycodelin-A protein interacts with siglec-6 protein to suppress trophoblast invasiveness by down-regulating extracellular signal-regulated kinase (ERK)/c-Jun signaling pathway. J. Biol. Chem. 286, 37118–37127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee C. L., Chiu P. C., Pang P. C., Chu I. K., Lee K. F., Koistinen R., Koistinen H., Seppälä M., Morris H. R., Tissot B., Panico M., Dell A., Yeung W. S. (2011) Glycosylation failure extends to glycoproteins in gestational diabetes mellitus: evidence from reduced α2–6 sialylation and impaired immunomodulatory activities of pregnancy-related glycodelin-A. Diabetes 60, 909–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Riittinen L., Julkunen M., Seppälä M., Koistinen R., Huhtala M. L. (1989) Purification and characterization of endometrial protein PP14 from mid-trimester amniotic fluid. Clin. Chim. Acta 184, 19–29 [DOI] [PubMed] [Google Scholar]
- 17. So K. H., Lee C. L., Yeung W. S., Lee K. F. (2012) Glycodelin suppresses endometrial cell migration and invasion but stimulates spheroid attachment. Reprod. Biomed. Online 24, 639–645 [DOI] [PubMed] [Google Scholar]
- 18. Hashimoto S., Suzuki T., Dong H. Y., Yamazaki N., Matsushima K. (1999) Serial analysis of gene expression in human monocytes and macrophages. Blood 94, 837–844 [PubMed] [Google Scholar]
- 19. Martínez E., Sureda A., Dalmases C. D., Sánchez J. A., Amill B., Tugues D., Sardá P., Miralles A., Brunet S., Domingo-Albós A., García J. (1996) Mobilization of peripheral blood progenitor cells by cyclophosphamide and rhGM-CSF in multiple myeloma. Bone Marrow Transplant. 18, 1–7 [PubMed] [Google Scholar]
- 20. Lehtonen A., Ahlfors H., Veckman V., Miettinen M., Lahesmaa R., Julkunen I. (2007) Gene expression profiling during differentiation of human monocytes to macrophages or dendritic cells. J. Leukoc. Biol. 82, 710–720 [DOI] [PubMed] [Google Scholar]
- 21. Ulevitch R. J. (1993) Recognition of bacterial endotoxins by receptor-dependent mechanisms. Adv. Immunol. 53, 267–289 [DOI] [PubMed] [Google Scholar]
- 22. Vellenga E., Tuyt L., Wierenga B. J., Müller M., Dokter W. (1999) Interleukin-6 production by activated human monocytic cells is enhanced by MK-571, a specific inhibitor of the multidrug resistance protein-1. Br. J. Pharmacol. 127, 441–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Prieto J., Eklund A., Patarroyo M. (1994) Regulated expression of integrins and other adhesion molecules during differentiation of monocytes into macrophages. Cell. Immunol. 156, 191–211 [DOI] [PubMed] [Google Scholar]
- 24. Haugen T. S., Skjonsberg O. H., Nakstad B., Lyberg T. (1999) Modulation of adhesion molecule profiles on alveolar macrophages and blood leukocytes. Respiration 66, 528–537 [DOI] [PubMed] [Google Scholar]
- 25. Vigne J. L., Hornung D., Mueller M. D., Taylor R. N. (2001) Purification and characterization of an immunomodulatory endometrial protein, glycodelin. J. Biol. Chem. 276, 17101–17105 [DOI] [PubMed] [Google Scholar]
- 26. Alok A., Mukhopadhyay D., Karande A. A. (2009) Glycodelin-A, an immunomodulatory protein in the endometrium, inhibits proliferation and induces apoptosis in monocytic cells. Int. J. Biochem. Cell Biol. 41, 1138–1147 [DOI] [PubMed] [Google Scholar]
- 27. Tseng S. S., Van Duuren B. L., Solomon J. J. (1977) Synthesis of 4aα-phorbol 9-myristate 9a-acetate and related esters. J. Org. Chem. 42, 3645–3649 [DOI] [PubMed] [Google Scholar]
- 28. Kohro T., Tanaka T., Murakami T., Wada Y., Aburatani H., Hamakubo T., Kodama T. (2004) A comparison of differences in the gene expression profiles of phorbol 12-myristate 13-acetate-differentiated THP-1 cells and human monocyte-derived macrophage. J. Atheroscler. Thromb. 11, 88–97 [DOI] [PubMed] [Google Scholar]
- 29. Daigneault M., Preston J. A., Marriott H. M., Whyte M. K., Dockrell D. H. (2010) The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS ONE 5, e8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mukhopadhyay D., Sundereshan S., Rao C., Karande A. A. (2001) Placental protein 14 induces apoptosis in T-cells but not in monocytes. J. Biol. Chem. 276, 28268–28273 [DOI] [PubMed] [Google Scholar]
- 31. Tee M. K., Vigne J. L., Yu J., Taylor R. N. (2008) Natural and recombinant human glycodelin activate a pro-apoptotic gene cascade in monocyte cells. J. Leukoc. Biol. 83, 843–852 [DOI] [PubMed] [Google Scholar]
- 32. Seppälä M., Koistinen H., Koistinen R., Chiu P. C., Yeung W. S. (2007) Glycosylation-related actions of glycodelin: gamete, cumulus cell, immune cell, and clinical associations. Hum. Reprod. Update 13, 275–287 [DOI] [PubMed] [Google Scholar]
- 33. Link A., Hummel B., Schwerdt H., Schwamborn J., Jung F., Schieffer H. (1997) Influence of neutrophil separation on the expression of adhesion molecules. Clin. Hemorheol. Microcirc. 17, 175–180 [PubMed] [Google Scholar]
- 34. Breckpot K., Corthals J., Heirman C., Bonehill A., Michiels A., Tuyaerts S., De Greef C., Thielemans K. (2004) Activation of monocytes via the CD14 receptor leads to the enhanced lentiviral transduction of immature dendritic cells. Hum. Gene Ther. 15, 562–573 [DOI] [PubMed] [Google Scholar]
- 35. Jauniaux E., Gulbis B., Schandene L., Collette J., Hustin J. (1996) Distribution of interleukin-6 in maternal and embryonic tissues during the first trimester. Mol. Hum. Reprod. 2, 239–243 [DOI] [PubMed] [Google Scholar]
- 36. Dimitriadis E., White C. A., Jones R. L., Salamonsen L. A. (2005) Cytokines, chemokines, and growth factors in endometrium related to implantation. Hum. Reprod. Update 11, 613–630 [DOI] [PubMed] [Google Scholar]
- 37. Li C., Houser B. L., Nicotra M. L., Strominger J. L. (2009) HLA-G homodimer-induced cytokine secretion through HLA-G receptors on human decidual macrophages and natural killer cells. Proc. Natl. Acad. Sci. U.S.A. 106, 5767–5772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Houser B. L., Tilburgs T., Hill J., Nicotra M. L., Strominger J. L. (2011) Two unique human decidual macrophage populations. J. Immunol. 186, 2633–2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Laird S. M., Tuckerman E., Li T. C., Bolton A. E. (1994) Stimulation of human endometrial epithelial cell interleukin-6 production by interleukin-1 and placental protein 14. Hum. Reprod. 9, 1339–1343 [DOI] [PubMed] [Google Scholar]
- 40. Silver R. M., Schwinzer B., McGregor J. A. (1993) Interleukin-6 levels in amniotic fluid in normal and abnormal pregnancies: preeclampsia, small-for-gestational-age fetus, and premature labor. Am. J. Obstet. Gynecol. 169, 1101–1105 [DOI] [PubMed] [Google Scholar]
- 41. Meisser A., Cameo P., Islami D., Campana A., Bischof P. (1999) Effects of interleukin-6 (IL-6) on cytotrophoblastic cells. Mol. Hum. Reprod. 5, 1055–1058 [DOI] [PubMed] [Google Scholar]
- 42. Smith S. D., Dunk C. E., Aplin J. D., Harris L. K., Jones R. L. (2009) Evidence for immune cell involvement in decidual spiral arteriole remodeling in early human pregnancy. Am. J. Pathol. 174, 1959–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nishino E., Matsuzaki N., Masuhiro K., Kameda T., Taniguchi T., Takagi T., Saji F., Tanizawa O. (1990) Trophoblast-derived interleukin-6 (IL-6) regulates human chorionic gonadotropin release through IL-6 receptor on human trophoblasts. J. Clin. Endocrinol. Metab. 71, 436–441 [DOI] [PubMed] [Google Scholar]
- 44. Dominguez F., Martínez S., Quiñonero A., Loro F., Horcajadas J. A., Pellicer A., Simón C. (2008) CXCL10 and IL-6 induce chemotaxis in human trophoblast cell lines. Mol. Hum. Reprod. 14, 423–430 [DOI] [PubMed] [Google Scholar]
- 45. Zhao S., Gu Y., Dong Q., Fan R., Wang Y. (2008) Altered interleukin-6 receptor, IL-6R and gp130, production and expression and decreased SOCS-3 expression in placentas from women with preeclampsia. Placenta 29, 1024–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Saito S., Nakashima A., Shima T., Ito M. (2010) Th-1/Th-2/Th-17 and regulatory T-cell paradigm in pregnancy. Am. J. Reprod. Immunol. 63, 601–610 [DOI] [PubMed] [Google Scholar]
- 47. Piccinni M. P. (2007) Role of T-cell cytokines in decidua and in cumulus oophorus during pregnancy. Gynecol. Obstet. Invest. 64, 144–148 [DOI] [PubMed] [Google Scholar]
- 48. Diehl S., Rincón M. (2002) The two faces of IL-6 on Th-1/Th-2 differentiation. Mol. Immunol. 39, 531–536 [DOI] [PubMed] [Google Scholar]
- 49. Challis J. R., Lockwood C. J., Myatt L., Norman J. E., Strauss J. F., 3rd, Petraglia F. (2009) Inflammation and pregnancy. Reprod. Sci. 16, 206–215 [DOI] [PubMed] [Google Scholar]
- 50. Murphy S. P., Tayade C., Ashkar A. A., Hatta K., Zhang J., Croy B. A. (2009) Interferon γ in successful pregnancies. Biol. Reprod. 80, 848–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Laird S. M., Tuckerman E. M., Cork B. A., Linjawi S., Blakemore A. I., Li T. C. (2003) A review of immune cells and molecules in women with recurrent miscarriage. Hum. Reprod. Update 9, 163–174 [DOI] [PubMed] [Google Scholar]
- 52. Kim J. H., Studer R. K., Vo N. V., Sowa G. A., Kang J. D. (2009) p38 MAPK inhibition selectively mitigates inflammatory mediators and VEGF production in AF cells co-cultured with activated macrophage-like THP-1 cells. Osteoarthritis Cartilage 17, 1662–1669 [DOI] [PubMed] [Google Scholar]
- 53. Olsnes C., Olofsson J., Aarstad H. J. (2011) MAPKs ERK and p38, but not JNK phosphorylation, modulate IL-6 and TNF-α secretion following OK-432 in vitro stimulation of purified human monocytes. Scand. J. Immunol. 74, 114–125 [DOI] [PubMed] [Google Scholar]
- 54. Libermann T. A., Baltimore D. (1990) Activation of interleukin-6 gene expression through the NF-κB transcription factor. Mol. Cell. Biol. 10, 2327–2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chiu P. C., Wong B. S., Lee C. L., Lam K. K., Chung M. K., Lee K. F., Koistinen R., Koistinen H., Gupta S. K., Seppälä M., Yeung W. S. (2010) Zona pellucida-induced acrosome reaction in human spermatozoa is potentiated by glycodelin-A via down-regulation of extracellular signal-regulated kinases and up-regulation of zona pellucida-induced calcium influx. Hum. Reprod. 25, 2721–2733 [DOI] [PubMed] [Google Scholar]
- 56. Varki A. (2009) Essentials of Glycobiology, 2nd Ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY: [PubMed] [Google Scholar]
- 57. Rachmilewitz J., Borovsky Z., Riely G. J., Miller R., Tykocinski M. L. (2003) Negative regulation of T-cell activation by placental protein 14 is mediated by the tyrosine phosphatase receptor CD45. J. Biol. Chem. 278, 14059–14065 [DOI] [PubMed] [Google Scholar]
- 58. Poornima B. L., Karande A. A. (2007) Differential sialylation regulates the apoptotic activity of glycodelin-A. FEBS Lett. 581, 4366–4370 [DOI] [PubMed] [Google Scholar]
- 59. Lam K. K., Chiu P. C., Chung M. K., Lee C. L., Lee K. F., Koistinen R., Koistinen H., Seppälä M., Ho P. C., Yeung W. S. (2009) Glycodelin-A as a modulator of trophoblast invasion. Hum. Reprod. 24, 2093–2103 [DOI] [PubMed] [Google Scholar]
- 60. Chiu P. C., Chung M. K., Koistinen R., Koistinen H., Seppälä M., Ho P. C., Ng E. H., Lee K. F., Yeung W. S. (2007) Cumulus oophorus-associated glycodelin-C displaces sperm-bound glycodelin-A and -F and stimulates spermatozoa-zona pellucida binding. J. Biol. Chem. 282, 5378–5388 [DOI] [PubMed] [Google Scholar]
- 61. Yeung W. S., Lee K. F., Koistinen R., Koistinen H., Seppälä M., Chiu P. C. (2009) Effects of glycodelins on functional competence of spermatozoa. J. Reprod. Immunol. 83, 26–30 [DOI] [PubMed] [Google Scholar]
- 62. Crocker P. R., Paulson J. C., Varki A. (2007) Siglecs and their roles in the immune system. Nat. Rev. Immunol. 7, 255–266 [DOI] [PubMed] [Google Scholar]
- 63. Patel K. D., Cuvelier S. L., Wiehler S. (2002) Selectins: critical mediators of leukocyte recruitment. Semin. Immunol. 14, 73–81 [DOI] [PubMed] [Google Scholar]
- 64. Juliano R. L. (2002) Signal transduction by cell adhesion receptors and the cytoskeleton: functions of integrins, cadherins, selectins, and immunoglobulin superfamily members. Annu. Rev. Pharmacol. Toxicol. 42, 283–323 [DOI] [PubMed] [Google Scholar]
- 65. Crockett-Torabi E. (1998) Selectins and mechanisms of signal transduction. J. Leukoc. Biol. 63, 1–14 [PubMed] [Google Scholar]
- 66. Grailer J. J., Kodera M., Steeber D. A. (2009) L-selectin: role in regulating homeostasis and cutaneous inflammation. J. Dermatol. Sci. 56, 141–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Laudanna C., Constantin G., Baron P., Scarpini E., Scarlato G., Cabrini G., Dechecchi C., Rossi F., Cassatella M. A., Berton G. (1994) Sulfatides trigger increase of cytosolic free calcium and enhanced expression of tumor necrosis factor-α and interleukin-8 mRNA in human neutrophils. Evidence for a role of L-selectin as a signaling molecule. J. Biol. Chem. 269, 4021–4026 [PubMed] [Google Scholar]
- 68. Po J. L., Mazer B., Jensen G. S. (1995) The L-selectin antibody FMC46 mediates rapid, transient increase in intracellular calcium in human peripheral blood mononuclear cells and Daudi lymphoma cells. Biochem. Biophys. Res. Commun. 217, 1145–1150 [DOI] [PubMed] [Google Scholar]
- 69. Waddell T. K., Fialkow L., Chan C. K., Kishimoto T. K., Downey G. P. (1995) Signaling functions of L-selectin. Enhancement of tyrosine phosphorylation and activation of MAP kinase. J. Biol. Chem. 270, 15403–15411 [DOI] [PubMed] [Google Scholar]
- 70. Smolen J. E., Petersen T. K., Koch C., O'Keefe S. J., Hanlon W. A., Seo S., Pearson D., Fossett M. C., Simon S. I. (2000) L-selectin signaling of neutrophil adhesion and degranulation involves p38 mitogen-activated protein kinase. J. Biol. Chem. 275, 15876–15884 [DOI] [PubMed] [Google Scholar]
- 71. Brenner B., Gulbins E., Schlottmann K., Koppenhoefer U., Busch G. L., Walzog B., Steinhausen M., Coggeshall K. M., Linderkamp O., Lang F. (1996) L-selectin activates the Ras pathway via the tyrosine kinase p56lck. Proc. Natl. Acad. Sci. U.S.A. 93, 15376–15381 [DOI] [PMC free article] [PubMed] [Google Scholar]